Abstract
Pompe disease (OMIM 232300) is an autosomal recessive disorder caused by mutations in the gene encoding acid α-glucosidase (GAA) (EC 3.2.1.20), the enzyme responsible for hydrolyzing lysosomal glycogen. The primary cellular pathology is lysosomal glycogen accumulation in cardiac muscle, skeletal muscle, and motor neurons, which ultimately results in cardiorespiratory failure. However, the severity of pathology and its impact on clinical outcomes are poorly described in smooth muscle. The advent of enzyme replacement therapy (ERT) in 2006 has improved clinical outcomes in infantile-onset Pompe disease patients. Although ERT increases patient life expectancy and ventilator free survival, it is not entirely curative. Persistent motor neuron pathology and weakness of respiratory muscles, including airway smooth muscles, contribute to the need for mechanical ventilation by some patients on ERT. Some patients on ERT continue to experience life-threatening pathology to vascular smooth muscle, such as aneurysms or dissections within the aorta and cerebral arteries. Better characterization of the disease impact on smooth muscle will inform treatment development and help anticipate later complications. This review summarizes the published knowledge of smooth muscle pathology associated with Pompe disease in animal models and in patients.
Keywords: Pompe disease, smooth muscle, airway, vasculature, gastrointestinal
Background
Pompe disease is a devastating glycogen storage disease caused by a deficiency in acid α-glucosidase (GAA), an enzyme that is essential for lysosomal glycogen hydrolysis (1). Lack of functional GAA leads to the accumulation of glycogen throughout many organ systems, most notably in the heart and skeletal muscle. Pompe disease is an autosomal recessive disorder of GAA enzyme deficiency. Currently, the Pompe Center at Erasmus University, Rotterdam, Netherlands has described and aggregated more than 550 mutations in the GAA gene. There are gradient of phenotypes due to the extensive number of possible mutations. Originally observed in a single patient by J.C. Pompe in 1932, Pompe disease is now broadly divided in two classifications - infantile-onset (IOPD) or late-onset (LOPD), which are distinguished by clinical severity and age at onset (2,3,4).
Patients with IOPD have mutations that reduce functional GAA to levels <1% of normal (4, 5). Symptoms begin to present within the first few months of life and are rapidly progressive. The disease results in cardiomegaly, respiratory failure, and ultimately death within two years of life if untreated (3, 6, 7). The infantile-onset group is further divided into classical and non-classical IOPD, the latter of which is distinguished by slower progression and less severe cardiomyopathy (4, 8). A retrospective analysis of the natural course of IOPD found a median age of symptom onset at 2 months and median age of diagnosis at 4.7 months. Patients most commonly present with hypotonia, respiratory distress, muscle weakness, failure to thrive, and feeding difficulties. As patients age, they require ventilator support due to respiratory muscle weakness and disease progression. The median age of first ventilator support is 5.9 months and of death is 8.7 months (3).
Late-onset Pompe disease encompasses a broader spectrum of disease than IOPD. This includes a larger range in age of onset, time of diagnosis, and need for ventilator support. This spectrum is due to variation in active GAA levels, which is reported to be 1 – 40% (7, 9, 10). Late-onset patients typically manifest with proximal muscle weakness that begins in early childhood through adulthood, with some patients diagnosed as late as 60 years of age (11, 12). Patients with LOPD have a much slower disease course defined by decreasing muscular and respiratory function that leads to the use of walking aides, wheelchairs, and respiratory support (13, 14). Respiratory failure is the leading cause of death in LOPD patients (15). Interestingly, fatal cerebral aneurysm rupture has also been described and is attributed to glycogen buildup in the smooth muscle of cerebral arteries. Life expectancy is more variable in LOPD as opposed to IOPD due to the decreased severity of the overall disease. The best prognostic indicator for both IOPD and LOPD is the age of symptom onset. In both populations, patients with later onset have less severe symptoms and slower disease course than those with an earlier diagnosis (3, 13, 15).
Naturally occurring generalized glycogenosis and Pompe-like phenotypes have been described in a wide variety of animals including Japanese quail (16), Finnish and Swedish Lapphunds (17), Brahman and Shorthorm cattle species (18, 19), and Corriedale sheep (20, 21). Laboratory generated Gaa−/− mouse models are an instrumental resource for understanding the mechanisms of disease pathology and treatment evaluation (22,23,24).
Following nearly 30 years of preclinical development, two seminal clinical trials in the late 1990s evaluated the efficacy of the now sole FDA-approved treatment for PD. This treatment is known as enzyme replacement therapy (ERT) and is comprised of recombinant human GAA (rhGAA) (25,26,27,28,29). Also known as alglucosidase alfa or Lumizyme® (Genzyme, Cambridge, Massachusetts), ERT has substantially prolonged survival in many IOPD patients, predominantly by reducing cardiac hypertrophy (30, 31). However, ERT is less effective in patients who are cross reactive immunologic material (CRIM) negative, an indicator that they produce no residual GAA, due to an immune response against the enzyme (32). For patients whose symptoms are primarily in skeletal muscle, ERT is less effective due to less endocytic receptors compared to cardiac muscle and impaired autophagy (33). The major alternative treatment currently under investigation is gene therapy, in which recombinant adeno-associated viral (rAAV) vectors are used to deliver a functional copy of hGAA (34, 35). Clinical trials assessing this method are completed, ongoing, or enrolling (36,37,38,39).
Respiratory insufficiency is common in Pompe disease, with nearly 50% of all patients eventually requiring ventilator assistance, and the associated pathophysiology has many facets (8, 40,41,42,43). Skeletal muscle dysfunction results in macroglossia and weakness in the tongue, which then obstructs the upper airway. Diaphragm and accessory muscle weakness reduce respiratory capacity. Additionally, decreased hypoglossal and phrenic nerve innervation of the tongue and diaphragm, respectively, exacerbates the dysfunction in these muscles (44,45,46,47,48). Recently, new respiratory system symptoms, such as bronchomalacia and tracheomalacia, have been reported in patients surviving longer on ERT (49). These symptoms indicate that glycogen accumulation in smooth muscle also contributes to respiratory insufficiency.
While extensive attention is given to cardiac and skeletal muscle, few studies have comprehensively investigated how glycogen accumulation in smooth muscle plays out in the pathophysiology. This review summarizes available preclinical data and case studies indicating smooth muscle dysfunction in the respiratory, vascular, gastrointestinal, and genitourinary systems. Our aim is to examine the current literature to generate an up-to-date and comprehensive understanding of the impact of Pompe disease on smooth muscle throughout the organ systems. A breakdown of findings by system is shown in Table 1. Additionally, we have included original figures as examples of representative smooth muscle sections in the Pompe disease mouse model.
Table 1. Summary of Pompe disease clinical reports citing smooth muscle pathology.
| Smooth muscle location | Findings | Clinical correlate | Frequency of symptoms | References | |
| Aorta | Glycogen deposition in the media of aorta with large ascending aneurysm | Cerebrovascular events, Aneurysms, Dilative arteriopathy | >20 patients | (11, 88, 89, 92) | |
| Coronary artery | Glycogen deposition | (11) | |||
| Carotid artery | Glycogen deposition | (56, 65, 87) | |||
| Arteries and veins | Glycogen deposition in smooth muscle cells only Sparing other cell types |
(11, 14, 65, 74,75,76,78, 79, 82, 88, 89, 91,92,93,94,95, 105, 118) | |||
| Eye | Glycogen accumulation in the iris sphincter muscle and ciliary body | Strabismus, Ptosis, Myopia | >10 patients | (65, 108, 119) | |
| GI tract | Glycogen deposition in the tunica muscularis and muscularis mucosa of the GI tract, as well as esophagus and stomach | Nausea, vomiting, Diarrhea, Abdominal pain, Dysphagia | >5 patients | (11, 54, 65, 96,105, 118) | |
| Genitourinary | Glycogen deposition in the muscularis propria of the bladder | Urinary incontinence | >5 patients | (11, 65, 67, 96,102, 105, 107) | |
| Arrector Pili | Glycogenosomes and autophagic vacuoles | Not described | >5 patients | (109) | |
| Airway | Glycogen deposition in the trachea, bronchi and bronchioles, Reduced bronchoconstriction, Impaired calcium signaling in smooth muscle cells | Band-like atelectasis, Bronchomalacia, Tracheomalacia | <5 patients | (36, 49, 113) | |
| Uterus | Glycogen deposition | Not described | Only in mice | (67) | |
| Spleen | Glycogen deposition in the capsule and trabeculae | Not described | Only in mice | (67) | |
Methods
Selection of manuscripts
Manuscripts were identified using the search term (((((airway[Title/Abstract]) OR vasc*[Title/Abstract]) OR gastrointest*[Title/Abstract]) OR urin*[Title/Abstract]) OR smooth muscle[Title/Abstract]) AND pompe*[Title/Abstract] in PubMed.
Periodic acid-Schiff staining
Heterozygous Gaatm1Rabn mice were obtained from The Jackson Laboratory (24). They were bred to generate Gaa−/− and B6;129 WT littermates that were maintained in accordance with Duke University Institutional Animal Care and Use Committee (IACUC). They were euthanized at six months of age and smooth muscle (esophagus and bladder) was fixed in glutaraldehyde solution. The samples were processed, embedded into plastic, and sectioned by the Duke University Research Electron Microscopy Core. The 2μm sections were stained with Periodic acid-Schiff’s reagent (Sigma) according to manufacturer’s instructions. Briefly, sections were hydrated for 5 min, then incubated with preheated Periodic Acid, followed by incubation with preheated Schiff’s reagent for 15 min. Sections were counterstained with Toluidine Blue, then dehydrated in an increasing gradient of ethanol. Tissues were imaged using a Leica DMRA2 Compound Microscope with OpenLab Software.
Results
Literature search
Our search yielded 106 results published in English. Of these, 37 related directly to smooth muscle pathology in Pompe disease and are referenced here. The remaining 69 manuscripts were excluded for the following reasons: not pertaining to Pompe disease, not pertaining to smooth muscle symptoms or biochemical and histological analysis, and/or the manuscript was a review. We also included 11 manuscripts that were not obtained by the original search, but were referenced within the manuscripts of the original search.
Airway smooth muscle
Respiratory insufficiency is among the most common symptom that Pompe disease patients experience; often it is the first that raises concern of a problem (8, 40, 50). In a study of both IOPD and LOPD patients, respiratory distress was the reported presenting symptom in at least 50% of patients (50). Both tachypnea and dyspnea or rapid and labored breathing are reported (51,52,53). Pulmonary function tests (PFTs) are used to evaluate respiratory muscle strength and disease progression (8).
The sequela of respiratory system dysfunction include increased respiratory infections and reduced sleep quality (8, 54,55,56,57). Aspiration pneumonia can occur and can be a serious infection with long lasting effects. Even when appropriately treated, patients who contract pneumonia in their first three years of life have reduced PFTs (58). Patients also have significantly higher risks for sleep disordered breathing and obstructive sleep apnea (OSA) (59), both of which decrease quality of life (QoL) scores (59). The treatment for respiratory insufficiency, distress, and OSA is respiratory support at levels ranging from non-invasive ventilation (NIV) to tracheostomy and continuous ventilator support. Non-invasive ventilation encompasses continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP). Both CPAP and BiPAP require masks, which can be uncomfortable. The treatment itself can cause airway dryness, congestion, ulceration of the skin where the mask contacts the face, and abdominal distention or flatulence as air enters the stomach incidentally. As the disease progresses, NIV is no longer sufficient to maintain adequate ventilation of the lungs. Once continuous ventilator support is required, a patient may undergo a tracheostomy to have a tube inserted into their upper airway. This allows for mechanical ventilation without the need for orotracheal intubation. Early complications include obstruction of the tube and pneumothorax or subcutaneous emphysema. Late complications include tracheal stenosis, ulceration around the trachea tube or fistula formation, decreased ability to speak, and readmission (60,61,62).
According to a comprehensive study of 225 patients, respiratory failure is the most common cause of death among non-classical IOPD and LOPD patients (15). Respiratory failure was reported in more than 72% of patients of those analyzed in this study (15). While respiratory insufficiency is traditionally attributed to weakness of the diaphragm and tongue as well as respiratory motor neuron pathology (40, 44, 46,47,48, 63, 64), recent studies of surviving IOPD patients and the Gaa−/− mouse reveal the importance of respiratory smooth muscle involvement (49, 65, 66).
Pena et al. reports the only findings regarding glycogen accumulation in the human airway smooth muscle. In the postmortem autopsies they found mild to moderate glycogen accumulation within the bronchi of the three patients (65). All of these children experienced respiratory illnesses that contributed to their deaths. Two required ventilation assistance to address obstructive apnea and hypoventilation.
In cattle with generalized glycogenosis, two affected calves died within the first year of life. Both calves showed symptoms of severe respiratory distress and red froth in the trachea (18). Bijvoet et al. performed histological analyses using their Pompe disease mouse model and observed glycogen accumulation within the mice bronchioles (67).
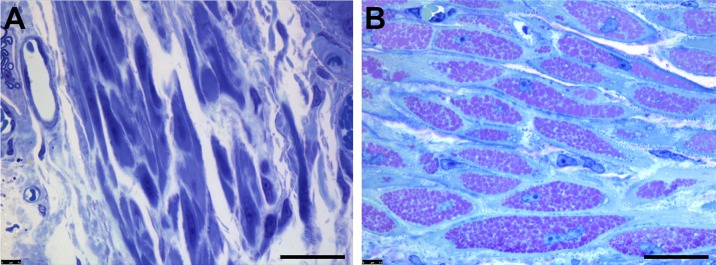
Keeler et al. provides the first in depth look at the impact of Gaa-deficiency on airway smooth muscle in the most commonly used Gaa−/− mouse model generated by Raben et al (24, 66). Glycogen accumulation within smooth muscle cells of the trachea and bronchi disrupted the cellular architecture. Electron micrographs also revealed enlarged lysosomes within the trachealis smooth muscle in Gaa−/− mice which were clearly absent in controls (66). In addition, the airway of Gaa−/− mice were hyporesponsive to the bronchoconstrictive agent methacholine, indicating that the glycogen accumulation within smooth muscle cells impaired their contractility. Finally, at a molecular level, there was reduced calcium signaling in Gaa−/− airway smooth muscle cells. The researchers hypothesize that reductions in sustained contractility were secondary to impaired calcium influx to the cell (66). Periodic acid-Schiff positive (PAS+) staining indicated accumulation in the smooth muscle layer of the trachealis from adult Gaa−/− mice, whereas wildtype mice were devoid of glycogen deposits (Fig. 1A, B).
Fig. 1.
Glycogen Deposits found within Airway Smooth Muscle of Gaa−/− Mouse.
Representative images of fixed and plastic embedded tissue, sectioned, and stained with Periodic Acid-Schiff reagents to detect glycogen, then counterstained with Toluidine Blue. 2μm sections from the trachealis of six-month-old (A) wildtype and (B) Gaa−/− mice are indicative of the smooth muscle found along the airway musculature. Aggregates of pink puncta indicate large deposits of glycogen within lysosomes. Scale bar = 20 μm.
Effects of ERT
Despite the advent of ERT, case reports indicate that many patients still require some form of mechanical ventilation (31, 49, 54, 65). Pena et al. describes three IOPD patients who received ERT for 2.5, 4, and 15 months yet still suffered cardiorespiratory failure (65). Upon autopsy, glycogen accumulation was found within the respiratory tracts of each child (65). A female patient diagnosed at 9 years old began experiencing deterioration of her respiratory system after five years on ERT. Her FVC and FEV1 levels had decreased more than 30% over the preceding three years (49). Investigation of her airways led to the novel finding of tracheomalacia and bronchomalacia. This deterioration of the lower airway luminal diameter increases the work of breathing and can precipitate respiratory distress. Tracheomalacia/bronchomalacia is also associated with more frequent respiratory illnesses with longer subsequent recovery times (68). Her left bronchus was weakened so greatly that more than 90% of the lumen was collapsed, which required a metallic stent be implanted (49). A similar finding was noted in a teenage male who required an emergency bronchoscopy while under anesthesia (69). He had complete effacement of his distal trachea and bronchi. Evaluation of tracheo- and bronchomalacia by bronchoscopy is not routinely performed in Pompe disease patients on ERT but should be considered in patients with progressive respiratory distress prior to initiating positive pressure ventilation. In long-term studies of LOPD patients on ERT, reported respiratory system responses range from minor improvements to deterioration plateaus to diminished benefits after an initial improvement (70, 71). Thus, despite ERT, airway smooth muscle glycogen accumulation may continue to be a problem and further studies are needed to investigate the impact on respiratory function in this population.
Vascular smooth muscle
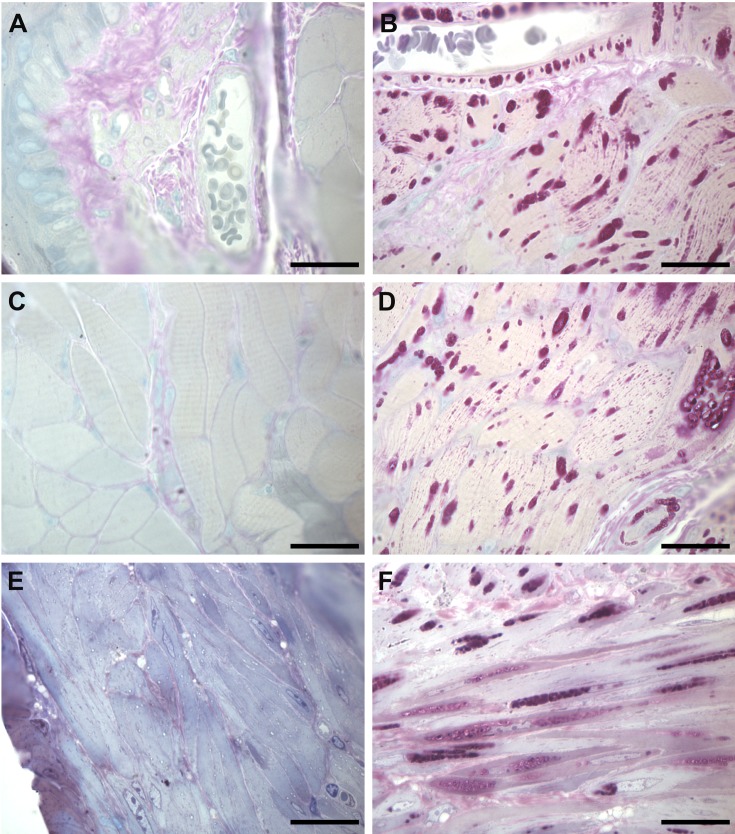
In 1975, Manktelow and Hartley presented the first report of naturally occurring generalized glycogenosis in an animal, which described necropsy of Corriedale sheep in New Zealand (20). They observed large PAS+ deposits, indicative of glycogen accumulation, in both small and large blood vessels including the aorta. Since then, similar findings have been described in cattle and Japanese quail (16, 72). The pathology of PAS+ aggregation also occurs in the smooth muscle layers of Gaa−/− mice arteries, significant in comparison to its complete absence in wildtype mice (Fig. 2A, B).
Fig. 2.
Glycogen Deposits found within Vascular, GI, and GU Smooth Muscle of Gaa−/− Mouse.
Representative images of fixed and plastic embedded tissue, sectioned to, and stained with Periodic Acid-Schiff reagents to detect glycogen, then counterstained with Toluidine Blue. 2μm sections from the esophagus of six-month-old (A and C) wildtype and (B and D) Gaa−/− mice are indicative of the smooth muscle found along the vascular system (A and B) as well as within the gastrointestinal tract (C and D). 2μm sections from the bladder of (E) wildtype and (F) Gaa−/− mice represent the pathology present in the genitourinary. Aggregates of pink puncta indicate large deposits of glycogen within lysosomes. Scale bar = 20 μm.
A vast body of published data on Pompe disease and smooth muscle focuses on the smooth muscle within small arteries and arterioles (11, 65, 67, 73). Clinically, the vascular smooth muscle pathology presents as a cerebral aneurysm, aortic aneurysm, aortic dissection, or dilative arteriopathy. In LOPD patients, both with and without generalized myopathy, magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) reveal arteriopathy within vertebrobasilar arteries, renal and iliac arteries, cerebral artery, and carotid arteries (14, 74,75,76,77). Interestingly, in most reports of patients with vertebrobasilar dolichoectasia, there are no neurological abnormalities (78). Many experience life-threatening comas, aneurysms, strokes, and microhemorrhages (70, 74, 75, 79, 80). These arteriopathies are frequently a presenting sign of disease, and as a result authors suggest that MRI and MRA be included in work-ups of all adult Pompe disease patients (74, 75, 79).
Dilation of the basilar artery is among the most common symptoms (74, 80,81,82,83,84,85). The basilar artery supplies the brain with oxygen-rich blood. When cerebral arteries deteriorate and micro-hemorrhage, patients experience frequent headaches as well as numbness along one side of the face that continues down the shoulder and arm (85,86,87). Weakened, glycogen-filled muscle myocytes may be the cause of dolichoectasia, which reduces blood flow and in one reported case caused stenosis of the cerebral artery (87). In addition to blood vessel occlusion, cerebral artery walls become fragile and rupture, leading to coma and fatal aneurysms (80, 81). Winkel et al. compiles the natural histories of more than 200 patients with non-classic IOPD and LOPD, and finds that cerebral aneurysm rupture caused 3% of deaths - an astonishing number for an under-recognized symptom (15).
El Gharbawy et al. reports 5 females with LOPD who had aortic aneurysms between 3.5 – 4.5 cm in length, predominantly in the ascending aorta and aortic arch (88). Furthermore, aortic stiffness leading to increased blood pressure, a predictor of cardiovascular-related fatality, was found in a cohort of 17 LOPD patients (89). In autopsies of IOPD patients, glycogen is observed within blood vessels (65, 90).
On a cellular level, glycogen build-up and vacuoles are found in smooth muscle cells (SMCs) within the tunica media of larger vessels such as the carotid artery and aorta, as well as in capillaries and small vessels throughout multiple organ systems (11, 91, 92). In the arteries of a 40 year-old male patient, PAS+ material was found within lysosomes and the cytoplasm, indicating that the glycogen burden within lysosomes was too great and led to rupture (93). The walls of an artery with a small aneurysm were decorated with PAS+ material. It is likely that as glycogen accumulates the vasculature weakens and small aneurysms form, leading to further weakening of the vessel walls (93).
Effects of ERT
Only a few studies report vascular pathology in patients receiving ERT. A follow-up to the initial ERT clinical trial using rhGAA purified from rabbit milk reports that one in four patients saw reduced cytoplasmic and lysosomal glycogen in arteries and veins (94). In one young man receiving ERT, microhemorrhages and diffuse ectasia of intracranial vessels were present (86). A prospective study of 8 LOPD patients receiving ERT reveal that hypertension is present in patients despite ERT (76). This is corroborated by a larger study of 84 non-classical IOPD patients, 82% of whom had been receiving ERT for an average of 5 years. In these patients, increased aortic stiffness correlated with an increase in blood pressure (95). Furthermore, Corti et al. describes an adolescent male with IOPD who began ERT immediately upon diagnosis at 15 months of age. Despite prolonged ERT administration, at 12 years old he suffered an intracranial hemorrhage resulting in death (37). In the Bijvoet et al. mouse model, glycogen decreased somewhat but not completely in arteries and veins following 14–15 weeks of rhGAA delivery (96). This indicates that despite ERT with rhGAA, the vascular smooth muscle pathology persists and can result in fatal outcomes such as arterial dissections, strokes, and aneurysms.
Gastrointestinal system smooth muscle
In IOPD, reported gastrointestinal (GI)-related symptoms include gastroesophageal reflux (GER), oropharyngeal dysphagia, and feeding difficulties (3, 54, 90, 97). Oropharyngeal dysphagia is reported in a patient as young as 15-days old (98). These symptoms result in poor weight gain, failure to thrive, inability to be fed by mouth, and a need for nutritional support (8). Poor feeding, GER, use of a gastrostomy tube, use of a nasogastric tube, and dysphagia are also present in many of these young patients (65).
Symptoms of nausea, vomiting, chronic diarrhea, intestinal incontinence, abdominal pain, bloating, loss of appetite, and early satiety are less acknowledged but still present in LOPD (54). Patients not receiving ERT report chronic bloating, abdominal discomfort or pain, cramps, vomiting, bowel incontinence, uncontrolled diarrhea, poor appetite, hypercalcemia, use of a feeding tube, sensitivity to dairy, intolerance to cold, weight loss, gastroesophageal reflux, and chronic constipation (54, 91, 99). In a post-mortem study, one patient experienced disease onset at 4 years old but did not begin ERT until 28 years old, and was found to have a dilated esophagus (52). Pre-mortem, the patient suffered from severe emaciation and low albumin levels, secondary to GI involvement (52).
Glycogen accumulation is reported throughout the gastrointestinal tract (16, 22, 54, 65, 100). In autopsy studies, glycogen accumulation is found in the stomach, esophagus, small intestine, and in both infantile onset and late onset Pompe disease. Autopsies of two Lapland canines with glycogenosis demonstrated vacuolation of the center of smooth muscle fibers within the GI tract, which likely caused the reported vomiting (101). A biodistribution study of the Bijvoet et al. mouse model reveals glycogen accumulation within the tunica muscularis and muscularis mucosae; smooth muscle layers which line the GI tract from the small intestine to the colon (96). Researchers also note abnormal glycogen deposition in the gallbladders and within the capsule and trabeculae of spleens in Pompe mice (67, 96). Smooth muscle PAS+ staining in the stomach, cecum, and intestines is also found in mice (67). Such glycogen accumulation likely influences the gastrointestinal symptoms of the disease. A study using Gaa−/− mice notes that intestinal blockage may have been the cause of death in the animals (67). Researchers found glycogen deposits within the muscularis mucosa and muscularis propria layers of the esophagus (Fig. 2C, D). In LOPD, glycogen accumulation in the smooth muscle may cause constipation and diminished bowel motility (67). Similarly, glycogen accumulation explains the intestinal dysmotility and impaired bowel control found in a group of IOPD patients (65).
Lysosomal dysfunction leads to the increased vacuolation of endosomes and autophagosomes that are detected in gastrointestinal smooth muscles (16, 20, 82, 100). In sheep, vacuolation has been observed in the rumen and intestine (20). Likewise, PAS+ staining-identifies vacuolation changes in the esophagus, stomach, and intestines of quail (16). An autopsy of a patient with LOPD showed vacuolated muscle within the lower esophagus, ileum, and bladder (100). Lastly, another study finds vacuolation of the gastric wall and stroma (82).
Effects of ERT
In a mouse model, glycogen accumulation was significantly reduced in the smooth muscle of the stomach and salivary glands after initiating ERT (96). PAS+ staining in the smooth muscle of the stomach and digestive tract was also diminished (96). Early initiation of ERT could swiftly improve GI symptoms. Since GI smooth muscle cells have inherently less glycogen accumulation, they may take longer to progress to a severe accumulation stage. This is unlike cardiac muscle, type II skeletal muscle fibers, and airway smooth muscle (54, 66, 96).
Glycogen reduction following ERT administration ameliorates GI-related symptoms. Sacconi et al. notes three LOPD patients that reported gastrointestinal symptoms; two of these had chronic constipation and the other had gastrointestinal reflux (91). Interestingly, all of the patients with GI-related symptoms also had vascular irregularities (91). One year after the start of ERT, GI symptoms had resolved in all three patients (91). In another LOPD patient, fecal incontinence had resolved and internal sphincter pressures had alleviated after four months of ERT (102). Likewise, Bernstein et al. reports that all GI related symptoms in three LOPD patients resolved after ERT for 3, 5, and 10 months (54). The most notable symptoms that are reported to be resolved after ERT are chronic constipation, gastroesophageal reflux, incontinence, abdominal discomfort, and poor appetite (54, 91, 103, 104). However, a report on another LOPD patient who only survived 21 months after the start of ERT identifies unresolved smooth muscle pathology of an enlarged esophagus (52).
Genitourinary tract smooth muscle
Glycogen accumulation and vacuolated smooth muscle is found in the muscularis propria of both the urinary bladder and urethra of IOPD patients (100, 105). In mouse models, glycogen accumulation is found in the kidney, urinary bladder, and epididymis of the testes (67). Clinically, LOPD patients report urinary incontinence at all stages of the disease (11, 12, 67, 102). The reported urinary incontinence in a cohort of Pompe disease patients was higher than that of the general population (106). However, Remiche et al. does not report similar findings in its cohort (102). Other reported symptoms include weak stream, post-void dribbling, bladder infections, and inability to stop stream (106). Lower urinary tract symptoms and urinary incontinence is also associated with IOPD (107). Children have daytime incontinence, nocturia, intermittency, hesitancy, giggle incontinence, stress incontinence, and voiding dysfunction (107). These urinary symptoms are likely the result of glycogen accumulation in the smooth muscle of the GU system (106). In Fig. 2E, F, we show PAS+ glycogen accumulation in Gaa−/− mouse bladders.
Effects of ERT on GU smooth muscle
After the onset of ERT delivery in mice, degradation of glycogen in the bladder has been observed using EM microscopy (96). In one patient, nocturnal urinary incontinence resolved after 3 months of ERT (54). This contrasts with LOPD patients who did not see relief (99). In a post-mortem analysis of this patient the smooth muscle was examined and the muscularis propria of the bladder showed no clearance of glycogen (105).
Ocular Smooth Muscle
Children with IOPD have vacuolar myopathy of the ciliary body smooth muscle and the iris sphincter smooth muscle (65, 108). The clinical correlation of the iris smooth muscle accumulation is unclear. However, the involvement of the ciliary body smooth muscle increases the risk of myopia, or nearsightedness, in patients with Pompe disease. Ciliary muscle involvement can also increase the risk of astigmatism, which is prominent in patients surviving long term with infantile onset Pompe disease (65).
Dermatologic smooth muscle
Within the skin, glycogen accumulation and vacuolization occurs in the arrector pili muscles and eccrine glands (65, 109). Katona et al. finds that levels of accumulated glycogen correlate with the disease severity. More glycogen deposition and vacuolization correlates with poorer prognosis and heavier disease burden. As skin biopsies are relatively non-invasive and practical to perform in an outpatient setting, they may yield more utility in helping patients to understand their disease and give more accurate prognoses (109).
Discussion & Conclusions
As described here, the impact of Pompe disease, once thought to be limited to the cardiopulmonary system and limb-girdle muscles, has a broader impact on the body that involves smooth muscle to varying degrees. Furthermore, we provide evidence of glycogen accumulation in the smooth muscle layers of several relevant tissues (trachealis, esophagus, and bladder) in the most commonly used Pompe disease mouse model, which was developed by Nina Raben (24). The pathology present in these tissues mimics that found in patients, which makes these models a useful tool for investigating the disease mechanisms and the development of improved therapies.
The advent of ERT has increased the lifespan of infantile onset Pompe disease by preventing heart failure and infantile death (110,111,112). Despite ERT, there is still an incidence of cerebral artery aneurysms and death, (37) and the disease continues to progress in the respiratory system (8). For instance, patients receiving ERT show greater improvement in motor outcomes relative to respiratory outcomes, and this discrepancy is attributed to diaphragmatic involvement (113). However, given the pathology noted in airway smooth muscles, the tracheomalacia, and the flaccidity of the airway in Pompe patients, it is possible that glycogen accumulation and weakness in lower airway smooth muscles contributes to differences in ERT responses (49, 113). Further research is needed to clarify how airway smooth muscle compromise impacts pulmonary function and disease progression.
Dysfunction in the gastrointestinal and genitourinary systems impact patient QoL. In one cohort nearly half of the patients reported “frustration”, “interference with activities”, and “overall bother” due to GU-related symptoms (106). Similarly, lower reported QoL in LOPD patients may be due to a host of GI symptoms, such as gastroesophageal reflux and abdominal discomfort (54). Generally, urinary and fecal incontinence are related to reduced QoL. In the case of LOPD, incontinence is also associated with decreased Qol (102). Urinary incontinence is both underdiagnosed and undertreated in LOPD patients (106).
Autophagy dysregulation is a widely described pathology that occurs subsequent to glycogen accumulation, and significantly burdens the cellular architecture, particularly in skeletal muscle. As originally proposed by Fukuda et al, autophagic flux ultimately disrupts from both the back and the front (114, 115). At first, autophagosomes lose their ability to fuse with glycogen-filled lysosomes, creating an accumulation of vesicles that cannot be cleared. Increased autophagosome formation is initiated when glycogen is not degraded and the cell senses glucose shortage. The abundance of lysosomes and autophagosomes within the muscle fiber disrupts contractility and places a physical burden on the sarcolemma, eventually leading to breakage (33, 116). When this occurs in many fibers in a single muscle, the muscle as a whole weakens. While no histological or molecular case studies in either Pompe patients or mouse models have reported the presence of autophagosomes in smooth muscle, a similar mechanism is potentially in effect.
Another potential cause of smooth muscle weakness in Pompe disease patients is decreased calcium handling. Influx of calcium ions (Ca2+) is crucial for the initiation of contractile force among actin and myosin cross-bridges (117). Keeler et al. demonstrates that Ca2+ influx from sarcoplasmic reticulum reserves is relatively unchanged in Gaa−/− mouse bronchi compared to WT (66). However, the influx of extracellular Ca2+ through ion channels within the plasma membrane is significantly reduced (66). The exact mechanism of reduction of ion channel activity has not been delineated, but could be due to tensile changes placed on the membrane from the amassing glycogen-filled vesicles.
In summary, as more long-term data has emerged on the phenotypes of LOPD patients and IOPD patients on ERT, it has become clear that the impact of Pompe disease is widespread throughout the body. Smooth muscle plays a vital role in normal physiologic function and Pompe disease clearly harms its homeostasis. With many of the disease symptoms attributed to smooth muscle dysfunction, more research is needed to elucidate how glycogen accumulation disrupts normal smooth muscle function. Not only is a greater understanding of the cellular mechanism and pathophysiology of the disease in smooth muscle crucial, but evaluation of the effectiveness of ERT and novel therapies in these tissues should be taken into consideration. Further studies are needed to evaluate the efficacy of treatments on smooth muscle in both the animal model and Pompe disease patients.
Authors’ contributions
ALM completed mouse work presented here and was a key contributor to the manuscript writing and editing. JS was a major contributor to manuscript writing. PB compiled literature review notes and was a minor contributor to the manuscript writing. LMS was a key manuscript editor. MKE conceptualized the manuscript, and acted as supervisor and editor of the work by all other authors. All authors read and approved the final manuscript.
Ethics approval
The usage of mice in this study was approved by the Duke University Institutional Animal Care and Use Committee (A232-17–10).
Competing interests
The authors declare that they have no competing interests.
Funding
Funding from K08HD077040-07 (MKE), R21 NS098131-02 (MKE), Derfner Foundation Award (ALM) supported the novel findings presented in this review. The authors confirm independence from the sponsors; the consent of the article has not been influenced by sponsors.
Acknowledgments
The authors would like to thank Marina Zeiger, PhD for her contributions to the development and preparation of the trachea staining. We would also like to acknowledge the Duke University Electron Microscopy Core, specifically Sara Miller, PhD and Ricardo Vancini, PhD for their expertise and skill in the preparation of tissues.
References
- 1.Hers HG. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe’s disease). Biochem J. 1963; 86: 11–6. doi: 10.1042/bj0860011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hout HMP, Hop W, van Diggelen OP, Smeitink JAM, Smit GPA, Poll-The BTT, Bakker HD, Loonen MCB, de Klerk JBC, Reuser AJJ, van der Ploeg AT. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003; 112(2): 332–40. doi: 10.1542/peds.112.2.332 [DOI] [PubMed] [Google Scholar]
- 3.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D, Infantile-Onset Pompe Disease Natural History Study Group. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006; 148(5): 671–6. doi: 10.1016/j.jpeds.2005.11.033 [DOI] [PubMed] [Google Scholar]
- 4.Kishnani PS, Steiner RD, Bali D, Berger K, Byrne BJ, Case LE, Crowley JF, Downs S, Howell RR, Kravitz RM, Mackey J, Marsden D, Martins AM, Millington DS, Nicolino M, O’Grady G, Patterson MC, Rapoport DM, Slonim A, Spencer CT, Tifft CJ, Watson MS. Pompe disease diagnosis and management guideline. Genet Med. 2006; 8(5): 267–88. doi: 10.1097/01.gim.0000218152.87434.f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beratis NG, LaBadie GU, Hirschhorn K. Genetic heterogeneity in acid alpha-glucosidase deficiency. Am J Hum Genet. 1983; 35(1): 21–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Slonim AE, Bulone L, Ritz S, Goldberg T, Chen A, Martiniuk F. Identification of two subtypes of infantile acid maltase deficiency. J Pediatr. 2000; 137(2): 283–5. doi: 10.1067/mpd.2000.107112 [DOI] [PubMed] [Google Scholar]
- 7.Reuser AJ, Koster JF, Hoogeveen A, Galjaard H. Biochemical, immunological, and cell genetic studies in glycogenosis type II. Am J Hum Genet. 1978; 30(2): 132–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne BJ, Kishnani PS, Case LE, Merlini L, Müller-Felber W, Prasad S, van der Ploeg A. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab. 2011; 103(1): 1–11. doi: 10.1016/j.ymgme.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Kroos MA, Pomponio RJ, Hagemans MLC, Keulemans JLM, Phipps M, DeRiso M, Palmer RE, Ausems MG, Van der Beek NAME, Van Diggelen OP, Halley DJJ, Van der Ploeg AT, Reuser AJ. Broad spectrum of Pompe disease in patients with the same c.-32-13T->G haplotype. Neurology. 2007; 68(2): 110–5. doi: 10.1212/01.wnl.0000252798.25690.76 [DOI] [PubMed] [Google Scholar]
- 10.Wokke JHJ, Escolar DM, Pestronk A, Jaffe KM, Carter GT, van den Berg LH, Florence JM, Mayhew J, Skrinar A, Corzo D, Laforet P. Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve. 2008; 38(4): 1236–45. doi: 10.1002/mus.21025 [DOI] [PubMed] [Google Scholar]
- 11.Hobson-Webb LD, Proia AD, Thurberg BL, Banugaria S, Prater SN, Kishnani PS. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab. 2012; 106(4): 462–9. doi: 10.1016/j.ymgme.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 12.Chancellor AM, Warlow CP, Webb JN, Lucas MG, Besley GT, Broadhead DM. Acid maltase deficiency presenting with a myopathy and exercise induced urinary incontinence in a 68 year old male. J Neurol Neurosurg Psychiatry. 1991; 54(7): 659–60. doi: 10.1136/jnnp.54.7.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagemans MLC, Winkel LPF, Van Doorn PA, Hop WJC, Loonen MCB, Reuser AJJ, Van der Ploeg AT. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005; 128(Pt 3): 671–7. doi: 10.1093/brain/awh384 [DOI] [PubMed] [Google Scholar]
- 14.Montagnese F, Granata F, Musumeci O, Rodolico C, Mondello S, Barca E, Cucinotta M, Ciranni A, Longo M, Toscano A. Intracranial arterial abnormalities in patients with late onset Pompe disease (LOPD). J Inherit Metab Dis. 2016; 39(3): 391–8. doi: 10.1007/s10545-015-9913-x [DOI] [PubMed] [Google Scholar]
- 15.Winkel LPF, Hagemans MLC, van Doorn PA, Loonen MCB, Hop WJC, Reuser AJ, van der Ploeg AT. The natural course of non-classic Pompe’s disease; a review of 225 published cases. J Neurol. 2005; 252(8): 875–84. doi: 10.1007/s00415-005-0922-9 [DOI] [PubMed] [Google Scholar]
- 16.Matsui T, Kuroda S, Mizutani M, Kiuchi Y, Suzuki K, Ono T. Generalized glycogen storage disease in Japanese quail (Coturnix coturnix japonica). Vet Pathol. 1983; 20(3): 312–21. doi: 10.1177/030098588302000307 [DOI] [PubMed] [Google Scholar]
- 17.Seppälä EH, Reuser AJ, Lohi H. A nonsense mutation in the acid α-glucosidase gene causes Pompe disease in Finnish and Swedish Lapphunds. PLoS One. 2013; 8(2): e56825. doi: 10.1371/journal.pone.0056825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell JM, Dorling PR, Cook RD, Robinson WF, Bradley S, Gawthorne JM. Infantile and late onset form of generalised glycogenosis type II in cattle. J Pathol. 1981; 134(4): 267–77. doi: 10.1002/path.1711340403 [DOI] [PubMed] [Google Scholar]
- 19.Jolly RD, Van-de-Water NS, Richards RB, Dorling PR. Generalized glycogenosis in beef shorthorn cattle--heterozygote detection. Aust J Exp Biol Med Sci. 1977; 55(2): 14U–50. doi: 10.1038/icb.1977.11 [DOI] [PubMed] [Google Scholar]
- 20.Manktelow BW, Hartley WJ. Generalized glycogen storage disease in sheep. J Comp Pathol. 1975; 85(1): 139–45. doi: 10.1016/0021-9975(75)90092-4 [DOI] [PubMed] [Google Scholar]
- 21.Walvoort HC. Glycogen storage diseases in animals and their potential value as models of human disease. J Inherit Metab Dis. 1983; 6(1): 3–16. doi: 10.1007/BF02391186 [DOI] [PubMed] [Google Scholar]
- 22.Bijvoet AGA, van de Kamp EH, Kroos MA, Ding JH, Yang BZ, Visser P, Bakker CE, Verbeet MP, Oostra BA, Reuser AJ, van der Ploeg AT. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum Mol Genet. 1998; 7(1): 53–62. doi: 10.1093/hmg/7.1.53 [DOI] [PubMed] [Google Scholar]
- 23.Raben N, Lu N, Nagaraju K, Rivera Y, Lee A, Yan B, Byrne B, Meikle PJ, Umapathysivam K, Hopwood JJ, Plotz PH. Conditional tissue-specific expression of the acid alpha-glucosidase (GAA) gene in the GAA knockout mice: implications for therapy. Hum Mol Genet. 2001; 10(19): 2039–47. doi: 10.1093/hmg/10.19.2039 [DOI] [PubMed] [Google Scholar]
- 24.Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B, Plotz P. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem. 1998; 273(30): 19086–92. doi: 10.1074/jbc.273.30.19086 [DOI] [PubMed] [Google Scholar]
- 25.Hug G, Schubert WK. Lysosomes in type II glycogenosis. Changes during administration of extract from Aspergillus niger. J Cell Biol. 1967; 35(1): C1–6. doi: 10.1083/jcb.35.1.C1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Ploeg AT, Loonen MC, Bolhuis PA, Busch HM, Reuser AJ, Galjaard H. Receptor-mediated uptake of acid alpha-glucosidase corrects lysosomal glycogen storage in cultured skeletal muscle. Pediatr Res. 1988; 24(1): 90–4. doi: 10.1203/00006450-198807000-00021 [DOI] [PubMed] [Google Scholar]
- 27.Van der Ploeg AT, Kroos MA, Willemsen R, Brons NH, Reuser AJ. Intravenous administration of phosphorylated acid alpha-glucosidase leads to uptake of enzyme in heart and skeletal muscle of mice. J Clin Invest. 1991; 87(2): 513–8. doi: 10.1172/JCI115025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Hout H, Reuser AJ, Vulto AG, Loonen MCB, Cromme-Dijkhuis A, Van der Ploeg AT. Recombinant human α-glucosidase from rabbit milk in Pompe patients. Lancet. 2000; 356(9227): 397–8. doi: 10.1016/S0140-6736(00)02533-2 [DOI] [PubMed] [Google Scholar]
- 29.Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, Mackey J, Kishnani P, Smith W, McVie-Wylie A, Sullivan JA, Hoganson GE, Phillips JA, 3rd, Schaefer GB, Charrow J, Ware RE, Bossen EH, Chen YT. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001; 3(2): 132–8. [PubMed] [Google Scholar]
- 30.Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, McDonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, De la Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007; 68(2): 99–109. doi: 10.1212/01.wnl.0000251268.41188.04 [DOI] [PubMed] [Google Scholar]
- 31.Nicolino M, Byrne B, Wraith JE, Leslie N, Mandel H, Freyer DR, Arnold GL, Pivnick EK, Ottinger CJ, Robinson PH, Loo JCA, Smitka M, Jardine P, Tatò L, Chabrol B, McCandless S, Kimura S, Mehta L, Bali D, Skrinar A, Morgan C, Rangachari L, Corzo D, Kishnani PS. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009; 11(3): 210–9. doi: 10.1097/GIM.0b013e31819d0996 [DOI] [PubMed] [Google Scholar]
- 32.Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010; 99(1): 26–33. doi: 10.1016/j.ymgme.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shea L, Raben N. Autophagy in skeletal muscle: implications for Pompe disease. Int J Clin Pharmacol Ther. 2009; 47(Suppl 1): S42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME, Collins SW, Conlon TJ, Clement N, Cleaver BD, Cloutier DA, Porvasnik SL, Islam S, Elmallah MK, Martin A, Smith BK, Fuller DD, Lawson LA, Mah CS. Pompe disease gene therapy. Hum Mol Genet. 2011; 20(R1): R61–8. doi: 10.1093/hmg/ddr174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keeler AM, Zieger M, Todeasa SH, McCall AL, Gifford JC, Birsak S, Choudhury SR, Byrne BJ, Sena-Esteves M, ElMallah MK. Systemic delivery of AAVB1-GAA clears glycogen and prolongs survival in a mouse model of Pompe disease. Hum Gene Ther. 2019; 30(1): 57–68. doi: 10.1089/hum.2018.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith BK, Collins SW, Conlon TJ, Mah CS, Lawson LA, Martin AD, Fuller DD, Cleaver BD, Clément N, Phillips D, Islam S, Dobjia N, Byrne BJ. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther. 2013; 24(6): 630–40. doi: 10.1089/hum.2012.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corti M, Liberati C, Smith BK, Lawson LA, Tuna IS, Conlon TJ, Coleman KE, Islam S, Herzog RW, Fuller DD, Collins SW, Byrne BJ. Safety of Intradiaphragmatic Delivery of Adeno-Associated Virus-Mediated Alpha-Glucosidase (rAAV1-CMV-hGAA) Gene Therapy in Children Affected by Pompe Disease. Hum Gene Ther Clin Dev. 2017; 28(4): 208–18. doi: 10.1089/humc.2017.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti M, Cleaver B, Clément N, Conlon TJ, Faris KJ, Wang G, Benson J, Tarantal AF, Fuller D, Herzog RW, Byrne BJ. Evaluation of Readministration of a Recombinant Adeno-Associated Virus Vector Expressing Acid Alpha-Glucosidase in Pompe Disease: Preclinical to Clinical Planning. Hum Gene Ther Clin Dev. 2015; 26(3): 185–93. doi: 10.1089/humc.2015.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bond JE, Kishnani PS, Koeberl DD. Immunomodulatory, liver depot gene therapy for Pompe disease. Cell Immunol. 2017; S0008-8749(17)30238-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuller DD, ElMallah MK, Smith BK, Corti M, Lawson LA, Falk DJ, Byrne BJ. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol. 2013; 189(2): 241–9. doi: 10.1016/j.resp.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagemans MLC, Winkel LPF, Hop WCJ, Reuser AJ, Van Doorn PA, Van der Ploeg AT. Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. 2005; 64(12): 2139–41. doi: 10.1212/01.WNL.0000165979.46537.56 [DOI] [PubMed] [Google Scholar]
- 42.Haley SM, Fragala MA, Skrinar AM. Pompe disease and physical disability. Dev Med Child Neurol. 2003; 45(9): 618–23. doi: 10.1111/j.1469-8749.2003.tb00966.x [DOI] [PubMed] [Google Scholar]
- 43.Marsden D. Infantile onset Pompe disease: a report of physician narratives from an epidemiologic study. Genet Med. 2005; 7(2): 147–50. doi: 10.1097/01.GIM.0000154301.76619.5C [DOI] [PubMed] [Google Scholar]
- 44.DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH, Jr, Mah C, Reier PJ, Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA. 2009; 106(23): 9419–24. doi: 10.1073/pnas.0902534106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ElMallah MK, Falk DJ, Lane MA, Conlon TJ, Lee KZ, Shafi NI, Reier PJ, Byrne BJ, Fuller DD. Retrograde gene delivery to hypoglossal motoneurons using adeno-associated virus serotype 9. Hum Gene Ther Methods. 2012; 23(2): 148–56. doi: 10.1089/hgtb.2012.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KZ, Qiu K, Sandhu MS, Elmallah MK, Falk DJ, Lane MA, Reier PJ, Byrne BJ, Fuller DD. Hypoglossal neuropathology and respiratory activity in pompe mice. Front Physiol. 2011; 2: 31. doi: 10.3389/fphys.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner SM, Hoyt AK, ElMallah MK, Falk DJ, Byrne BJ, Fuller DD. Neuropathology in respiratory-related motoneurons in young Pompe (Gaa(-/-)) mice. Respir Physiol Neurobiol. 2016; 227: 48–55. doi: 10.1016/j.resp.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmallah MK, Falk DJ, Nayak S, Federico RA, Sandhu MS, Poirier A, Byrne BJ, Fuller DD. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice. Mol Ther. 2014; 22(4): 702–12. doi: 10.1038/mt.2013.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang CF, Niu DM, Jeng MJ, Lee YS, Taso PC, Soong WJ. Late-onset Pompe disease with left-sided bronchomalacia. Respir Care. 2015; 60(2): e26–9. doi: 10.4187/respcare.03419 [DOI] [PubMed] [Google Scholar]
- 50.Fukuhara Y, Fuji N, Yamazaki N, Hirakiyama A, Kamioka T, Seo JH, Mashima R, Kosuga M, Okuyama T. A molecular analysis of the GAA gene and clinical spectrum in 38 patients with Pompe disease in Japan. Mol Genet Metab Rep. 2017; 14: 3–9. doi: 10.1016/j.ymgmr.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito T, Sasaki T, Ono I. Secondary endocardial fibroelastosis associated with Pompe disease and multicystic dysplastic kidney. Heart Vessels. 2000; 15(5): 240–2. doi: 10.1007/s003800070014 [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi H, Shimada Y, Ikegami M, Kawai T, Sakurai K, Urashima T, Ijima M, Fujiwara M, Kaneshiro E, Ohashi T, Eto Y, Ishigaki K, Osawa M, Kyosen SO, Ida H. Prognostic factors for the late onset Pompe disease with enzyme replacement therapy: from our experience of 4 cases including an autopsy case. Mol Genet Metab. 2010; 100(1): 14–9. doi: 10.1016/j.ymgme.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 53.Baqir M, Ryu JH, Sorenson EJ, Olson EJ. A. A 62-year-old man with dyspnea. Respir Med Case Rep. 2016; 17: 50–3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernstein DL, Bialer MG, Mehta L, Desnick RJ. Pompe disease: dramatic improvement in gastrointestinal function following enzyme replacement therapy. A report of three later-onset patients. Mol Genet Metab. 2010; 101(2-3): 130–3. doi: 10.1016/j.ymgme.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 55.Burrow TA, Bailey LA, Kinnett DG, Hopkin RJ. Acute progression of neuromuscular findings in infantile Pompe disease. Pediatr Neurol. 2010; 42(6): 455–8. doi: 10.1016/j.pediatrneurol.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 56.Bembi B, Cerini E, Danesino C, Donati MA, Gasperini S, Morandi L, Musumeci O, Parenti G, Ravaglia S, Seidita F, Toscano A, Vianello A. Management and treatment of glycogenosis type II. Neurology. 2008; 71(23 Suppl 2): S12–36. doi: 10.1212/WNL.0b013e31818da93f [DOI] [PubMed] [Google Scholar]
- 57.Boentert M, Karabul N, Wenninger S, Stubbe-Dräger B, Mengel E, Schoser B, Young P. Sleep-related symptoms and sleep-disordered breathing in adult Pompe disease. Eur J Neurol. 2015; 22(2): 369–76, e27. doi: 10.1111/ene.12582 [DOI] [PubMed] [Google Scholar]
- 58.Chan JYC, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015; 135(4): 607–16. doi: 10.1542/peds.2014-3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boentert M, Dräger B, Glatz C, Young P. Sleep-disordered breathing and effects of noninvasive ventilation in patients with late-onset pompe disease. J Clin Sleep Med. 2016; 12(12): 1623–32. doi: 10.5664/jcsm.6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trottier SJ, Ritter S, Lakshmanan R, Sakabu SA, Troop BR. Percutaneous tracheostomy tube obstruction: warning. Chest. 2002; 122(4): 1377–81. doi: 10.1378/chest.122.4.1377 [DOI] [PubMed] [Google Scholar]
- 61.Fikkers BG, van Veen JA, Kooloos JG, Pickkers P, van den Hoogen FJA, Hillen B, van der Hoeven JG. Emphysema and pneumothorax after percutaneous tracheostomy: case reports and an anatomic study. Chest. 2004; 125(5): 1805–14. doi: 10.1378/chest.125.5.1805 [DOI] [PubMed] [Google Scholar]
- 62.Spataro E, Durakovic N, Kallogjeri D, Nussenbaum B. Complications and 30-day hospital readmission rates of patients undergoing tracheostomy: A prospective analysis. Laryngoscope. 2017; 127(12): 2746–53. doi: 10.1002/lary.26668 [DOI] [PubMed] [Google Scholar]
- 63.Mah C, Fraites TJ, Jr, Cresawn KO, Zolotukhin I, Lewis MA, Byrne BJ. A new method for recombinant adeno-associated virus vector delivery to murine diaphragm. Mol Ther. 2004; 9(3): 458–63. doi: 10.1016/j.ymthe.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 64.Falk DJ, Mah CS, Soustek MS, Lee KZ, Elmallah MK, Cloutier DA, Fuller DD, Byrne BJ. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther. 2013; 21(9): 1661–7. doi: 10.1038/mt.2013.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pena LDM, Proia AD, Kishnani PS. Postmortem Findings and Clinical Correlates in Individuals with Infantile-Onset Pompe Disease. JIMD reports, vol. 23. Springer; 2015. p. 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keeler AM, Liu D, Zieger M, Xiong L, Salemi J, Bellvé K, Byrne BJ, Fuller DD, ZhuGe R, ElMallah MK. Airway smooth muscle dysfunction in Pompe (Gaa-/- ) mice. Am J Physiol Lung Cell Mol Physiol. 2017; 312(6): L873–81. doi: 10.1152/ajplung.00568.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bijvoet AGA, Van Hirtum H, Vermey M, Van Leenen D, Van Der Ploeg AT, Mooi WJ, Reuser AJ. Pathological features of glycogen storage disease type II highlighted in the knockout mouse model. J Pathol. 1999; 189(3): 416–24. doi: [DOI] [PubMed] [Google Scholar]
- 68.Masters IB, Zimmerman PV, Pandeya N, Petsky HL, Wilson SB, Chang AB. Quantified tracheobronchomalacia disorders and their clinical profiles in children. Chest. 2008; 133(2): 461–7. doi: 10.1378/chest.07-2283 [DOI] [PubMed] [Google Scholar]
- 69.Brenn BR, Theroux MT, Shah SA, Mackenzie WG, Heinle R, Scavina MT. Critical Airway Stenosis in an Adolescent Male With Pompe Disease and Thoracic Lordosis: A Case Report. A Case Rep. 2017; 9(7): 199–203. [DOI] [PubMed] [Google Scholar]
- 70.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, Herson S, Kishnani PS, Laforet P, Lake SL, Lange DJ, Leshner RT, Mayhew JE, Morgan C, Nozaki K, Park DJ, Pestronk A, Rosenbloom B, Skrinar A, van Capelle CI, van der Beek NAME, Wasserstein M, Zivkovic SA. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010; 362(15): 1396–406. doi: 10.1056/NEJMoa0909859 [DOI] [PubMed] [Google Scholar]
- 71.Anderson LJ, Henley W, Wyatt KM, Nikolaou V, Waldek S, Hughes DA, Lachmann RH, Logan S. Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: results from the NCS-LSD cohort study. J Inherit Metab Dis. 2014; 37(6): 945–52. doi: 10.1007/s10545-014-9728-1 [DOI] [PubMed] [Google Scholar]
- 72.Richards RB, Edwards JR, Cook RD, White RR. Bovine Generalized Glyocogenosis. Neuropathol Appl Neurobiol. 1977; 3(1): 45–56. doi: 10.1111/j.1365-2990.1977.tb00568.x [DOI] [PubMed] [Google Scholar]
- 73.de Gijt JP, van Capelle CI, Oosterhuis JW, van der Ploeg AT, van der Wal KGH. Gingival overgrowth in Pompe disease: a case report. J Oral Maxillofac Surg. 2011; 69(8): 2186–90. doi: 10.1016/j.joms.2011.03.070 [DOI] [PubMed] [Google Scholar]
- 74.Refai D, Lev R, Cross DT, Shimony JS, Leonard JR. Thrombotic complications of a basilar artery aneurysm in a young adult with Pompe disease. Surg Neurol. 2008; 70(5): 518–20. doi: 10.1016/j.surneu.2007.05.049 [DOI] [PubMed] [Google Scholar]
- 75.Quenardelle V, Bataillard M, Bazin D, Lannes B, Wolff V, Echaniz-Laguna A. Pompe disease presenting as an isolated generalized dilative arteriopathy with repeated brain and kidney infarcts. J Neurol. 2015; 262(2): 473–5. [DOI] [PubMed] [Google Scholar]
- 76.Hensel O, Hanisch F, Stock K, Stoevesandt D, Deschauer M, Müller T. Morphology and function of cerebral arteries in adults with pompe disease. JIMD Rep. 2015; 20: 27–33. doi: 10.1007/8904_2014_385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pappa E, Papadopoulos C, Grimbert P, Laforêt P, Bassez G. Renal artery fibromuscular dysplasia in Pompe disease: A case report. Mol Genet Metab Rep. 2018; 16: 64–5. doi: 10.1016/j.ymgmr.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pichiecchio A, Sacco S, De Filippi P, Caverzasi E, Ravaglia S, Bastianello S, Danesino C. Late-onset Pompe disease: a genetic-radiological correlation on cerebral vascular anomalies. J Neurol. 2017; 264(10): 2110–8. doi: 10.1007/s00415-017-8601-1 [DOI] [PubMed] [Google Scholar]
- 79.Huded V, Bohra V, Prajapati J, DeSouza R, Ramankutty R. Stroke in Young-Dilative Arteriopathy: A Clue to Late-Onset Pompe’s Disease? J Stroke Cerebrovasc Dis. 2016; 25(4): e50–2. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 80.Laforêt P, Petiot P, Nicolino M, Orlikowski D, Caillaud C, Pellegrini N, Froissart R, Petitjean T, Maire I, Chabriat H, Hadrane L, Annane D, Eymard B. Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology. 2008; 70(22): 2063–6. doi: 10.1212/01.wnl.0000313367.09469.13 [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto Y, Etoh Y, Joh R, Noda K, Ohya I, Morimatsu M. Adult-onset acid maltase deficiency in siblings. Acta Pathol Jpn. 1985; 35(6): 1533–42. [DOI] [PubMed] [Google Scholar]
- 82.Makos MM, McComb RD, Hart MN, Bennett DR. Alpha-glucosidase deficiency and basilar artery aneurysm: report of a sibship. Ann Neurol. 1987; 22(5): 629–33. doi: 10.1002/ana.410220512 [DOI] [PubMed] [Google Scholar]
- 83.Braunsdorf WE. Fusiform aneurysm of basilar artery and ectatic internal carotid arteries associated with glycogenosis type 2 (Pompe’s disease). Neurosurgery. 1987; 21(5): 748–9. doi: 10.1227/00006123-198711000-00030 [DOI] [PubMed] [Google Scholar]
- 84.Matsuoka Y, Senda Y, Hirayama M, Matsui T, Takahashi A. Late-onset acid maltase deficiency associated with intracranial aneurysm. J Neurol. 1988; 235(6): 371–3. doi: 10.1007/BF00314237 [DOI] [PubMed] [Google Scholar]
- 85.Zhang B, Zhao Y, Liu J, Li L, Shan J, Zhao D, Yan C. Late-onset Pompe disease with complicated intracranial aneurysm: a Chinese case report. Neuropsychiatr Dis Treat. 2016; 12: 713–7. doi: 10.2147/NDT.S94892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandhu D, Rizvi A, Kim J, Reshi R. Diffuse cerebral microhemorrhages in a patient with adult-onset Pompe’s disease: a case report. J Vasc Interv Neurol. 2014; 7(5): 82–5. [PMC free article] [PubMed] [Google Scholar]
- 87.Anneser JMH, Pongratz DE, Podskarbi T, Shin YS, Schoser BGH. Mutations in the acid α-glucosidase gene (M. Pompe) in a patient with an unusual phenotype. Neurology. 2005; 64(2): 368–70. doi: 10.1212/01.WNL.0000149528.95362.20 [DOI] [PubMed] [Google Scholar]
- 88.El-Gharbawy AH, Bhat G, Murillo JE, Thurberg BL, Kampmann C, Mengel KEE, Kishnani PS. Expanding the clinical spectrum of late-onset Pompe disease: dilated arteriopathy involving the thoracic aorta, a novel vascular phenotype uncovered. Mol Genet Metab. 2011; 103(4): 362–6. doi: 10.1016/j.ymgme.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 89.Nemes A, Soliman OII, Geleijnse ML, Anwar AM, van der Beek NAME, van Doorn PA, Gavallér H, Csajbók E, ten Cate FJ. Increased aortic stiffness in glycogenosis type 2 (Pompe’s disease). Int J Cardiol. 2007; 120(1): 138–41. doi: 10.1016/j.ijcard.2006.07.215 [DOI] [PubMed] [Google Scholar]
- 90.Clement DH, Godman GC. Glycogen disease resembling mongolism, cretinism, and amytonia congenita; case report and review of literature. J Pediatr. 1950; 36(1): 11–30 illust. doi: 10.1016/S0022-3476(50)80174-9 [DOI] [PubMed] [Google Scholar]
- 91.Sacconi S, Bocquet JD, Chanalet S, Tanant V, Salviati L, Desnuelle C. Abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease. J Neurol. 2010; 257(10): 1730–3. doi: 10.1007/s00415-010-5618-0 [DOI] [PubMed] [Google Scholar]
- 92.Goeber V, Banz Y, Kaeberich A, Carrel T. Huge aneurysm of the ascending aorta in a patient with adult-type Pompe’s disease: histological findings mimicking fibrillinopathy. Eur J Cardiothorac Surg. 2013; 43(1): 193–5. [DOI] [PubMed] [Google Scholar]
- 93.Kretzschmar HA, Wagner H, Hübner G, Danek A, Witt TN, Mehraein P. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci. 1990; 98(2-3): 169–83. [DOI] [PubMed] [Google Scholar]
- 94.Van den Hout JM, Kamphoven JHJ, Winkel LPF, Arts WFM, De Klerk JBC, Loonen MC, Vulto AG, Cromme-Dijkhuis A, Weisglas-Kuperus N, Hop W, Van Hirtum H, Van Diggelen OP, Boer M, Kroos MA, Van Doorn PA, Van der Voort E, Sibbles B, Van Corven EJ, Brakenhoff JP, Van Hove J, Smeitink JA, de Jong G, Reuser AJ, Van der Ploeg AT. Long-term intravenous treatment of Pompe disease with recombinant human alpha-glucosidase from milk. Pediatrics. 2004; 113(5): e448–57. doi: 10.1542/peds.113.5.e448 [DOI] [PubMed] [Google Scholar]
- 95.Wens SCA, Kuperus E, Mattace-Raso FUS, Kruijshaar ME, Brusse E, van Montfort KCAGM, de Boer MS, Sijbrands EJG, van der Ploeg AT, van Doorn PA. Increased aortic stiffness and blood pressure in non-classic Pompe disease. J Inherit Metab Dis. 2014; 37(3): 391–7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bijvoet AGA, Van Hirtum H, Kroos MA, Van de Kamp EH, Schoneveld O, Visser P, Brakenhoff JPJ, Weggeman M, van Corven EJ, Van der Ploeg AT, Reuser AJ. Human acid alpha-glucosidase from rabbit milk has therapeutic effect in mice with glycogen storage disease type II. Hum Mol Genet. 1999; 8(12): 2145–53. doi: 10.1093/hmg/8.12.2145 [DOI] [PubMed] [Google Scholar]
- 97.Prater SN, Banugaria SG, DeArmey SM, Botha EG, Stege EM, Case LE, Jones HN, Phornphutkul C, Wang RY, Young SP, Kishnani PS. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. 2012; 14(9): 800–10. doi: 10.1038/gim.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones HN, Muller CW, Lin M, Banugaria SG, Case LE, Li JS, O’Grady G, Heller JH, Kishnani PS. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia. 2010; 25(4): 277–83. doi: 10.1007/s00455-009-9252-x [DOI] [PubMed] [Google Scholar]
- 99.Karabul N, Skudlarek A, Berndt J, Kornblum C, Kley RA, Wenninger S, Tiling N, Mengel E, Plöckinger U, Vorgerd M, Deschauer M, Schoser B, Hanisch F. Urge incontinence and gastrointestinal symptoms in adult patients with pompe disease: a cross-sectional survey. JIMD Rep. 2014; 17: 53–61. doi: 10.1007/8904_2014_334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Walt JD, Swash M, Leake J, Cox EL. The pattern of involvement of adult-onset acid maltase deficiency at autopsy. Muscle Nerve. 1987; 10(3): 272–81. doi: 10.1002/mus.880100311 [DOI] [PubMed] [Google Scholar]
- 101.Walvoort HC, Dormans JAMA, van den Ingh TS. Comparative pathology of the canine model of glycogen storage disease type II (Pompe’s disease). J Inherit Metab Dis. 1985; 8(1): 38–46. doi: 10.1007/BF01805484 [DOI] [PubMed] [Google Scholar]
- 102.Remiche G, Herbaut AG, Ronchi D, Lamperti C, Magri F, Moggio M, Bresolin N, Comi GP. Incontinence in late-onset Pompe disease: an underdiagnosed treatable condition. Eur Neurol. 2012; 68(2): 75–8. doi: 10.1159/000338776 [DOI] [PubMed] [Google Scholar]
- 103.Gesquière-Dando A, Attarian S, Maues De Paula A, Pouget J, Salort-Campana E. Fibromyalgia-like symptoms associated with irritable bowel syndrome: A challenging diagnosis of late-onset Pompe disease. Muscle Nerve. 2015; 52(2): 300–4. doi: 10.1002/mus.24618 [DOI] [PubMed] [Google Scholar]
- 104.Pardo J, García-Sobrino T, López-Ferreiro A. Síntomas gastrointestinales en la enfermedad de Pompe de inicio tardío: respuesta rápida a la terapia sustitutiva enzimática. Gastrointest Symptoms Late-onset Pompe Dis Early Response Enzyme Replacement Ther J Neurol Sci. 2015; 353(1–2): 181–2 . [DOI] [PubMed] [Google Scholar]
- 105.Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai ACH, Bossen E, Kishnani PS, O’Callaghan M. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006; 86(12): 1208–20. doi: 10.1038/labinvest.3700484 [DOI] [PubMed] [Google Scholar]
- 106.McNamara ER, Austin S, Case L, Wiener JS, Peterson AC, Kishnani PS. Expanding our understanding of lower urinary tract symptoms and incontinence in adults with pompe disease. JIMD Rep. 2015; 20: 5–10. doi: 10.1007/8904_2014_381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ajay D, McNamara ER, Austin S, Wiener JS, Kishnani P. Lower Urinary Tract Symptoms and Incontinence in Children with Pompe Disease. JIMD Rep. 2016; 28: 59–67. doi: 10.1007/8904_2015_492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prakalapakorn SG, Proia AD, Yanovitch TL, DeArmey S, Mendelsohn NJ, Aleck KA, Kishnani PS. Ocular and histologic findings in a series of children with infantile pompe disease treated with enzyme replacement therapy. J Pediatr Ophthalmol Strabismus. 2014; 51(6): 355–62. doi: 10.3928/01913913-20140813-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katona I, Weis J, Hanisch F. Glycogenosome accumulation in the arrector pili muscle in Pompe disease. Orphanet J Rare Dis. 2014; 9(1): 17. doi: 10.1186/1750-1172-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Meijden JC, Kruijshaar ME, Harlaar L, Rizopoulos D, van der Beek NAME, van der Ploeg AT. Long-term follow-up of 17 patients with childhood Pompe disease treated with enzyme replacement therapy. J Inherit Metab Dis. 2018; 41(6): 1205–14. doi: 10.1007/s10545-018-0166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ripolone M, Violano R, Ronchi D, Mondello S, Nascimbeni A, Colombo I, Fagiolari G, Bordoni A, Fortunato F, Lucchini V, Saredi S, Filosto M, Musumeci O, Tonin P, Mongini T, Previtali S, Morandi L, Angelini C, Mora M, Sandri M, Sciacco M, Toscano A, Comi GP, Moggio M. Effects of short-to-long term enzyme replacement therapy (ERT) on skeletal muscle tissue in late onset Pompe disease (LOPD). Neuropathol Appl Neurobiol. 2017. [DOI] [PubMed] [Google Scholar]
- 112.Chen M, Zhang L, Quan S. Enzyme replacement therapy for infantile-onset Pompe disease. Cochrane Database Syst Rev. 2017; 11: CD011539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaeta M, Barca E, Ruggeri P, Minutoli F, Rodolico C, Mazziotti S, Milardi D, Musumeci O, Toscano A. Late-onset Pompe disease (LOPD): correlations between respiratory muscles CT and MRI features and pulmonary function. Mol Genet Metab. 2013; 110(3): 290–6. doi: 10.1016/j.ymgme.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 114.Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E, Plotz PH, Raben N. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006; 59(4): 700–8. doi: 10.1002/ana.20807 [DOI] [PubMed] [Google Scholar]
- 115.Fukuda T, Roberts A, Ahearn M, Zaal K, Ralston E, Plotz PH, Raben N. Autophagy and lysosomes in Pompe disease. Autophagy. 2006; 2(4): 318–20. doi: 10.4161/auto.2984 [DOI] [PubMed] [Google Scholar]
- 116.Nascimbeni AC, Fanin M, Masiero E, Angelini C, Sandri M. Impaired autophagy contributes to muscle atrophy in glycogen storage disease type II patients. Autophagy. 2012; 8(11): 1697–700. doi: 10.4161/auto.21691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003; 27(1-4): 201–6. doi: 10.1152/advan.00025.2003 [DOI] [PubMed] [Google Scholar]
- 118.Chan J, Desai AK, Kazi ZB, Corey K, Austin S, Hobson-Webb LD, Case LE, Jones HN, Kishnani PS. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol Genet Metab. 2017; 120(3): 163–72. doi: 10.1016/j.ymgme.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 119.Yanovitch TL, Banugaria SG, Proia AD, Kishnani PS. Clinical and histologic ocular findings in pompe disease. J Pediatr Ophthalmol Strabismus. 2010; 47(1): 34–40. doi: 10.3928/01913913-20100106-08 [DOI] [PubMed] [Google Scholar]