Abstract
Objective:
We aim to characterize the qualities of estimation approaches for individual exposure to ambient-origin fine particulate matter (PM2.5), for use in epidemiological studies.
Methods:
The analysis incorporates personal, home indoor, and home outdoor air monitoring data and spatio-temporal model predictions for 60 participants from the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). We compared measurement-based personal PM2.5 exposure with several measured or predicted estimates of outdoor, indoor, and personal exposures.
Results:
The mean personal 2-week exposure was 7.6 (standard deviation 3.7) μg/m3. Outdoor model predictions performed far better than outdoor concentrations estimated using a nearest-monitor approach (R=0.63 versus R=0.43). Incorporating infiltration indoors of ambient-derived PM2.5 provided better estimates of the measurement-based personal exposures than outdoor concentration predictions (R=0.81 versus R=0.63) and better scaling of estimated exposure (mean difference 0.4 versus 5.4 μg/m3 higher than measurements), suggesting there is value to collecting data regarding home infiltration. Incorporating individual-level time-location information into exposure predictions did not increase correlations with measurement-based personal exposures (R=0.80) in our sample consisting primarily of retired persons.
Conclusions:
This analysis demonstrates the importance of incorporating infiltration when estimating individual exposure to ambient air pollution. Spatio-temporal models provide substantial improvement in exposure estimation over a nearest monitor approach.
Keywords: Air pollution, epidemiology, exposure modeling, personal exposure, population-based studies
Introduction
Exposure estimation in epidemiological research of ambient air pollutants typically relies—directly or indirectly—upon data collected from sparsely distributed networks of regulatory-directed ambient monitors. While personal monitoring of exposure may in many ways be considered superior to these estimation approaches, such monitoring is impractical for large cohorts and for long-term studies. Further, if the goal is to understand ambient-origin exposures, personal monitoring introduces misclassification by including pollutants generated indoors and by personal activity.(1) As a result, prediction is preferred for ambient exposure assessment even as concerns remain about the degree of exposure misclassification. Several investigator groups have recently recommended improvements in exposure predictions used in epidemiology, including the testing of multiple exposure metrics with each health dataset(2) and the incorporation of pollutant-specific infiltration and detailed time-activity information.(3) This paper addresses these needs in the context of a recent epidemiological study.
While historical air pollution studies often used simpler exposure assignment approaches such as assignment of measured concentration from a single nearest monitor, many modern epidemiological studies use modeled ambient pollutant concentrations from spatial or spatio-temporal models assigned to discrete spatial locations (e.g., outside a given subject’s home). Outdoor air pollutant concentrations generated for geocoded residence locations are usually assumed to be representative of an individual’s true exposure. While much of outdoor fine particulate matter (PM2.5) air pollution may infiltrate indoors, infiltration rates can vary by season, geographic location, and with housing or behavioral characteristics including building age, air conditioning usage, and window opening.(4–8) Variability in PM2.5 infiltration is further affected by particle size distribution and by chemical composition; larger particles, ultrafine particles, and more volatile species all have reduced infiltration efficiency.(7, 9, 10) Further, people spend time at locations other than immediately outside or inside their homes, such as at work or commuting, which are not necessarily well-represented by residence-based predictions.
Understanding exposure misclassification is a key component of measurement error analysis. As part of the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air),(11) we examined sources of exposure misclassification associated with various approaches to exposure estimation for air pollution epidemiological research. This analysis incorporates personal, home indoor, and home outdoor air monitoring data collected for this study. Using particulate sulfur measurements, we created a “reference approach” for personal ambient-derived PM2.5 exposure and compared this measurement-based personal exposure metric to a number of additional exposure estimation metrics including spatio-temporal exposure model predictions developed for the entire multi-city MESA Air cohort. These comparisons allowed us to understand different aspects of exposure misclassification by comparing personal to outdoor and indoor exposures and measured to predicted exposures. We anticipated that outdoor concentrations predicted with the spatio-temporal model would be more aligned with our reference personal exposure metric than concentrations estimated with a simpler “nearest monitor” approach. Additionally, we hypothesized that incorporating information on pollutant infiltration and personal time-location patterns would further improve this relationship.
Methods
Approach
The primary goal of this paper was to evaluate and understand differences in estimates of personal exposure to ambient-origin air pollution. Eight different exposure metrics are presented, including several residence-specific measurement-based concentrations, predictions derived from highly resolved spatio-temporal models, and concentrations using data from the nearest regulatory monitors. These metrics were developed to reflect the range of exposure metrics presented in the epidemiological literature in order to better understand and characterize exposure misclassification that may be associated with each approach.
Study Population
MESA Air is an ancillary study to the Multi-Ethnic Study of Atherosclerosis (MESA),(12) a prospective cohort study of the incidence and progression of cardiovascular diseases (CVD) in adults. MESA included 6,814 participants from six US communities: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN. Participants were aged 45–84 years at enrollment, which occurred between 2000-2002, with an approximately equal sex ratio, and were free of recognized CVD at baseline. Four ethnic/racial groups were targeted for inclusion: non-Hispanic white, non-Hispanic black, Hispanic, and Chinese. MESA Air includes 7,551 participants, of which the majority are from the parent MESA cohort. Additional participants were recruited from a second ancillary study to MESA, the MESA Family Study (n=490), and directly for MESA Air (n=257) in three additional areas near existing MESA communities, two areas in the Los Angeles basin and one area near New York City.(11)
Between 2005-2008, a subset of 90 MESA Air participants completed a two-week personal monitoring study. All of the participating centers’ institutional review boards approved the study, and all study participants gave written informed consent before data collection. These participants completed concurrent indoor, outdoor, and personal PM2.5 monitoring and recorded their locations for the duration of the personal monitoring period in detailed time-location diaries; 80 of these participants completed the monitoring protocol twice, in two distinct seasons.(13) Participation in this personal monitoring study was restricted to individuals living in nonsmoking households.
Data Sources
Residential and Personal Measurements of Fine Particulate Matter (PM2.5)
MESA Air collected two-week concurrent outdoor, indoor, and personal measurements of PM2.5 using previously described methods.(13) Briefly, outdoor sampling was typically conducted in a participant’s backyard, away from all structures, and indoor samplers were deployed in the participant’s main activity room away from pollutant sources or ventilation systems. Harvard personal environmental monitors (HPEM, Cambridge, MA) were worn during the day, with an inlet to the sampler worn in the breathing zone, with the air sampling pump (TSI SidePak SP530, Shoreview, MN) carried in a backpack or fanny pack, running on battery power. PM2.5 mass concentrations were gravimetrically determined from Teflon filters at the University of Washington in a temperature- and humidity-controlled environment(14) using standard filter weighing procedures.(15) In addition to determining PM2.5 mass, filters were also analyzed for sulfur content by x-ray fluorescence. Sulfur has been found to be a reasonable tracer of ambient particle infiltration, with a similar infiltration rate to particles as a whole, but with very few indoor sources.(16)
Modeled PM2.5 Exposures
Outdoor fine particulate matter concentrations were modeled on the two-week scale for each MESA city using area-specific finely resolved spatio-temporal methods.(17) These models were developed by determining a temporal trend from MESA Air study-specific and government agency monitors, incorporating land-use information such as distance to road, population in buffers, elevation, vegetative cover, industrial emissions, and other variables through partial-least squares (PLS) regression, and universal kriging of the spatio-temporal residuals between model predictions generated by the time-trend and PLS components and two-week measurements. Measurement data were derived from monitors at regional regulatory agency sites (13-45 sites in each area with 51-346 2-week observations per site), MESA Air stationary sites (3-7 per area with 6-93 2-week observations per site), and MESA Air home monitoring locations (86-136 in each area with 1-5 observations per site), including the homes of personal monitoring study participants. The prediction models use data derived from participant-specific home outdoor measurements, so predictions at personal monitoring participants’ homes would have been expected to be better on average than those for a randomly selected cohort members. This could have led to potentially biased misclassification assessments, as the majority of cohort members did not have a monitor placed outside at their home. To address this possibility, predictions used in this paper were derived from models developed for cross-validation exercises which excluded the home under study. Spatio-temporal models were re-fit 10 times, each time leaving out 10% of the monitors and generating predictions for those locations based on the remaining 90% of the monitoring data. Cross-validated R2 for each model were calculated based on these out-of-sample predictions, and ranged from 0.54 to 0.85 for prediction at home sites. The RMSE ranged from 1.00 to 2.92 μg/m3. Predictions were generated for non-overlapping two-week periods that generally aligned well with residential monitoring.
Personal Time-Activity Patterns
Time activity patterns were assessed using two different questionnaires.(18) Two-week specific time-location information was provided by personal monitoring study participants using a time-location diary (TLD) that was completed concurrently with personal exposure monitoring. In the TLD, participants recorded their location (the number of minutes in each hour) for each hour of each study day spent in one of seven microenvironments: home indoors, home outdoors, motorized vehicle, work indoors, work outdoors, other indoors, and other outdoors. A second questionnaire, the “MESA Air Questionnaire” (MAQ) was administered to the entire MESA Air cohort. The MAQ included an assessment of a typical week in July (warm weather) and in January (cold weather), with time-location behavior (hours per day) recorded for each day in a similar set of seven microenvironments (home indoors, home outdoors, work/volunteer/school indoors, work/volunteer/school outdoors, in transit (e.g., car, bike, public transit), other indoors, and other outdoors) as had been documented in the TLD.
Modeled Infiltration Efficiency (Finf(p))
As part of the MAQ, participants reported on home characteristics and behaviors that impact air flow into the home, such as window-opening frequency and air conditioning use. These responses together with ambient temperature data were used to develop an infiltration model generalizable to the entire cohort, including participants that were not included in the environmental monitoring subset.(4)
Regulatory Monitor Measurements of PM2.5
A common approach to exposure estimation is to assign each participant pollutant concentration data measured at the air monitoring agency station closest to their residence. Station monitors are brought online or decommissioned according to national and local regulatory priorities, which could introduce additional variability if the “nearest monitor” assigned to a particular participant changes. Therefore, for our nearest-monitor approach, we included only monitors that had measurements available during the entire study period. The list of monitors is provided in Supplementary Table S1.
Exposure Metrics
Table 1 summarizes the eight measured and modeled exposure metrics compared in this study, which each cover the intended two-week study-specific sampling period.
Table 1.
Summary of exposure metrics with abbreviations used throughout the article.
| Description | Abbreviation | Calculation/Source |
|---|---|---|
| 1. Measurement-based personal ambient- derived PM2.5 (Reference Approach) |
PM2.5-pers(m) | (Sulfur2.5-pers / Sulfur2.5-out) * PM2.5-out(m) |
| 2. Measured outdoor PM2.5 | PM2.5-out(m) | Measured |
| 3. Predicted outdoor PM2.5 | PM2.5-out(p) | Modeled |
| 4. Nearest monitor outdoor PM2.5 | PM2.5-out(nm) | Measured by regulatory agencies |
| 5. Measurement-based indoor ambient- derived PM2.5 |
PM2.5-in(m) | Finf(m) * PM2.5-out(m) |
| 6. Prediction-based indoor ambient-derived PM2.5 |
PM2.5-in(p) | Finf(p) * PM2.5-out(p) |
| 7. Measurement-based, individually weighted ambient-derived PM2.5 |
PM2.5-pers(m-alt) | PM2.5-out(m) * tout-TLD + Finf(m) * PM2.5-out(m) * tin-TLD |
| 8. Prediction-based, individually weighted ambient-derived PM2.5 |
PM2.5-pers(p) | PM2.5-out(p) * tout-MAQ + Finf(p) * PM2.5-out(p) * tin-MAQ |
Notes: pers: personal; out: outdoor; in: indoor; m: measured; p: predicted; nm: nearest monitor; t: time; TLD: time-location diary; MAQ: MESA Air Questionnaire; Finf(p): Measured Infiltration Efficiency, Sulfur2.5-in / Sulfur2.5-out; Finf(p): Modeled Infiltration Efficiency, described by Allen et al.(4)
1. Measurement-based Personal Ambient-Derived PM2.5 (Reference Approach)
The measurement-based reference approach estimates personal exposure to PM2.5 of ambient origin by multiplying measured outdoor PM2.5 by the ratio of personal sulfur to outdoor sulfur. This sulfur ratio is used to estimate the proportion of measured personal PM2.5 that is thought to be of ambient origin.
2. Measured Outdoor PM2.5
This metric represents the two-week integrated measurements of PM2.5 collected outside participant residences as described above.
3. Predicted Outdoor PM2.5
Predicted ambient concentrations of PM2.5 at each subject’s home concurrent with measured concentrations are available from the spatio-temporal model developed for the entire cohort.(17) Ten of the 170 personal monitoring rounds did not occur on this schedule due to logistical conflicts, and their measurements overlap two consecutive predictions. In these cases, the two predictions were averaged for comparison to the measurements.
4. Nearest Monitor Outdoor PM2.5
EPA Air Quality System (AQS) monitors were selected to represent ambient community-scale exposure. A list of the selected monitors is provided in the Supplemental Materials. Daily or one-in-three day 24-hour integrated measurements collected during the two-week study-specific sampling period were averaged to yield this metric.
5. Measurement-Based Indoor Ambient-Derived PM2.5
The measurement-based indoor approach estimates indoor exposure to PM2.5 of ambient origin by multiplying the measured outdoor PM2.5 by the ratio of indoor sulfur to outdoor sulfur. This sulfur ratio is used to estimate the proportion of measured indoor PM2.5 that is thought to be of ambient origin (measured infiltration efficiency, Finf(m)).
6. Prediction-Based Indoor Ambient-Derived PM2.5
The prediction-based indoor approach estimates indoor exposure to PM2.5 of ambient origin by adjusting the predicted outdoor PM2.5 using the predicted two-week specific predicted infiltration efficiency modeled in MESA Air (Finf(p)).(4)
7. Measurement-Based Individually-Weighted Ambient-Derived PM2.5
This metric is an alternative, measurement-based approach that uses two-week specific measurements. These measurements are analogous to the components used in the prediction-based approach, described below. For this metric, measured outdoor PM2.5 and measurement-based indoor ambient-derived PM2.5 are averaged with weighting by time reported spent indoors and outdoors on the TLD. In calculating this metric, we assumed that the home indoor and outdoor concentrations are representative of all indoor and all outdoor locations, respectively.
8. Prediction-Based Individually-Weighted Ambient-Derived PM2.5
This metric included both outdoor predictions and the indoor predictions, which incorporate Finf(p). (4) Individual-level predictions were made by calculating time-weighted air pollution predictions incorporating the indoor and outdoor predictions and reported time-location information from the MAQ. For this analysis, we assumed that concentrations measured at home indoors are representative of all indoor locations, and concentrations measured at home outdoors are representative of all outdoor locations.
For purposes of evaluation in the context of our epidemiological study, metrics 3, 4, 6, and 8 can be estimated for most cohort members while the other methods require the information from the personal monitoring study described in this paper.
Analysis Methods
For all measurement-based exposure metrics, we excluded rounds of sampling that did not contain a complete set of measurements (outdoor PM2.5, outdoor sulfur, indoor sulfur, personal sulfur, and time-location data). Results were also excluded if the sulfur ratios (personal to outdoor or indoor to outdoor) were higher than 1.05 to avoid samples that represent rare or isolated atypical exposures.(4)
For the individual-level calculations, the percent of time spent outdoors was based on the sum of the time spent outdoors at home, outdoors at work, outdoors at other locations, and in transit/in vehicle. Data from the TLD and MAQ were used for the measurement-based and predicted exposure metrics, respectively.
Summary statistics (mean, median, standard deviation, and range) were calculated for each exposure metric. Our analysis focused on a series of comparisons among the eight exposure metrics (Table 2). This allowed us to assess the contributions of different layers of estimation to the measurement error present in exposure estimates generated for unmeasured locations and times. For each of these comparisons, we calculated the following between-exposure metrics: Pearson correlation coefficient (R), mean relative percent difference (RPD; |X - Y|/mean(X,Y)), and root mean square error (RMSE). For primary analyses, summary statistics and comparisons were calculated using all two-week sampling periods. Additional analyses were limited to data from participants with two sampling rounds; these results were presented as both averaged and unaggregated. All analyses were conducted using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria).
Table 2.
Summary of exposure metric comparisons.
| Primary Metric | Comparison Metric | Goal for Comparison is to Understand Differences Between: |
|---|---|---|
| 1. PM2.5-pers(m) | 2. PM2.5-out(m) | personal and outdoor, measured concurrently |
| 1. PM2.5-pers(m) | 3. PM2.5-out(p) | personal and predicted outdoor |
| 1. PM2.5-pers(m) | 4. PM2.5-out(nm) | personal and predicted outdoor using a simplified approach |
| 1. PM2.5-pers(m) | 5. PM2.5-in(m) | personal and ambient-derived infiltrated indoor |
| 1. PM2.5-pers(m) | 6. PM2.5-in(p) | personal and predicted ambient-derived infiltrated indoor |
| 1. PM2.5-pers(m) | 7. PM2.5-pers(m-alt) | two measurement-based approaches for estimating personal exposures |
| 1. PM2.5-pers(m) | 8. PM2.5-pers(p) | personal and predicted time-weighted indoor and outdoor |
| 7. PM2.5-pers(m-alt) | 8. PM2.5-pers(p) | measured and predicted time-weighted indoor and outdoor |
As part of a sensitivity analysis, the comparisons between our reference exposure metric (1) and the prediction-based individually-weighted ambient-derived PM2.5 (8), were evaluated to explore the impact of city, race/ethnicity, sex, participant age, building age, time away from home, time in transit, season (cold, warm, very warm), and month of sampling. We also conducted sensitivity analyses where we included all available valid data in the comparisons. Further, the infiltration model was re-fit excluding homes in the personal monitoring study and Finf re-calculated using results of this model; R was computed among personal monitoring homes comparing predicted Finf from both versions of the model.
Results
Individuals who participated in personal monitoring were more likely to be white and tended to be younger than the overall MESA Air cohort. Although 90 individuals participated in personal monitoring, several were missing at least one of the indoor, outdoor, or personal measurements, primarily due to problems with sampling equipment performance. There were also a number of participants living in New York City who could not wear the active samplers (due to security concerns) while riding the subway. Five two-week sampling episodes were excluded because the measured ratio of indoor/outdoor sulfur or personal/outdoor sulfur was greater than 1.05. Sixty individuals met the complete data criterion for primary analyses. Of these, 29 provided one valid monitoring session and 31 provided two valid sessions. Participants from New York City and Los Angeles were most likely to have missing data, while participants from Baltimore and St. Paul were least likely. Measurement counts and participant characteristics are provided in Table 3 with additional detail provided in Supplementary Table S2. Summary statistics for measured data are provided in Table S3.
Table 3.
Participant characteristics and number of measurements among participants with a complete set of valid metrics for at least one round of air monitoring.
| W-S | NY | Baltimore | St. Paul | Chicago | LA | Total | |
|---|---|---|---|---|---|---|---|
| N | 10 | 8 | 13 | 12 | 7 | 10 | 60 |
| Age (years)a | 64 [58-74] |
72 [63-86] |
67 [54-81] |
62 [54-75] |
68 [56-76] |
65 [54-77] |
66 [54-86] |
| Femalea | 9 (90%) | 3 (40%) | 5 (40%) | 7 (60%) | 1 (14%) | 6 (60%) | 31 (52%) |
| Whitea | 7 (70%) | 5 (63%) | 10 (77%) | 10 (83%) | 5 (71%) | 4 (40%) | 41 (68%) |
| Chinesea | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 1 (2%) |
| Blacka | 3 (30%) | 2 (25%) | 3 (23%) | 0 | 2 (29%) | 1 (10%) | 11 (18%) |
| Hispanica | 0 | 1 (13%) | 0 | 2 (17%) | 0 | 4 (40%) | 7 (12%) |
| Year Home | 1964 | 1948 | 1964 | 1964 | 1960 | 1964 | 1956 |
| Builtb | [1937-1998] | [1925-1970] | [1938-1994] | [1887-1985] | [1915-1965] | [1945-2004] | [1887-2004] |
| Percent of Time in Microenvironments | |||||||
| Home Indoor | 72% [50-97] |
79% [70-89] |
73% [54-94] |
74% [54-90] |
74% [49-95] |
81% [63-92] |
75% [49-97] |
| Home Total (In and Out) |
75% [51-98] |
86% [71-100] |
74% [58-95] |
77% [59-91] |
75% [51-95] |
86% [63-99] |
79% [51-100] |
| Indoor Total (Home and Other) |
90% [84-97] |
85% [72-92] |
89% [78-96] |
88% [66-96] |
89% [69-99] |
89% [81-95] |
88% [66-99] |
| In Vehicle | 5% [1-9] |
5% [0-18] |
6% [2-17] |
6% [1-27] |
4% [1-14] |
3% [0-8] |
5% [0-27] |
| Home Type | |||||||
| Single Family | 10 (100%) | 5 (63%) | 11 (85%) | 8 (67%) | 4 (57%) | 8 (80%) | 45 (75%) |
| Apartment or Condo |
0 | 2 (25%) | 1 (8%) | 0 | 2 (29%) | 2 (20%) | 7 (12%) |
| Rowhouse, Du-/Triplex |
0 | 1 (13%) | 1 (8%) | 4 (33%) | 1 (14%) | 0 | 8 (13%) |
| Sampling Month (n = 91) | |||||||
| December- February |
3 (3%) | 1 (1%) | 4 (4%) | 4 (4%) | 1 (1%) | 4 (4%) | 17 (19%) |
| March – May | 4 (4%) | 2 (2%) | 9 (10%) | 8 (9%) | 3 (3%) | 2 (2%) | 28 (31%) |
| June – August | 6 (7%) | 6 (7%) | 6 (7%) | 5 (5%) | 5 (5%) | 1 (1%) | 29 (32%) |
| September – November |
2 (2%) | 2 (2%) | 4 (4%) | 2 (2%) | 2 (2%) | 5 (5%) | 17 (19%) |
Mean and range are provided for age and percent of time spent in microenvironments. Median and range are provided for year home built; all other statistics are provided as count (%).
Approximately 50% of participants did not provide information on the age of the building.
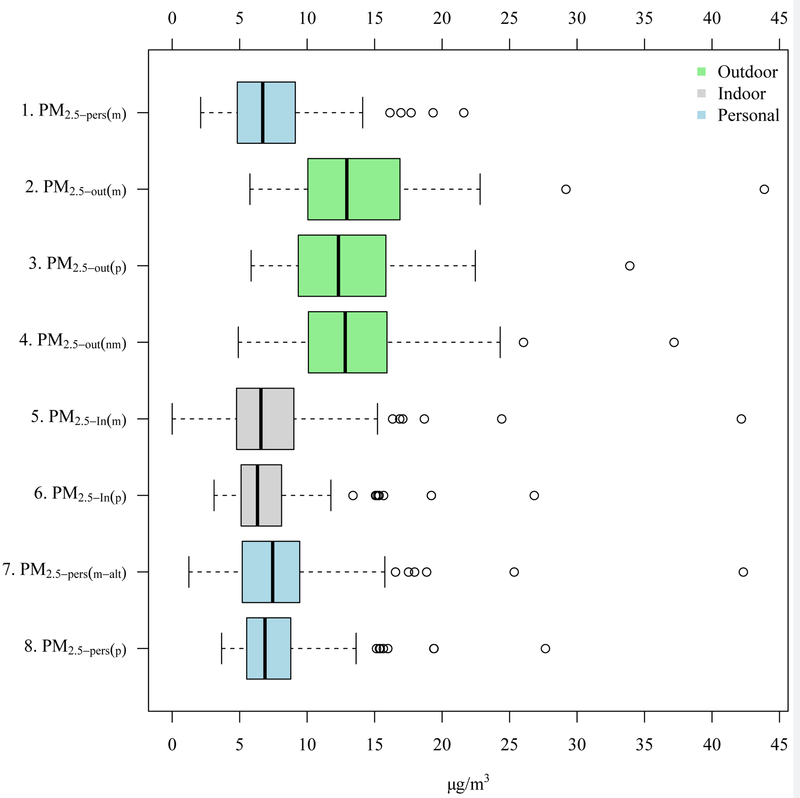
Exposure metric distributions are shown in Figure 1 and Supplementary Table S4, with results for the subset with two sampling rounds provided in the Supplementary Table S5. The range and variability of all outdoor metrics were similar, though on average slightly lower for the predicted outdoor concentrations (# 3) compared to the measured concentrations (# 2). When comparing measurement-based and prediction-based metrics, the distribution and scale of the reference metric (#1) was most similar to the distribution of predicted indoor exposure (#6). On average, the values of the measurement-based individually-weighted method (#7) and of the prediction-based individually-weighted, ambient-derived exposure (#8) were both slightly higher than the reference method.
Figure 1.
Exposure metrics (n=91 total 2-week rounds, including 31 participants with 2 rounds (62 rounds) and 29 participants with 1 round of sampling). All metrics are ambient origin PM2.5, not total PM2.5.
Strong correlations (Table 4), low RPD (Table 5), and low RMSE (Supplementary Table S6) were observed when comparing the reference method (#1) with prediction-based ambient-derived individually-weighted (#8) and indoor exposures (#6). The reference method also compared very well with measurement-based ambient-derived individually-weighted (#7) and indoor exposures (#5), and there was good agreement between the two individually-weighted exposures (#7 and #8).
Table 4.
Pearson correlations between exposure metrics. Correlations are those among all measurements (n = 91 rounds of 2-week sampling).
| 1. PM2.5- pers(m) |
2. PM2.5- out(m) |
3. PM2.5- out(p) |
4. PM2.5- out(nm) |
5. PM2.5- in(m) |
6. PM2.5- in(p) |
7. PM2.5- pers(m-alt) |
|
|---|---|---|---|---|---|---|---|
| 2. PM2.5-out(m) | 0.69 | 1.00 | |||||
| 3. PM2.5-out(p) | 0.63 | 0.80 | 1.00 | ||||
| 4. PM2.5-out(nm) | 0.43 | 0.70 | 0.87 | 1.00 | |||
| 5. PM2.5-in(m) | 0.88 | 0.76 | 0.55 | 0.42 | 1.00 | ||
| 6. PM2.5-in(p) | 0.81 | 0.62 | 0.77 | 0.63 | 0.76 | 1.00 | |
| 7. PM2.5-pers(m-alt) | 0.88 | 0.79 | 0.58 | 0.46 | 0.99 | 0.77 | 1.00 |
| 8. PM2.5-pers(p) | 0.80 | 0.64 | 0.79 | 0.65 | 0.76 | 1.00 | 0.77 |
Notes: Primary comparisons are shaded (see Table 2).
Table 5.
Relative percent difference (RPD, |X-Y|/mean(X, Y)) between metrics among all 2-week measurements (n = 91).
| 1. PM2.5- pers(m) |
2. PM2.5- out(m) |
3. PM2.5- out(p) |
4. PM2.5- out(nm) |
5. PM2.5- in(m) |
6. PM2.5- in(p) |
7. PM2.5- pers(m-alt) |
|
|---|---|---|---|---|---|---|---|
| 2. PM2.5-out(m) | 59% | ||||||
| 3. PM2.5-out(p) | 55% | 11% | |||||
| 4. PM2.5-out(nm) | 59% | 17% | 14% | ||||
| 5. PM2.5-in(m) | 18% | 61% | 59% | 62% | |||
| 6. PM2.5-in(p) | 21% | 60% | 56% | 60% | 27% | ||
| 7. PM2.5-pers(m-alt) | 17% | 53% | 50% | 54% | 12% | 25% | |
| 8. PM2.5-pers(p) | 22% | 55% | 50% | 54% | 27% | 6% | 24% |
Notes: Primary comparisons are shaded
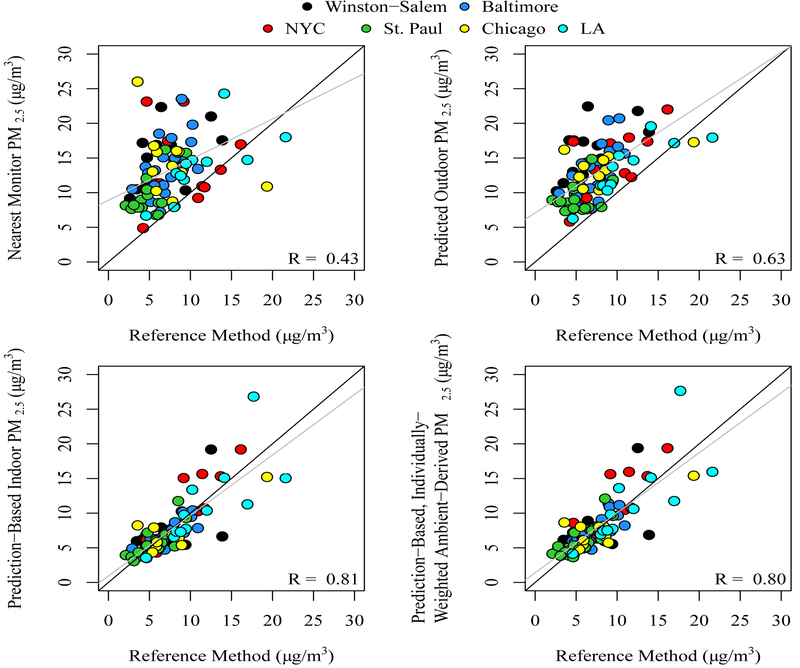
Weaker correlations and larger RPD and RMSE were observed when comparing the reference method to the outdoor predictions (#3), although the correlation between measured (#2) and predicted outdoor (#3) was high. Figure 2 shows the correlation plot between the reference metric and the nearest monitor (#4), the predicted outdoor (#3), the predicted indoor (#6), and the predicted personal (#8) metrics. Variability was greatest and correlation weakest with the nearest monitor metric.
Figure 2.
Comparison between reference method ((Sulfur2.5-pers/ Sulfur2.5-out) * PM2.5-out(m)) and each of four exposure metrics: 1) Average concentration at nearest regulatory monitor, 2) outdoor (PM2.5-out(p)), 3) indoor (PM2.5-in(p)), and 4) individually-weighted, ambient derived (PM2.5-out(p) * tout-MAQ)) + Finf(p) * PM2.5-out(p) * (1 - tout-MAQ)) for 91 rounds of 2-week sampling. All metrics are ambient origin PM2.5, not total PM2.5.
Notes: Predicted outdoor PM2.5 is expected to be higher than personal exposure due to the attenuation by infiltration and time spent indoors. The 1-1 line (black) and best fit line (gray) are shown for reference.
Results including aggregated data for participants with two sampling rounds are presented in Supplementary Tables S7-S9 and Figure S1. When restricted to participants with two rounds of sampling, RMSE values tended to be lower but correlations and RPD values were similar. Averaging the results from the two sampling rounds did not substantially change RMSE, RPD, or correlation values.
For sensitivity analyses including all available data, correlations were substantially similar to those observed in the main analyses (Supplementary Tables S10-S11). Among personal monitoring homes, predicted Finf generated from both versions of the infiltration model were highly correlated (R2=0.997;Supplementary Table S12 and Figure S2). There was no impact of city, race/ethnicity, sex, participant age, building age, time away from home, time in transit, season, or month of sampling on comparisons between the primary exposure metric (#1) and the predicted personal exposures (#8) (see table S13 of the supplement).
Discussion
Using personal monitoring measurements and predictions from a highly-spatially resolved exposure model, this study provides a novel evaluation of the characteristics of exposure misclassification found in a variety of air pollution exposure estimation approaches used in epidemiological research. Comparisons with a reference method that robustly estimated personal exposure to ambient-derived fine particles revealed several key observations. First, the predicted and measured outdoor concentration approaches were comparable in estimating personal exposure, while the nearest monitor approach was clearly worse. Second, both measured and predicted home outdoor concentrations introduced notable error in terms of scale (having higher concentrations) and poor correlation with personal exposure. Third, accounting for predicted residential particulate infiltration corrected the scaling problem (as both infiltrated and personal exposure had similar concentration values) and also improved correlations. Fourth, further accounting for personal time-activity data did not appear to add predictive value for this cohort.
Previous personal monitoring studies, with varying designs and objectives, have compared personal measurements to those inside and outside a subject’s residence.(5, 19–27) However, few personal PM2.5 monitoring studies have been nested within cohorts, where estimates from exposure metrics applied to the entire cohort population can be compared to personal ambient-derived PM2.5 measurements. Personal monitoring within a cohort setting, as was done in this study, can inform questions of exposure measurement error within the same study and in other studies. Adjustments for spatial misalignment can be incorporated into epidemiological analyses,(28-30) although care must be taken to account for bias and inflated standard errors due to limitations in the size of validation data sets.(31, 32)
Compared with the nearest monitor method, predicted outdoor PM2.5 from the spatio-temporal model showed a stronger relationship with both the reference personal exposure method and with measured outdoor concentrations (Tables 4 and 5). This confirms our hypothesis that the advanced methods used to model outdoor air pollution provide improved spatial resolution of the exposure concentration contrasts. The relative strengths of these relationships were tested through the use of cross-validation models, which excluded measurements specific to the participants monitored in this study.
Predicted indoor exposure was as highly correlated with the personal exposure reference method as was predicted personal exposure. These results imply that for this older cohort (likely representative of individuals who spend a great deal of time indoors at home), there may be little benefit in assessing detailed time-location patterns. This observation may not be true for other populations, particularly those with individuals who spend a large portion of their time away from home. For example, a simulation study using the London Hybrid Exposure Model(33) found that personal predictions correlated best with home outdoor predictions among individuals spending all their time at home and for those using primarily active transport. In contrast to the time-location information, there was an exposure prediction benefit in our population to asking participants a few simple questions about their home characteristics and typical ventilation behavior, which predict infiltration.
A recent study conducted in central North Carolina also explored the potential for measurement error from variations in infiltration and time activity patterns.(27) Breen et al. compared exposure metrics based on personal, indoor, and outdoor monitoring; their study operated a central site rather than using data from a regulatory monitor. Infiltration was determined two ways: 1) via a sulfur tracer (as in the current study) and 2) via a mechanistic model. Their infiltration modeling approach differed from ours (mechanistic versus regression), but both approaches accounted for housing characteristics and climate information. As was done in our MESA Air study, Breen et al. emphasized the importance of including infiltration and time-activity pattern data in exposure estimates for epidemiologic research. However, in contrast to our study, they did not find substantial differences between the nearest monitor and measured outdoor concentrations. The increased variability between nearest monitor and measured (or predicted) outdoor concentrations in our study relative to the Breen et al. investigation is likely due to the larger geographic area represented and conditions encountered in the MESA Air study. We investigated PM2.5 in six cities varying in urbanicity and in relative contribution of regional background PM2.5 to residential concentrations. The smallest differences between nearest monitor and the other measures were seen in the MESA Air city of St. Paul, where concentrations of PM2.5 tended to be fairly homogeneous.
The spatio-temporal model predictions used in this study were those derived from a ten-fold cross-validation excluding 10% of monitors in turn. This was done as a conservative measure to avoid creating predictions in this subset that were closer to the true exposure than those available in the rest of the cohort, and as such to enhance generalizability of these results to the remainder of the MESA Air cohort. It is possible that the omission of 10% of the monitoring data actually created predictions that are slightly worse than the average predictions in the entire cohort when non-cross-validated models are used. In this case, true correlations between personal exposure and all exposure metrics incorporating outdoor predictions might be stronger than those reported.
Sulfur is commonly used as a tracer of indoor infiltration of fine particulate matter (4, 16, 34–37) and can be used to quantify the ambient-derived portion of personal PM2.5. The use of sulfur as a tracer to characterize ambient-origin particles is a strength of this study. This requires at least two assumptions: 1) no indoor sources of sulfur are present (e.g., smoking, candles, or incense) and 2) infiltration efficiencies are equivalent for sulfur and PM2.5. Using a sulfur tracer may actually overestimate PM2.5 infiltration, because sulfate-containing particles tend to be smaller than many fine particles and thus may have higher infiltration rates than PM2.5 as a whole.(36) If infiltration were over-estimated, the difference in scale between outdoor and personal exposure would be even greater than reported in this research.
The modeled infiltration factor (Finf(p)) was derived using a dataset that included participant data from the personal monitoring campaign.(4) To ensure that the incorporation of data from these participants did not overfit the estimate of infiltration for the current analysis, the infiltration model was refit excluding the personal monitoring participants. The resulting model output was very similar to the previously derived model (see Supplementary Materials). Therefore, the original model(4) was retained for the analyses reported in this paper.
Our primary analysis only included rounds of sampling with complete data for all exposure measurements (outdoor PM2.5, outdoor sulfur, indoor sulfur, personal sulfur, and time-location data). However, missingness of data was greater in cities with higher and more variable PM2.5 concentrations, which may have influenced the estimated correlation and RSME. Results for participants with two sampling rounds tended to be less variable in this study. This observation seems explainable, because participants in New York and Los Angeles, where observed concentrations were the most variable, were more likely to have only one valid sampling round. Results of RPD calculations were similar among participants with one sampling round compared to those with two; as a relative metric, RPD is robust when comparing predictions across varying scales.
Data collected on a two-week time scale were utilized in this study; predictions used in epidemiological studies of the MESA Air cohort typically aggregate two-week predictions from the spatio-temporal model to long-term exposure predictions by averaging up to the annual time scale. Greater natural exposure variability occurs on the two-week scale compared to the annual scale, so it is challenging to quantify the magnitude of exposure misclassification that may occur in a long-term study based on the analysis presented in this paper. However, the aggregated analyses among the subset of participants with two rounds of sampling in different seasons provide a surrogate for a long-term exposure, and results in this subset did not provide materially different conclusions compared to the non-aggregated analysis.
Our study focused on ambient-derived particles; we did not attempt to incorporate a model of indoor PM2.5 that included indoor sources. Non-smoking homes were chosen to enhance internal study validity. Consequently, homes with significant indoor sources of particles (e.g., from smoking) were not evaluated. If there are important differences between smoking and non-smoking homes not captured in our predictive modeling, our results may be less generalizable to participants who smoke or live with smokers.
This study provides important evidence that personal ambient-origin exposure levels are substantially lower than outdoor predictions, among an older general population sample with little occupational exposure. This finding has significant implications for air pollution regulation, where current regulatory practice is based on measured outdoor concentrations. The results of our study imply that the outdoor predictions commonly used in epidemiology studies overestimate the true ambient-origin personal exposure. This, in turn, may underestimate health effect parameters. On a per unit scale, the true hazard ratio associated with ambient PM2.5 exposure may be larger than typically reported in the epidemiological literature, but formal measurement error analysis is required to estimate the bias.
Conclusions
In conclusion, this paper advances the understanding of exposure misclassification in epidemiological research of fine particulate air pollution, using systematic evaluation of personal monitoring measurements compared with multiple exposure metrics generated by a combination of state-of-the-art air monitoring data and spatio-temporal modeling. We found that among a sample from a cohort of older individuals who spend much of their time at home, estimated individual-level exposure which incorporates modeled infiltration improves exposure prediction over outdoor predictions measurements.
Supplementary Material
Acknowledgments
This publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S Environmental Protection Agency. Funding was also provided by EPA grant No. RD-83479601-0 (CCAR) and RD-83830001 (MESA Air Next Stage). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, by grants UL1-TR-000040 and UL1-RR-025005 from the National Center for Research Resources (NCRR), and P30ES007033 from the National Institute of Environmental Health Sciences. Funding for MESA Family is provided by grants R01-HL-071051, R01-HL-071205, R01-HL-071250, R01-HL-071251, R01-HL-071252, R01-HL-071258, R01-HL-071259, UL1-TR-001079, by NCRR, Grant UL1RR033176, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000124. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supplementary information is available at the Journal of Exposure Science & Environmental Epidemiology’s website.
Conflict of Interest
All authors declare they have no actual or potential competing financial interest.
References
- 1.Wilson WE, Mage DT, Grant LD. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: Why and how. Journal of the Air & Waste Management Association. 2000. July;50(7):1167–83. [DOI] [PubMed] [Google Scholar]
- 2.Dionisio KL, Baxter LK, Burke J, Ozkaynak H. The importance of the exposure metric in air pollution epidemiology studies: When does it matter, and why? Air Quality, Atmosphere & Health. 2016;9(5):495–502. [Google Scholar]
- 3.Baxter LK, Dionisio KL, Burke J, Sarnat SE, Sarnat JA, Hodas N, et al. Exposure prediction approaches used in air pollution epidemiology studies: key findings and future recommendations. Journal of Exposure Science and Environmental Epidemiology. 2013;23(6):654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen RW, Adar SD, Avol E, Cohen M, Curl CL, Larson T, et al. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ Health Perspect. 2012. June;120(6):824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Science of the total environment. 2009;407(12):3754–65. [DOI] [PubMed] [Google Scholar]
- 6.Clark NA, Allen RW, Hystad P, Wallace L, Dell SD, Foty R, et al. Exploring variation and predictors of residential fine particulate matter infiltration. International journal of environmental research and public health. 2010;7(8):3211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodas N, Meng Q, Lunden MM, Rich DQ, Ozkaynak H, Baxter LK, et al. Variability in the fraction of ambient fine particulate matter found indoors and observed heterogeneity in health effect estimates. Journal of Exposure Science and Environmental Epidemiology. 2012;22(5):448–54. [DOI] [PubMed] [Google Scholar]
- 8.MacNeill M, Wallace L, Kearney J, Allen R, Van Ryswyk K, Judek S, et al. Factors influencing variability in the infiltration of PM2.5 mass and its components. Atmos Environ. 2012;61:518–32. [Google Scholar]
- 9.Meng QY, Turpin BJ, Lee JH, Polidori A, Weisel CP, Morandi M, et al. How does infiltration behavior modify the composition of ambient PM2.5 in indoor spaces? An analysis of RIOPA data. Environmental science & technology. 2007;41(21):7315–21. [DOI] [PubMed] [Google Scholar]
- 10.Sarnat SE, Coull BA, Ruiz PA, Koutrakis P, Suh HH. The influences of ambient particle composition and size on particle infiltration in Los Angeles, CA, residences. Journal of the Air & Waste Management Association. 2006;56(2):186–96. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). American Journal of Epidemiology. 2012. November 1;176(9):825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, Detrano R, ez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. AmJEpidemiol. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MA, Adar SD, Allen RW, Avol E, Curl CL, Gould T, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ Sci Technol. 2009. July 1;43(13):4687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen R, Box M, Liu L-JS, Larson TV. A cost-effective weighing chamber for particulate matter filters. Journal of the Air & Waste Management Association. 2001;51(12):1650–3. [DOI] [PubMed] [Google Scholar]
- 15.EPA US. Quality Assurance Guidance Document 2.12. Monitoring PM2.5 in Ambient Air Using Designated Reference or Class I Equivalent Methods.Washington, D.C.: U.S. Environmental Protection Agency; 1998. [Google Scholar]
- 16.Sarnat JA, Long CM, Koutrakis P, Coull BA, Schwartz J, Suh HH. Using sulfur as a tracer of outdoor fine particulate matter. Environmental Science & Technology. 2002;36(24):5305–14. [DOI] [PubMed] [Google Scholar]
- 17.Keller JP, Olives C, Kim S-Y, Sheppard L, Sampson PD, Szpiro AA, Oron AP, Lindstrom J, Vedal S, and Kaufman JD A unified spatiotemporal modeling approach for prediction of multiple air pollutants in the Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect. 2015;123(4):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spalt EW, Curl CL, Allen RW, Cohen M, Adar SD, Stukovsky KH, et al. Time-location patterns of a diverse population of older adults: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Journal of Exposure Science and Environmental Epidemiology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen R, Wallace L, Larson T, Sheppard L, Liu L-JS. Estimated hourly personal exposures to ambient and nonambient particulate matter among sensitive populations in Seattle, Washington. Journal of the Air & Waste Management Association. 2004;54(9):1197–211. [DOI] [PubMed] [Google Scholar]
- 20.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor-and outdoor-generated particles in children with asthma. Environmental health perspectives. 2005;113(4):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler AJ, Wallace LA, Kearney J, Van Ryswyk K, You H, Kulka R, et al. Personal, indoor, and outdoor concentrations of fine and ultrafine particles using continuous monitors in multiple residences. Aerosol Science and Technology. 2011;45(9):1078–89. [Google Scholar]
- 22.Schembari A, Triguero-Mas M, de Nazelle A, Dadvand P, Vrijheid M, Cirach M, et al. Personal, indoor and outdoor air pollution levels among pregnant women. Atmos Environ 2013;64:287–95. [Google Scholar]
- 23.Weisel CP, Zhang J, Turpin B, Morandi M, Colome S, Stock T, et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. Research Report (Health Effects Institute). 2005(130 Pt 1):1–107; discussion 9–27. [PubMed] [Google Scholar]
- 24.Rojas-Bracho L, Suh HH, Koutrakis P. Relationships among personal, indoor, and outdoor fine and coarse particle concentrations for individuals with COPD. Journal of Exposure Analysis & Environmental Epidemiology. 2000;10(3):294–306. [DOI] [PubMed] [Google Scholar]
- 25.Lachenmyer C Urban measurements of outdoor-indoor PM2.5 concentrations and personal exposure in the deep south. Part I. Pilot study of mass concentrations for nonsmoking subjects. Aerosol Science & Technology. 2000;32(1):34–51. [Google Scholar]
- 26.Rodes CE, Lawless PA, Evans GF, Sheldon LS, Williams RW, Vette AF, et al. The relationships between personal PM exposures for elderly populations and indoor and outdoor concentrations for three retirement center scenarios. Journal of exposure analysis and environmental epidemiology. 2001;11(2):103–15. [DOI] [PubMed] [Google Scholar]
- 27.Breen MS, Long TC, Schultz BD, Williams RW, Richmond-Bryant J, Breen M, et al. Air pollution exposure model for individuals (EMI) in health studies: evaluation for ambient PM2.5 in central North Carolina. Environmental science & technology. 2015;49(24):14184–94. [DOI] [PubMed] [Google Scholar]
- 28.Kioumourtzoglou MA, Spiegelman D, Szpiro AA, Sheppard L, Kaufman JD, Yanosky JD, et al. Exposure measurement error in PM2.5 health effects studies: A pooled analysis of eight personal exposure validation studies. Environ Health-Glob 2014. January 13;13(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegelman D Approaches to uncertainty in exposure assessment in environmental epidemiology. Annual review of public health. 2010;31:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnat JA, Wilson WE, Strand M, Brook J, Wyzga R, Lumley T. Panel discussion review: session 1--exposure assessment and related errors in air pollution epidemiologic studies. J Expo Sci Environ Epidemiol 2007. December;17 Suppl 2:S75–82. [DOI] [PubMed] [Google Scholar]
- 31.Sheppard L, Burnett RT, Szpiro AA, Kim S-Y, Jerrett M, Pope III CA, et al. Confounding and exposure measurement error in air pollution epidemiology. Air Quality, Atmosphere & Health. 2012;5(2):203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szpiro AA, Paciorek CJ. Measurement error in two-stage analyses, with application to air pollution epidemiology. Environmetrics. 2013;24(8):501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JD, Mitsakou C, Kitwiroon N, Barratt BM, Walton HA, Taylor JG, et al. London Hybrid Exposure Model: Improving Human Exposure Estimates to NO2 and PM2.5 in an Urban Setting. Environ Sci Technol 2016. November 01;50(21):11760–8. [DOI] [PubMed] [Google Scholar]
- 34.Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: a comparison of health effects. Epidemiology. 2005;16(3):396–405. [DOI] [PubMed] [Google Scholar]
- 35.Sarnat JA, Brown KW, Bartell SM, Sarnat SE, Wheeler AJ, Suh HH, et al. The relationship between averaged sulfate exposures and concentrations: results from exposure assessment panel studies in four US cities. Environmental science & technology. 2009;43(13):5028–34. [DOI] [PubMed] [Google Scholar]
- 36.Wallace L, Williams R. Use of personal-indoor-outdoor sulfur concentrations to estimate the infiltration factor and outdoor exposure factor for individual homes and persons. Environmental science & technology. 2005;39(6):1707–14. [DOI] [PubMed] [Google Scholar]
- 37.Wilson WE, Brauer M. Estimation of ambient and non-ambient components of particulate matter exposure from a personal monitoring panel study. Journal of Exposure Science and Environmental Epidemiology. 2006;16(3):264–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.