Abstract
Interactions between bacterial microbiota and mosquitoes play an important role in mosquitoes’ capacity to transmit pathogens. However, microbiota assemblages within mosquitoes and the impact of microbiota in environments on mosquito development and survival remain unclear. This study examined microbiota assemblages and the effects of aquatic environment microbiota on the larval development of the Aedes albopictus mosquito, an important dengue virus vector. Life table studies have found that reducing bacterial load in natural aquatic habitats through water filtering and treatment with antibiotics significantly reduced the larva-to-adult emergence rate. This finding was consistent in two types of larval habitats examined—discarded tires and flowerpots, suggesting that bacteria play a crucial role in larval development. Pyrosequencing of the bacterial 16S rRNA gene was used to determine the diversity of bacterial communities in larval habitats and the resulting numbers of mosquitoes under both laboratory and field conditions. The microbiota profiling identified common shared bacteria among samples from different years; further studies are needed to determine whether these bacteria represent a core microbiota. The highest microbiota diversity was found in aquatic habitats, followed by mosquito larvae, and the lowest in adult mosquitoes. Mosquito larvae ingested their bacterial microbiota and nutrients from aquatic habitats of high microbiota diversity. Taken together, the results support the observation that Ae. albopictus larvae are able to utilize diverse bacteria from aquatic habitats and that live bacteria from aquatic habitats play an important role in larval mosquito development and survival. These findings provide new insights into bacteria’s role in mosquito larval ecology.
Keywords: 16S rRNA gene, Aedes albopictus, bacteria, microbiota
1 |. INTRODUCTION
The past several decades have seen a growing interest in the impact of microbiota—a collection of microbial populations that reside on and in the host—on the physiology, development, and reproduction of invertebrates and vertebrates. In insects, the natural bacterial microbiota (hereafter referred to as microbiota) has been shown to contribute to nutritional metabolism (Coon, Vogel, Brown, & Strand, 2014), immunity, and vector competence to parasites and pathogens (Engel & Moran, 2013; Jupatanakul, Sim, & Dimopoulos, 2014). For example, termite gut microbiota is critical to cellulose digestion, providing important carbon and nitrogen to the host, which is often deficient in decomposing plant diets (Sapountzis et al., 2016). In Drosophila, microbiota and the innate immune system interact closely, and the microbiota is required for antiviral defence (Sansone et al., 2015). The gut microbial community also plays an important role in inducing biological larvicides’ killing effects in the cotton leafworm (Caccia et al., 2016).
Because of the microbiota’s potentially important function in organismal physiology and development, extensive research has been conducted to characterize microbiota diversity in insects. In a number of insect species, the microbiota has been documented to be extremely diverse (Douglas, 2015; Engel & Moran, 2013; Manirajan et al., 2016; Minard, Mavingui, & Moro, 2013); however, the factors shaping insect microbiota composition and structure are not well understood. Insects acquire many of their gut microbiota from the natural environment (Strand, 2017), but the extent of microbiota fluctuations in the environment and their impact on insect micro-biota are largely unknown. Furthermore, the effects of environmental microbiota on insect development and reproduction are not clear (Dickson et al., 2017; Minard, Mavingui, et al., 2013). For example, Coon et al. (2014) reported that Aedes, Culex and Anopheles mosquito larvae required living bacteria for development, although larval development appeared not to depend on particular bacterial species. On the other hand, Chouaia et al. (2012) reported that An. stephensi mosquito larvae survived in rearing water treated with rifampicin and successfully developed into adults, though with a delay.
The bacterial microbiota has been examined in a number of mosquito species, including Ae. albopictus, Ae. aegypti, Ae. japonicus, Ae. triseriatus, An. gambiae, An. stephensi, Cx. pipiens, Cx. nigripalpus and Cx. quinquefasciatus. A variety of bacterial phyla were present in mosquito guts and bodies and shared among mosquito species, including Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria and Proteobacteria, though whether the bacteria shared by different mosquito species or by different mosquito populations of the same species are core bacteria remains unknown (see review by Guegan et al., 2018). Throughout their life cycle, mosquitoes continuously encounter microorganisms, particularly in the larval stage, where their aquatic habitat harbours a high diversity of bacteria. Some evidence suggests that hosts exert strong control over their microbiota through their innate immune response in insects (Martinson, Douglas, & Jaenike, 2017; Smith et al., 2015), but microbes may directly engage the immune system and actively shape beneficial host immune responses (Napflin & Schmid-Hempel, 2016). On the other hand, environmental factors, such as diet (Belda et al., 2011; Hu, Lukasik, Moreau, & Russell, 2014), and even microenvironmental factors such as gut intestinal pH, oxygen status and residence time of digesta play an important role in gut microbiota structure (Mikaelyan, Meuser, & Brune, 2017).
This study’s objective was to determine bacterial microbiota’s diversity and stability in larval habitats of the Asian tiger mosquito, Ae. albopictus, and the impact of microbiota on larval mosquito development. Aedes albopictus is an important vector of dengue, Zika (Liu et al., 2017), chikungunya (Bonilauri et al., 2008) and other viruses (Gratz, 2004). Aedes albopictus breeds primarily in artificial containers (e.g., tires, cemetery urns and water storage containers; Li et al., 2014). Due to its high physiological and ecological plasticity and ability to utilize containers, Ae. albopictus has spread globally from its native Asia during the past three decades, and is now considered to be the most invasive mosquito species in the world (Benedict, Levine, Hawley, & Lounibos, 2007; Bonizzoni, Gasperi, Chen, & James, 2013). Aedes albopictus spread across continents primarily through shipment of commodities, such as used tires and house plants, that contained stagnant water (Knudsen, 1995). We addressed three major questions: (a) Would bacterial clearance or abundance reduction through the use of antibiotics in larval habitats and through filtering of water of natural larval habitats inhibit or negatively affect larval mosquito development and survival? (b) Would the mosquito microbiota assemblage be modulated by environmental bacteria and mosquito physiological status, such as development stage and age? And (c) would microbiota fluctuation in larval habitats affect mosquito microbiota assemblage? Answers to these questions will provide important information about environmental modulations of mosquito microbiota and the role of microbiota in mosquito larval ecology. Such information may be valuable for the development of new vector control tools.
2 |. MATERIALS AND METHODS
2.1 |. Study sites
The study was conducted in Guangzhou (113°20′E, 23°10′N), Guangdong Province, China, and in Nabang (97°32′E, 24°45′N), Yingjiang County, Yunnan Province, China, on the China–Myanmar border area. For the past three decades, the two sites have been experiencing major dengue outbreaks, and Ae. albopictus is the major dengue vector species (Gratz, 2004; Wu, Lun, James, & Chen, 2010). During the experimental period (June to August), the annual average temperature in these two sites was comparable, ranging from 27.8 to 29.9°C, within the optimal temperature for mosquito development.
2.2 |. Ethics statement
No specific permits were required for the described field studies. For mosquito collection in residential areas, oral consent was obtained from homeowners in each location. These locations were not protected lands, and the field studies did not involve endangered or protected species.
2.3 |. Study design
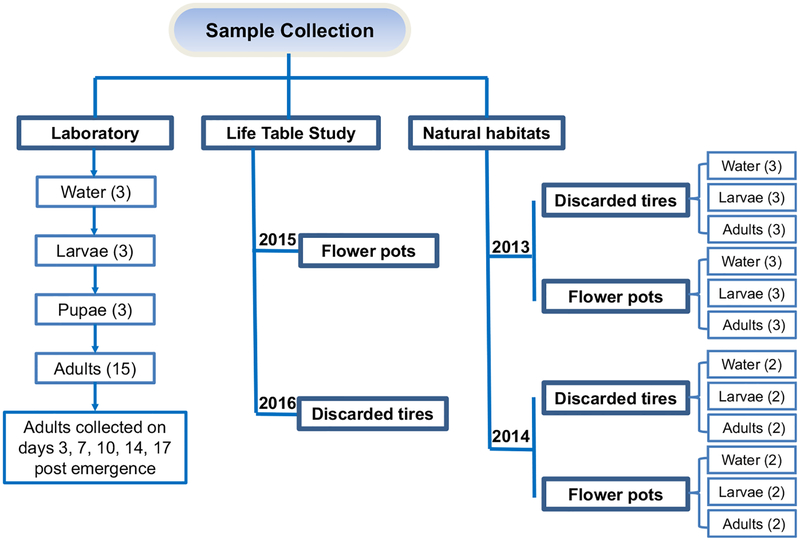
2.3.1 |. Life table studies under seminatural conditions
To examine habitat bacteria’s impact on Ae. albopictus larval development and survival, life table studies were conducted using microcosms under seminatural conditions in Guangzhou in 2015 and Nabang in 2016 (Figure 1). The microcosms were made of sterilized metal bowls (15 cm diameter and 5 cm deep) and 200 ml of water. In Guangzhou, we designed three treatments: (a) water from natural flowerpots; (b) natural flowerpot water filtered by a 0.22-μm Milli-pore filter, which should have filtered >95% bacteria (Wang, Hammes, Boon, & Egli, 2007); and (c) ampicillin added to water from natural flowerpots, with a final antibiotic concentration of 100 μg/ml. We used ampicillin as it is a wide-spectrum antibiotic and has been used in several studies on mosquito microbiota (Coon, Brown, & Strand, 2016; Coon et al., 2017). Fifty newly hatched larvae of the Ae. albopictus Foshan strain were added to each microcosm, and the number of surviving larvae was recorded daily until all larvae pupated or died. The Foshan Ae. albopictus strain was originated in Foshan city, Guangdong Province, and has been maintained in the laboratory since 1981. Each treatment had six replicates or 18 microcosms in total. To determine whether food limitations contributed to low larval survivorship in natural habitats, and to test the effect of bacterial depletion in larval habitats on larval survival in the context of larval food enrichment, we set up 18 additional microcosms by adding autoclaved mosquito larval food (brewer’s yeast Saccharomyces cerevisiae cells) to each treatment. Overall, a total of 36 microcosms were used.
TABLE 1.
Pseudo F table of PERMANOVA based on Bray–Curtis dissimilarities in the field Aedes albopictus populations
| Source of variance | df | Sum of squares | Mean square | F | R2 | P |
|---|---|---|---|---|---|---|
| Year | 1 | 0.751 | 0.751 | 4.141 | 0.086 | 0.057 |
| Development stage | 2 | 2.313 | 1.157 | 6.378 | 0.263 | 0.008 |
| Habitat type | 1 | 0.274 | 0.274 | 1.510 | 0.031 | 0.235 |
| Year × Development stage | 2 | 0.972 | 0.486 | 2.680 | 0.111 | 0.096 |
| Year × Habitat | 1 | 0.285 | 0.285 | 1.571 | 0.032 | 0.226 |
| Development stage × Habitat | 2 | 0.451 | 0.226 | 1.245 | 0.051 | 0.312 |
| Year × Development stage × Habitat | 2 | 0.476 | 0.238 | 1.312 | 0.054 | 0.294 |
| Residuals | 18 | 3.264 | 0.181 | 0.372 | ||
| Total | 29 | 8.785 |
In 2016, we conducted a life table study in Yunnan province with water from discarded tires and the same Foshan strain of Ae. albopictus mosquitoes to determine the findings’ generalizability. There were two treatments: (a) water from natural discarded tires and (b) natural discarded tire water filtered by a 0.22-μm Millipore filter. There were 10 replicates per treatment. Similar to the Guangzhou experiment, additional equal numbers of microcosms were set up to test the effect of bacterial depletion on larval survival when larval food was not limited. In 2016, we used a total of 40 microcosms.
To verify whether filtration and antibiotics have eliminated bacteria from larval habitats, on Days 1, 5 and 10 since the initiation of the life table study, 50 ml of surface microlayer water was collected from each microcosm using sterile plastic syringes and membrane filtration. Membranes were preserved in 100% ethanol in a −20°C freezer for subsequent quantification of total bacteria by qPCR (see below).
2.3.2 |. Bacterial microbiota assemblage in laboratory mosquito populations
We conducted experiments using the Foshan strain of Ae. albopictus under well-controlled laboratory conditions to determine the impact of habitat microbiota and mosquito developmental stage on mosquito microbiota assemblage (Figure 1). Newly hatched Ae. albopictus larvae were reared with a density of 300 larvae in 3 L water in an insectary regulated at 27°C and 70% relative humidity. Larval food (brewer’s yeast cells) was fed to larvae daily, and adult mosquitoes were reared in 10% sucrose. Three independent replicates were used. Microbiota analysis was conducted on water samples, third-instar larvae, pupae and adults of different ages (Days 3, 7, 10, 14 and 17 postemergence). Water samples were 50 ml of surface microlayer water from a larval rearing tray in the Day 5 life table study. A total of 50 third-instar larvae and 30 pupae were collected, rinsed in double-distilled water and 100% ethanol three times, and then preserved in 100% ethanol in a −20°C freezer. Adult mosquitoes of different ages were collected, rinsed and preserved in the same manner as the larval samples. Three biological replicates were used for each development stage and each time point in the adult population.
2.3.3 |. Bacterial microbiota diversity and stability in natural mosquito populations
To determine the diversity of microbiota in aquatic habitats and the relationship between aquatic habitat microbiota and microbiota assemblages in the mosquitoes, water samples from larval habitats, third-instar larvae and adult mosquitoes were collected from natural habitats in Guangzhou in 2013 and 2014 (Figure 1). Two types of natural habitats were examined—discarded tires and flowerpots—the predominant larval habitat types of Ae. albopictus in the area. For each habitat type, more than 300 larvae were collected from a minimum of 50 aquatic habitats, rinsed and preserved in the same manner described above. In a similar manner, surface microlayer water (within 5 mm from the surface) from these habitats was also collected, filtered and stored in 100% ethanol for microbiota analysis. Aedes albopictus mosquitoes were collected using BG traps alongside the larval habitats.
2.3.4 |. Bacterial 16S rRNA gene library construction and pyrosequencing
For microbiota analysis, genomic DNA was extracted from pooled water samples and pooled mosquito specimens (10 larvae or adults per pool for each developmental stage or habitat type), using the ZR Fungal/Bacterial DNA MicroPrep™ kit following the manufacturer’s instructions (Zymo Research, CA). Pooling of samples from multiple habitats was important because it enabled us to determine the bacterial diversity of various larval habitats in the community and the resulting mosquitoes.
We used the 16S rRNA gene V4 region for microbiota analysis because the V4 region has been shown to be a sensitive marker for bacterial phylogenetic analysis and is employed commonly (Caporaso et al., 2011; Kozich, Westcott, Baxter, Highlander, & Schloss, 2013; Yang, Wang, & Qian, 2016). In brief, the 16S rRNA gene was amplified using PCR primers 515F (5′-GTGCCAGCMGCCGCG GTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) with Illumina library adaptors. The 25 μl PCR included 0.6 μl forward and reverse primers, 2 μl template DNA (10 ng/μl), 10 μl ThermoMix and 6.8 μl PCR-grade water. Reaction conditions were as follows: 95°C for 10 min to denature the DNA, with 30 cycles of amplification at 95°C for 30 s, 50°C for 60 s and 72°C for 90 s; and a final extension of 10 min at 72°C. The amplification was performed in triplicate. Golay barcodes were added using the second PCR. The 25 μl amplification reaction included 0.5 μl forward and reverse primers, 5 μl DNA template (10 ng/μl), 12.5 ll Green PCR Mix (Thermo Scientific) and 6.5 μl PCR-grade water. Reactions were held at 95°C for 10 min to denature the DNA, with amplification proceeding for eight cycles at 95°C for 45 s, 50°C for 60 s and 72°C for 90 s, followed by a final extension of 10 min at 72°C. The amplicon for each sample was examined on an agarose gel, quantified with Picogreen and then cleaned using an Ampure XP PCR purification kit (Beckman Coulter, Inc.). Amplicon sequencing was conducted by Laragen (Culver City, CA) using an Illumina MiSeq platform with a MiSeq 2 × 250 kit, following the manufacturer’s instructions. For each development stage and water sample, we used three biological replicates, except that two biological replicates were used in 2014. Therefore, a total of 54 samples were sequenced on Illumina, including 24 samples for the laboratory populations (8 developmental stages × 3 replicates = 24), 18 samples for 2013 (2 habitat types × 3 developmental stages × 3 replicates = 18) and 12 samples for 2014 (2 habitat types × 3 developmental stages × 2 replicates = 12).
2.3.5 |. Quantitative PCR (qPCR) for quantification of total bacteria
We conducted qPCR to quantify the total bacteria load in mosquito samples used in the microbiota analysis described above, following the method of Lazarevic, Gaia, Girard, and Schrenzel (2016). In brief, the qPCR used the 515F/806R primer pair that targets the V4 region of the 16S rRNA gene, mosquito rpS7 gene, as the reference gene (Wang, Gilbreath, Kukutla, Yan, & Xu, 2011). Bacterial quantity was expressed as the ratio of 16S rRNA gene copy number to rpS7 gene copy number. The amplification was conducted in triplicate, each in a 20 μl reaction mixture containing 2 μl of genomic DNA, 10 μl 2XSYBR Green qPCR Master Mix (Thermo Scientific) and 0.5 μM primer. Reactions were performed in a CF × 96 Touch™ Real-Time PCR Detection System (Bio-Rad), with an initial denaturation at 95°C for 3 min, followed by 45 cycles at 94°C for 30 s, 55°C for 30 s and 68°C for 1 min, with a final step of 95°C for 10 s. This was then followed by a melting curve step analysis with the temperature ranging from 65°C to 95°C at 0.5°C increments to determine each amplified product’s melting temperature. Because total bacterial load was determined relative to mosquito DNA, and mosquito DNA varied among larvae (0.70 μg/larva), pupae (2.65 μg/pupa) and adults (0.31 μg/adult), we used total mosquito DNA measured from Nanodrop to normalize the bacterial load calculation.
Quantification of total bacteria in the water samples followed the method of Lazarevic et al. (2016). Series dilutions of E. coli DH5α strain genomic DNA of known concentration were used for qPCR to build the reference curve. The qPCR amplified the 16S rRNA V4 region gene using a mixture of 12.5 μl Maxima SYBR Green qPCR Master Mix (2X; Thermo Scientific), 1 μl of each 10 μM forward primer (515F) and reverse primer (806R), 1 μl template DNA extracted above and 9.5 μl PCR water. The qPCR assay was performed on a Bio-Rad CFX96 Touch™ Real-Time PCR system with an initial denaturation at 95°C for 10 min, followed by 45 cycles at 94°C for 15 s, 55°C for 30 s and 72°C for 30 s. After a step of 72°C for 10 min for extension, a melting curve step of temperature ranging from 65°C to 95°C at 0.5°C increments was used to determine each amplified product’s melting temperature. All reactions were carried out in triplicate. Bacterial load in a water sample was calculated against the reference curves obtained with E. coli DH5α genomic DNA, and expressed as the number of E. coli genome equivalents in a 1 ml water sample.
2.3.6 |. Wolbachia strain quantification
Bacterial microbiota analysis identified Wolbachia as the most abundant bacteria in the adult mosquito specimens. Previous studies documented the presence of two Wolbachia strains (wAlbA and wAlbB) in Ae. albopictus (Zhou, Rousset, & O’Neill, 1998). To quantify the relative abundance of each Wolbachia strain, we performed qPCR analysis following the method of Minard et al. (2014). In brief, Wolbachia strains were initially confirmed using diagnostic primers that amplify a region of the gene encoding the Wolbachia outer surface protein wAlbA and wAlbB, using the primers and PCR conditions previously described by Zouache et al. (2011). Quantification of each Wolbachia strain was based on a standard curve constructed from a dilution series (101–106 molecules) of the pQuantAlb plasmid containing wsp and actin fragments kindly provided by Weill Mylene, of the University of Montpellier (Tortosa, Courtiol, Moutailler, Failloux, & Weill, 2008). Three technical replicates were conducted per sample. The abundance of the wAlbA and wAlbB strain was calculated as the ratio of Wolbachia wsp gene to Ae. albopictus actin gene copies (Minard et al., 2014). Total Wolbachia abundance was calculated as the sum of wAlbA and wAlbB abundance.
2.4 |. Statistical analyses
2.4.1 |. Survival analysis of life table data
The emergence rate was calculated as the proportion of first-instar larvae that developed into adults. The average emergence rate for each treatment was calculated. To determine the effects of bacterial removal by filtration and using antibiotics or adding larval food, non-parametric Wilcoxon tests were conducted to compare the differences in emergence rates among treatments.
2.4.2 |. Pyrosequencing data processing
All analyses were conducted in mother v.1.38.0, a software package that combines a variety of tools designed to process 16S rRNA gene sequence data (Kozich et al., 2013). In brief, two sets of reads for each sample were combined using the make.contigs command. The sequences were further filtered for sequence length (shorter than 250 bp or longer than 275 bp) or presence of more than one ambiguous base using the screen.seqs command, and unique sequences were identified using the unique.seqs command. Processed sequences were aligned against the SILVA reference database (https://www.mothur.org/w/images/9/98/Silva.bacteria.zip), followed by screen.seqs to remove sequences with more than eight homopolymers and the overhangs at both ends. Redundant sequences were examined and removed using unique.seqs. At last, the pre.cluster scripts in MOTHUR were used to denoise sequences, and chimera.vsearch and remove.seqs commands were used to remove chimeric sequences.
2.4.3 |. Operational taxonomic units (OTUs) determination and α-diversity
Preprocessed sequences were aligned against the SILVA reference database, normalized and then used to generate an uncorrected pairwise distance matrix. Using the distance matrix and the furthest neighbour algorithm, all preprocessed sequences were clustered to detect OTUs at 0.03 (species), 0.05 (genus) and 0.2 (phylum) distance levels according to Schloss and Handelsman (Schloss & Handelsman, 2004). Following the method of Coon et al. (2014), singleton reads assigned to OTUs were discarded from the downstream analysis if their abundance was less than five reads because singletons may result from sequencing errors and thus lead to an overestimation of diversity. Rarefaction curves were constructed to determine sample coverage. Based on the OTU picker data, richness estimators (Chao1) and diversity indices (Shannon) were calculated for each mosquito or water sample. Chao1 measures diversity based on only the number of OTUs present, whereas Shannon also takes into consideration each OTU’s relative abundance. These analyses were carried out using the mother software.
2.4.4 |. β-diversity analysis
Permutational multivariate analysis of variance (PERMANOVA) was used to determine whether microbiota assemblage varied among mosquito developmental stages, different larval habitat types and sampling years (Chen et al., 2016). PERMANOVA tests (999 mutations) were performed using adonis functions in the vegan package in R 3.2.3. Nonmetric multidimensional scaling (NMDS) was used to visualize the pairwise Bray–Curtis distances among samples (Dixon, 2003; Oksanen et al., 2015). Both analyses used a Bray–Curtis dissimilarity matrix calculated from the OTU abundance table as input. The Bray–Curtis dissimilarity matrix was calculated from a PHYLIP-formatted distance matrix based on OTU abundance using MOTHUR. Metastats (http://metastats.cbcb.umd.edu) was used to identify OTUs that exhibited significant differences in abundance between treatment at genus level (White, Nagarajan, & Pop, 2009). This analysis pooled sequences from the biological replicates to determine significant differences in relative abundance between larvae and adults, or between young and old adults.
Because Wolbachia was overwhelmingly abundant in the mosquito samples, we conducted two separate a- and b-diversity analyses, one using all processed sequences and the other with Wolbachia sequences excluded. This is because the microbiota outside cells is functionally and spatially distinct from Wolbachia, which is an endosymbiont. The two separate analyses would reveal whether a large number of Wolbachia sequence reads will skew the analysis of microbiota diversity.
2.4.5 |. Bacterial load data analysis
To determine the impact of filtration and antibiotics treatment on total bacterial load in the water and larval mosquitoes, a one-way ANOVA with repeated measures was used. Tukey–Kramer honest significant difference (HSD) tests were used to compare the means of different treatments and time points.
3 |. RESULTS
3.1 |. Impact of bacteria on larval survivorship and development in life table study
The larva-to-adult emergence rate for Aedes albopictus larvae reared in the water from natural flowerpots in 2015 in Guangzhou was 11.0% and was 3.3% for those reared in water with depleted bacteria through filtering by 0.22-lm filter (Figure 2a). The difference was marginally significant (χ2 = 3.16, df = 1, p = 0.07). In habitats treated with ampicillin, no Ae. albopictus larvae successfully developed into pupae, suggesting that antibiotic depletion of bacteria in aquatic habitats significantly inhibited mosquito larvae development. The inhibition of larval development was likely caused by the lack of critical nutrients in the microcosms or the larvae’s inability to digest nutrients, because when autoclaved, and larval food was added to the rearing condition, the larvae emergence rate increased to 58.0–79.3% (χ2 = 12.36, df = 1, p < 0.001; Figure 2a). Nonsignificant differences in the larvae’s emergence rates among the three treatments supplemented with food (χ2 = 3.31, df = 2, p > 0.05) suggested that the antibiotic itself in the rearing water was not detrimental to the development of mosquito larvae when nutrients were not limiting.
FIGURE 2.
Emergence rates of Aedes albopictus in larval life table study in 2015 (a) and 2016 (b). In 2015, the study used microcosms made of water from natural flowerpots, water with bacteria removed by filtering and treated by ampicillin. In 2016, microcosms made of water from natural discarded tires and bacteria removed by filtering were used. Significant improvements in emergence rate were observed in the corresponding treatments supplemented with sterilized larval food. NS, not significant; *p = 0.07; **p < 0.01 by the nonparametric Wilcoxon test
In 2016, we conducted similar life table studies in Yunnan using water from natural discarded tires and found that the emergence rate of Ae. albopictus larvae reared in microcosms with bacteria-depleted waters through filtering by 0.22-μm filters was 3.0%. This emergence rate was significantly lower than that in the microcosms made of water from natural discarded tires (82.0%; χ2 = 7.21, df = 1, p < 0.01; Figure 2b). When autoclaved larval food was added to microcosms of filtered water, the larvae emergence rate increased significantly, to a level similar to that of the unfiltered natural discarded tire water (90.7%; χ2 = 2.87, df = 2, p > 0.05; Figure 2b).
3.2 |. Bacterial quantification in microcosms
The qPCR analysis was performed to determine the total bacterial load in water samples from microcosms in the life table studies at three time points: 1, 5 and 10 days since the first-instar larvae were introduced. In the 2015 study, we found that filtering natural flowerpot water with 0.22-μm filters reduced the total bacterial load by 3.9-fold (p < 0.01) and antibiotics treatment by 14.1-fold on Day 1 (p < 0.001; Figure 3a). The total bacteria load was significantly reduced, by more than 90%, in Days 5 and 10 after mosquito larvae were introduced to the microcosms in comparison with Day 1, and this phenomenon was consistent among the three microcosm types (natural water, filtered water and antibiotic-treated water; Figure 3a). In comparison with microcosms without larval food supplement, bacterial loads in Day 1 in water samples from microcosms supplemented with autoclaved mosquito larval food (yeasts) were increased by 5.1-, 34.3- and 117.7-fold for natural water, filtered water and antibiotic-treated water (p < 0.0001 for the three comparisons; Figure 3a). Similar to the microcosms without food supplementation, bacterial loads were reduced by >95% in Days 5 and 10 relative to Day 1 (Figure 3a). The pattern of bacterial loads in Day 1 in microcosms of different water treatments remarkably mirrored the larva-to-adult emergence rate pattern shown in Figure 2a.
FIGURE 3.
Dynamics of total bacterial load in water samples of Aedes albopictus larval life table study in 2015 (a) and 2016 (b). The experiments in 2015 used microcosms made of water from natural flowerpots, water with bacteria removed by filtering and water treated by ampicillin. The experiments in 2016 used microcosms made of water from natural discarded tires and water with bacteria removed by filtering. Corresponding treatments supplemented with sterilized larval food were conducted in parallel. Total bacteria abundance was quantified in Day 1, Day 5, and Day 10 using qPCR. NS, not significant; *p < .05, **p < .01, ***p < .001
In the 2016 study with discarded tire water, filtering with 0.22-μm filters reduced the total bacterial load of larval microcosms by 2.4-fold (p < 0.01; Figure 3b), paralleling the dramatic reduction in larva-to-adult emergence rate in the filtered microcosms (from 82.0% to 3.0%). The addition of autoclaved larval food to the microcosms with filtered water increased the total bacterial load by 6.6-fold (p < 0.0001), echoing the large increase in the larva-to-adult emergence rate (3.0–90.7%). The total bacteria loads decreased over time, and this was consistent for all treatments regardless of food supplementation (Figure 3b). Overall, these results suggest that live bacteria from aquatic habitats played an important role in Ae. albopictus larval development and survival in natural habitats where nutrients for mosquito larvae were limiting.
3.3 |. Pyrosequencing of bacterial 16S rRNA gene
To determine bacterial species composition in larval habitats and mosquito larvae, we examined microbiota in laboratory microcosms and natural habitats by pyrosequencing the bacterial 16S rRNA V4 gene. Among the 54 samples examined, a total of 19.9 million sequence reads were obtained, and 9.2 million high-quality sequences were generated after quality filtering. The coverage of the sequences was 99.9 ± 0.1%, indicating very few singletons in the sequences obtained. The rarefaction curve with asymptotes suggests that samples from mosquitoes and larval habitat water had reached saturation and that the obtained OTUs represented the majority of microbiota in the populations, except for one sample (Day 3 adult; Supporting information Figure S1). Therefore, the data for one Day 3 adult sample were excluded from the subsequent analysis. Overall, a total of 2,179 OTUs were identified for mosquitoes and water samples from laboratory microcosms (Supporting information Table S1), and 6,003 OTUs for natural habitat samples (Supporting information Table S2).
For the Ae. albopictus colony reared in laboratory microcosms, the main bacterial phyla across all developmental stages were Proteobacteria (87.9%), Actinobacteria (4.3%), Bacteroidetes (3.6%), Planctomycetes (2.7%) and Firmicutes (0.8%). At the class level, the most common bacteria included α-Proteobacteria (58.8%), β-Proteobacteria(7.7%), γ-Proteobacteria (21.2%), Actinobacteria (4.3%), Planctomycetacia (2.7%), Flavobacteria (1.9%) and Sphingobacteria (1.7%). For the mosquitoes collected from the field, the main bacterial phyla included Proteobacteria (78.7%), Bacteroidetes (11.7%), Acidobacteria(4.9%), Planctomycetes (2.2%) and Verrucomicrobia (1.0%). Similar to the laboratory colony, α-, β-, and γ-Proteobacteria were the most common bacteria class found in the field mosquitoes, constituting 35.4%, 25.9% and 16.1% of the bacteria.
3.4 |. Bacterial load and microbiota diversity in laboratory microcosms
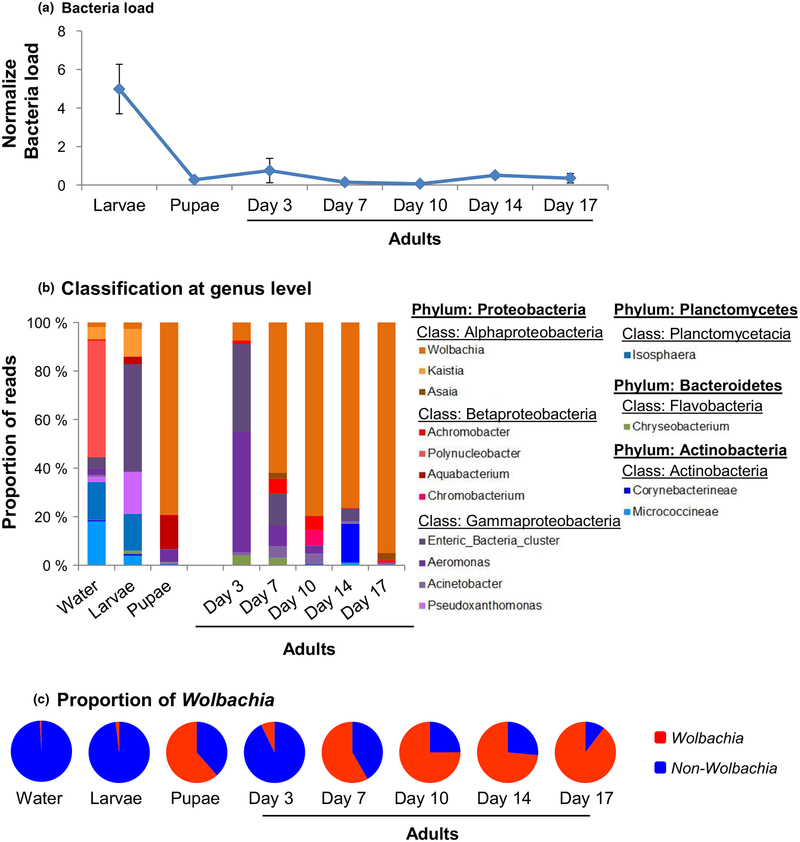
To determine the impact of mosquito development stage on micro-biota composition and quantity, we used Ae. albopictus laboratory colonies reared in microcosms under well-controlled laboratory conditions. We found that the total bacteria load in mosquitoes changed significantly over developmental stages (ANOVA; F(6,35) = 48.09, p < 0.0001). In particular, the larval stage exhibited the highest load, 18.2-, and 13.6-fold higher than pupae and adults, respectively (Figure 4a; Tukey–Kramer HSD test; p < 0.001 between larvae and pupae, and between larvae and adults).
FIGURE 4.
Mosquito bacterial microbiota dynamics across different developmental stages in laboratory-reared Aedes albopictus. (a) qPCR quantification of total bacterial load across different developmental stages and adult mosquito ages. Day 3, Day 7, Day 10, Day 14 and Day 17 represented adults in days postemergence. The total bacteria load was expressed as the fold change of bacterial 16S rRNA gene to the rPS7 gene in the mosquitoes. (b) Bacterial composition dynamics at the genus levels. Unclassified and uncultured bacteria genera were not presented. And (c) The proportion of Wolbachia in laboratory Ae. albopictus strain in different development stages and adult ages
The bacteria’s composition varied significantly across mosquito development stages. At the phylum level, Proteobacteria was always the predominant bacteria for larvae, pupae and adults, but its proportion increased from 81.8% in larvae to 93.7% in young adults (3 days old; Metastats, p = 0.010; Figure 4b) and to 97.8% in old adults (17 days old; Metastats, p = 0.009; Figure 4b). Planctomycetes abundance decreased by ~11% (p < 0.05 for both comparisons) and Actinobacteria by 4% in adults in comparison with larvae (p < 0.001 for both comparisons). Water samples exhibited the highest diversity, followed by larvae and pupae, and adult mosquitoes exhibited the lowest diversity, as measured by the Shannon index and inverse Simpson index (Supporting information Table S1).
At the genus level, microbiota assemblages showed no significant differences among water, larval, and pupal samples (PERMANOVA; F1,7 = 1.887, p > 0.05); however, a significant difference was found between immatures and adults (Figure 4c; PERMANOVA; F1,19 = 6.176, p = 0.022). Bacterial microbiota assemblage varied significantly among adults of different ages. For example, in young (3 days old) adults, Aeromonas (49.8%), enteric bacteria (36.1%) and Wolbachia (7.3%) were the most common bacteria, but in older mosquitoes, Wolbachia became the predominant bacteria (Figure 4d). The enteric bacteria cluster included bacteria from a number of genera, such as Brenneria, Buttiauxella, Citrobacter, Erwinia, Escherichia, Klebsiella, Kluyvera, Pantoea, Pectobacterium, Raoultella, Serratia and Yersinia. Bacterial composition among the three independent replicates was similar in all mosquito developmental stages (Supporting information Figure S2).
We estimated the abundance of Wolbachia and non-Wolbachia bacteria using the total reads of Wolbachia and non-Wolbachia sequences from pyrosequencing, and Wolbachia-specific qPCR (Supporting information Figure S3). We found that the total non-Wolbachia reads were the highest in larvae, and decreased as mosquitoes aged (Supporting information Figure S3A). The number of Wolbachia reads was the lowest and increased substantially in older female adults (Supporting information Figure S3B). The qPCR found significantly higher Wolbachia abundance in adult mosquitoes in than larvae or pupae, and in old mosquitoes (>10 days old) than in young adults (<7 days old; Supporting information Figure S3C). These results demonstrated higher Wolbachia abundance in older mosquitoes than in larvae, pupae and young adults. Using diagnostic primers for Wolbachia, two strains of Wolbachia (wAlbA and wAlbB) were detected, and strain wAlbB was found more abundant in larvae, pupae and adults of all ages (Supporting information Figure S4).
3.5 |. Diversity and temporal stability of bacterial microbiota of mosquitoes from natural habitats
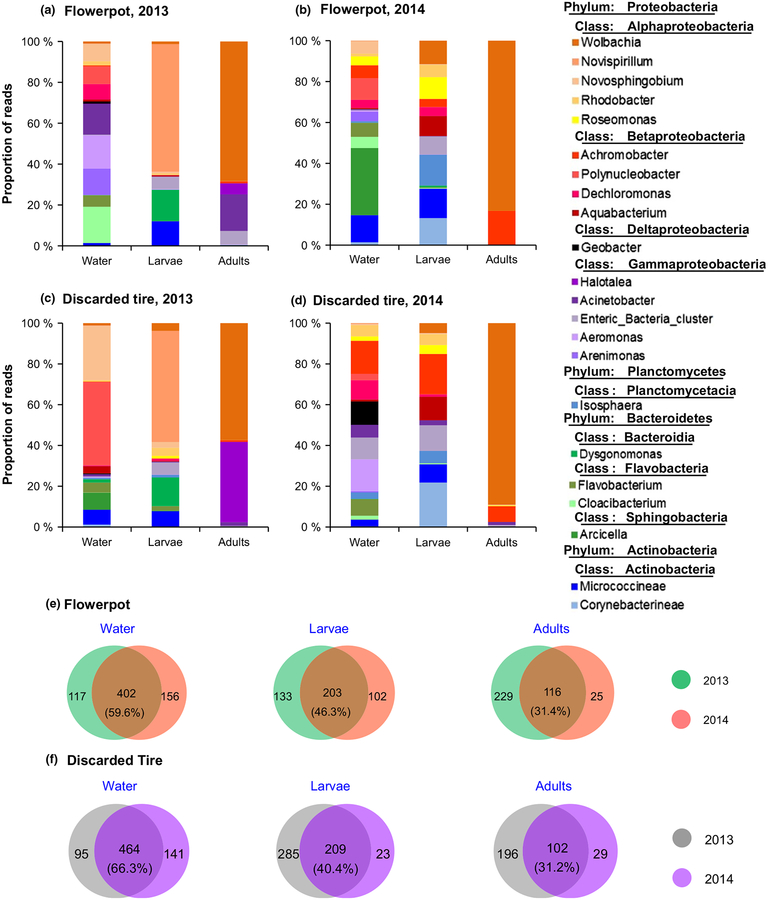
Consistent with the laboratory microcosm study, water samples from natural mosquito habitats exhibited the highest bacterial diversity, followed by larvae, and adult mosquitoes showed significantly reduced diversity (Supporting information Table S2). This finding was consistent for both flowerpot and discarded tire habitat types in the two sampling years.
We performed PERMANOVA to determine the temporal stability of microbiota in water samples, and mosquito larvae and adults from natural habitats, using data from flowerpots and discarded tires for the two sampling years. The analysis based on Bray–Curtis dissimilarities revealed a marginally significant effect of study year on microbial assemblage (PERMANOVA; F1,18 = 4.14, p = 0.057; Table 1), suggesting a lack of temporal stability in microbiota assemblage in larval habitats and mosquitoes. For example, the top two most common genera in larvae from flowerpots belonged to Novispirillum (62.5%) and Dysgonomonas (15.3%) in 2013, but to Micrococcineae (14.4%) and Corynebacterineae (13.2%) in 2014 (Figure 5a,b). The predominant bacteria were Wolbachia (68.3%) and Acinetobacter (17.9%) for adult mosquitoes collected in 2013, and Wolbachia (83.2%) and Achromobacter (16.6%) for those collected in 2014 (PERMANOVA; F1,7 = 1.887, p > 0.05). A lack of temporal stability was found for mosquitoes from discarded tire habitats (Figure 5c,d). Overall, there was a modest overlap in OTUs between the two study years in mosquito larvae, adults and water samples in the two habitat types, ranging from 31.4% to 59.6% for flowerpots (Figure 5e) and 31.2–66.3% for discarded tires (Figure 5f). The difference in microbiota species composition between mosquito larvae and adults was highly significant (PERMANOVA; F2,18 = 6.38, p < 0.01).
FIGURE 5.
Composition of bacterial microbiota of field-collected Aedes albopictus larvae and adults, and habitat water. Main bacterial genera from flowerpots in 2013 (a) and 2014 (b), and from discarded tires in 2013 (c) and 2014 (d). Venn diagrams show the numbers of shared or unique bacterial OTUs among larval and adult mosquitoes and among habitat water from flowerpots (e) and discarded tires (f)
NMDS based on the Bray–Curtis distances was used to visualize the relationships shared among water and mosquito samples from natural environments (Supporting information Figure S5). Aedes albopictus larvae, adults and habitat water samples had cohesive clusters individually despite having different origins, of flowerpots or tires. The water and larvae samples had more similar bacterial community composition with a higher proximity in the NMDS plot, while adults had a more unique community. The influence of developmental stage factors in the microbial composition can be observed.
Because Wolbachia reside in the insect cells, while the microbiota are present on the outside of the body or within the gut and have a more transient association, including Wolbachia sequences in the analysis may skew the analysis of microbiota diversity. We therefore performed separate β-diversity analysis of field mosquitoes and water samples with all Wolbachia sequences removed. The bacterial composition is shown in Supporting information Figure S6. With the new data set, the PERMANOVA found a marginally significant effect of study year (p = 0.051) and a significant effect of developmental stage (p < 0.01) on microbial assemblage (Supporting information Table S3), similar to the above analysis with all Wolbachia sequences included. Therefore, including or excluding Wolbachia sequences did not affect the conclusions about mosquito β-diversity.
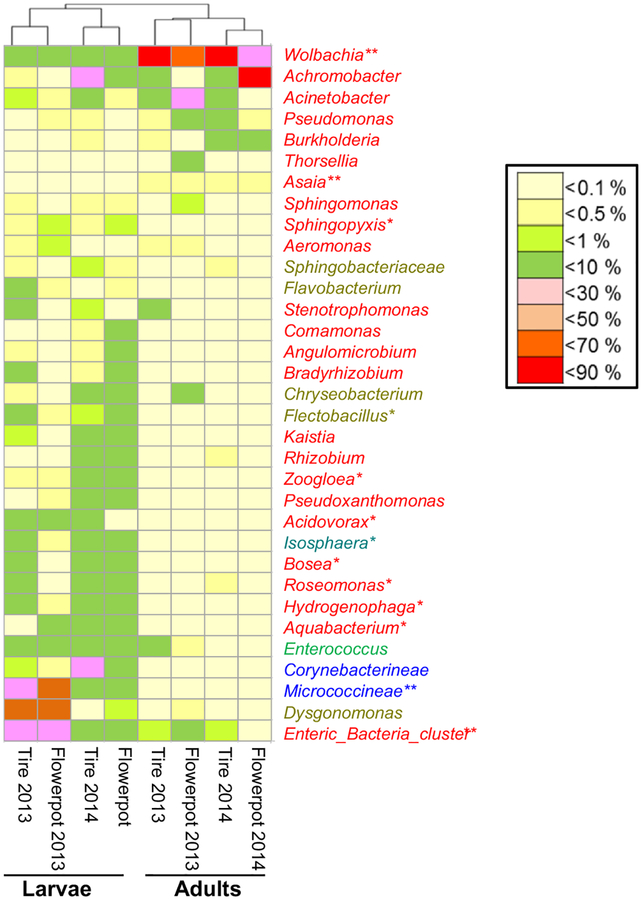
3.6 |. Common shared bacteria
A total of 50 bacteria genera shared between Ae. albopictus larvae or adults in the two sampling years with a minimum relative abundance of 0.1% were identified (Figure 6a). These bacteria were mostly within the phylum of Proteobacteria (33 genera) and Bacteroidetes (8 genera). Among them, 33 genera (66%) were shared between larvae and adults in the two study years. Clustering analysis based on the relative abundance of these 33 shared bacteria found two major clades corresponding to mosquito developmental stage (larva vs. adult), but not to habitat type or sampling years (Figure 6b), indicating that mosquito developmental stage was a major determinant of microbiota assemblage. Among the shared bacteria, the relative abundance of Wolbachia and Asaia was significantly greater in adults than in larvae (Metastats, both p < 0.01), whereas 11 genera, including Enteric bacteria, Micrococcineae, Aquabacterium, Hydrogenophaga, Roseomonas, Bosea, Isosphaera, Acidovorax, Zoogloea, Flectobacillus and Sphingopyxis, were more abundant in larvae (Metastats, all p < 0.05).
FIGURE 6.
Heat map of relative abundance of shared bacterial microbiota in Aedes albopictus mosquitoes across developmental stages, habitat types and sampling years. A genus marked by “*” indicates the bacterial genera that differed in abundance between larval and adult stages at p < 0.05. Bacterial genera were represented in colors based on their phylum classification
4 |. DISCUSSION
In the current study, we demonstrated that live bacteria in aquatic habitats play a critical role in the development and survivorship of Ae. albopictus mosquito larvae under natural conditions when food for mosquito larvae was limited. Bacteria’s important role in mosquito larval development is evidenced by three experimental observations. First, through our life table study in the Guangzhou site in 2015 using natural flowerpot habitats, we found a significant reduction (from 11.0% to 3.3%) in emergence rate when water was filtered using 0.22-μm filters and total bacterial content was dramatically reduced. Bacterial depletion of the same water using ampicillin antibiotics resulted in a 0% pupation rate. In 2016, a 79% reduction in emergence rate was found in the Yunnan study site when water from discarded tires was filtered. Second, there appeared to be a dose–response relationship between mosquito emergence rate and bacterial load in aquatic habitats. For example, we observed a six- to nine-fold higher total bacterial load in the treatments with larval food supplementation compared to the respective controls. In parallel, larval survivorship in these treatments also increased dramatically. Higher bacterial load in microcosms with water from natural flowerpots and discarded tires compared to those with 0.22-μm filtered water corresponded to an increased pupation rate. Third, by Day 5 and Day 10 total bacterial load was significantly reduced from Day 1 in nearly all microcosms used in our study. Such a reduction in total bacterial load likely resulted from mosquito larvae’s ingestion and subsequent removal of bacteria. Together with the findings of Coon et al. (2014) and other earlier literature (Kaufman, Bland, Worthen, Walker, & Klug, 2001; Kaufman et al., 2006), we conclude that live bacteria are critical to the larval development of Ae. albopictus mosquitoes in nature when nutrients for mosquito larvae are generally limited.
Our results were consistent with an earlier study on An. Stephensi, which reported that the pupation time of mosquito larvae reared in rifampicin-treated water was delayed by more than 4 days and that the pupation rate was reduced by 88% (Chouaia et al., 2012). We observed a zero pupation rate in our experiments with Ae. albopictus in habitats with ampicillin-treated water and an 11% pupation rate in the control. The difference in pupation rate between our experiments and those reported by Chouaia et al. (2012) was due to different experimental conditions and different mosquito species used by these two studies: We used water from natural flowerpots under seminatural microcosm conditions, whereas Chouaia et al. (2012) used an insectary setting with minced commercial mouse food as larval food (Chouaia et al., 2012). Chouaia et al. (2012) demonstrated the important role of Asaia in mosquito larval survival by removing Asaia using rifampicin and restoring Asaia by supplementing rifampicin-resistant Asaia in the diet. Our study did not investigate the significance of Asaia in Ae. albopictus larval survival, but Asaia was rare, constituting <0.01% of the total bacterial microbiota reads. The reason for the large increases in bacterial load in the microcosms with the brewer’s yeast addition is not clear. The food was autoclaved, and autoclaving destroyed >99.5% of bacterial DNA. It is possible that the increase in bacterial load in microcosms supplemented with larval food originated from heterotrophic proliferation in the microcosms, particularly those with large 16S rRNA gene copy numbers (Louca, Doebeli, & Parfrey, 2018). Similar to the microcosms without food supplementation, bacterial loads were reduced by >95% in Day 5 and Day 10 relative to Day 1. The pattern of bacterial loads in Day 1 in microcosms of different water treatments remarkably mirrored the larva-to-adult emergence rate pattern, suggesting that bacteria play a critical role in larval development and survivorship.
We detected a higher emergence rate in microcosms made of natural water from discarded tires than in microcosms of flowerpot water (82% vs. 11%). This difference may be explained by factors related to detritus and microorganisms in the water and differences in rearing conditions, such as temperature. First, the water from discarded tires contained visible detritus (dead particulate organic material) from dead plants and animals, whereas no detritus was found in flowerpot water. Detritus is often colonized by various microorganisms which facilitate its decomposition (Holguin, Vazquez, & Bashan, 2001), as evidenced by the 2.2-fold higher total bacterial load in discarded tire water than in flowerpot water. Small particulates in the detritus can be directly ingested by mosquito larvae, and ingestion of small particulates is considered an efficient means for larvae to obtain nutrients (Yee & Juliano, 2006). Second, higher bacterial loads in the discarded tire water may have helped larval development and survivorship, particularly when detritus was available in the discarded tire water. Yee, Yee, Kneitel, and Juliano (2007) found that larval survival to adulthood was significantly higher when larvae had access to both microorganisms and animal detritus than to water microorganisms or to detritus alone. The finding of high larval survivorship in the discarded tire water was consistent with the finding that discarded tires typically showed a higher larval positivity rate than flowerpots and other types of aquatic habitats in nature (Li et al., 2014).
We consistently detected significantly higher microbiota diversity in aquatic water samples than in larval or adult mosquitoes under the laboratory and field conditions. This is expected, as larvae acquired their microbiota from the habitats and are permissive to only a subset of bacteria to survive and propagate. Significant differences in micro-biota detected between larvae and adult mosquitoes observed in our study may be due to immune selection of bacterial fauna by different mosquito developmental stages and by adult mosquitoes’ mobile foraging behaviour. In particular, Wolbachia was extremely common in adult Ae. albopictus mosquitoes, constituting >50% bacterial abundance. Wolbachia can cause cytoplasmic incompatibility to Ae. albopictus, reduce mosquito lifespan and alter vector competence to viruses (Blagrove, Arias-Goeta, Failloux, & Sinkins, 2012; Fraser et al., 2017; van Tol & Dimopoulos, 2016). Thus, Wolbachia have been proposed as a biopesticide for population suppression (O’Connor et al., 2012) and as a potential candidate to drive pathogen-blocking genes into natural mosquito populations (Caragata et al., 2013). We found that Wolbachia was increasingly abundant in older mosquitoes compared to younger mosquitoes, and all the field-collected adults were infected with Wolbachia. The mechanism for increasing Wolbachia abundance in older mosquitoes is not clear, but this phenomenon may have important implications for Wolbachia’s transmission-blocking capacity. Wolbachia colonization and proliferation in the adult mosquito host is highly dynamic during the period when pathogens were ingested through blood meal. Pathogen development must encounter micro-biota perturbation, which may subsequently impact the mosquitoes’ antipathogen immune response (Weiss & Aksoy, 2011).
Among all the shared bacterial genera in Ae. albopictus larvae and adults, Wolbachia was the dominant genus, especially in adults. Consistent with our results, the high prevalence of Acinetobacter has been previously reported in Ae. albopictus (Minard, Tran, et al., 2013). Acinetobacter was stably associated with several Aedes and Culex species and was found to be synergistic with Asaia in Ae. albopictus (Minard, Tran et al., 2013). Acinetobacter was also found to be involved in blood digestion (Minard, Mavingui et al., 2013). The enteric bacteria Aeromonas and Micrococcineae are common in environmental water and can be transmitted from larvae to adults as nutrients (Minard, Mavingui et al., 2013). Mutual exclusion between Asaia and Wolbachia was reported in Aedes and Anopheles mosquitoes (Rossi et al., 2015), which may explain why our mosquito samples found a low abundance of Asaia, with very high Wolbachia abundance. Overall, there is very limited knowledge of the biological function of the shared bacteria identified in the current study.
In summary, the current study demonstrated bacteria’s important role in Ae. albopictus larvae development and survivorship. Mosquito larvae acquired their microbiota from their habitats, and mosquito microbiota was strongly modulated by mosquito physiological status, such as developmental stage and mosquito age. Aedes albopictus larvae survive well in natural aquatic habitats with highly dynamic microbiota. This species’ ability to use diverse bacteria for its larval development and survival has contributed its successful utilization of containers as larval habitats and facilitated its global expansion.
Supplementary Material
FIGURE 1.
Experimental design for bacterial microbiota diversity study of Aedes albopictus mosquitoes. Laboratory mosquito strain was used to determine the effects of mosquito development stage and adult age on microbiota diversity. Mosquitoes were collected from the field in 2013 and 2014 to determine microbiota in the natural environments and temporal stability. The numbers in parenthesis indicate the number of independent biological samples analyzed
ACKNOWLEDGMENTS
We thank Yiji Li, Tengfei Zhou, Xiaoling Wang, Hongrang Zhou, Ying Che and Chao Huang for assistance with the field sample collection and larval habitat surveys, and Elizabeth Hemming-Schroeder and Beth Riley for proofreading the manuscript. The three anonymous reviewers provided constructive comments. This research was supported by grants from the National Institutes of Health (NIH) (grants U19 AI129326, R01 AI050243 and R01 AI136850), the National Natural Science Foundation of China (81420108024, 81528013) and the Natural Science Foundation of Guangdong Province (2014A030312016).
Funding information
Natural Science Foundation of Guangdong Province, Grant/Award Number: 2014A030312016; National Institutes of Health, Grant/Award Number: R01 AI050243, R01 AI136850, U19 AI129326; National Natural Science Foundation of China, Grant/Award Number: 81420108024, 81528013
Footnotes
DATA ACCESSIBILITY
The data sets with 16S rRNA gene Illumina MiSeq sequences reported in this study are available with NCBI SRA Accession no. SRP131914. The R-code and MOTHUR command lines used in the statistical analyses are available at Dryad, https://doi.org/10.5061/dryad.dg11vn5.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Belda E, Pedrola L, Pereto J, Martinez-Blanch JF, Montagud A, Navarro E, … Porcar M (2011). Microbial diversity in the midguts of field and lab-reared populations of the European corn borer Ostrinia nubilalis. PLoS ONE, 6(6), e21751 10.1371/journal.pone.0021751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, & Lounibos LP (2007). Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis, 7(1), 76–85. 10.1089/vbz.2006.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove MSC, Arias-Goeta C, Failloux AB, & Sinkins SP (2012). Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proceedings of the National Academy of Sciences of the United States of America, 109(1), 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilauri P, Bellini R, Calzolari M, Angeflni R, Venturi L, Fallacara F, … Dottori M (2008). Chikungunya virus in Aedes albopictus, Italy. Emerging Infectious Diseases, 14(5), 852–854. 10.3201/eid1405.071144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X, & James AA (2013). The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends in Parasitology, 29(9), 460–468. 10.1016/j.pt.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia S, Di Lelio I, La Storia A, Marinelli A, Varricchio P, Franzetti E, … Pennacchio F (2016). Midgut microbiota and host immuno-competence underlie Bacillus thuringiensis killing mechanism. Proceedings of the National Academy of Sciences of the United States of America, 113(34), 9486–9491. 10.1073/pnas.1521741113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, … Knight R (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 108, 4516–4522. 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, O’Neill SL, & McGraw EA (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathogens, 9(6), e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Zhao JC, Joshi D, Xi ZY, Norman B, & Walker ED (2016). Persistent infection by Wolbachia wAlbB has no effect on composition of the gut microbiota in adult female Anopheles stephensi. Frontiers in Microbiology, 7, 1485 10.3389/fmicb.2016.01485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaia B, Rossi P, Epis S, Mosca M, Ricci I, Damiani C, … Favia G (2012). Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiology, 12(Suppl 1), S2 10.1186/1471-2180-12-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KL, Brown MR, & Strand MR (2016). Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Molecular Ecology, 25(22), 5806–5826. 10.1111/mec.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KL, Valzania L, McKinney DA, Vogel KJ, Brown MR, & Strand MR (2017). Bacteria-mediated hypoxia functions as a signal for mosquito development. Proceedings of the National Academy of Sciences of the USA, 114(27), E5362–E5369. 10.1073/pnas.1702983114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KL, Vogel KJ, Brown MR, & Strand MR (2014). Mosquitoes rely on their gut microbiota for development. Molecular Ecology, 23(11), 2727–2739. 10.1111/mec.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson LB, Jiolle D, Minard G, Moltini-Conclois I, Volant S, Ghozlane A, … Lambrechts L (2017). Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Science Advances, 3(8), e1700585 10.1126/sciadv.1700585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon P (2003). VEGAN, a package of R functions for community ecology. Journal of Vegetation Science, 14(6), 927–930. 10.1111/j.1654-1103.2003.tb02228.x [DOI] [Google Scholar]
- Douglas AE (2015). Multiorganismal insects: Diversity and function of resident microorganisms. Annual Review of Entomology, 60, 17–34. 10.1146/annurev-ento-010814-020822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, & Moran NA (2013). The gut microbiota of insects - diversity in structure and function. Fems Microbiology Reviews, 37(5), 699–735. 10.1111/1574-6976.12025 [DOI] [PubMed] [Google Scholar]
- Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, Flores HA, & O’Neill SL (2017). Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathogens, 13(12), e1006751 10.1371/journal.ppat.1006751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG (2004). Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology, 18(3), 215–227. 10.1111/j.0269-283X.2004.00513.x [DOI] [PubMed] [Google Scholar]
- Guegan M, Zouache K, Demichel C, Minard G, Van Tran V, Potier P, … Valiente Moro C (2018). The mosquito holobiont: Fresh insight into mosquito-microbiota interactions. Microbiome, 6(1), 49 10.1186/s40168-018-0435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin G, Vazquez P, & Bashan Y (2001). The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biology and Fertility of Soils, 33(4), 265–278. 10.1007/s003740000319 [DOI] [Google Scholar]
- Hu Y, Lukasik P, Moreau CS, & Russell JA (2014). Correlates of gut community composition across an ant species (Cephalotes varians) elucidate causes and consequences of symbiotic variability. Molecular Ecology, 23(6), 1284–1300. 10.1111/mec.12607 [DOI] [PubMed] [Google Scholar]
- Jupatanakul N, Sim S, & Dimopoulos G (2014). The insect microbiome modulates vector competence for arboviruses. Viruses-Basel, 6(11), 4294–4313. 10.3390/v6114294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Bland SN, Worthen ME, Walker ED, & Klug MJ (2001). Bacterial and fungal biomass responses to feeding by larval Aedes triseriatus (Diptera: Culicidae). Journal of Medical Entomology, 38(5), 711–719. 10.1603/0022-2585-38.5.711 [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Wanja E, Maknojia S, Bayoh MN, Vulule JM, & Walker ED (2006). Importance of algal biomass to growth and development of Anopheles gambiae larvae. Journal of Medical Entomology, 43(4), 669–676. 10.1603/0022-2585(2006)43[669:Ioabtg]2.0.Co;2 [DOI] [PubMed] [Google Scholar]
- Knudsen AB (1995). Global distribution and continuing spread of Aedes albopictus. Parassitologia, 37(2–3), 91–97. [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, & Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Applied and Environmental Microbiology, 79(17), 5112–5120. 10.1128/Aem.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Gaia N, Girard M, & Schrenzel J (2016). Decontamination of 16S rRNA gene amplicon sequence datasets based on bacterial load assessment by qPCR. BMC Microbiology, 16, 10.1186/s12866-016-0689-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y, … Chen XG (2014). Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Neglected Tropical Diseases, 8(11), e3301 10.1371/journal.pntd.0003301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZZ, Zhou TF, Lai ZT, Zhang ZH, Jia ZR, Zhou GF, … Chen XG (2017). Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus mosquitoes as Zika Virus Vectors, China. Emerging Infectious Diseases, 23(7), 1085–1091. 10.3201/eid2307.161528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S, Doebeli M, & Parfrey LW (2018). Correcting for 16S rRNA gene copy numbers in microbiome surveys remains an unsolved problem. Microbiome, 6(1), 41 10.1186/s40168-018-0420-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manirajan BA, Ratering S, Rusch V, Schwiertz A, Geissler-Plaum R, Cardinale M, & Schnell S (2016). Bacterial microbiota associated with flower pollen is influenced by pollination type, and shows a high degree of diversity and species-specificity. Environmental Microbiology, 18(12), 5161–5174. 10.1111/1462-2920.13524 [DOI] [PubMed] [Google Scholar]
- Martinson VG, Douglas AE, & Jaenike J (2017). Community structure of the gut microbiota in sympatric species of wild Drosophila. Ecology Letters, 20(5), 629–639. 10.1111/ele.12761 [DOI] [PubMed] [Google Scholar]
- Mikaelyan A, Meuser K, & Brune A (2017). Microenvironmental heterogeneity of gut compartments drives bacterial community structure in wood- and humus-feeding higher termites. FEMS Microbiology Ecology, 93(1), fiw210 10.1093/femsec/fiw210 [DOI] [PubMed] [Google Scholar]
- Minard G, Mavingui P, & Moro CV (2013). Diversity and function of bacterial microbiota in the mosquito holobiont. Parasites & Vectors, 6, 146 10.1186/1756-3305-6-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard G, Tran FH, Dubost A, Van TV, Mavingui P, & Moro CV (2014). Pyrosequencing 16S rRNA genes of bacteria associated with wild tiger mosquito Aedes albopictus: A pilot study. Frontiers in Cellular and Infection Microbiology, 4, 59 10.3389/fcimb.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard G, Tran FH, Raharimalala FN, Hellard E, Ravelonandro P, Mavingui P, & Moro CV (2013). Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. Fems Microbiology Ecology, 83(1), 63–73. 10.1111/j.1574-6941.2012.01455.x [DOI] [PubMed] [Google Scholar]
- Napflin K, & Schmid-Hempel P (2016). Immune response and gut microbial community structure in bumblebees after microbiota transplants. Proceedings. Biological sciences, 283(1831), 20160312 10.1098/rspb.2016.0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L, Plichart C, Sang AC, Brelsfoard CL, Bossin HC, & Dobson SL (2012). Open release of male mosquitoes infected with a wolbachia biopesticide: Field performance and infection containment. PLoS Neglected Tropical Diseases, 6(11), e1797 10.1371/journal.pntd.0001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, … Wagner H (2015). Vegan: community ecology package . R package vegan, vers. 2.2–1 In. [Google Scholar]
- Rossi P, Ricci I, Cappelli A, Damiani C, Ulissi U, Mancini MV, … Favia G (2015). Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasites & Vectors, 8, 278 10.1186/s13071-015-0888-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone CL, Cohen J, Yasunaga A, Xu J, Osbom G, Subramanian H, … Cherry S (2015). Microbiota-dependent priming of antiviral intestinal immunity in Drosophila. Cell Host & Microbe, 18(5), 571–581. 10.1016/j.chom.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzis P, de Verges J, Rousk K, Cilliers M, Vorster BJ, & Poulsen M (2016). Potential for nitrogen fixation in the fungus-growing termite symbiosis. Frontiers in Microbiology, 7, 1993 10.3389/fmicb.2016.01993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, & Handelsman J (2004). Status of the microbial census. Microbiology and Molecular Biology Reviews, 68(4), 686 10.1128/Mmbr.68.4.686-691.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Lukasik P, O’Connor MP, Lee A, Mayo G, Drott MT, … Russell JA (2015). Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Molecular Ecology, 24(5), 1135–1149. 10.1111/mec.13095 [DOI] [PubMed] [Google Scholar]
- Strand MR (2017). The gut microbiota of mosquitoes: Diversity and function. Arthropod Vector: Controller of Disease Transmission, Vol 1: Vector Microbiome and Innate Immunity of Arthropods, 1, 185–199. 10.1016/B978-0-12-805350-8.00011-8 [DOI] [Google Scholar]
- van Tol S, & Dimopoulos G (2016). Influences of the mosquito micro-biota on vector competence. Progress in Mosquito Research, 51, 243–291. 10.1016/bs.aiip.2016.04.006 [DOI] [Google Scholar]
- Tortosa P, Courtiol A, Moutailler S, Failloux AB, & Weill M (2008). Chikungunya-Wolbachia interplay in Aedes albopictus. Insect Molecular Biology, 17(6), 677–684. 10.1111/j.1365-2583.2008.00842.x [DOI] [PubMed] [Google Scholar]
- Wang Y, Gilbreath TM, Kukutla P, Yan GY, & Xu JN (2011). Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE, 6(9), e24767 10.1371/journal.pone.0024767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hammes F, Boon N, & Egli T (2007). Quantification of the filterability of freshwater bacteria through 0.45, 0.22, and 0.1 mu m pore size filters and shape-dependent enrichment of filterable bacterial communities. Environmental Science & Technology, 41(20), 7080–7086. 10.1021/es0707198 [DOI] [PubMed] [Google Scholar]
- Weiss B, & Aksoy S (2011). Microbiome influences on insect host vector competence. Trends in Parasitology, 27(11), 514–522. 10.1016/j.pt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Nagarajan N, & Pop M (2009). Statistical methods for detecting differentially abundant features in clinical metagenomic samples. Plos Computational Biology, 5(4), e1000352 10.1371/journal.pcbi.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Lun ZR, James AA, & Chen XG (2010). Dengue fever in mainland China. American Journal of Tropical Medicine and Hygiene, 83(3), 664–671. 10.4269/ajtmh.2010.09-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Wang Y, & Qian PY (2016). Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinformatics, 17, 135 10.1186/s12859-016-0992-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, & Juliano SA (2006). Consequences of detritus type in an aquatic microsystem: Effects on water quality, micro-organisms and performance of the dominant consumer. Freshwater Biology, 51(3), 448–459. 10.1111/j.1365-2427.2005.01504.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Yee SH, Kneitel JM, & Juliano SA (2007). Richness-productivity relationships between trophic levels in a detritus-based system: Significance of abundance and trophic linkage. Oecologia, 154(2), 377–385. 10.1007/s00442-007-0837-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WG, Rousset F, & O’Neill S (1998). Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society B-Biological Sciences, 265(1395), 509–515. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LHR, Ravelonandro P, & Mavingui P (2011). Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiology Ecology, 75(3), 377–389. 10.1111/j.1574-6941.2010.01012.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.