Abstract
Background/Objectives:
Antibody detection is commonly used for diagnosis of histoplasmosis, cross-reactions have been recognized due to endemic mycoses but not cryptococcosis. We observed cross-reactions in an anti-Histoplasma antibody enzyme immunoassay (EIA) in the cerebrospinal fluid (CSF) from a patient with cryptococcal meningitis and sought to assess the risk for cross-reactive anti-Histoplasma antibodies in persons with cryptococcal meningitis.
Methods:
An anti-cryptococcal antibody EIA was developed to measure CSF antibody response in HIV-infected subjects from Kampala, Uganda and previously healthy, HIV-negative subjects at the National Institutes of Health (NIH) with cryptococcal meningitis. Specimens were tested for cross reactivity in assays for IgG anti-Histoplasma, anti-Blastomyces, and anti-Coccidioides antibodies.
Results:
Among 61 subjects with cryptococcal meningitis (44 Kampala cohort, 17 NIH cohort), elevated CSF anti-cryptococcal antibody levels existed in 38% (23/61). Of the 23 CSF specimens containing elevated anti-cryptococcal antibodies, falsely positive results were detected in antibody EIAs for histoplasmosis (8/23, 35%), coccidioidomycosis (6/23, 26%), and blastomycosis (1/23, 4%). Overall, 2% (2/81) of control CSF specimens had elevated anti-cryptococcal antibody detected, both from Indiana.
Conclusions:
Cryptococcal meningitis may cause false-positive results in the CSF for antibodies against Histoplasma, Blastomyces and Coccidioides. Fungal antigen testing should be performed to aid in differentiating true and false positive antibody results in the CSF.
Keywords: Cryptococcosis, Histoplasmosis, Antibody detection, Cross-reactivity, coccidioidomycosis, Blastomycosis
Introduction
Antigen and antibody detection are widely used for diagnosis of histoplasmosis, providing the initial basis for diagnosis in the majority of subjects with pulmonary or disseminated histoplasmosis.1, 2 One limitation of these methods is cross-reactivity caused by other endemic mycoses, most commonly blastomycosis and coccidioidomycosis.3 Cryptococcosis and histoplasmosis have not been demonstrated to cause cross-reactions in either Cryptococcus or Histoplasma antigen detection assays,4, 5 although both tests have been reported as positive in persons with dual infections5. More recently, positive tests for anti-Histoplasma IgG antibodies using a newly described enzyme immunoassay2 were observed in the serum of 1.3% (2/151) Ugandans surviving cryptococcal meningitis.4 Whether these were caused by cross-reactivity or prior infection was undeterminable.
Subsequently, we observed positive results for anti-Histoplasma antibodies in the CSF of a HIV-negative, previously healthy 47 year-old patient who had presented with gait abnormalities and emotional lability. CSF fungal cultures were initially negative and a ventriculoperitoneal (VP) shunt was placed for hydrocephalus. Months later, the patient was found unconscious by a friend, VP shunt malfunction was noted and CSF culture grew Cryptococcus neoformans. CSF analysis at that time included glucose of 56 mg/dL, a protein of 28 mg/dL, a leukocyte count of 297 cells/mL (97% lymphocytes) and few yeast on gram stain. Cryptococcal antigen (CrAg) testing by a latex agglutination assay (Meridian Bioscience, Cincinnati, OH) was negative but subsequently positive (1:1 titer) by a CrAg lateral flow assay (ImmunoMycologics, Norman, OK). Lateral flow assay testing was done due to discordant CrAg and culture results in the setting of know increased sensitivity compared to latex agglutination. Serum CrAg testing was negative. Due to discrepant latex agglutination CrAg and fungal culture results, additional diagnostic testing was performed for histoplasmosis. CSF, urine, serum, and bronchoalveolar lavage fluid were tested for Histoplasma antigen, all of which were negative. Anti-Histoplasma antibody testing of the CSF by EIA was strongly positive for IgG antibodies (>80 units) while testing by immunodiffusion was negative. Anti-Histoplasma serum compliment fixation was negative. Using the IgG anti-cryptococcal antibody assay described below, positive results were observed in the CSF (16.9 units). This case was instructive as to the clinical consequences of fungal diagnostic test cross-reactivity.
This case prompted further investigation to assess cross-reactivity of antibodies induced by cryptococcal meningitis with antibody detection assays for histoplasmosis and endemic mycosis that are common in the United States. A second objective was to determine if immune compromising conditions such as AIDS, impair anti-cryptococcal antibody production compared to that of non-immunocompromised subjects.
Methods
NIH cohort:
Since 1993, cases of cryptococcosis in non-HIV, non-solid organ transplant subjects have been recruited globally to the NIH with informed consent, for participation in an active IRB-approved protocol (NIAID Protocol #93-I-0106; available at: https://clinicaltrials.gov/ct2/show/NCT00001352). In 2013, another cohort study of non-HIV cryptococcosis was initiated in 25 U.S. centers, referring subjects with no immunosuppression to the NIH. The purpose of the NIH study is to characterize the natural history, study genetic susceptibilities and improve outcomes of cryptococcosis in previously healthy adults. Cryptococcal meningitis was confirmed in each case by CSF cryptococcal antigen detection by latex agglutination (Meridian Bioscience) and/or fungal culture. Seventeen subjects were included in the present study from this cohort.
Kampala cohort:
Subjects were prospectively enrolled in a study at Mulago National Referral Hospital in Kampala, Uganda with appropriate IRB approvals and the written informed consent of the subjects or their surrogates. From November 2010 until December 2013, HIV-infected persons with CD4<200 cells/μl suspected to have meningitis were enrolled.6–10 Cryptococcal meningitis was diagnosed by CSF cryptococcal antigen detection via latex agglutination or lateral flow assay (ImmunoMycologics) and/or fungal culture. Forty-four subjects were included in the present study from this cohort.
Controls:
CSF samples from 24 individuals with HIV infection from the Kampala cohort that were determined not to have cryptococcal meningitis were included as controls (tuberculous meningitis n=3, aseptic meningitis n=8, no meningitis n=13).4 CSF specimens from six subjects with cryptococcosis not involving the CNS from the NIH were also tested as controls. Additional controls included CSF specimens from 51 subjects at Indiana University Medical Center in whom fungal meningitis was not suspected clinically and in whom Histoplasma antigen testing was not performed from an IRB approved study.11 Additional CSF specimens were included from 10 subjects with Histoplasma meningitis and 10 subjects with Coccidioides meningitis with high levels of Histoplasma and Coccidioides antigen and anti-Histoplasma or anti-Coccidioides antibodies by EIA.2, 12 Cryptococcal meningitis was excluded by CSF fungal culture and/or CSF cryptococcal antigen testing via latex agglutination (ImmunoMyologics or Meridian Biosciences) in these subjects.
Anti-Cryptococcal antibody EIA.
C. neoformans strain H99 (ATCC298821), obtained from Dr. John Perfect, Duke University, was grown to mid log phase, centrifuged at 12,000 g, subjected to 1 minute of vortexing with 0.46 mm acid-washed glass beads followed by centrifugation. Antigen in the supernatant following centrifugation was assayed for protein by a Bradford reaction according to the manufacturer’s directions (BioRad Laboratories, Redmond, WA).
Separate pooled samples of CSF from subjects with and without cryptococcal meningitis were tested in serial dilutions against a Nunc Maxisort microplate (Thermo Fisher Scientific) coated with cryptococcal antigen in order to determine the optimal concentration of cryptococcal antigen to use in coating microplates. A 1:25 dilution of the positive CSF control pool and of the negative CSF control pool were tested on four occasions for evaluation of reproducibility. The mean OD of the positive control pool was 1.288 milli-absorbance units (mau), the standard deviation was 0.154 mau and the coefficient of variation was 12.0%. The mean OD of the negative control pool was 0.015 mau, the standard deviation was 0.0004 mau and the coefficient of variation was 2.8%. The optimum OD cutoff was 0.076 mau.
Microplates were coated with cryptococcal antigen at a concentration of 2 μg/mL and then blocked with StartingBlock blocking buffer (Thermo Scientific, Rockford, IL). Between each step the plates were washed with phosphate-buffered saline-Tween (Bioreba, Reinach, Switzerland). 100 μL of the CSF specimen, which was diluted to 1:25 was added to each well and incubated at 37°C for 1 hour, after which bound antibody was detected with biotinylated goat anti-human IgG antibody (Vector Laboratories, Burlingame, CA) by incubation at 37°C for 1 hour. Plates were then incubated with 100μl of streptavidin-horseradish peroxidase at 37°C for 1 hour, followed by tetramethylbenzidine (SurModics, Eden Prairie, MN) for 8 minutes at room temperature in the dark. Sulfuric acid was then added to each well to stop the reaction, after which plates were read in a microplate reader at 450 nm with a 620 nm reference filter. Results were expressed as IgG anti-cryptococcal antibody units by dividing the optical density (OD) of the specimen by the cutoff OD. All specimens were tested in the same assay.
Other fungal antibody EIAs.
CSF specimens from subjects with cryptococcal meningitis were also tested via previously reported MiraVista enzyme immunoassays for anti-Blastomyces antibodies,13 anti-Histoplasma antibodies,2 and anti-Coccidioides antibodies.12 Unlike the anti-cryptococcal antibody EIA, these assays have been validated for clinical use and contain standard curves, from which results are calculated, and reported semi-quantitatively as units. The cutoff for a positive result is 10 units in each case and the highest standard used in the assays is 80 units.
Statistics.
Fisher’s exact tests were used to compare diagnostic methods in the clinical and epidemiological groups (SPSS,version 21, Armonk, NY, USA ) and T tests were used for categorical variables.. P values of <0.05 were considered statistically significant.
Results
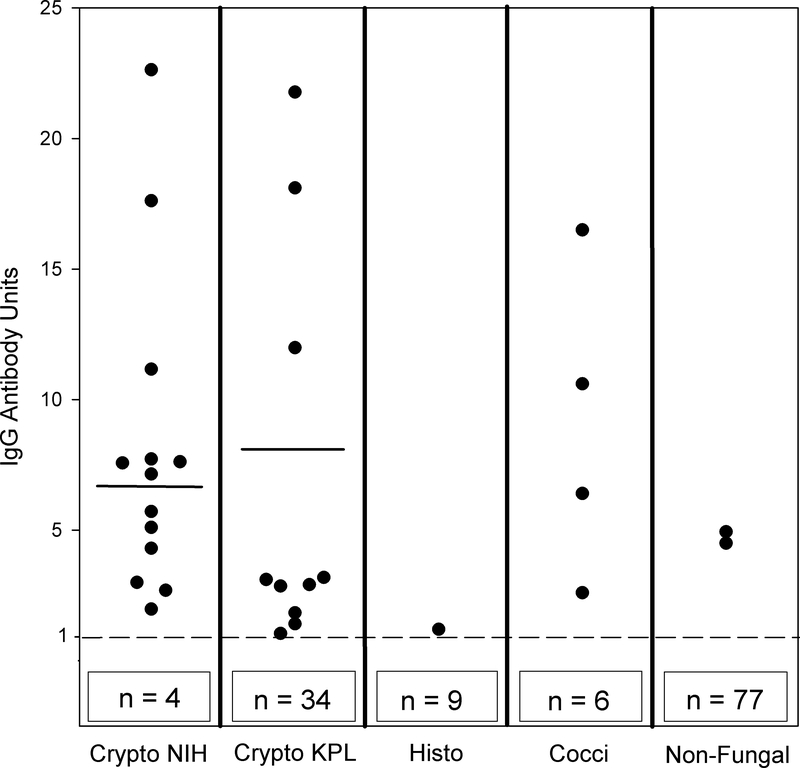
CSF was tested for cryptococcal antibody from 61 subjects with cryptococcal meningitis and 81 controls. Elevated anti-cryptococcal antibody levels were present in 22.7% (10 of 44) of AIDS subjects from Kampala and 76.4% (13 of 17) of non-immunocompromised subjects from NIH (p=<0.001) and in 37.7% (23/61) of cases of cryptococcal meningitis overall. The mean antibody level for meningitis cases with positive antibody results was 6.8 units in the Kampala cohort and 8.0 units in the NIH cohort (p=0.677). Only five (11%) of the Kampala cases were receiving antiretroviral therapy, and all had CD4 counts <100 cells/μL while CD4 counts were normal in all of the NIH cases.
No anti-cryptococcal antibody was detected in the CSF among 24 AIDS control subjects from Kampala (71% of whom were receiving antiretroviral therapy) or six controls from the NIH cohort. Elevated levels of anti-cryptococcal antibodies were detected in the CSF in two of 51 (3.9%) Indianapolis controls. One had tuberculous meningitis and the IgG anti-cryptococcal antibody was 4.5 units while the IgG anti-Histoplasma antibody level was 37.7 units. The other patient had neurosarcoidosis, and the anti-cryptococcal IgG antibody level was 4.9 units while anti-Histoplasma antibody level was negative.
We also assessed cross-reactivity in the IgG anti-cryptococcal antibody assay in subjects with Histoplasma or Coccidioides meningitis. Cross-reactions were noted in the CSF in one of 10 (10%) subjects with Histoplasma meningitis and in four of 10 (40%) with Coccidioides meningitis, Figure 1. Specimens were not available from subjects with Blastomyces meningitis.
Figure 1. IgG anti-cryptococcal antibody levels in CSF among cases and controls.
The cutoff for positivity of OD of 0.076 milli-absorbance units is indicated by the broken horizontal line and the numbers in the box below the broken line represent the number of subjects with negative results. Horizontal lines in columns one and two represent mean values of 6.8 units and 8.0 units, respectively. Means were not calculated for other columns due to too few data points. The boxes below the broken line indicate the additional numbers of data points below the cutoff. For example, in the “Crypto NIH” column, 13 individual dots are shown above the cutoff and an additional four are represented by the box labeled n=4 for a total of 17 data points. Abbreviations: Crypto = cryptococcosis; Histo = histoplasmosis; Cocci = coccidioidomycosis; NIH = National Institutes of Health, KPL = Kampala
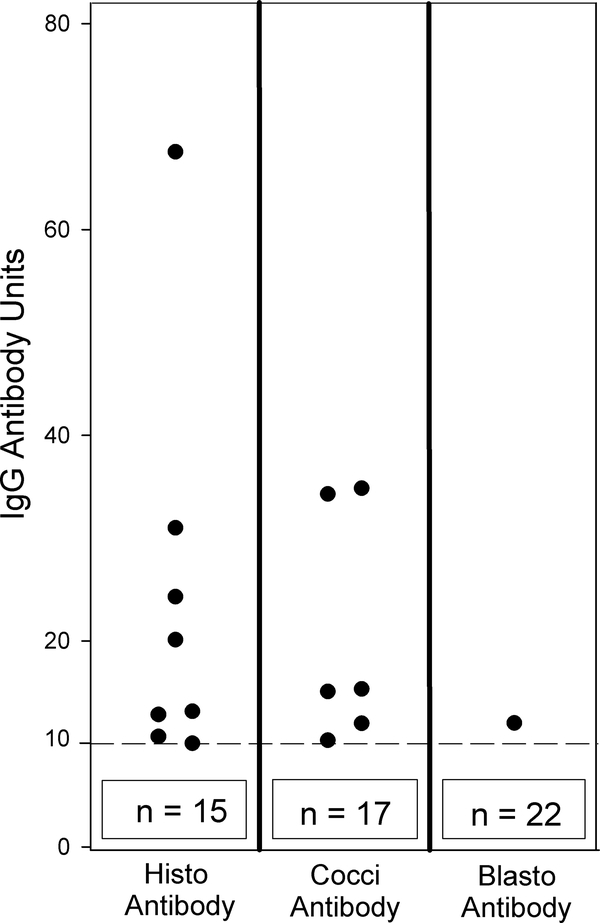
CSF from the 23 subjects with cryptococcal meningitis and elevated levels of anti-cryptococcal antibodies were tested for antibodies in IgG antibody EIAs to Histoplasma,2 Blastomyces,13 and Coccidioides.12 Of these 23 subjects, cross-reactions for anti-Histoplasma antibodies were detected in eight (34.8%), anti-Coccidioides antibodies in six (26.1%), and anti-Blastomyces antibodies in one (4.3%) (Table 1). Individual cases are shown in Figure 2.
Table 1.
Cross-reactivity for IgG anti-Histoplasma, anti-Coccidioides and anti-Blastomyces antibodies in subjects with cryptococcal meningitis.
| Antibody EIA | NIH cohort | Kampala cohort | P value | Total |
|---|---|---|---|---|
| Histoplasma | 6/13 (46.2%) | 2/10 (20.0%) | 0.379 | 8/23 (34.8%) |
| Coccidioides | 3/13 (23.1%) | 3/10 (30.0%) | 1.000 | 6/23 (26.1%) |
| Blastomyces | 1/13 (7.7%) | 0/10 (0%) | 1.000 | 1/23 (4.3%) |
In cerebrospinal fluid (CSF) samples from subjects known to have cryptococcal meningitis with positive anti-cryptococcal antibodies detected, there was no different in frequency of cross-reaction with anti-histoplasma, anti-coccidioides, or anti-blastomyces enzyme immunoassays. P values obtained via Fisher’s exact tests comparing NIH and Kampala cohorts.
Figure 2. Cross-reactivity of IgG anti-cryptococcal antibodies in EIAs for IgG anti-Histoplasma, IgG anti-Blastomyces and IgG anti-Coccidioides antibodies.
The cutoff for positivity is indicated by the broken horizontal line at 10 antibody units and the numbers in the box below the broken line represent the number of subjects with negative results. Abbreviations: Histo = IgG anti-Histoplasma EIA, Cocci = IgG anti-Coccidioides EIA, Blasto=IgG anti-Blastomyces EIA. The ‘n” noted at the base of each column represents the additional data points that were below the cut-off. For example, in the H capsulatum column, eight dots are shown above the cutoff and 15 are represented by the box, for a total of 23 data points.
Discussion
We identified false positive tests for anti-Histoplasma antibodies in the CSF in a non-immunocompromised patient with cryptococcal meningitis. This was not suspected based on prior studies reporting the absence of cross-reactivity in the antigen detection assays.4, 5, 14 These observations prompted a larger evaluation of cross-reactivity in CSF specimens from subjects with cryptococcal meningitis with assays for antibody detection from other endemic fungi.
Anti-cryptococcal antibodies were lower in persons infected with HIV compared to those without HIV infection (22.7% vs. 76.4%). Decreased CD4+ T cell helper function and subsequent reduction in B cell activation may have suppressed antibody response, as reported for CNS toxoplasmosis.15 This assay was not developed for use in clinical testing and we do not feel an anti-cryptococcal antibody test has a role in clinical diagnosis.
CSF specimens from subjects with cryptococcal meningitis containing high levels of anti-cryptococcal antibodies frequently cross-reacted with tests for other fungi. Additionally, CSF specimens from subjects with meningitis caused by coccidioidomycosis and histoplasmosis also cross-reacted with anti-cryptococcal antibodies. Cross-reactive antibodies may be a function of polyclonal reactivation in people who have relatively preserved immune function. A majority of subjects with elevated anti-cryptococcal antibodies developed cryptocococcosis in the setting of no apparent immunosuppression. These subjects had normal CD4 lymphocyte counts and no past histories of complex infections. While underlying deficits remained unclear in most, two had documented autoantibodies directed against granulocyte-monocyte stimulating factor, suppressing macrophage clearance.16 In addition, two of 81 controls had elevated levels of anti-cryptococcal antibodies detected in the CSF. One case had TB meningitis and a second had neurosarcoidosis. In both cases the anti-cryptococcal antibodies were likely falsely elevated.
These findings emphasize the potential for cross-reactive antibody production that may mislead diagnosis when used in isolation. Here, high amounts of cross-reactivity seen in relatively immunocompetent people with cryptococcosis may be either a function of the host or the infection. Of note, the antigens detected in the CSF in subjects with histoplasmosis and cryptococcosis are not cross-reactive,4, 5 offering a way to aid in assessment of cross-reactivity in the antibody assays.
Several potential limitations of this study should be recognized. First, only 17 non-immunocompromised subjects with cryptococcal meningitis were tested. Second, the control group was not matched for the cases. Third, the anti-cryptococcal antibody EIA was not optimized for sensitivity and specificity, but used here as a qualitative research test. Fourth, although unlikely, given that cases of histoplasmosis and coccidiomycosis were diagnosed by antigen and antibody detection alone (e.g. not cultures) it is possible that another fungi could have falsely caused these positive results. Lastly, no blood testing was done and so we cannot comment on possible cross-reactivity among these fungi in the blood based on this study. Positive tests for anti-Histoplasma IgG antibodies using a newly described enzyme immunoassay2 were observed in the serum of 1.3% (2/151) Ugandans surviving cryptococcal meningitis.4 However it’s not clear whether these were due to cross-reactivity.
In conclusion, people with cryptococcosis, primarily developed in context of relatively preserved immunity, were observed to have relatively high amounts of cross-reactive antibodies to Histoplasma and Coccidioides, emphasizing the importance of establishing specific diagnosis based on culture and antigen testing. Further studies will be necessary to determine the mechanism(s) of cross-reactive antibody production, and its clinical significance.
Acknowledgments
Funding: This study was supported, in part, by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (AI001123, AI001124) as well as extramural grants from the National Institute of Allergy and Infectious Diseases (T32AI055433, U01AI089244, UO1AI109657, RO1 AIO59681, and RO1AI127704). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Conflicts of interest. L.J.W is a medical director and part owner of MiraVista Diagnostics and M.M.D and M.L.S. are employees of MiraVista Diagnostics, a company that offers tests for diagnosis of fungal infections commercially. K.A.M is part owner of MycoMed Technologies. All other authors report no potential conflicts of interest. MiraVista Diagnostics donated laboratory technologist time, reagents for laboratory testing (on the cryopreserved samples only), and paid for shipping of cryopreserved samples in the Uganda cohort to MiraVista Diagnostics. MiraVista did not compensate those authors that are not MiraVista employees and did not fund the parent studies in from which the samples were initially procured.
References:
- 1.Lackner M, Lass-Florl C. Commercial Molecular Tests for Fungal Diagnosis from a Practical Point of View. Methods Mol Biol 2017; 1508: 85–105. [DOI] [PubMed] [Google Scholar]
- 2.Richer SM, Smedema ML, Durkin MM, et al. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin Infect Dis 2016; 62(7): 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheat LJ, Azar MM, Bahr NC, et al. Histoplasmosis. Infect Dis Clin North Am 2016; 30(1): 207–27. [DOI] [PubMed] [Google Scholar]
- 4.Bahr NC, Sarosi GA, Meya DB, et al. Seroprevalence of histoplasmosis in Kampala, Uganda. Med Mycol 2016; 54(3): 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang D, Hage C, De Jesus M, et al. Cryptococcal glucoxylomannan does not exhibit cross-reactivity in the MVista Histoplasma antigen enzyme immunoassay. Clin Vaccine Immunol 2008; 15(2): 392–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med 2010; 7(12): e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulware DR, Meya DB, Bergemann TL, et al. Antiretroviral therapy down-regulates innate antiviral response genes in patients with AIDS in sub-saharan Africa. J Acquir Immune Defic Syndr 2010; 55(4): 428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370(26): 2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson RD, Rolfes MA, Birkenkamp KE, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis 2014; 29(2): 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis 2008; 46(11): 1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloch KC, Myint T, Raymond-Guillen L, et al. Improvement in diagnosis of Histoplasma meningitis by combined testing for Histoplasma antigen and IgG and IgM anti-Histoplasma antibody in cerebrospinal fluid. Clin Infect Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malo J, Holbrook E, Zangeneh T, et al. Enhanced Antibody Detection and Diagnosis of Coccidioidomycosis with the MiraVista IgG and IgM Detection Enzyme Immunoassay. J Clin Microbiol 2017; 55(3): 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richer SM, Smedema ML, Durkin MM, et al. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin Vaccine Immunol 2014; 21(2): 143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tone K, Umeda Y, Makimura K. Cross-reactivity in Cryptococcus antigen latex agglutination test in two commercial kits. Med Mycol 2016; 54(4): 439–43. [DOI] [PubMed] [Google Scholar]
- 15.Machala L, Maly M, Hrda S, et al. Antibody response of HIV-infected patients to latent, cerebral and recently acquired toxoplasmosis. Eur J Clin Microbiol Infect Dis 2009; 28(2): 179–82. [DOI] [PubMed] [Google Scholar]
- 16.Rosen LB, Freeman AF, Yang LM, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 2013; 190(8): 3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]