Abstract
In this perspective, we evaluate key and emerging epidemiological and toxicological data concerning immunotoxicity of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) and seek to reconcile conflicting conclusions from two reviews published in 2016. We summarize ways that immunosuppression and immunoenhancement are defined and explain how specific outcomes are used to evaluate immunotoxicity in humans and experimental animals. We observe that different approaches to defining immunotoxicological outcomes, particularly those that do not produce clinical disease, may lead to different conclusions from epidemiological and toxicological studies. The fundamental point that we make is that aspects of epidemiological studies considered as limitations can be minimized when data from toxicological studies support epidemiological findings. Taken together, we find that results of epidemiological studies, supported by findings from toxicological studies, provide strong evidence that humans exposed to PFOA and PFOS are at risk for immunosuppression.
Keywords: Emerging contaminants, perfluorinated chemicals, population based studies
Introduction
In 2006, the U.S. Environmental Protection Agency Science Advisory Board (EPA SAB) reviewed an EPA document entitled “Draft Risk Assessment of Potential Human Health Effects Associated with Perfluorooctanoic Acid (PFOA) and Its Salts.” One recommendation of the EPA SAB was that “immunotoxicity should be considered as an endpoint addressed quantitatively in the [revised/final] risk assessment” (1). The SAB indicated that additional data in experimental animals were necessary for incorporation of immunotoxicity data into a PFOA risk assessment. This recommendation was based on studies by Yang et al. (2–4) indicating that PFOA was a potent immunosuppressant in mice. Since then, additional studies addressing immunotoxicity of PFOA and perfluorooctane sulfonate (PFOS) provide evidence that PFOA and PFOS are immunosuppressive in humans and experimental animals. A smaller body of evidence demonstrates that these compounds can modulate other aspects of immunity, including hypersensitivity responses, further supporting that these agents target the immune system.
PFOA and PFOS are two of the most frequently studied members of a broader class of chemicals called per- and polyfluoroalkyl substances (PFASs). These synthetic and highly persistent chemicals have been integrated into myriad industrial processes and consumer products for the past 50–60 years. PFASs are mainly used as surfactants or surface protection agents due to their water- and oil-repellency and the chemical and thermal stability of their characteristic carbon-fluorine bonds (5). PFASs are resistant to environmental degradation and when combined with their ability to bioaccumulate into living organisms and their widespread use, they are now present globally in environmental media and biota, including humans (6). PFAS exposure has been associated with adverse health outcomes in humans, including certain types of cancer, developmental toxicity, liver toxicity, endocrine disruption, and immunotoxicity. Studies of experimental animals support a causal role for PFASs in the induction of these toxicities (7).
In 2016, two reviews of PFOA/PFOS immunotoxicity were published. One was a systematic review by the U.S. National Toxicology Program (NTP) to evaluate immunotoxicity of PFOA and PFOS (8). An NTP “systematic review uses a predefined, multistep process to identify, select, critically assess, and synthesize evidence from scientific studies to reach a conclusion” and a transparent process to document the basis for scientific judgments (9). This process derives a confidence rating in the scientific data that is translated into a level of evidence for health effects (9). The NTP determined that 33 human studies, 93 experimental animal studies, and 27 in vitro/mechanistic studies were relevant for addressing immunotoxicity. Based on available data across these study types, the NTP concluded that both PFOA and PFOS were “presumed to be an immune hazard to humans.” This conclusion was based on a high level of evidence from studies of experimental animals and a moderate level of evidence from epidemiological studies that exposure suppresses antigen-specific antibody responses. The report also concluded that a moderate level of evidence from studies of experimental animals supported that exposure to PFOA increases hypersensitivity-related outcomes. Other types of immune-related health effects have been reported, but the NTP concluded that lines of evidence were strongest for suppression of antibody responses.
The second publication was by Chang et al. (10) and was a critical review of 24 epidemiological studies of PFOA and PFOS immunotoxicity, with some consideration of data from studies of experimental animals. The authors assessed the methodology of the studies, consistency of results, and whether or not the studies excluded confounding, bias, or chance as an explanation for observed associations. They concluded that available evidence from epidemiological studies was insufficient to reach a conclusion about a causal relationship between exposure to PFOA and PFOS and immune-related health conditions in humans. These conclusions were based, in part, on generally weak associations, no specific endpoints with consistent findings across all relevant studies, uncertainty about critical durations of exposure, mixed exposure-response trends, and a scarcity of supportive animal and mechanistic data. The authors noted that PFOA and PFOS could cause immunosuppression in experimental animals, but due to inconsistencies in responses across species and strains, internal serum concentrations in exceedance of those measured in humans, and relevance of outcome measures in animals to humans, application of these findings to human health outcomes was uncertain.
The focus of this perspective is to evaluate evidence that PFOA and PFOS are immune hazards to humans in light of the different conclusions reached by the NTP (8) and Chang et al. (10) reviews, discuss how immunotoxicity is defined, and discuss additional evidence from studies of PFOA and PFOS-induced immunotoxicity published since 2016. Rather than conduct a new critical review of the literature, our goal is to 1) highlight findings from epidemiological and experimental animal studies where antibody responses and/or hypersensitivity responses were evaluated, 2) posit whether these findings are consistent with commonly accepted definitions of immunotoxicity, and 3) emphasize some of the fundamental differences in methodology employed by the NTP (8) and Chang et al. (10) reviews.
Overview of immunotoxicity
Immunotoxic effects following exposure to exogenous agents generally are classified as immunosuppression or immunoenhancement (11). Immunosuppression typically has been the focus of regulatory testing for pharmaceutical and chemical agents due to risks of increased infection (12). Immunosuppression is defined as a reduced ability of the immune system to respond to a challenge from a level considered normal, regardless of whether clinical disease is present (13–16). Impacts of immunosuppression on the general health of an afflicted individual can be mild, such as slightly reduced responses to vaccinations that do not impact resistance to disease, to severe, such as greatly increased susceptibility to common and opportunistic pathogens as well as certain cancers (13–16).
While immune function can be evaluated with multiple assays, the T cell-dependent antibody response (TDAR) is considered a “gold standard” by regulatory agencies for evaluation of immunotoxic potential and is reportedly the most sensitive functional assay for evaluating immunosuppression (17). This particular assay focuses on the humoral arm of adaptive immunity and because a response requires antigen recognition and presentation, T and B cell signaling, and class switching, its particular strength lies in its ability to detect immunosuppression across a range of cell types and signals (11). To measure the TDAR, experimental animals are given an antigen injection and some days later (typically 4–5 days for rodents) blood is collected and primary (IgM) antibodies specific to the antigen are measured (17). The assay can be modified to detect secondary (IgG) antibodies, which requires a second antigen injection. Certainly, other types of immunosuppression are possible and may fall outside of the TDAR detection range, such as effects on innate immunity, but the TDAR is widely regarded as robust and sensitive, and translatable to humans (18). The analogous human response is antibodies generated toward a specific vaccine, which can be measured in human populations exposed to particular exogenous agents.
Immunoenhancement can broadly be defined as inappropriate activation of the immune system (11) and may result in hypersensitivity responses such as allergy or asthma or as autoimmune reactions where the immune system responds to self-antigens (12). It has typically been more challenging to assess hypersensitivity and autoimmunity in humans and experimental animals because screening for immunotoxicity, as previously discussed, classically was designed to measure loss of function, i.e., suppression.
Hypersensitivity reactions can be classified into four types that differ by immune reactant (e.g., immunoglobulin or T cells), antigen form, and mechanisms of action. Allergic diseases such as allergic rhinitis and sinusitis, conjunctivitis, and asthma, are Type I hypersensitivity reactions, and can have significant impacts on the quality of life of those afflicted (19). A hallmark of Type I hypersensitivity in a mouse model is increased levels of antigen-specific IgE antibodies (19). Unlike immunosuppression, which can include a variety of clinical or sub-clinical manifestations that can be self-diagnosed, Type I hypersensitivity reactions, such as asthma, cannot be self-diagnosed. Findings in humans can be supported by non-specific IgE levels and in experimental animals by antigen-specific IgE levels. These type of hypersensitivity reactions include a sensitization phase and an elicitation phase. In certain individuals, antigens stimulate IgE antibody production (sensitization) rather than an antibody response that would clear the antigen without production of allergic symptoms. Upon subsequent exposure to the same antigen (elicitation), IgE antibodies again modulate the reaction, which leads to characteristic signs of Type I hypersensitivity reactions (20). Exogenous agents may act at both sensitization and/or elicitation phases, making it challenging to define the precise role of exogenous agents in human studies.
On the other side of the immunoenhancement spectrum are T cell-mediated Type IV hypersensitivity reactions, which include contact dermatitis and autoimmune diseases (e.g., Type I diabetes, multiple sclerosis, and lupus). Testing for Type IV hypersensitivity disorders and autoimmunity in rodents is limited to a fairly rudimentary assessment of contact hypersensitivity or serological biomarkers of autoimmunity (autoantibodies). This means that toxicants that promote autoimmune disease may rarely be flagged in assessments of immunotoxicity. Clinically, a challenge of assessing autoimmune reactions in humans is that no single blood test of immunologic assessment can confirm an autoimmune disease diagnosis. For most autoimmune diseases, histopathological findings represent the gold standard of diagnosis and monitoring of disease progression, but as many autoimmune diseases affect multiple organs, they often are difficult to diagnose.
Evaluating immune-related health effects in exposed human populations can therefore span the continuum of immunotoxicological outcomes. Of particular importance related to definitions of immunotoxicity, whether effects are classified as suppression or enhancement, is that clinical manifestation of immune-related diseases are not required for an agent to be classified as immunotoxic.
Key epidemiological studies of antibody suppression discussed by the NTP (8) and/or Chang et al. (10) reviews
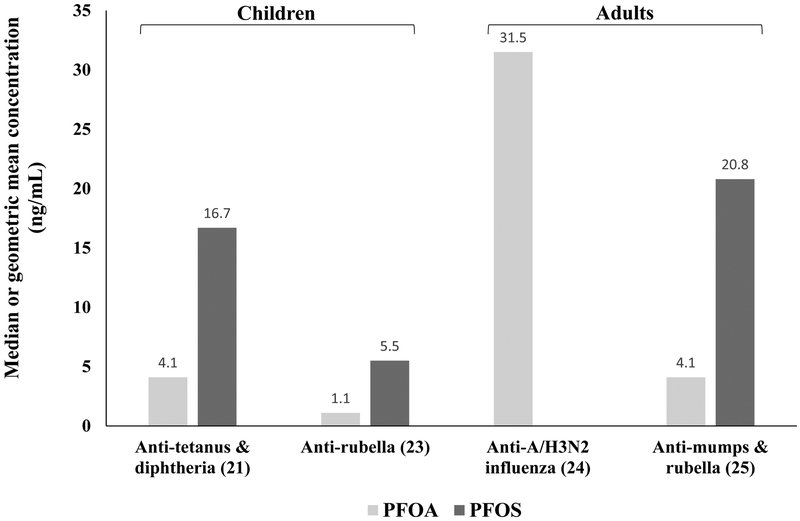
Several epidemiological studies addressing immune-related health effects in humans exposed to PFASs have focused on functional outcomes, notably, responses to specific vaccines. Here, we summarize prospective studies first and then follow with cross-sectional studies. One of the first prospective studies of PFASs and immune-related health effects evaluated vaccine responses in children developmentally exposed to PFASs (21). This study was done in the Faroe Islands, where dietary intake of marine food is associated with increased exposures to PFASs. They observed that a two-fold increase in PFOA and PFOS serum concentrations (geometric means of 4.06 and 16.7 ng/mL, respectively) in five-year-old children was associated with an odds ratio between 2.38 and 4.20 for falling below a clinically protective level for both tetanus and diphtheria antibodies in seven-year-old children (N=464). This study also observed that maternal PFOS serum concentrations (geometric mean of 27.3 ng/mL) collected during the last trimester of pregnancy were negatively associated with anti-diphtheria antibody concentrations in five-year-old children (N=532). Using data collected from seven-year-old children in this same cohort, Mogensen et al. (22) used a combined exposure model that included PFOA, PFOS, and perfluorohexane sulfonate (PFHxS) and reported that combining PFASs in their model led to a stronger negative association between PFASs and the antibody response than individual PFASs. In a separate prospective study from the Norwegian Mother and Child Cohort Study, which is representative of general population exposures in Norway, Granum et al. (23) observed that concentrations of four PFASs, PFOA, PFOS, PFHxS, and perfluorononanoate (PFNA), in maternal blood (median concentrations were 1.1, 0.3, 0.3, and 5.5 ng/mL respectively) collected at the time of delivery, were all inversely correlated with the level of anti-rubella antibodies measured in three-year-old children (N=56). They also observed that maternal levels of PFOA and PFNA were positively correlated with the number of episodes of common colds in the children and that PFOA and PFHxS were positively correlated with the number of episodes of gastroenteritis in the children. In a prospective study (known as the “C8 Health Project”) of 403 adults in Ohio/West Virginia of the U.S. who were exposed to PFOA via water contaminated from a nearby PFAS manufacturing facility, serum concentrations of PFOA (median concentrations of 31.5 ng/mL) were negatively associated with a reduced antibody response to the A/H3N2 influenza vaccine (24).
In a large cross-sectional analysis of data (N=1,191) from the U.S. National Health and Nutrition Examination Survey (NHANES), Stein et al. (25) reported (not included in the Chang et al. (10) review) that decreases in anti-mumps antibodies were associated with increases in serum concentrations of PFOA and PFOS (geometric means of 20.8 and 4.13 ng/mL respectively) and anti-rubella antibodies were associated with increases in serum concentrations of PFOA, PFOS, and PFHxS (geometric mean of 2.47 ng/mL). In very small cross-sectional study of 12 adult volunteers from the general human population in Denmark, Kielsen et al. (26), reported a negative association between serum concentrations of most of the eight different PFASs they measured and anti-tetanus and diphtheria antibodies. While this study was from a small sample size, it is noteworthy that the authors found statistically significant associations consistent with results from larger study populations.
Key experimental animal studies of antibody suppression included in the NTP (8) and/or Chang et al. (10) reviews
While numerous studies of PFOA or PFOS in rodents have evaluated immunotoxicological outcomes, a smaller subset evaluated the TDAR and are briefly discussed here. Oral exposure to a dose of 20 mg PFOA/kg body weight/day given for a duration of 10 or 21 days profoundly suppressed the TDAR (2, 27; reference 27 was not included in the Chang et al. (10) review). Additional studies of PFOA have supported this initial finding, demonstrating dose-responsive suppression of the TDAR (28,29). A lowest observed adverse effect level was identified as 3.75 mg PFOA/kg body weight/day and a benchmark dose of 3 mg PFOA/kg body weight/day also was calculated (28). While systemic stress likely plays a role in TDAR suppression at relatively high doses, such as 15 mg PFOA/kg body weight/day and above (28, 29), adrenalectomized animals still exhibited TDAR suppression, indicating lack of systemic stress at lower doses (30; this reference was not included in the Chang et al. (10) review). Similarly to PFOA, PFOS administered orally also suppresses the TDAR, albeit at a much wider range of doses and with less consistent responses, from 0.002 to 40 mg of PFOS/kg body weight/day when given during development or from seven to 60 days in duration (27, 31–37). Two of these studies reported that PFOS did not suppress the TDAR. In male and female Sprague-Dawley rats given 2–100 mg PFOS/kg body weight/day in the diet for 28 days, Lefebvre et al. (32) reported dose-responsive elevations in antigen-specific IgG antibodies in male animals and no changes in female animals. Male B6C3F1 mice given 7 mg PFOS/kg body weight/day in the diet for 28 days had no change in the TDAR (37). While a mode/mechanism of action for this immunosuppressive effect has not yet been determined, the TDAR is a robust and sensitive assay for detecting effects on the immune system. These toxicological studies of PFOA and PFOS support findings in exposed humans that these compounds are capable of suppressing adaptive immune function.
Key epidemiological studies of immunoenhancement discussed by the NTP (8) and/or Chang et al. (10) reviews
As previously stated, immunoenhancement is more challenging to assess relative to immunosuppression as biomarkers in humans can vary by type of hypersensitivity reaction (i.e., immune reactant, antigen form, and mechanisms of action). Several prospective and cross-sectional studies have evaluated Type I hypersensitivity reactions, including serum IgE and/or clinical hypersensitivity reactions following gestational or childhood exposure to PFASs. In general, prospective studies of birth cohorts from the general human population (N ranging from ~200 to 2,000+ subjects/study) did not find associations between PFOA/PFOS levels in maternal serum or cord blood and Type I hypersensitivity reactions in the children (23, 38–41) or IgE levels were inconsistent among studies (38, 39, 42). A small number of cross-sectional studies evaluated associations between serum PFAS levels and serum IgE and/or clinical Type I hypersensitivity reactions in children. In three separate analyses of NHANES data, Humblet et al. (43) and Stein et al. (25) reported positive associations of PFOA and PFOS with several respiratory hypersensitivity outcomes (N=1,877 and 638, respectively) and Buser and Scinicariello (44) reported that serum PFOA and PFOS were associated with an increase in self-reported food allergies (references 25 and 44 were not included in the Chang et al. (10) review). Stein et al. (25) measured total serum IgE and levels of antigen-specific IgE and reported mixed outcomes; PFOA was positively associated with total serum IgE and PFOS results varied depending on the antigen, but was not associated with total serum IgE. In one additional study of children enrolled in the Genetic and Biomarkers Study for Childhood Asthma in Taiwan, the authors reported positive associations between current levels of PFOA and PFOS and serum IgE and asthma severity scores in asthmatic children (45).
Anderson-Mahoney et al. (46) questioned adult (N=566) residents in Ohio/West Virginia of the U.S. who consumed drinking water contaminated by a nearby PFAS manufacturing facility and reported that exposed subjects had a greater prevalence of self-reported asthma. However, in a C8 Health Project study of workers (N=1,881) at the PFAS manufacturing facility who were highly exposed to PFOA (median measured serum concentration = 113 ng/mL) and where self-reported diseases were validated with medical records, Steenland et al. (47) reported a negative association between PFOA exposure and workers who had asthma and were taking medication for that disease.
The number of studies evaluating the relationship between PFAS and Type IV hypersensitivity reactions such as autoimmune diseases or markers of autoimmune reactions is limited. Among adults in the C8 Health Project (N=32,254), the incidence of ulcerative colitis, a type of autoimmune disease producing chronic digestive tract inflammation, was associated with PFOA exposure (median measured serum concentration in entire cohort = 26 ng/mL; 48). In a related study of workers (N=1,881) exposed to PFOA (median measured serum concentration = 113 ng/mL), ulcerative colitis and rheumatoid arthritis were positively associated with exposure (47). In a small study of children (N=37) from the Faroe Islands, prenatal concentrations of PFOS (geometric mean of 3.1 ng/mL), but not PFOA (geometric mean of 0.68 ng/mL), were negatively associated with an antibody directed against a self-protein (49).
Key experimental animal studies of immunoenhancement discussed by the NTP (8) and/or Chang et al. (10) reviews
Relatively few toxicological studies have evaluated hypersensitivity reactions following PFOA or PFOS exposure. In a mouse model of asthma, Fairley et al. (50) tested dermal PFOA exposure on modulation of the hypersensitivity response to ovalbumin (OVA), an egg protein often used in such models. Four days of dermal exposure to PFOA (0.25–50 mg PFOA/kg of body weight/day) enhanced the hypersensitivity response to OVA as measured by changes in IgE and lung histopathology, but PFOA exposure alone did not increase IgE, suggesting to the authors that PFOA may enhance the allergenicity of environmental allergens. Ryu et al. (51) exposed mice to a single dose of PFOA or PFOS (4 mg PFOA or PFOS/kg of food) from gestational day two through 12 weeks of age and reported that while neither compound affected OVA-induced airway hyperresponsiveness, PFOA alone increased airway inflammation and function. An additional study in adult mice demonstrated that 60 days of oral exposure to 50 mg PFOS/kg of body weight/day increased antigen-specific IgE serum levels (36). Studies of hypersensitivity reactions in experimental animals do not provide consistent evidence that exposure to these compounds are able to induce hypersensitivity.
Recent studies
Stein et al. (52) examined the relationship between serum concentrations of eight different PFASs, including PFOA and PFOS, and vaccination to FluMist intranasal live attenuated influenza vaccine in a small group of healthy adults from the general U.S. population (N=78). No consistent pattern between PFAS concentrations and anti-A H1N1 antibody responses were observed. However, authors noted that their results do not rule out impaired vaccine responses to other vaccines or vaccine components. Using a cohort of children from the Faroe Islands, similar to the cohorts used by Grandjean et al. (21) and Mogensen et al. (22), Timmerman et al. (53) evaluated whether PFAS concentrations measured in maternal serum or serum of five- and 13-year-old children were associated with serum IgE levels (measured in cord blood and at seven years of age) and/or Type I hypersensitivity reactions. In a subset of children that did not receive a measles, mumps, and rubella (MMR) vaccination (N=22), higher PFAS levels at age five were associated with increased odds of asthma at ages five and 13. This risk did not exist in MMR-vaccinated children, suggesting that the MMR vaccine may modify effects of PFAS exposure. In a prospective birth cohort (N=687) study from the general population in China, Chen et al. (54) reported that cord blood PFOA concentrations were positively associated with development of atopic dermatitis (a type of dermatitis associated with asthma and allergic rhinitis) in female children during the first 24 months of life. These recent epidemiological studies demonstrate that immune outcomes and PFAS exposures are not consistent across vaccine types and that sex and timing of data collection may influence associations.
The Agency for Toxic Substances and Disease Registry (ATSDR) recently released a revised draft Toxicological Profile for Perfluoroalkyls for public comment (7). The ATSDR evaluated available data for 14 different PFASs, including PFOA and PFOS, and determined that based on consistency of findings across studies, available epidemiological studies suggest associations between exposure and a decreased antibody response to vaccines for PFOA, PFOS, and three other PFASs evaluated, including PFHxS. They also determined that immunotoxicity was one of the primary health effects observed in experimental animals exposed to PFOA or PFOS. While they used developmental toxicity as points of departure for deriving minimal risk levels (an estimate of daily human exposure to a hazardous substance likely to be without appreciable risk of adverse noncancer health effects for a specific route and duration of exposure) for PFOA and PFOS, they included an additional modifying factor for PFOS as some of the lowest administered doses associated with adverse effects were from immunotoxicity studies (33, 35, 36). Data from these studies were not chosen as points of departure due to lack of pharmacokinetic model parameters for the two mouse strains tested (7).
Exposure considerations
Measured PFOA and PFOS serum concentrations (geometric means or medians) in epidemiological studies that found suppression of antigen-specific antibody responses from a subset of epidemiological studies are depicted in Figure 1. Although the number of studies is low, they demonstrate that serum concentrations of PFOA and PFOS that induced antibody suppression in children were lower than serum concentrations that induced antibody suppression in adults; the exception was that measured concentrations of PFOA were similar in a study of children from the Faroe Islands (21) and a study of adults from the general U.S. population (25). Recently, Grandjean et al. (55) determined that estimated serum concentration at three and six months of age had a stronger inverse association with antibody concentrations at five years of age, particularly for anti-tetanus antibodies, when compared to measured concentrations at later ages. These estimates suggest that the developing immune system is particularly vulnerable to PFAS exposures, especially when exposures occur during the first six months of life and when the transfer of PFASs via breast feeding are likely to be highest (55). The timing of exposure assessments for immune-related health effects is therefore critical, especially for developmental effects that may produce changes throughout an exposed person’s lifetime. This is particularly important for the developing immune system as exposure during different times of development can result in different effects on the immune system and alter the risk of developing later-life diseases (56).
Figure 1.
Serum PFOA and PFOS concentrations in epidemiological studies showing inhibited antibody responses to vaccines. (21): PFAS measured in serum of five-year-old children and antibodies measured in seven-year-old children (N=464) in the Faroe Islands; (23): PFAS measured in maternal serum at birth and antibodies measured in three-year-old children (N=56) in Norway; (24): PFAS measured in adults (N=403) from the C8 Health Project and antibodies measured after 21±3 days after vaccination; (25): PFAS measured in adults (N=1,191) in the National Health and Nutrition Examination Survey in the U.S. and antibodies to childhood vaccinations.
Approaches that led to different conclusions between the NTP (8) and the Chang et al. (10) reviews
One reason for discrepancies between the conclusions of the NTP (8) and Chang et al. (10) reviews is differences in their definitions of immunosuppression. The NTP defined primary immune outcomes of immunosuppression as, for example, increases in infections or decreased vaccine antibody responses in humans and decreased host resistance to an influenza vaccine or a decreased TDAR in experimental animals (8). A clinical abnormality was not necessary for classification as a primary outcome of immunosuppression. Contrary to the NTP’s approach, Chang et al. (10) questioned whether it was appropriate to identify PFOA and/or PFOS as immunotoxicants when there was no observable abnormality. Chang et al. (10) stated that “an immunodeficiency should not be presumed to exist when there is no evidence of a clinical abnormality” and rely on a set of ten warning signs for primary immunodeficiency diagnosis in children and adults for defining clinical abnormalities. In children, these include, for example, four or more new ear infections within a year, recurrent, deep skin or organ abscesses, and/or a family history of primary immunodeficiency. This definition of a clinical abnormality comes from the Jeffrey Modell Foundation, which educates people about diseases classified as primary immunodeficiencies, a set of 350 immune system disorders (57). According to the American Academy of Allergy, Asthma, and Immunology, when the cause of an immunodeficiency is hereditary or genetic, it is classified as a primary immunodeficiency disease; when the cause of an immunodeficiency is environmental factors, it is classified as a secondary immunodeficiency disease (58). Therefore, the definition of a clinical abnormality used by Chang et al. (10) is not highly relevant for immunodeficiencies induced by environmental factors.
Chang et al. (10) includes a section on vaccine responses and evaluated four studies (21–24; reviewed above) and concluded that on the whole, these studies did not provide consistent evidence of a significant association between PFOA or PFOS exposure and vaccine responses. Some of the concerns raised by Chang et al. (10) regarding these vaccine studies included: none of the studies demonstrated a clinically recognizable increased risk of infectious disease, results were inconsistent by vaccine type, authors failed to provide a priori biological hypotheses to explain why PFOA or PFOS exposure would produce different effects across different vaccines, and some of the associations were not statistically significant. However, the authors did acknowledge that although the results of these studies were insufficient for a causal effect of PFOA or PFOS, the positive associations warranted replication in additional studies.
The NTP (8) approach considered decreased vaccine antibody responses in humans or a decreased TDAR in experimental animals as evidence of immunosuppression. Testing the response of the immune system to an antigen challenge, such as with a vaccine, is the best way to identify deficits in the immune response and an increasing number of studies demonstrate that when deficits are observed in the TDAR in exposed experimental animals, immunotoxicity can be predicted in exposed humans (59). Further, epidemiological studies of exposed humans at the extremes of age, those with existing immunodeficiencies, and those exposed to chronic stress, demonstrate that what is considered mild to moderate immunosuppression in the general human population leads to increased risk of infections to commonly encountered pathogens in these more susceptible populations (59). Therefore, well-accepted biomarkers of immunosuppression, such as a decreased response to vaccines in relatively healthy populations, should not be dismissed because clinical abnormalities also have not been observed or measured in the exposed populations.
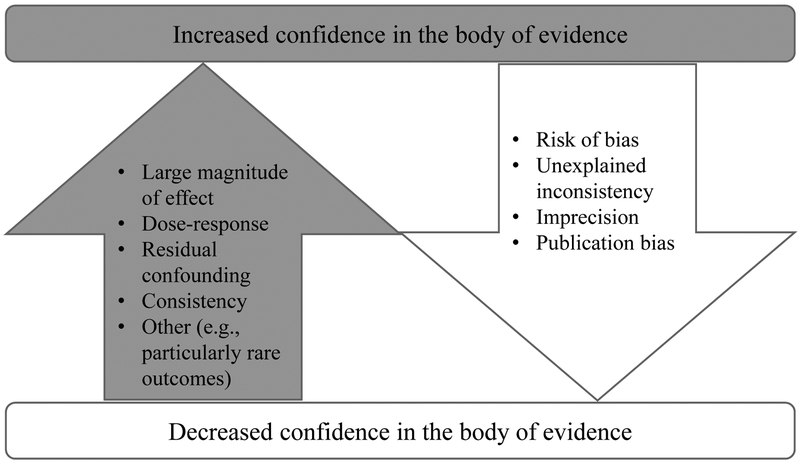
Another reason for the discrepancy between the conclusions of NTP (8) and Chang et al. (10) reviews was the types of studies included in the overall evaluation. Chang et al. assessed only epidemiologic studies and based their assessment on validity and reliability of outcome assessment and exposure assessment, control of confounding, potential for selection bias, and appropriateness of the statistical approach. The NTP (8) used a similar approach using primary outcomes as a line of evidence and determined a confidence rating (see Figure 2) in the entire body of evidence selected for consideration. The difference between the two approaches was that the NTP applied this method to include not only epidemiological studies but studies in experimental animals and support from mechanistic studies. The NTP concluded that evidence for suppression of antibody responses for both PFOA and PFOS was high in experimental animals and moderate in humans. Levels of confidence were low or very low for human and experimental animal data for all other immune-related health outcomes except for hypersensitivity reactions in experimental animals.
Figure 2.
Factors used by the U.S. National Toxicology Program in assessing confidence in the body of evidence concerning the immunotoxic effects of PFOA and PFOS (adopted from 8).
Chang et al. (10) and the NTP (8) reached similar conclusions with respect to hypersensitivity reactions that the degree of consistency among epidemiological studies was low. In part, this is likely due to the lack of accepted biomarkers for hypersensitivity reactions. Chang et al. concluded that the weight of epidemiologic evidence revealed only weak associations that were insufficient to conclude that a causal relationship had been established between PFOA or PFOS and immune conditions, in general, in humans. These conclusions were partially based on epidemiologic studies that included asthma, a particular immune-related health condition that Chang et al. determined to be a clinical immune abnormality. The NTP (8) also determined that the evidence for hypersensitivity-related outcomes for PFOA and PFOS was “low” or “very low” for human data and “moderate” or “low” for experimental animal data. Although the NTP determined that their level of confidence in the hypersensitivity data was overall low, they did note that the collective human and animal bodies of evidence present a consistent pattern of findings that higher exposure to PFOA was associated with an increase in hypersensitivity outcomes.
Conclusions
Immunotoxicity can manifest in myriad ways and typically is classified as immunosuppression or immunoenhancement; these outcomes are not always associated with clinically defined diseases. An immunotoxicological outcome in humans and experimental animals therefore is not limited to clinical outcomes and includes biomarkers, such as antigen-specific antibody responses (i.e., responses to vaccinations or the TDAR), that are well-accepted and robust markers of immune function. To date, evidence supports that PFOA and PFOS, members of the PFAS family of compounds, are immunotoxic with respect to antigen-specific antibody responses. This conclusion is supported by data from epidemiological and toxicological studies and emphasizes that when such studies are considered jointly, aspects of epidemiological studies that are considered limitations, such as timing of exposures compared to effects, concerns about mixtures, and poorly constrained exposure data, can be minimized when data from toxicological studies are supportive.
Acknowledgements
We gratefully acknowledge comments and suggestions on this manuscript provided by Dr. Philippe Grandjean of the University of Southern Denmark and Department of Environmental Health, Harvard T.H. Chan School of Public Health.
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES027706 to LAS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
Dr. DeWitt reports that she was a protocol reviewer and a draft report reviewer for the NTP Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid or Perfluorooctane Sulfonate.
Contributor Information
Jamie C. DeWitt, Department of Pharmacology & Toxicology, Brody School of Medicine, East Carolina University, 600 Moye Blvd., Greenville, NC 27834, USA
Sarah J. Blossom, Department of Pediatrics, University of Arkansas for Medical Sciences, College of Medicine, Arkansas Children’s Research Institute, 13 Children’s Way, Little Rock, AR 72202, USA
Laurel A. Schaider, Silent Spring Institute, 320 Nevada Street, Suite 302, Newton, MA 02460, USA
References
- 1.Science Advisory Board (SAB). SAB Review of EPA’s Draft Risk Assessment of Potential Human Health Effects Associated with PFOA and Its Salts. 2006.
- 2.Yang Q, Xie Y, Depierre JW. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin Exp Immunol. 2000;122(2):219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Xie Y, Eriksson AM, Nelson BD, DePierre JW. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem Pharmacol. 2001;62(8):1133–40. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Abedi-Valugerdi M, Xie Y, Zhao X-YY, Möller G, Dean Nelson B, et al. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int Immunopharmacol. 2002;2(2–3):389–97. [DOI] [PubMed] [Google Scholar]
- 5.Organisation for Economic Cooperation and Development (OECD). Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs). 2018. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JMMONO(2018)7&doclanguage=en.
- 6.Wang Z, DeWitt JC, Higgins CP, Cousins IT. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ Sci Technol. 2017;51(5):2508–18. [DOI] [PubMed] [Google Scholar]
- 7.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Perfluoroalkyls Draft for Public Comment. 2018. (June). https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. [PubMed]
- 8.National Toxicology Program (NTP). NTP Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid or Perfluorooctane Sulfonate. 2016. https://ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf.
- 9.NTP. Systematic Review Fact Sheet. 2015. https://www.niehs.nih.gov/health/materials/systematic_review_508.pdf.
- 10.Chang ET, Adami HO, Boffetta P, Wedner HJ, Mandel JS. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit Rev Toxicol. 2016;46(4):279–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peyton Myers L Clinical Immunotoxicology In: Methods in molecular biology (Clifton, NJ: ). 2018. p. 15–26. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SE, Shane HL. Investigative Immunotoxicology In: Methods in molecular biology (Clifton, NJ: ). 2018. p. 27–46. [DOI] [PubMed] [Google Scholar]
- 13.Luster MI. Immunotoxicology: Clinical consequences. Tox Ind Health. 1996;12(3/4):533–5. [DOI] [PubMed] [Google Scholar]
- 14.Luster MI, Germolec DR, Parks CG, Blaciforti L, Kashon M, Luebke R. Associating Changes in the Immune System with Clinical Diseases for Interpretation in Risk Assessment. Curr Protoc Toxicol. 2004; Unit 18.1. [DOI] [PMC free article] [PubMed]
- 15.Luebke RW, Parks C, Luster MI. Suppression of immune function and susceptibility to infections in humans: Association of immune function with clinical disease. J Immunotoxicol. 2004;1(1):15–24. [DOI] [PubMed] [Google Scholar]
- 16.DeWitt JC, Germolec DR, Luebke RW, Johnson VJ. Associating Changes in the Immune System with Clinical Diseases for Interpretation in Risk Assessment. Curr Protoc Toxicol. 2016;67:18.1.1–18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladics GS. The Sheep Erythrocyte T-Dependent Antibody Response (TDAR) In: Methods in molecular biology (Clifton, NJ: ). 2018. p. 83–94. [DOI] [PubMed] [Google Scholar]
- 18.Luster MI, Portier C, Pait DG, White KL, Gennings C, Munson AE, et al. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam Appl Toxicol. 1992;18(2):200–10. [DOI] [PubMed] [Google Scholar]
- 19.Ward MDW, Copeland LB. Evaluating Antigen-Specific IgE Using the Rat Basophil Leukemia Cell (RBL) Assay In: Methods in molecular biology (Clifton, NJ: ). 2018. p. 371–81. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan BLF, Sulentic CEW, Holsapple MP Kaminski NE. Chapter 12: Toxic Responses of the Immune System In: Casarett and Doull’s essentials of toxicology. 2015. p 177–94. [Google Scholar]
- 21.Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogensen UB, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jorgensen E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates Children’s Environmental Health. Environ Heal A Glob Access Sci Source. 2015;14(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10(4):373–9. [DOI] [PubMed] [Google Scholar]
- 24.Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG, et al. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci. 2014;138(1):76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS. Perfluoroalkyl and Polyfluoroalkyl Substances and Indicators of Immune Function in Children Aged 12 – 19 years: National Health and Nutrition Examination Survey. Pediatr Res. 2015;79(2):348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz-Jørgensen E, et al. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J Immunotoxicol. 2016;13(2):270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vetvicka V, Vetvickova J. Reversal of perfluorooctanesulfonate-induced immunotoxicity by a glucan-resveratrol-vitamin C combination. Orient Pharm Exp Med. 2013;13(1):77–84. [Google Scholar]
- 28.DeWitt JC, Copeland CB, Strynar MJ, Luebke RW. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ Health Perspect. 2008;116(5):644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loveless SE, Hoban D, Sykes G, Frame SR, Everds NE. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol Sci. 2008;105(1):86–96. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt JCJCCopeland CBCB, Luebke RWRW. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicol Sci. 2009;109(1):106–12. [DOI] [PubMed] [Google Scholar]
- 31.Keil DE, Mehlmann T, Butterworth L, Peden-Adams MM. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci. 2008;103(1):77–85. [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre DE, Curran I, Armstrong C, Coady L, Parenteau M, Liston V, et al. Immunomodulatory effects of dietary potassium perfluorooctane sulfonate (PFOS) exposure in adult Sprague-Dawley rats. J Toxicol Environ Health A. 2008;71(23):1516–25. [DOI] [PubMed] [Google Scholar]
- 33.Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol Sci. 2008;104(1):144–54. [DOI] [PubMed] [Google Scholar]
- 34.Zheng L, Dong GH, Jin YH, He QC. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch Toxicol. 2009;83(7):679–89. [DOI] [PubMed] [Google Scholar]
- 35.Dong G-H, Zhang Y-H, Zheng L, Liu W, Jin Y-H, He Q-C. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch Toxicol. 2009;83(9):805–15. [DOI] [PubMed] [Google Scholar]
- 36.Dong G-H, Liu M-M, Wang D, Zheng L, Liang Z-F, Jin Y-H. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch Toxicol. 2011;85(10):1235–44. [DOI] [PubMed] [Google Scholar]
- 37.Qazi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M. 28-Day dietary exposure of mice to a low total dose (7 mg/kg) of perfluorooctanesulfonate (PFOS) alters neither the cellular compositions of the thymus and spleen nor humoral immune responses: Does the route of administration play a pivotal role in PFOS-induced immunotoxicity? Toxicology. 2010;267(1–3):132–9. [DOI] [PubMed] [Google Scholar]
- 38.Wang IJ, Hsieh WS, Chen CY, Fletcher T, Lien GW, Chiang HL, et al. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ Res. 2011;111(6):785–91. [DOI] [PubMed] [Google Scholar]
- 39.Okada E, Sasaki S, Saijo Y, Washino N, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res. 2012;112:118–25. [DOI] [PubMed] [Google Scholar]
- 40.Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ Int. 2014;65:127–34. [DOI] [PubMed] [Google Scholar]
- 41.Smit LAM, Lenters V, Høyer BB, Lindh CH, Pedersen HS, Liermontova I, et al. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy. 2015;70(6):653–60. [DOI] [PubMed] [Google Scholar]
- 42.Ashley-Martin J, Dodds L, Levy AR, Platt RW, Marshall JS, Arbuckle TE. Prenatal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL-33. Environ Res. 2015;140:360–8. [DOI] [PubMed] [Google Scholar]
- 43.Humblet O, Diaz-Ramirez LG, Balmes JR, Pinney SM, Hiatt RA. Perfluoroalkyl chemicals and asthma among children 12–19 years of age: NHANES (1999–2008). Environ Health Perspect. 2014;122(10):1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buser MC, Scinicariello F. Perfluoroalkyl substances and food allergies in adolescents. Environ Int. 2016;88:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong G-H, Tung K-Y, Tsai C-H, Liu M-M, Wang D, Liu W, et al. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ Health Perspect. 2013;121(4):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson-Mahoney P, Kotlerman J, Takhar H, Gray D, Dahlgren J. Self-Reported Health Effects among Community Residents Exposed to Perfluorooctanoate. J Environ Occup Heal Policy. 2008;18(2):129–43. [DOI] [PubMed] [Google Scholar]
- 47.Steenland K, Zhao L, Winquist A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med. 2015;72(5):373–80. [DOI] [PubMed] [Google Scholar]
- 48.Steenland K, Zhao L, Winquist A, Parks C. Ulcerative Colitis and Perfluorooctanoic Acid (PFOA) in a Highly Exposed Population of Community Residents and Workers in the Mid-Ohio Valley. Environ Health Perspect. 2013;121(8):900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osuna CE, Grandjean P, Weihe P, El-Fawal HAN. Autoantibodies associated with prenatal and childhood exposure to environmental chemicals in Faroese children. Toxicol Sci. 2014;142(1):158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairley KJ, Purdy R, Kearns S, Anderson SE, Meade BJ. Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol Sci. 2007;97(2):375–83. [DOI] [PubMed] [Google Scholar]
- 51.Ryu MH, Jha A, Ojo OO, Mahood TH, Basu S, Detillieux KA, et al. Chronic exposure to perfluorinated compounds: Impact on airway hyperresponsiveness and inflammation. Am J Physiol Cell Mol Physiol. 2014;307(10):L765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein CR, Ge Y, Wolff MS, Ye X, Calafat AM, Kraus T, et al. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environ Res. 2016;149:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmermann CAG, Budtz-Jørgensen E, Jensen TK, Osuna CE, Petersen MS, Steuerwald U, et al. Association between perfluoroalkyl substance exposure and asthma and allergic disease in children as modified by MMR vaccination. J Immunotoxicol. 2017;14(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Q, Huang R, Hua L, Guo Y, Huang L, Zhao Y, et al. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and childhood atopic dermatitis: a prospective birth cohort study. Environ Heal. 2018;17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A, et al. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol. 2017. J;14(1):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeWitt JC, Peden-Adams MM, Keil DE, Dietert RR. Current status of developmental immunotoxicity: early-life patterns and testing. Toxicol Pathol. 2012;40(2):230–6. [DOI] [PubMed] [Google Scholar]
- 57.Jeffrey Modell Foundation. (2013). Jeffrey Modell Foundation. Educational Materials. 10 Warning Signs. Available at: http://www.info4pi.org/library/educational-materials/10-warning-signs. Last visited October 9, 2018.
- 58.American Academy of Allergy, Asthma, and Immunology. 2018. Primary Immunodeficiency Disease. https://www.aaaai.org/conditions-and-treatments/primary-immunodeficiency-disease. Last visited October 9, 2018.
- 59.Selgrade MK. Immunotoxicity: the risk is real. Toxicol Sci. 2007;100(2):328–32. [DOI] [PubMed] [Google Scholar]