Abstract
Objectives:
Although epidemiological evidence suggests that short sleep duration may affect renal function, the influence of long sleep and risk of end-stage renal disease (ESRD) is unclear. We examined the association between sleep duration and risk of ESRD.
Methods:
We investigated sleep duration and ESRD risk in the Singapore Chinese Health Study, a prospective population-based cohort of 63,257 Chinese in Singapore, who were aged 45–74 years at recruitment (1993–1998). Information on daily sleep duration (including naps), diet, medical history and other lifestyle factors was collected at recruitment from in-person interviews. ESRD cases were identified via linkage with the nationwide Singapore Renal Registry through year 2014. We used the Cox proportional hazards regression method to estimate hazard ratio (HR) and 95% confidence interval (CI) of ESRD in relation to sleep duration.
Results:
After an average 16.8 years of follow-up, 1,143 (1.81%) ESRD cases were documented. Sleep duration had a U-shaped association with risk of ESRD (P for quadratic trend < 0.001). Compared with participants with 7 hours/day of sleep, the multivariable adjusted HR (95% CI) of ESRD was 1.43 (1.18–1.74) for short sleep (≤ 5 hours/day) and 1.28 (1.03–1.60) for long sleep duration (≥ 9 hours/day). The increased risk was stronger in participants with more than 10 years of follow-up compared to those with shorter follow-up time, especially for long sleep (P for interaction = 0.003).
Conclusions:
Our findings demonstrated that both short and long sleep durations were associated with a higher risk of ESRD in this Asian population.
Keywords: Sleep duration, end-stage renal disease, epidemiology, Singapore Chinese Health Study
1. Introduction
Sleep duration is associated with an increased risk of cardiovascular disease, diabetes and hypertension [1–3], and these comorbidities are well-known risk factors for the development and progression of chronic kidney disease (CKD) [4]. Specifically, a U-shaped association has been found between sleep duration and diabetes [2], cardiovascular disease mortality [5, 6] and all-cause mortality [7]. Meta-analyses of prospective cohort studies have also suggested that both short sleep [8] and long sleep durations [9] are associated with a higher risk of diabetes and cardiovascular disease, with additional risk associated with hypertension for short sleep [8].
Sleep duration has also been studied in association with risk of CKD and eGFR level in epidemiologic studies. Compared with sleep duration of 7 to 8 hours, short sleep duration has been associated with 28% increased risk in the development of proteinuria [10], and 79% higher risk of faster decline in renal function in cohort studies [11]. Short sleep duration has also been associated with a greater eGFR decline among patients with hypertension and CKD [12, 13]. Conversely, the association between long sleep duration and decline in renal function is not consistent, with some cross-sectional studies showing long sleep to be associated with higher prevalence of low eGFR [14] or CKD [15], while other studies showed long sleep to have non-significant associations with lower prevalence of reduced eGFR [12] or lower risk of rapid decline in eGFR [11]. Furthermore, sleep disturbance, as a symptom of advanced CKD, may affect sleep quality and sleep duration [16–18]. Hence, previous studies with relatively short follow-up period may not be able to differentiate the effect of sleep on renal function from the reverse causality effect of renal disease on sleep duration.
Using data from Singapore Chinese Health Study, we have previously shown that both short and long sleep durations were associated with higher risk of all-cause mortality [19], as well as mortality due to coronary artery disease or stroke [5, 6]. To our best knowledge, there is no epidemiologic study on sleep duration and risk of end stage renal disease (ESRD) in a general population-based cohort. Thus, in this study, we investigated the association between sleep duration and risk of ESRD in the population-based Singapore Chinese Health Study cohort.
2. Method
2.1. Study population
The Singapore Chinese Health Study is a population-based prospective cohort study consists of 63,257 Chinese adults (27,959 men and 35,298 women), aged 45–74 years during recruitment between April 1993 and December 1998. The participants were recruited from government-built housing estates, where 86% of the Singapore population resided at the time of recruitment, and they were restricted to the two major dialect groups of Chinese in Singapore, the Hokkien and Cantonese, who originated from the contiguous provinces of Fujian and Guangdong in the southern part of China, respectively [20]. All participants gave written informed consent. The study was approved by the Institutional Review Board of the National University of Singapore.
2.2. Ascertainment of sleep duration and covariates
At recruitment, the trained interviewers did face-to-face interviews with a structured questionnaire and obtained information on demographics, sleep duration, height, weight, lifetime use of tobacco, alcohol intake, habitual physical activity, self-reported medical history, including physician-diagnosed hypertension, coronary artery disease, stroke and diabetes. Diet was assessed by a validated 165-item, semi-quantitative food frequency questionnaire. Body mass index (BMI, kg/m2) was calculated by body weight in kilogram divided by square of height in meter. Sleep duration of the participants was assessed by asking the question “On the average, during the last year, how many hours did you sleep in a day, including naps?”, with the following response categories: “≤ 5 hours”, “6 hours”, “7 hours”, “8 hours”, “9 hours”, and “≥ 10 hours”.
2.3. Ascertainment of incident ESRD cases
We identified ESRD cases by linking the cohort database with the population-based Singapore Renal Registry, which has been comprehensive in the ESRD recording since 1999. The registry identified ESRD cases through laboratory records, hospital records, and listings of patients on dialysis [21]. The cases were registered in the National registry of diseases office if they met ≥ one of the following criteria: (1) serum creatinine level ≥ 880 μmol/L (10 mg/dl); (2) eGFR < 15 ml/min per 1.73 m2 (based on either the Modification of Diet in Renal Disease Study equation; Cockcroft Gault equation, or 24-hour creatinine clearance); (3) undergoing hemodialysis or peritoneal dialysis; or (4) has undergone kidney transplant. The first three criteria have to be persistent over three months for qualifying as ESRD [21]. As of Dec 31 2014, only 57 subjects were lost to follow-up due to migration out of Singapore or for other reasons from our cohort, which suggests that emigration among the participants was negligible and that follow-up via linkage with the nationwide registry was virtually complete. The current analysis included data from 63,147 participants after excluding 110 participants with ESRD who were diagnosed with ESRD before enrollment.
2.4. Statistical analysis
We counted person-years from the date of recruitment to date of reported ESRD, loss to follow-up, death, or December 31st 2014, whichever occurred first. The differences in baseline characteristics by sleep duration were examined using the chi-squared test for categorical variables and ANOVA test for continuous variables. Kaplan-Meier survival curves were used to estimate the survival function of ESRD by sleep duration. We used multivariable Cox proportional hazards regression models to compute the HR and 95% CI for the association between sleep duration and risk of ESRD. We chose 7 hours/day as reference group based on the recommended sleep amount for healthy older adults by the American Academy of Sleep Medicine and Sleep Research Society [22].
We selected the potential confounders based on prior consideration of the associations with risk of ESRD in this population [23–26]. Experimental studies in animal models suggest that long-term incense use may have deleterious effects on kidney function [27], while the use of Chinese herbal medicine and the risk of ESRD has been described in human studies [28], Conversely, ginseng may have important bioactive constituents that can control pathological conditions associated with diabetic nephropathy [29]. Since information on incense use and intake of ginseng and medicinal soup was available in this population, we had also included these as covariates in the model. Model 1 was adjusted for the following factors: age (years), gender, dialect (Hokkien / Cantonese), year of baseline interview (19931995, 1996–1998), and education level (none, primary school, secondary school or higher), body mass index (kg/m2), smoking status (never, ever), physical activity (defined as any weekly moderate activity, vigorous activity or strenuous sports lasting ≥ 0.5 hours), alcohol consumption (none, occasionally, weekly, daily), total energy intake (kcal/day), total protein intake (g/day, in quartiles), red meat intake (g/day, in quartiles), coffee consumption (none to < 1 cup/day, 1 cup/day, ≥ 2 cups/day), domestic incense use (current or non-current), as well as taking ginseng (yes or no) or medicinal soup (yes or no) at least once a week. In addition to model 1 adjustment, model 2 was adjusted for baseline self-reported history of physiciandiagnosed hypertension, coronary artery disease, stroke and diabetes (yes or no for each disease).
We further performed stratified analysis by follow-up time (≤ 10 years vs. > 10 years), BMI (< 25 kg/m2 vs. ≥ 25 kg/m2), gender (men vs. women), and baseline history of comorbidities (with at least one of the comorbidities vs. without any of the comorbidities). The heterogeneity of the sleep-ESRD associations in each sleep duration category by different factors was tested by including an interaction term (product between each category of sleep duration and interaction factor) in the Cox model. We also included linear and quadratic terms of sleep duration in the Cox regression model to test the curvilinear relation.
All analyses were performed using Stata statistical software, release 14.0 (StataCorp LP, College Station, Texas), and two-sided p-value of < 0.05 were considered statistically significant.
3. Results
Among the 63,147 participants, 9.7% reporting short sleep duration with ≤ 5 hours/day, 23.3% reported sleeping 6 hours/day, 32.6% of them reported sleeping 7 hours/day, 27.4% reported sleeping 8 hours/day and 6.9% reporting long sleep duration with ≥ 9 hours/day. As shown in Table 1, compared to participants with 7 hours/day of sleep, participants with short or long sleep durations were older and had a lower educational level. They were more likely to have ever been smokers or daily drinkers, but less likely to be physically active. They also had a higher prevalence of baseline history of hypertension, coronary artery disease, stroke and diabetes. The mean BMI was comparable across all the groups.
Table 1.
Baseline characteristics of the population according to sleep duration in the Singapore Chinese Health Study (n = 63,147, 1,143 cases)
| Characteristics | Daily sleep duration |
P-value | ||||
|---|---|---|---|---|---|---|
| ≤ 5 hours | 6 hours | 7 hours | 8 hours | ≥ 9 hours | ||
| Number of participants, n | 6,133 | 14,712 | 20,599 | 17,317 | 4,386 | |
| ESRD cases, n | 152 | 234 | 325 | 328 | 104 | |
| Age at recruitment, year | 58.8 ± 8.1 | 56.7 ± 7.9 | 55.8 ± 7.7 | 55.9 ± 8.1 | 58.3 ± 8.4 | < 0.001 |
| Body mass index, kg/m2 | 23.1 ± 3.3 | 23.2 ± 3.3 | 23.1 ± 3.2 | 23.1 ± 3.3 | 23.1 ± 3.5 | 0.06 |
| Men (%) | 2,378 (38.8) | 6,484 (44.1) | 9,066 (44.0) | 8,004 (46.2) | 1,964 (44.8) | < 0.001 |
| Cantonese dialect (%) (%) | 3,066 (50.0) | 6,727 (45.7) | 9,462 (45.9) | 7,906 (45.7) | 2,071 (47.2) | < 0.001 |
| Level of education | < 0.001 | |||||
| No formal education | 2,111 (34.4) | 4,155 (28.2) | 5,391 (26.2) | 4,350 (25.1) | 1,295 (29.5) | |
| Primary school | 2,738 (44.6) | 6,400 (43.5) | 9,023 (43.8) | 7,761 (44.8) | 2,073 (47.3) | |
| Secondary school or higher | 1,284 (20.9) | 4,157 (28.3) | 6,185 (30.0) | 5,206 (30.1) | 1,018 (23.2) | |
| Ever smoked (%) | 1,907 (31.1) | 4,449 (30.2) | 5,921 (28.7) | 5,413 (31.3) | 1,611 (36.7) | < 0.001 |
| Weekly physical activitya (%) | 1,785 (29.1) | 4,871 (33.1) | 6,904 (33.5) | 5,870 (33.9) | 1,334 (30.4) | < 0.001 |
| Alcohol intake (%) | < 0.001 | |||||
| Non/occasionally drinkers | 5,448 (88.8) | 12,971 (88.2) | 18,290 (88.8) | 15,254 (88.1) | 3,874 (88.3) | |
| Weekly drinkers | 410 (6.7) | 1,233 (8.4) | 1,709 (8.3) | 1,433 (8.3) | 320 (7.3) | |
| Daily drinkers | 275 (4.5) | 508 (3.5) | 600 (2.9) | 630 (3.6) | 192 (4.4) | |
| Coffee consumption (%) | < 0.001 | |||||
| None to < 1 cup/day | 2,026 (33.0) | 4,464 (30.3) | 6,019 (29.2) | 4,924 (28.4) | 1,345 (30.7) | |
| 1 cup/day | 2,111 (34.4) | 5,069 (34.5) | 7,397 (35.9) | 6,586 (38.0) | 1,594 (36.3) | |
| ≥ 2 cups/day | 1,996 (32.6) | 5,179 (35.2) | 7,183 (34.9) | 5,807 (33.5) | 1,447 (33.0) | |
| Total protein intake g/day | 58.8 ± 10.1 | 59.0 ± 9.7 | 59.2 ± 10.0 | 59.2 ± 10.1 | 59.3 ± 10.2 | 0.0107 |
| Red meat intake g/day | 30.3 ± 19.2 | 30.3 ± 18.6 | 30.4 ± 18.5 | 30.5 ± 18.7 | 31.6 ± 19.2 | 0.0015 |
| Weekly ginseng intake (%) | 183 (3.0) | 399 (2.7) | 510 (2.5) | 460 (2.7) | 106 (2.4) | 0.19 |
| Weekly medicinal soup intake (%) | 667 (10.9) | 1,549 (10.5) | 1,982 (9.6) | 1,674 (9.7) | 474 (10.8) | 0.001 |
| Current daily incense users (%) | 4,637 (75.6) | 11,329 (77.0) | 15,747 (76.5) | 13,420 (77.5) | 3,412 (77.8) | 0.008 |
| Comorbidities (%) | ||||||
| Hypertension | 1,731 (28.2) | 3,437 (23.4) | 4,591 (22.3) | 4,002 (23.1) | 1,211 (27.6) | < 0.001 |
| Coronary artery disease | 370 (6.0) | 580 (3.9) | 719 (3.5) | 656 (3.8) | 262 (6.0) | < 0.001 |
| Stroke | 135 (2.2) | 170 (1.2) | 223 (1.1) | 269 (1.6) | 149 (3.4) | < 0.001 |
| Diabetes | 671 (11.0) | 1,268 (8.6) | 1,586 (7.7) | 1,565 (9.0) | 581 (13.3) | < 0.001 |
Data are shown as n (%) for categorical variables and mean ± SD for continuous variables.
Physical activity defined as at least 30 minutes of moderate activity, vigorous activity or strenuous sports.
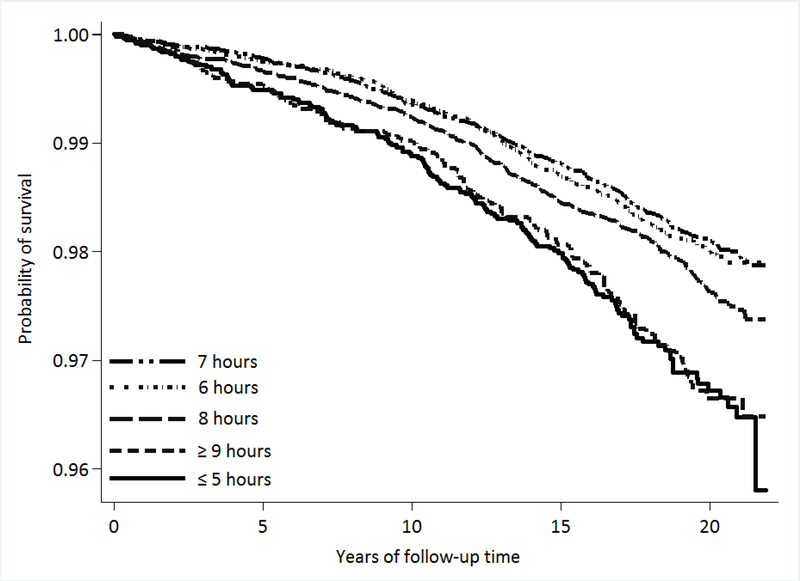
After a mean (SD) follow-up of 16.8 (5.1) years among 63,147 participants, there were 1,143 incident ESRD cases. Figure 1 shows the Kaplan-Meier survival curves of ESRD by sleep duration. Participants with 6 hours/day of sleep and 7 hours/day of sleep had comparable survival function. Compared with participants who reported 7 hours/day of sleep, participants who reported either ≤ 5 hours/day (short sleep) or ≥ 9 hours/day of sleep (long sleep) had the lowest survival function (both Ps for log-rank test < 0.001).
Figure 1.
Kaplan Meier survival curves for ESRD by sleep duration.
Sleep duration had a U-shaped association with risk of ESRD (P for quadratic trend < 0.001). In multivariable Model 1 that adjusted for potential confounders in age, gender, BMI, diet and lifestyle, compared with participants who reported 7 hours/day of sleep, the hazard ratios (HRs) of ESRD were 1.53 (95% confidence interval [95% CI], 1.26–1.86) among participants who reported ≤ 5 hours/day of sleep and 1.50 (1.20–1.88) among participants with ≥ 9 hours/day of sleep, respectively. After additional adjustment for the baseline comorbidities of hypertension, coronary artery disease, stroke and diabetes in Model 2, the risk estimates were attenuated by 14.7% for long sleep and by 6.5% for short sleep duration. Compared with 7 hours/day of sleep, the multivariable adjusted HRs (95% CI) of ESRD risk were 1.43 (1.18–1.74) for ≤ 5 hours/day, 1.00 (0.84–1.18) for 6 hours/day, 1.19 (1.02–1.39) for 8 hours/day and 1.28 (1.03–1.60) for ≥ 9 hours/day of sleep, respectively (Table 2).
Table 2.
Hazard ratio (95% confidence intervals) of ESRD according to sleep duration: Singapore Chinese Health Study (n = 63,147, 1,143 cases)
| Daily sleep duration |
|||||
|---|---|---|---|---|---|
| ≤ 5 hours | 6 hours | 7 hours | 8 hours | ≥ 9 hours | |
| Person-years | 97,496 | 247,706 | 354,731 | 292,877 | 68,340 |
| Cases | 152 | 234 | 325 | 328 | 104 |
| HR (95% CI)a | 1.53 (1.26–1.86) | 0.99 (0.84–1.18) | 1.00 | 1.21 (1.04–1.42) | 1.50 (1.20–1.88) |
| HR (95% CI)b | 1.43 (1.18–1.74) | 1.00 (0.84–1.18) | 1.00 | 1.19 (1.02–1.39) | 1.28 (1.03–1.60) |
The estimates were generated using Cox proportional hazards models.
Hazard ratios were adjusted for age at recruitment (years), gender, dialect (Cantonese, Hokkien), education level (no formal education, primary school, ≥ secondary school), year of interview (1993–1995, 1996–1998), body mass index (kg/m2), physical activity (any weekly moderate activity, vigorous activity or strenuous sports lasting at least 30 minutes: yes or no), smoking status (never-, ever-smokers), alcohol use (none, monthly, weekly, daily), total energy intake (kcal/day), total protein intake (g/day, quartiles), red meat consumption (g/day, quartiles) and coffee consumption (none to < 1 cup/day, 1 cup/day, ≥ 2 cups/day), weekly ginseng intake (yes or no), weekly medicinal soup intake (yes or no) and incense use (current users, non-current users).
In addition to above, hazard ratios were adjusted for self-reported history of physician-diagnosed hypertension, diabetes, coronary artery disease and stroke (yes or no).
When we stratified the analysis by baseline history of comorbidities, we did not find significant heterogeneity in the risk estimates between those with and without any of the preexisting comorbidities (all P for interaction ≥ 0.09). There was also no significant interaction between sleep duration and gender or baseline overweight status (< 25 kg/m2 vs. ≥ 25 kg/m2) (all P for interaction ≥ 0.29). In the analysis stratified by follow-up time (≤ 10 years versus > 10 years), the risks of short sleep duration and long sleep duration with ESRD were stronger in participants with longer follow-up of more than 10 years compared to those with shorter follow-up time. The difference in risk for long sleep duration by follow-up time was statistically significant (P for interaction = 0.003); however, the heterogeneity in risk for short sleep duration was not significant (P for interaction = 0.09) (Table 3).
Table 3.
Hazard ratio (95% confidence intervals) of ESRD according to sleep duration, stratified by baseline comorbidities (hypertension, stroke, coronary artery disease, and diabetes), BMI, gender, follow-up years (10 years)
| Daily sleep duration |
|||||
|---|---|---|---|---|---|
| ≤ 5 hours | 6 hours | 7 hours | 8 hours | ≥ 9 hours | |
| Comorbidities | |||||
| Any of four diseases | |||||
| Cases/person-years | 118/32,403 | 161/68,373 | 239/91,010 | 243/79,438 | 81/22,256 |
| HR (95% CI) a | 1.39 (1.11–1.73) | 0.90 (0.74–1.10) | 1.00 | 1.16 (0.97–1.38) | 1.24 (0.96–1.60) |
| None of four diseases | |||||
| Cases/person-years | 34/65,093 | 73/179,333 | 86/263,720 | 85/213,438 | 23/46,084 |
| HR (95% CI) a | 1.46 (0.98–2.18) | 1.20 (0.88–1.64) | 1.00 | 1.21 (0.91–1.63) | 1.40 (0.88–2.22) |
| P for interaction | 0.58 | 0.09 | 0.76 | 0.47 | |
| Body mass index (BMI) | |||||
| BMI < 25 kg/m2 | |||||
| Cases/person-years | 107/75,850 | 146/191,639 | 218/277,434 | 229/226,983 | 67/52,036 |
| HR (95% CI) a | 1.53 (1.21–1.94) | 0.94 (0.76–1.15) | 1.00 | 1.25 (1.04–1.50) | 1.34 (1.02–1.76) |
| BMI ≥ 25 kg/m2 | |||||
| Cases/person-years | 45/21,647 | 88/56,067 | 107/77,297 | 99/65,893 | 37/16,304 |
| HR (95% CI) a | 1.19 (0.84–1.68) | 1.12 (0.85–1.48) | 1.00 | 1.05 (0.81–1.37) | 1.18 (0.81–1.72) |
| P for interaction | 0.35 | 0.29 | 0.35 | 0.52 | |
| Gender | |||||
| Men | |||||
| Cases/person-years | 56/34,927 | 107/103,997 | 145/149,998 | 155/129,849 | 49/28,307 |
| HR (95% CI) a | 1.39 (1.02–1.91) | 1.04 (0.81–1.33) | 1.00 | 1.22 (0.97–1.53) | 1.36 (0.98–1.89) |
| Women | |||||
| Cases/person-years | 96/62,570 | 127/143,708 | 180/204,733 | 173/163,027 | 55/40,033 |
| HR (95% CI) a | 1.45 (1.13–1.87) | 0.96 (0.77–1.21) | 1.00 | 1.17 (0.95–1.45) | 1.24 (0.92–1.68) |
| P for interaction | 0.79 | 0.66 | 0.82 | 0.73 | |
| Follow-up time | |||||
| ≤ 10 years | |||||
| Cases/person-years | 63/5,308 | 83/9,979 | 125/12,477 | 123/11,560 | 39/4,473 |
| HR (95% CI) a | 1.26 (0.93–1.72) | 0.94 (0.71–1.25) | 1.00 | 1.04 (0.81–1.34) | 0.93 (0.64–1.33) |
| > 10 years | |||||
| Cases/person-years | 89/92,189 | 151/237,726 | 200/342,254 | 205/281,317 | 65/63,867 |
| HR (95% CI) a | 1.45 (1.13–1.87) | 1.05 (0.85–1.30) | 1.00 | 1.23 (1.01–1.50) | 1.41 (1.06–1.86) |
| P for interaction | 0.09 | 0.29 | 0.12 | 0.003 | |
Hazard ratios were adjusted for age at recruitment (years), gender, dialect (Cantonese, Hokkien), education level (no formal education, primary school, ≥ secondary school), year of interview (1993–1995, 1996–1998), body mass index (kg/m2), physical activity (any weekly moderate activity, vigorous activity or strenuous sports lasting at least 30 minutes: yes or no), smoking status (never-, ever-smokers), alcohol use (none, monthly, weekly, daily), total energy intake (kcal/day), total protein intake (g/day, quartiles), red meat consumption (g/day, quartiles) and coffee consumption (none to < 1 cup/day, 1 cup/day, ≥ 2 cups/day), weekly ginseng intake (yes or no), weekly medicinal soup intake (yes or no), incense use (current users, non-current users), self-reported history of physician-diagnosed hypertension, diabetes, coronary artery disease and stroke (yes or no) except the stratified factors.
4. Discussion
In this prospective cohort study of Singapore Chinese adults, we found that both short and long sleep durations were associated with an increased risk of developing ESRD. The estimates of sleep duration were attenuated after adjustment for baseline hypertension, diabetes, stroke and coronary artery disease. Furthermore, this attenuation was more substantial for long sleep than short sleep duration. In addition, the association between sleep duration and ESRD was stronger in participants with longer follow-up of more than 10 years, especially for long sleep. To our knowledge, this is the first prospective study to investigate the association of both short and long sleep durations with the risk of ESRD in an Asian population.
The relation between short sleep duration and renal function has been investigated in several studies. However, previous studies designed as cross-sectional [12] or retrospective studies [10], and some prospective study with a short follow-up time [13] or those that only included CKD patients [13] might be subject to recall bias or reverse causality, since renal disease is both a cause and symptom of sleep disturbances [16–18]. Moreover, most of the studies were conducted in Western populations; the results cannot be easily generalized to the Asian population. Our study is the first study to investigate sleep duration and risk of ESRD in an Asian population, and our prospective design in a population-based cohort with long duration of follow-up may overcome some of these methodologic limitations.
Our finding of short sleep duration being associated with higher risk of developing ESRD is largely consistent with other studies. A meta-analysis consisting of six observational studies showed a positive association between short sleep duration and proteinuria [30]. A retrospective cohort study in Japan with 6,834 participants of ages 20–65 years illustrated that sleep duration of 5 hours or less was associated with 28% increased risk of developing proteinuria [10]. Another cross-sectional study with 5,555 hypertensive participants in China showed that sleep duration with 6 or less per night was related with a higher prevalence of reduced eGFR (< 60 mL min−1 1.73 m2) [12]. A prospective cohort study which included 4,238 women from the Nurses’ Health Study (NHS) in US found that compared with sleep duration of 7–8 hours per night, the HR (95% CI) for the association between sleep duration of 5 hours or less and rapid eGFR decline was 1.79 (1.06 to 3.03) [11]. The Coronary Artery Disease in Young Adults (CARDIA) study in US investigate the association between objective assessment of sleep duration by wrist actigraphy and changes of eGFR in 463 healthy participants without hypertension, diabetes, cardiovascular disease or impaired kidney functions. The results suggested that shorter sleep was related to higher risk of kidney hyperfiltration over 10 years [31]. The Chronic Renal Insufficiency Cohort (CRIC) study involving 431 participants with CKD in US also assessed the association between sleep duration by wrist actigraphy and CKD progression, and reported that per hour decline in sleep duration was associated with a significant eGFR decline of 1.12 ml/min per 1.73m2 per year, and a non-significant higher risk of ESRD [13].
Our study focused on the relationship between sleep duration and incident of ESRD showed long sleep duration was associated with a higher risk of ESRD. Several studies have examined the relations between long sleep duration and renal function including risk of CKD and eGFR level, but the findings are not consistent. A cross-sectional study including 1,360 women conducted in Korea showed that long sleep duration (≥ 9 hours/day) was significantly associated with an increased prevalence of low eGFR, compared with the reference sleep group (7–8 hours/day) [14]. Another Korean cross-sectional study with 241,607 participants also showed that 9 or more hours of sleep was associated with a higher prevalence of CKD and glomerular hyperfiltration [15]. On the contrary, in a cross-sectional study with 5,555 hypertensive participants in China, long sleep duration was not significantly associated with eGFR [12]. The National Health Study (NHS) with 4,238 participants in US also found that compared with sleep duration 7–8 hours per night, participants with 9 or more hours had a decreased risk of rapid decline in eGFR, but this risk estimate was not statistically significant [11].
In our study, both the risk estimates of ESRD with short sleep and long sleep duration and ESRD were attenuated after adjustment for baseline history of hypertension, coronary artery disease, stroke and diabetes. The attenuation was more remarkable for long sleep, suggesting that some of the association between long sleep duration and ESRD could be confounded or mediated by these comorbidities that are also associated with long sleep [9]. Hence, long sleep duration might be a risk marker for these comorbidities [32], which in turn lead to higher risk of ESRD. Moreover, since the time-stratified analysis in our study showed that the association between sleep duration and ESRD was stronger in participants with longer follow-up of more than 10 years; our findings are less likely to be explained by reverse causality from the potential confounding effect of subclinical kidney disease on the sleep-related ESRD risk.
Although the mechanisms underlying the link between sleep duration and kidney function has not been fully elucidated, it is widely postulated that both short and long sleep durations may predispose to incident ESRD by increasing the risk of developing established CKD risk factors, including diabetes [2], hypertension [3] and cardiovascular disease [1], and these comorbidities subsequently have detrimental effects on renal function [4]. This concurs with our findings that individuals with short or long sleep had higher prevalence of these comorbidities and the risk estimates were attenuated after adjustment for these risk factors. In general, these comorbidities impair the renal function by contributing to chronic renal inflammatory changes and renal hemodynamic changes. Elevated blood pressure in hypertension could lead to glomerular hypertension, which will damage the nephron and lead to glomerular sclerosis [33, 34], hyperglycemia in diabetes can damage the glomerular mesangial cells and proximal tubular cells by increasing the production of reactive oxygen species [35], and cardiovascular disease can impair the renal hemodynamics through insufficient oxygenated blood supply [36]. Besides the indirect impact through comorbidities, activation of pro-inflammatory may represent a direct mechanism by which extreme sleep durations affect renal health since persistent low-grade inflammation has been recently recognized as an essential component of CKD [37]. Both short sleep and long sleep durations has been associated with elevations in C-reactive protein and interleukin-6 [38–40], with additional risk associated with elevated tumor necrosis factor (TNF)-α levels for short sleep [38]. Further studies are needed to elucidate these mechanisms.
The strengths of this study are its population-based prospective design, large sample size, long follow-up, the objective assessment of ESRD endpoints and the virtual completeness of follow-up by linkage with the nationwide Singapore Renal Registry [21]. In addition, we analyzed the association of sleep duration with risk of ESRD over time in order to observe the long-term from short-term effects more accurately [41]. Several limitations should be acknowledged. First, we did not collect the information on sleep disturbances and sleep quality, which have been associated with the development and progression of CKD [13, 18, 42]. Second, since we did not include any objective measurement to distinguish between naps and nocturnal sleep, we were unable to determine to what extent increased sleep duration represented daytime from night-time sleep. Third, the measurement of self-report and one-time assessment of sleep duration may lead to misclassification bias. However, given the prospective study design, this potential misclassification error is more likely to be non-differential, which could underestimate the true association between sleep duration and risk of ESRD. Fourth, the residual confounding cannot be completely ruled out in our study due to the limitation of the observational design. Finally, we did not measure the biomarkers for prognostication of CKD at recruitment, such as eGFR and proteinuria. Hence, we were unable to establish the temporal relationship between sleep duration and deteriorating renal function with certainty.
5. Conclusions
In conclusion, both short and long sleep durations could have long-term effects in increasing the risk of ESRD in the general population. Interventions to help individuals maintain recommended sleep duration could reduce the risk of ESRD, particularly in high risk populations. Future studies using serial measurements to look at effects of change in sleep duration on kidney function will determine the clinical usefulness of such interventions.
Supplementary Material
We examined the relation between sleep duration and risk of ESRD in the population.
Both short sleep and long sleep durations were associated with increased risk.
This increased risk was stronger in those with more than 10 years of follow-up.
Maintaining recommended sleep duration may reduce ESRD incidence in the population.
Acknowledgment:
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and Renwei Wang for the maintenance of the cohort study database. Finally, we acknowledge the founding Principal Investigator of the Singapore Chinese Health Study, Mimi C. Yu.
Funding:
This study was supported by the National Institutes of Health, USA (R01 CA144034 and UM1 CA182876). W-P Koh is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013)
Abbreviations:
- ESRD
end-stage renal disease
- CKD
chronic kidney disease
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- SD
standard deviation
Footnotes
Disclosure:
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Li W, Wang D, Cao S, Yin X, Gong Y, Gan Y, et al. Sleep duration and risk of stroke events and stroke mortality: A systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;223:870–6. [DOI] [PubMed] [Google Scholar]
- [2].Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529–37. [DOI] [PubMed] [Google Scholar]
- [3].Wang Y, Mei H, Jiang YR, Sun WQ, Song YJ, Liu SJ, et al. Relationship between Duration of Sleep and Hypertension in Adults: A Meta-Analysis . J Clin Sleep Med. 2015;11:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kazancioglu R Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011). 2013;3:368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pan A, De Silva DA, Yuan JM, Koh WP. Sleep duration and risk of stroke mortality among Chinese adults: Singapore Chinese health study. Stroke. 2014;45:1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population-based cohort study. Am J Epidemiol. 2008;168:1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang X, Liu X, Song Q, Wu S. Sleep duration and risk of myocardial infarction and all-cause death in a Chinese population: the Kailuan study. Sleep Med. 2016;19:13–6. [DOI] [PubMed] [Google Scholar]
- [8].Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. [DOI] [PubMed] [Google Scholar]
- [9].Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. [DOI] [PubMed] [Google Scholar]
- [10].Yamamoto R, Nagasawa Y, Iwatani H, Shinzawa M, Obi Y, Teranishi J, et al. Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis. 2012;59:343–55. [DOI] [PubMed] [Google Scholar]
- [11].McMullan CJ, Curhan GC, Forman JP. Association of short sleep duration and rapid decline in renal function. Kidney Int. 2016;89:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guo X, Yu S, Li Z, Guo L, Zheng L, Yang H, et al. Self-reported sleep duration is associated with reduced glomerular filtration rate among adults with hypertension: a population-based study from rural northeast China. J Sleep Res. 2015;24:351–8. [DOI] [PubMed] [Google Scholar]
- [13].Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, et al. The Association of Sleep Duration and Quality with CKD Progression. J Am Soc Nephrol. 2017;28:3708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choi H, Kim HC, Lee JY, Lee JM, Choi DP, Suh I. Sleep duration and chronic kidney disease: The Korean Genome and Epidemiology Study (KoGES)-Kangwha study. Korean J Intern Med. 2017;32:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim CW, Chang Y, Sung E, Yun KE, Jung HS, Ko BJ, et al. Sleep duration and quality in relation to chronic kidney disease and glomerular hyperfiltration in healthy men and women. PLoS One. 2017;12:e0175298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gigli GL, Adorati M, Dolso P, Piani A, Valente M, Brotini S, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5:309–15. [DOI] [PubMed] [Google Scholar]
- [17].Pugh-Clarke K, Read SC, Sim J. Symptom experience in non-dialysis-dependent chronic kidney disease: A qualitative descriptive study. J Ren Care. 2017;43:197–208. [DOI] [PubMed] [Google Scholar]
- [18].Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. 2012;60:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Soh AZ, Chee MWL, Yuan JM, Koh WP. Sleep lengthening in late adulthood signals increased risk of mortality. Sleep. 2018;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–95. [DOI] [PubMed] [Google Scholar]
- [21].National Registry of Diseases Office. Singapore Renal Registry Annual Registry Report 1999–2013 (Preliminary). Singapore 2014.
- [22].Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jin A, Koh WP, Chow KY, Yuan JM, Jafar TH. Smoking and risk of kidney failure in the Singapore Chinese health study. PLoS One. 2013;8:e62962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lew QJ, Jafar TH, Koh HW, Jin A, Chow KY, Yuan JM, et al. Red Meat Intake and Risk of ESRD. J Am Soc Nephrol. 2017;28:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lew QJ, Jafar TH, Talaei M, Jin A, Chow KY, Yuan JM, et al. Increased body mass index is a risk factor for end-stage renal disease in the Chinese Singapore population. Kidney Int. 2017;92:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jafar TH, Jin A, Koh WP, Yuan JM, Chow KY. Physical activity and risk of end-stage kidney disease in the Singapore Chinese Health Study. Nephrology (Carlton, Vic). 2015;20:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hussain T, Al-Attas OS, Alrokayan SA, Ahmed M, Al-Daghri NM, Al-Ameri S, et al. Deleterious effects of incense smoke exposure on kidney function and architecture in male albino rats. Inhal Toxicol. 2016;28:364–73. [DOI] [PubMed] [Google Scholar]
- [28].Lin MY, Chiu YW, Chang JS, Lin HL, Lee CT, Chiu GF, et al. Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. 2015;88:1365–73. [DOI] [PubMed] [Google Scholar]
- [29].Kang KS, Ham J, Kim YJ, Park JH, Cho EJ, Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheungpasitporn W, Thongprayoon C, Gonzalez-Suarez ML, Srivali N, Ungprasert P, Kittanamongkolchai W, et al. The effects of short sleep duration on proteinuria and chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32:991–6. [DOI] [PubMed] [Google Scholar]
- [31].Petrov ME, Kim Y, Lauderdale DS, Lewis CE, Reis JP, Carnethon MR, et al. Objective sleep, a novel risk factor for alterations in kidney function: the CARDIA study. Sleep Med. 2014;15:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marshall SS NS. Sleep duration: risk factor or risk marker for ill health? Oxford: Oxford University Press; 2010. [Google Scholar]
- [33].Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–11. [DOI] [PubMed] [Google Scholar]
- [34].Tedla FM, Brar A, Browne R, Brown C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. 2011;2011:132405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nishikawa T, Brownlee M, Araki E. Mitochondrial reactive oxygen species in the pathogenesis of early diabetic nephropathy. J Diabetes Investig. 2015;6:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. [DOI] [PubMed] [Google Scholar]
- [37].Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84–92. [DOI] [PubMed] [Google Scholar]
- [38].Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. [DOI] [PubMed] [Google Scholar]
- [40].Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74:994–7. [DOI] [PubMed] [Google Scholar]
- [42].Witkowski A, Kadziela J. Obstructive sleep apnoea, resistant hypertension and renal denervation. EuroIntervention. 2013;9 Suppl R:R105–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.