Abstract
Until recently, therapeutic development in psychiatry was targeted solely toward symptom reduction. While this is a worthwhile goal, it has yielded little progress in improved therapeutics in the last several decades in the field of mood disorders. Recent advancements in our understanding of pathophysiology suggests that an impairment of neuroplasticity may be a critical part of the development of neuropsychiatric disorders. Interventions that enhance or modulate neuroplasticity often reduce depressive symptoms when applied as stand-alone treatments. Unfortunately, when treatments are discontinued, the disease state often returns as patients relapse. However, treatments that enhance or modulate plasticity not only reduce symptom burden, but also may provide an opportune window wherein cognitive or behavioral interventions could be introduced to harness a state of enhanced neuroplasticity and lead to improved longer-term clinical outcomes. Here, we review the potential of synergistically combining plasticity-enhancing and behavioral therapies to develop novel translational treatment approaches for depression. After reviewing relevant neuroplasticity deficits in depression, we survey biological treatments that appear to reverse such deficits in humans, including N-methyl-D-aspartate receptor modulators (ketamine, D-cycloserine), electroconvulsive therapy, and transcranial brain stimulation. We then review evidence that either directly or indirectly supports the hypothesis that a robust enhancement of neuroplasticity through these methods might promote the uptake of cognitive and behavioral interventions to enhance longer-term treatment outcomes through a synergistic effect. We identify key missing pieces of evidence and discuss future directions to enhance this emerging line of research.
Keywords: Depression, Electroconvulsive therapy, Ketamine, Neuromodulation, Neuroplasticity, Transcranial magnetic stimulation
Neuroplasticity, or the brain’s capacity to flexibly adjust and reorganize itself in response to a changing environment, is fundamental to promoting adaptive functioning. Impairments of neuroplasticity characterize disorders of negative affect, including depression (1,2). Burgeoning evidence shows that traditional and novel treatments for depression exhibit plasticity-enhancing effects that at least partially underlie corresponding reductions in clinical symptoms observed in patients. While traditional pharmacological and psychotherapy approaches likely enhance neuroplasticity (3,4), novel treatments (5–7) seem to induce both clinical and neuroplastic effects more rapidly. Prominent among these newer approaches are glutamate-modulating agents [e.g., intravenous ketamine (1,2,8)]. In addition, noninvasive brain stimulation interventions (e.g., repetitive transcranial magnetic stimulation [rTMS]) may induce neuroplastic changes (9). One of the oldest and most efficacious treatments for depression, electroconvulsive therapy (ECT), also has potent acute plasticity-enhancing effects (10).
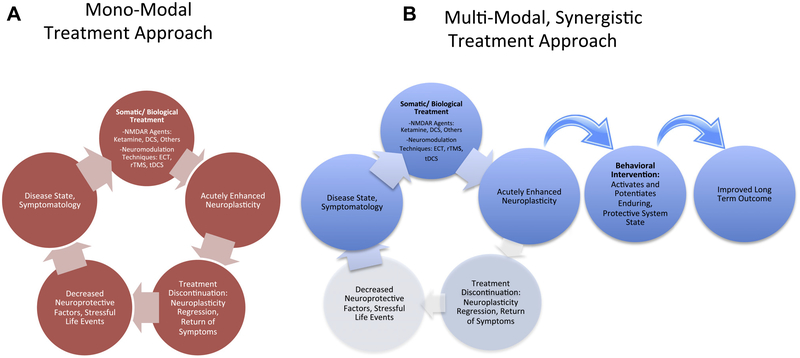
Each of these plasticity-enhancing approaches has the potential to exhibit therapeutic effects as monotherapies. However, clinical effects often dissipate once the intervention is removed (Figure 1A). This presents challenges for maintenance of gains, as long-term treatment may not be beneficial or feasible (11). Pharmacological/somatic therapies are challenging to maintain in the long term owing to patient discontinuation (11) and the rarity of follow-up opportunities in community practice (12). By contrast, findings suggest that gold-standard behavioral treatments such as cognitive behavioral therapy (CBT) can help prevent relapse of depression symptoms even in the absence of ongoing care (11,13–15), suggesting that the introduction of adaptive learning results in the long-term potential to buffer against negative affect. Finally, preclinical evidence suggests that unfavorable environmental factors may worsen mood symptoms in the setting of enhanced neuroplasticity by biological agents (16). Hence, a multimodal treatment approach may make use of this interaction between biological/somatic therapies that enhance neuroplasticity and cognitive behavioral interventions that harness, solidify, and guide this enhanced neuroplasticity, potentially resulting in lower rates of relapse (Figure 1B).
Figure 1.
(A) The monomodal approach often leads to a return of the symptomatic state once treatment is discontinued. (B) The proposed, multimodal approach may combine treatment modalities synergistically to enhance and subsequently harness a state of neuroplasticity to lead to improved longer-term outcomes. DCS, D-cycloserine; ECT, electroconvulsive therapy; NMDAR, N-methyl-D-aspartate receptor; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
This review summarizes the potential for plasticity-enhancing somatic/biological agents to be leveraged in this multimodal approach, i.e., as short-term enhancers of cognitive flexibility and adaptive learning that may be combined with learning-based approaches to foster long-term relief from depression. After reviewing the role of neuro-plasticity in depression, we survey existing data in support of the notion that the introduction and facilitation of new learning during somatically induced neuroplasticity “windows of opportunity” might provide an efficient path to enhance or extend symptom relief. We focus on therapies that have preclinical and clinical data supporting their potential to enhance neuroplasticity. We also identify key gaps in this emerging literature and conclude by proposing future research directions within this framework.
NEUROPLASTICITY DEFICITS IN DEPRESSION
At the molecular level, depression has been characterized as a failure of neuroplasticity, including neuronal atrophy and synaptic depression in the prefrontal cortex (PFC) and hippocampus (1,2). Chronic stress contributes to sustained decreases in neuroprotective factors (e.g., brain-derived neurotrophic factor expression and signaling) that damage plasticity, fostering neuronal atrophy and synaptic depression (1,2). This results in deficient adaptation to the environment, compromised learning and stress coping, and downstream gain of activity in some affective processing regions (e.g., amygdala) regulated by the PFC. Conversely, when neuroplasticity is enhanced (e.g., by treatment), synaptic contacts increase, enhancing adaptability by allowing activity-dependent competition to stabilize the neural structures that best represent internal and external conditions (17–19).
In corresponding patterns of human neurocognition, depression and related conditions are associated with impaired cognitive flexibility (20,21) and decreased regulation of stimulus-driven affective processing (22,23). These behavioral deficits are linked to altered functional integration across the PFC and affective circuits (24–27). These alterations are posited to produce the rigid negative biases evident in depressed patients across a wide range of implicit information processing domains [e.g., negative appraisals of self, the environment, and the future (28); preferential attention and memory for negative stimuli (23,29,30)], which in turn maintain and reinforce a state of high negative affect by fostering overestimation of the personal shortcomings, dangers, and misfortunes inherent to the individual’s life (31). Somatic therapies that address neuroplasticity deficits may hold the potential to relax rigid patterns of processing and cognitive inflexibility, facilitating adaptive learning and promoting acquisition of effective emotion regulation skills.
SEARCH STRATEGY
To identify ongoing or completed trials to include in this review, we performed a systematic search in April 2018 of ClinicalTrials.gov. Search terms were (“ketamine” or “ECT” or “electroconvulsive therapy” or “TMS” or “transcranial magnetic stimulation” or “tDCS” or “transcranial direct current stimulation” or “DCS” or “D-cycloserine”) AND (“CBT” or “cognitive therapy” or “cognitive behavioral therapy” or “behavioral activation” or “cognitive training” or “neurocognitive training”). We also included trials if they were found among the references of included studies or relevant reviews in this area. We included studies in which a somatic treatment was used in combination with a behavioral intervention for the treatment of a depressive disorder.
HISTORICAL PRECEDENT IN CONVENTIONAL THERAPIES
While in this review we focus on emerging approaches to biological-behavioral treatment combination, the search for synergy across pharmacological and behavioral modalities has historical precedent. Although conventional (e.g., monoamine-based) oral antidepressants show compelling neuroplasticity-enhancing effects in both animals and humans (18,32), findings regarding synergistic effects in patients are equivocal, with meta-analyses supporting, at best, a modest benefit for combination treatment over either modality alone (33–35). Furthermore, it remains unclear whether any observed effects are truly synergistic; alternatively, the combination could reflect a simple group-level additive pattern whereby distinct (or partially overlapping) subsets of patients benefit from each of the two monotherapies. Burgeoning approaches would therefore benefit from the use of research designs that explicitly tackle this question, seeking to establish a clear synergistic benefit by exploiting rapid-onset biological effects, in the hopes of documenting a more efficient time course of recovery than has been previously possible.
GLUTAMATE-MODULATING AGENTS
Ketamine
Monomodal Neuroplasticity and Clinical Effects.
Ketamine is an N-methyl-D-aspartate (NMDA) antagonist used routinely for anesthesia. In randomized controlled trials, subanesthetic doses (0.5 mg/kg given over 40 minutes) of intravenous ketamine exhibit well-replicated, rapid antidepressant effects (i.e., meta-analytic Cohen’s d = 1.4) (36), even in treatment-resistant depression (37) and bipolar depression(38). Antidepressant effects begin as soon as 2 hours post-infusion and continue far beyond the drug’s elimination half-life of 2.5 to 3 hours. However, effects typically dissipate within 7 to 14 days following exposure of a single infusion, demonstrating that when plasticity-enhancing treatments are withdrawn, patients often experience a return of symptoms (Figure 1A). To date, the only strategy shown in replicated datasets to extend ketamine’s rapid effects is to give repeated ketamine infusions (39–41). While an increasing number of clinicians now offer longer-term ketamine treatment in an effort to maintain antidepressant effects of the drug (42), there are feasibility and safety concerns for long-term use (43–45), including potential neurocognitive impact, neurotoxicity, and addiction/abuse liability.
The rapid nature of ketamine’s effects have been attributed to its ability to rapidly and profoundly reverse neuroplasticity deficits (1,2,8). Ketamine induces neuroplastic changes (increases in spine density, synaptic strengthening) over periods of hours to days following exposure in animals (5,46). As glutamate receptors are ubiquitous throughout the brain, such findings suggest the potential for far-reaching, rapid functional reorganization, as observed in patients (47) and monkeys (48) 24 hours postketamine administration. It was originally believed that these downstream effects were mediated through ketamine’s antagonism of NMDA receptors (5); however, recent evidence suggests that mechanisms independent of NMDA receptor antagonism could also mediate these effects (8).
Consistent with a broad effect on cognitive flexibility and plasticity in human patients, there is evidence that a single infusion of ketamine may enhance cognitive abilities or at least resolve depression-related cognitive impairment in the short term (49–51). Notably, this is in contrast to the detrimental effects of long-term exposure to ketamine on cognition in rodent models (52) and high-frequency (HF) substance abusers (53). Ketamine also exhibits delayed but enhancing effects on synaptic potentiation in humans (6). Furthermore, ketamine induces rapid plasticity in implicit processing patterns relevant in depression (54,55). At the neural network level, neuroimaging investigations in depressed patients have linked ketamine’s antidepressant effects to increased activity and connectivity in PFC and striatal/reward circuits (47,56–58). Connectivity decreases within affective and default mode networks have also been observed after ketamine [in magnetoencephalography (59) and in functional magnetic resonance imaging of primates (48)], interpreted as reversal of the maladaptive affective- and default mode network–driven hyperconnectivity that typifies depression (60).
Potential for Synergistic Effects.
Given that synaptic plasticity involving glutamatergic receptors is considered the major molecular substrate of learning and memory in the brain (61,62), ketamine-induced neuroplasticity could open a clinical “window of opportunity” for new, protective learning. A preliminary pilot study supports this notion, suggesting that CBT may sustain the antidepressant effects of ketamine. In a small sample (N = 16) of patients with treatment-resistant depression, in those who demonstrated clinical response to ketamine (n = 8) and then received 12 sessions of CBT over 10 weeks (open label), 75% maintained their response for 8 weeks following ketamine (63). This compares favorably to historical rates of 29% to 45% retaining ketamine response at 4 weeks in prior studies (40,64). More definitive tests of ketamine’s synergistic potential in combination with behavioral treatments are the focus of an ongoing randomized trial as follow-up to this study (NCT03027362) (see Table 1). Another ongoing study (NCT03237286) focuses on automated cognitive training as a potentially efficient, portable, dissemination-friendly, and low-cost behavioral intervention (65–67) using a computer-based paradigm [appetitive conditioning (68)]. The combination of intravenous ketamine followed promptly by active cognitive training is compared with relevant control treatments (saline followed by active training; ketamine followed by sham computer training). Results from these studies, as well as similar multimodal approaches in other affective conditions (69,70), will speak directly to the potential for ketamine to promote adaptive learning when combined with behavioral treatment paradigms.
Table 1.
Completed and Ongoing Clinical Trials Investigating Combinations of Somatic/Biological Treatments With Cognitive Behavioral Interventions in Depression
| NCT Identifier, Study Status | Trial Design and Description | Expected Completion Date, Projected/Actual Enrollment | Primary Outcome Measure or Results | RCT? | Includes Comparison Condition for Somatic Treatment Without Behavioral? | Includes Comparison Condition for Behavioral Treatment Without Somatic? |
|---|---|---|---|---|---|---|
| Ketamine | ||||||
| NCT03237286, ongoing | 3-arm RCT: IV ketamine + 4 days of computer-based cognitive training; IV ketamine + sham training; IV saline + cognitive training | Oct 2023, N = 150 |
|
Y | Y | Y |
| NCT03027362, ongoing | 2-arm RCT: ketamine responders randomized to CBT or psychoeducation | Jul 2019, N = 28 |
|
Y | Y | N |
| NCT02289248, completed | Open label, single arm: IV ketamine followed by CBT (8 weeks following ketamine) | Oct 2016, N = 16 | Among responders, 75% retained response 8 weeks following ketamine, though the majority eventually relapsed on longer-term, naturalistic follow-up (63) | N | N | N |
| D-cycloserine | ||||||
| NCT02376257, completed | 3-arm RCT: 250-mg DCS + CBT, 100-mg modafinil + CBT, or placebo + CBT | May 2017, N = 85 | Delayed memory recall for CBT content; results submitted but not yet published | Y | N | Y |
| ECT | ||||||
| NCT02176473, completed | Single arm, open label: ECT responders undergo computer-based CBT | Dec 2016, N = 15 | At 6 months, the relapse rate was 33% (100) | N | N | N |
| NCT00487500, completed | 3-arm RCT: ECT responders were randomized (1:1:1) to continuation phase medication (MED), CBT + MED, or ECT + MED |
2010, N = 60 | At 6 months, CBT/MED group had 77% sustained response, ECT/MED group had 40% sustained response, and MED-alone group had 44% sustained response (98) | Y | Y | N |
| No number, completed | Single arm, open label: ECT responders undergo CBT | 2005, N = 9 | At 12-month follow-up, 3 patients withdrew; mean BDI scores among completers were lower (11.7) compared to the end of ECT (18.8) (99) | N | N | N |
| rTMS | ||||||
| NCT03289923, ongoing | 2-arm RCT: active rTMS + CBT vs. sham rTMS + CBT | Jan 2019, N = 50 |
|
Y | N | Y |
| No number, completed | Single-arm, open-label, naturalistic study of rTMS + CBT (CBT performed during TMS sessions) | 2016, N = 196 | Posttreatment response and remission rates of 66% and 56%, respectively; sustained response and remission rates were seen in 65% and 60% of those originally attaining response/remission, respectively (121) | N | N | N |
| tDCS | ||||||
| NCT03548545, ongoing | 2-arm RCT: active tDCS 1 CBT vs. sham tDCS 1 CBT | Dec 2020, N = 72 | Change in depression severity (MADRS) at 4 weeks | Y | N | Y |
| NCT03518749, Ongoing | 3-arm RCT: 1-mA tDCS 1 CCT, 2-mA tDCS 1 CCT, sham tDCS 1 CCT | Mar 2019, N = 57 | Change in depression severity (MADRS) at 4 weeks | Y | N | Y |
| NCT02633449, ongoing | 3-arm RCT: active tDCS and CBT, sham tDCS and CBT, or CBT alone | Dec 2018, N = 192 | Primary outcome measure is change in MADRS scores from baseline to 6, 18, and 30 weeks (135) | Y | N | Y |
| NCT01974076, ongoing | 2-arm RCT: active tDCS 1 CBT vs. sham tDCS 1 CBT | Sep 2018, N = 135 | Depression severity (MADRS) at 3 weeks | Y | N | Y |
| NCT01875419, completed | 2-arm RCT: active tDCS 1 CBT vs. sham tDCS 1 CBT (tDCS immediately prior to CBT) | Oct 2017, N = 30 | Change in depression severity at 8 weeks (BDI and HDRS); results not yet published | Y | N | Y |
| ACTRN12613000050752a, completed | 3-arm RCT: tDCS 1 CCT, sham tDCS 1 CCT, vs. tDCS 1 sham CCT | June 2013, N = 27 | Depression severity (MADRS), with significant group-by-time interaction (p = .003), with tDCS 1 CCT group showing best clinical outcome at follow-up (133) | Y | Y | Y |
| NCT01434836, completed | 2-arm RCT: tDCS 1 CCT vs. sham tDCS 1 CCT | May 2013, N = 37 | Depression severity at 4 weeks; no group-by-time interaction was observed (134) | Y | N | Y |
BDI, Beck Depression Inventory; CBT, cognitive behavioral therapy; CCT, cognitive control training (a computer-based intervention); ECT, electroconvulsive therapy; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; HDRS, Hamilton Depression Rating Scale; IV, intravenous; MADRS, Montgomery–Åsberg Depression Rating Scale; MED, antidepressant medication; MEG, magnetoencephalography; N, no; NCT, national clinical trial; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; Y, yes.
Registered via the Australian New Zealand Clinical Trials Registry.
D-Cycloserine
Monomodal Treatment: Neuroplasticity and Clinical Effects.
D-cycloserine is a glutamate modulating agent that can act as either an NMDA partial agonist at low doses or an NMDA antagonist at higher doses. In patients, low and intermediate doses (50–250 mg/day) do not appear to have robust effects on mood (71). However, at higher doses (1000 mg/day), D-cycloserine exhibited robust antidepressant effects in a pilot trial of 26 depressed patients, with significant effects over placebo after 6 weeks (Cohen’s d range = 0.91–0.99) (72). This dose also shows initial promise in extending the rapid anti-depressant effects of ketamine when given subsequently (73), and several ongoing investigations are pursuing this sequenced approach (i.e., NCT02772211, NCT02974010, NCT03395392). In the cognitive domain, at an intermediate dose (250 mg), D-cycloserine has shown enhancement effects for declarative memory in healthy humans, which was linked to increased functional magnetic resonance imaging activation in the hippocampus (74), consistent with a potential facilitative effect on learning.
Potential for Synergistic Effects.
Though the current review focuses on depression, it is noteworthy that a large clinical literature has examined the potential for low-dose D-cycloserine (50 mg) to facilitate extinction learning during exposure therapy for anxiety conditions. Based on promising initial findings, this approach was pursued to synergistically capitalize on neuroplasticity by combining pharmacology with behavioral treatment (75). Unfortunately, subsequent research in this area suggests that D-cycloserine may not produce a reliable and clinically meaningful increase in the overall response rate above that achieved through gold-standard exposure therapy protocols alone (76). We are not aware of published findings that directly test whether D-cycloserine enhances behavioral treatments in the context of depression. One ongoing study (NCT02376257) is designed to test the hypothesis that an intermediate dose of D-cycloserine (250 mg) can enhance memory retention of computer-administered cognitive therapy session material among depressed patients (see Table 1) (77,78). While learning enhancement has been found at this intermediate dose, mood effects are robust only at higher doses. If such mood effects are an important clinical marker of neuroplasticity in depression, higher doses (i.e., 1000 mg) could be needed to maximize synergistic effects with behavioral learning, though this hypothesis has not been tested.
BRAIN STIMULATION THERAPIES
Electroconvulsive Therapy
Monomodal Treatment: Neuroplasticity and Clinical Effects.
ECT is the gold-standard therapy for severe depression (79). Whereas standard antidepressant therapies achieve remission in 13% to 14% of patients with treatment-resistant major depressive disorder (MDD) (80), ECT reliably achieves remission rates of 50% to 70% (81). ECT involves passing an electrical current through the brain to induce a generalized seizure under general anesthesia. During an acute treatment course, ECT is typically given several times per week (three times per week in the United States), with the average patient requiring a range of six to 12 treatments (81–83).
While the mechanism of action of ECT remains incompletely understood, its potent effects on neuroplasticity may underlie its antidepressant effects (10,84), though critical gaps in this literature remain. Electroconvulsive seizures (the analog of ECT in animal models) affect many neuroplastic processes, including gliogenesis, increased axospinous synapses in the CA1 pyramidal layer, increase in number of mushroom spines, axonal sprouting in the dentate gyrus, neurogenesis, as well as regulation of neurotrophic factors (85,86). Cognitive flexibility, operationalized as the ability to flexibly learn new and unlearn old associations as novel situations arise, has been shown to improve following electroconvulsive seizure in rodents (86). Studies of ECT in humans have also shown effects on neuroplasticity markers, including changes in hippocampal and amygdala volumes (10,84,87), peripheral brain-derived neurotrophic factor (88), and default mode network connectivity (89).
Notably, as with most antidepressant treatments, a major clinical problem related to ECT is the high probability of relapse following an index treatment course [as high as 84% during the subsequent 6 months when ECT is abruptly discontinued (90)]. Even with adjunctive continuation pharmacotherapy and other biological strategies, relapse rates approach 50% within the first year (91), with most patients relapsing 2 to 3 months following an index ECT course (90,92). These findings support the hypothesis that when plasticity-enhancing treatments are withdrawn, patients often experience a return of symptoms.
Potential for Synergistic Effects.
Early work on ECT did not typically probe the combined effect of psychotherapy (93), possibly reflecting the thinking that ECT patients were too impaired to effectively engage in psychotherapy. However, advancements in technique have improved cognitive outcomes substantially. In fact, many studies demonstrate that a number of cognitive domains improve post-ECT compared with baseline, likely as a result of improvements in mood and other related symptoms (92,94–96). A large meta-analysis of the effects of ECT on cognitive domains (N = 2981, k = 84) (97) found that while some cognitive domains show impairment in the subacute period (<3 days since last ECT), many domains showed improvement compared with baseline in the acute period (4–15 days) as well as on longer-term follow-up (>28 days) posttreatment. In fact, 1 month after treatment, approximately half of neurocognitive measures showed statistically significant improvement, with effect sizes (within subject) ranging from 0.37 to 0.75, and no assessment showed a decline compared with baseline (97).
Given potent effects of ECT on plasticity, the subacute post-ECT period may be an opportune time for cognitive and behavioral interventions to improve longer-term outcomes. Several studies suggest that the combination of ECT and psychotherapy may lead to improved longer-term outcomes (98–100) (see Table 1). The largest study to date (N = 60) showed that ECT followed by CBT was more efficacious at maintaining response (77% sustained response) than either continued ECT alone (40%) or pharmacotherapy alone (44%) (98).
Repetitive TMS
Monomodal Treatment: Neuroplasticity and Clinical Effects.
Transcranial magnetic therapy involves the noninvasive application of rapidly changing magnetic fields to induce focused electrical currents in the cortex. When stimulation is delivered in rapid succession (e.g., several pulses per second), this is called rTMS. Studies focused on stimulation of the motor cortex suggest that HF stimulation (5–20 Hz) leads to increased local cortical excitability whereas low-frequency stimulation (0.1–1.0 Hz) leads to local cortical inhibition (101).
The efficacy of rTMS for the treatment of depression was established in three large, multisite trials of adult patients with MDD who had failed to respond to one to four standard antidepressants. In these trials of HF rTMS, 703 subjects were randomized to active or sham rTMS (102–104), with modest effect sizes. Following a large industry-sponsored study (104,105), the Food and Drug Administration cleared the first rTMS device in 2008 for therapeutic clinical use in MDD. The Food and Drug Administration–approved treatment protocols involve 20 to 30 sessions of 10-Hz rTMS delivered to the left dorsolateral PFC (DLPFC). However, a small but substantial literature also supports the antidepressant efficacy of low-frequency rTMS applied to the right DLPFC (106) as well as bilateral rTMS (107). As with other acute treatments for depression, relapse following a successful course of rTMS is relatively high (108).
In depressed patients, a course of rTMS has been associated with improvement in cognitive function, though this is not clearly distinct from an overall antidepressant effect (109). TMS exhibits cognitive performance enhancement in healthy humans across numerous domains, including motor learning, attention, memory, and language (110). Another systematic review focused on HF rTMS targeting the PFC reported that rTMS was more likely to lead to cognitive improvements when applied over the left DLPFC (111).
It is hypothesized that TMS operates by modulating the function of neural circuits involved in emotion regulation, cognition, and attention control (112,113). As the primary target of TMS as a treatment for depression is the DLPFC, it has been suggested that TMS may specifically alter activity of a cognitive control network that includes this region, potentially enhancing cognitive control of emotion (114). Studies in human participants, animal models, and in vitro work have all demonstrated that rTMS affects synaptic plasticity in a relatively enduring way (9). In clinical populations, most but not all studies show that rTMS-induced changes in cortical excitability and brain activity (positron emission tomography, functional magnetic resonance imaging, electroencephalography) last beyond the immediate period of stimulation (9). Human brain imaging studies show effects of rTMS on regional cerebral blood flow (115), blood oxygen level–dependent activity patterns (116), and electroencephalography responses (117), which last up to several days beyond the stimulation period (118,119). In rodents, neuroplasticity markers—including brain-derived neurotrophic factor and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor phosphorylation—remain upregulated in the hippocampus 3 days following HF-rTMS (120).
Potential for Synergistic Effects.
Despite the potential synergistic effect of modulating the cognitive control network via rTMS and engaging patients in a cognitively based psychotherapy, few studies have combined the two approaches for any disorder. In depression, a large, nonrandomized naturalistic study treated patients for a minimum of 10 weeks with simultaneous rTMS and CBT (CBT sessions were conducted during TMS) and demonstrated relatively strong response (66%) and remission (56%) rates. A majority of individuals who achieved response or remission acutely maintained these outcomes at 6 months posttreatment (65% of responders retained response; 60% of remitters retained remission) (121). These findings await validation with a randomized controlled trial. While this review focuses on depression, it is worth noting that rTMS in combination with CBT in posttraumatic stress disorder showed enhanced clinical outcomes in two small (Ns = 9, 30) (122,123) and one relatively large (N = 103) (124) sham-controlled studies. Although still preliminary, these studies suggest that response to existing psychotherapeutic strategies for PTSD might be enhanced with concurrent focal brain stimulation.
Transcranial Direct Current Stimulation
Monomodal Treatment: Neuroplasticity and Clinical Effects.
Transcranial direct current stimulation (tDCS) passes a low-intensity electrical current through the brain between two electrodes (cathode and anode). tDCS does not directly depolarize cortical neurons but does result in lasting changes in cortical excitability, with increased cortical excitability occurring under the anode and decreased excitability occurring under the cathode. Some have proposed that tDCS induces neuroplasticity through NMDA-dependent mechanisms (125–127). Preliminary studies suggest that the NMDA antagonist dextromethorphan prevents lasting effects of tDCS on motor evoked potentials, while tDCS-induced excitability is potentiated by the partial NMDA agonist D-cycloserine (at a low dose of 100 mg) (127,128). tDCS has been studied as a potential treatment for several psychiatric disorders, including depression, and as a cognitive enhancer in healthy individuals. To date, the clinical data on tDCS are mixed. Although there is some evidence for antidepressant effects (129), these are modest at best, and a large clinical trial was negative (130). A quantitative review found no support for cognitive enhancing effects of a single session of tDCS in healthy individuals (131).
Potential for Synergistic Effects.
tDCS has been tested in combination with cognitive control training (CCT), an automated intervention designed to engage the PFC, increase cognitive control, and decrease symptoms through working memory exercises (132). A pilot study randomized 27 MDD participants into three groups: 1) tDCS combined with CCT, 2) sham brain stimulation combined with CCT, and 3) sham CCT plus active tDCS. In all three groups, there were similar immediate antidepressant effects, but the tDCS combined with the CCT group exhibited sustained and increased antidepressant effect at 3 weeks posttreatment (see Table 1) (133). In a similar double-blinded study (N = 37), depressed patients received active tDCS and CCT or sham tDCS and CCT for 10 consecutive days. Depressive symptoms posttreatment and at 2-week follow-up did not differ across groups; this may have been due to specific individual differences, though this hypothesis needs further confirmation (134). One additional, similar randomized controlled trial of tDCS combined with CCT is underway (see Table 1). Finally, three randomized controlled trials examining tDCS combined with CBT for depression are underway, with results anticipated shortly (see Table 1) (135).
tDCS combined with behavioral treatment has also been explored in other disorders of negative affect. For instance, using a fully crossed (2 × 2) randomized controlled design, tDCS (active or sham) over the DLPFC was combined with a single session of one of two forms of automated attention retraining (designed to train attention either toward or away from threat cues) in 77 participants with mild anxiety. Active tDCS facilitated the uptake of the trained attentional patterns (136). Additionally, a small case series (N = 4) has shown that tDCS combined with working memory training may improve cognitive and emotional function in patients with posttraumatic stress disorder and poor working memory (137).
SUMMARY AND FUTURE DIRECTIONS
The current review focuses on the potential of behavioral interventions to leverage plasticity-enhancing approaches to address pathological neural circuits in depression and hence improve longer-term outcomes. While the theoretical basis and indirect evidence for this strategy are compelling, few clinical studies directly test these approaches using critical control conditions, including each intervention component (somatic and behavioral) in the absence of the other (Table 1). Such studies are necessary to provide definitive evidence of synergy—ideally by showing not only that the combination treatment outperforms each intervention component on its own, but also that the clinical advantages of the multimodal treatment are not simply additive. These fully crossed designs are likely scarce because they present challenges to consider from both pragmatic (e.g., sufficient sample sizes required in each cell) and ethical (e.g., withholding gold-standard treatments) standpoints. Nevertheless, well-controlled designs are important to show definitively that neuroplasticity enhancement increases the impact of the behavioral treatment, and likewise that the behavioral treatment extends and/or magnifies the acute effect of the neuroplasticity intervention.
The somatic therapies reviewed above have the largest evidence base in humans with regard to stand-alone clinical and neuroplasticity effects and are the subject of preliminary or ongoing clinical studies testing their potential for synergistic combination with behavioral approaches. Countless other interventions may have similar plasticity-enhancing potential yet to be leveraged in clinical research. These include numerous other glutamate-modulating drugs that have been pursued in the wake of intravenous ketamine. The field anxiously anticipates the results of several phase II and III trials of these glutamate-modulating drugs with hypothesized potential for potent and rapid enhancements in neuroplasticity (esketamine, rapastinel, tulrampator/S-47445, AV-101, NRX-1074, CERC-301), many of which are completed or near completion [see (138)].
Nonpharmacological options to enhance plasticity are also important to consider. These include alternatives such as exercise, which exhibits plasticity and procognitive effects (139). Unfortunately, in the current system of Food and Drug Administration approval and new drug development, researchers investigating nonpharmacological approaches and older, well-established drugs without patent protection have considerably less opportunities for funding well-powered clinical trials compared with pharmacological agents with industry sponsorship.
In summary, a range of neuroplasticity enhancers have the potential to facilitate the uptake of adaptive cognitive patterns that may effectively buffer against depression over time. While this thesis is currently indirectly supported by robust animal and human literatures, the development and rigorous clinical validation of synergistic, neuroplasticity-based somatic-behavioral treatment combinations remains in an early stage.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported in part by Agency for Healthcare Research and Quality Grant No. K12HS023000 (to STW) (the content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality), the Brain and Behavior Research Foundation (formerly NARSAD) (to STW, PEH), the Robert E. Leet and Clara Guthrie Patterson Trust (to STW), the American Foundation for Suicide Prevention (to STW), National Institute of Mental Health Grant Nos. K23MH100259 and R01MH113857 (to RBP), and the Veterans Administration (PEH).
STW reports receiving research support from Janssen (administered through Yale) to conduct clinical trials as well as honoraria from Janssen. Over the past 12 months, PEH has received research support from Janssen, Neo-Sync, the National Institutes of Health, and the Veterans Administration. He receives royalties from Oxford University Press and UpToDate. SG, DSK, and RBP report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Samuel T. Wilkinson, Department of Psychiatry, Yale School of Medicine and Yale Psychiatric Hospital, New Haven, Connecticut;
Paul E. Holtzheimer, National Center for PTSD, Executive Division, White River Junction VA Medical Center, White River Junction, Vermont; Department of Psychiatry and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire;
Shan Gao, Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania..
David S. Kirwin, Department of Psychiatry, Yale School of Medicine and Yale Psychiatric Hospital, New Haven, Connecticut;
Rebecca B. Price, Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania.
REFERENCES
- 1.Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015): Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66:509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castren E (2013): Neuronal network plasticity and recovery from depression. JAMA Psychiatry 70:983–989. [DOI] [PubMed] [Google Scholar]
- 4.Sharpley CF (2010): A review of the neurobiological effects of psychotherapy for depression. Psychotherapy (Chic) 47:603–615. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. (2010): mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, et al. (2012): Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry 72:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. (2000): Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- 8.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. (2016): NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogendam JM, Ramakers GM, Di Lazzaro V (2010): Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3:95–118. [DOI] [PubMed] [Google Scholar]
- 10.Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, et al. (2014): Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A 111:1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bockting CL, ten Doesschate MC, Spijker J, Spinhoven P, Koeter MW, Schene AH, et al. (2008): Continuation and maintenance use of antidepressants in recurrent depression. Psychother Psychosom 77:17–26. [DOI] [PubMed] [Google Scholar]
- 12.Simon GE, Von Korff M, Rutter CM, Peterson DA (2001): Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Arch Gen Psychiatry 58:395–401. [DOI] [PubMed] [Google Scholar]
- 13.Bockting CL, Schene AH, Spinhoven P, Koeter MW, Wouters LF, Huyser J, et al. (2005): Preventing relapse/recurrence in recurrent depression with cognitive therapy: A randomized controlled trial. J Consult Clin Psychol 73:647–657. [DOI] [PubMed] [Google Scholar]
- 14.Hollon SD, Stewart MO, Strunk D (2006): Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annu Rev Psychol 57:285–315. [DOI] [PubMed] [Google Scholar]
- 15.Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. (2006): Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry 62:417–422. [DOI] [PubMed] [Google Scholar]
- 16.Alboni S, van Dijk RM, Poggini S, Milior G, Perrotta M, Drenth T, et al. (2017): Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psychiatry 22:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castrén E (2005): Is mood chemistry? Nat Rev Neurosci 6:241–246. [DOI] [PubMed] [Google Scholar]
- 18.Castren E, Hen R (2013): Neuronal plasticity and antidepressant actions. Trends Neurosci 36:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Changeaux JP, Danchin A (1976): Selective stabilization of developing synapses as a mechanism for the specification of neuronal networks. Nature 264:705–712. [DOI] [PubMed] [Google Scholar]
- 20.Kashdan TB, Rottenberg J (2010): Psychological flexibility as a fundamental aspect of health. Clin Psychol Rev 30:865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stange JP, Connolly SL, Burke TA, Hamilton JL, Hamlat EJ, Abramson LY, et al. (2016): Inflexible cognition predicts first onset of major depressive episodes in adolescence. Depress Anxiety 33:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joormann J (2010): Cognitive inhibition and emotion regulation in depression. Curr Dir Psychol Sci 19:161–166. [Google Scholar]
- 23.Gotlib IH, Joormann J (2010): Cognition and depression: Current status and future directions. Annu Rev Clin Psychol 6:285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price RB, Eldreth DA, Mohlman J (2011): Deficient prefrontal attentional control in late-life generalized anxiety disorder: An fMRI investigation. Transl Psychiatry 1:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Disner S, Beevers C, Haigh EAP, Beck AT (2011): Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 12:467–477. [DOI] [PubMed] [Google Scholar]
- 26.Price RB, Allen KB, Silk JS, Ladouceur CD, Ryan ND, Dahl RE, et al. (2016): Vigilance in the laboratory predicts avoidance in the real world: A dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Dev Cogn Neurosci 19:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price RB, Lane S, Gates KM, Kraynak TE, Horner MS, Thase ME, et al. (2017): Parsing heterogeneity in directed brain connectivity during positive mood: A community detection analysis in depressed and healthy adults. Biol Psychiatry 81:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dozois D, Beck A (2008): Cognitive schemas, beliefs and assumptions In: Dobson K, Dozois D, editors. Risk Factors in Depression. Oxford: Elsevier/Academic Press, 121–143. [Google Scholar]
- 29.de Raedt R, Koster EHW (2010): Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci 10:50–70. [DOI] [PubMed] [Google Scholar]
- 30.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH (2007): Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull 133: 1–24. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Bredemeier K (2016): A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clin Psychol Sci 4:596–619. [Google Scholar]
- 32.Kraus C, Castren E, Kasper S, Lanzenberger R (2017): Serotonin and neuroplasticity - Links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev 77:317–326. [DOI] [PubMed] [Google Scholar]
- 33.Craighead WE, Dunlop BW (2014): Combination psychotherapy and antidepressant medication treatment for depression: For whom, when, and how. Annu Rev Psychol 65:267–300. [DOI] [PubMed] [Google Scholar]
- 34.Cuijpers P, Dekker J, Hollon SD, Andersson G (2009): Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: A meta-analysis. J Clin Psychiatry 70:1219–1229. [DOI] [PubMed] [Google Scholar]
- 35.Ishak WW, Ha K, Kapitanski N, Bagot K, Fathy H, Swanson B, et al. (2011): The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry 19:277–289. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Hackett M, Carter G, Loo C, Galvez V, Glozier N, et al. (2015): Effects of low-dose and very low-dose dose ketamine among patients with major depression: A systematic review and meta-analysis. Int J Neuropsychopharmacol 19:pyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, et al. (2015): Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev (9):CD011612. [DOI] [PubMed] [Google Scholar]
- 38.McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, et al. (2015): Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev (9):CD011611. [DOI] [PubMed] [Google Scholar]
- 39.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. (2010): Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- 40.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. (2013): Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, et al. (2013): Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27:444–450. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson S, Toprak M, Turner M, Levine S, Katz R, Sanacora G (2017): A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry 174:695–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schatzberg AF (2014): A word to the wise about ketamine. Am J Psychiatry 171:262–264. [DOI] [PubMed] [Google Scholar]
- 44.Morgan CJ, Curran HV; Independent Scientific Committee on Drugs (2012): Ketamine use: A review. Addiction 107:27–38. [DOI] [PubMed] [Google Scholar]
- 45.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. (2015): Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- 46.Nagy D, Stoiljkovic M, Menniti FS, Hajos M (2015): Differential effects of an NR2B NAM and ketamine on synaptic potentiation and gamma synchrony: Relevance to rapid-onset antidepressant efficacy. Neuropsychopharmacology 41:1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. (2017): Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, et al. (2016): Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: Relevance to mood disorders. Biol Psychiatry 79:765–775. [DOI] [PubMed] [Google Scholar]
- 49.Murrough JW, Burdick KE, Levitch CF, Perez AM, Brallier JW, Chang LC, et al. (2014): Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: A randomized controlled trial. Neuropsychopharmacology 40:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Permoda-Osip A, Kisielewski J, Bartkowska-Sniatkowska A, Rybakowski JK (2015): Single ketamine infusion and neurocognitive performance in bipolar depression. Pharmacopsychiatry 48:78–79. [DOI] [PubMed] [Google Scholar]
- 51.Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO (2014): Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacology 17:1805–1813. [DOI] [PubMed] [Google Scholar]
- 52.Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RS, Siegel SJ (2012): Subchronic ketamine treatment leads to permanent changes in EEG, cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol Dis 47:338–346. [DOI] [PubMed] [Google Scholar]
- 53.Morgan CJ, Muetzelfeldt L, Curran HV (2009): Ketamine use, cognition and psychological wellbeing: A comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction 104:77–87. [DOI] [PubMed] [Google Scholar]
- 54.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. (2014): Effects of ketamine on explicit and implicit suicidal cognition: A randomized controlled trial in treatment-resistant depression. Depress Anxiety 31:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price RB, Nock MK, Charney DS, Mathew SJ (2009): Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, et al. (2015): Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry 5:e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li CT, Chen MH, Lin WC, Hong CJ, Yang BH, Liu RS, et al. (2016): The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: A randomized controlled study. Human Brain Mapp 37:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K, et al. (2016): Comparing the actions of lanicemine and ketamine in depression: Key role of the anterior cingulate. Eur Neuropsychopharmacol 26:994–1003. [DOI] [PubMed] [Google Scholar]
- 59.Nugent AC, Robinson SE, Coppola R, Zarate CA Jr (2016): Preliminary differences in resting state MEG functional connectivity preand post-ketamine in major depressive disorder. Psychiatry Res 254:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baudry M, Bi X, Gall C, Lynch G (2011): The biochemistry of memory: The twenty-six year journey of a “new and specific hypothesis201D. Neurobiol Learn Mem 95:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramirez A, Arbuckle MR (2016): Synaptic plasticity: The role of learning and unlearning in addiction and beyond. Biol Psychiatry 80:e73–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson ST, Wright D, Fasula MK, Fenton L, Griepp M, Ostroff RB, et al. (2017): Cognitive behavior therapy may sustain antidepressant effects of intravenous ketamine in treatment-resistant depression. Psychother Psychosom 86:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, et al. (2014): Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 155:123–129. [DOI] [PubMed] [Google Scholar]
- 65.Price RB, Wallace M, Kuckertz JM, Amir N, Graur S, Cummings L, et al. (2016): Pooled patient-level metaanalysis of children and adults completing a computer-based anxiety intervention targeting attentional bias. Clin Psychol Rev 50:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLeod C (2012): Cognitive bias modification procedures in the management of mental disorders. Curr Opin Psychiatry 25: 114–120. [DOI] [PubMed] [Google Scholar]
- 67.Mogoaşe C, David D, Koster EHW (2014): Clinical efficacy of attentional bias modification procedures: An updated meta-analysis. J Clin Psychol 70:1133–1157. [DOI] [PubMed] [Google Scholar]
- 68.Hofmann W, De Houwer J, Perugini M, Baeyens F, Crombez G (2010): Evaluative conditioning in humans: A meta-analysis. Psychol Bull 136:390–421. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez CI, Wheaton M, Zwerling J, Steinman SA, Sonnenfeld D, Galfalvy H, et al. (2016): Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? An open-label trial. J Clin Psychiatry 77:408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pradhan B, Mitrev L, Moaddell R, Wainer IW (2018): d-Serine is a potential biomarker for clinical response in treatment of post-traumatic stress disorder using (R,S)-ketamine infusion and TIMBER psychotherapy: A pilot study. Biochim Biophys Acta 1866: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, et al. (2006): Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord 93:239–243. [DOI] [PubMed] [Google Scholar]
- 72.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. (2013): A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol 16:501–506. [DOI] [PubMed] [Google Scholar]
- 73.Kantrowitz JT, Halberstam B, Gangwisch J (2015): Single-dose ketamine followed by daily D-Cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry 76:737–738. [DOI] [PubMed] [Google Scholar]
- 74.Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A, et al. (2010): The N-methyl-D-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry 67:1205–1211. [DOI] [PubMed] [Google Scholar]
- 75.Krystal JH, Tolin DF, Sanacora G, Castner SA, Williams GV, Aikins DE, et al. (2009): Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today 14:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mataix-Cols D, Fernandez de la Cruz L, Monzani B, Rosenfield D, Andersson E, Perez-Vigil A, et al. (2017): D-Cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: a systematic review and meta-analysis of individual participant data. JAMA Psychiatry 74:501–510. [DOI] [PubMed] [Google Scholar]
- 77.Antony MM (2011): Recent advances in the treatment of anxiety disorders. Can Psychol/Psychol canadienne 52:1–9. [Google Scholar]
- 78.Otto MW, Lee J, Hofmann SG, Hearon BA, Smits JA, Rosenfield D, et al. (2016): Examining the efficacy of d-cycloserine to augment therapeutic learning in depression. Contemp Clin Trials 48:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greenberg RM, Kellner CH (2005): Electroconvulsive therapy: A selected review. Am J Geriatr Psychiatry 13:268–281. [DOI] [PubMed] [Google Scholar]
- 80.Zisook S, Ganadjian K, Moutier C, Prather R, Rao S (2008): Sequenced Treatment Alternatives to Relieve Depression (STAR*D): Lessons learned. J Clin Psychiatry 69:1184–1185. [DOI] [PubMed] [Google Scholar]
- 81.Weiner R, Coffey C, Fochtmann L, Greenberg R, Isenberg K, Kellner C, et al. (2001): The Practice of Electroconvulsive Therapy. Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association, 2nd ed. Washington, DC: American Psychiatric Press. [Google Scholar]
- 82.Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, et al. (2006): Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: A multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry 63:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al. (2016): Right unilateral ultrabrief pulse ECT in geriatric depression: Phase 1 of the PRIDE study. Am J Psychiatry 173:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. (2016): Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 79:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilkinson ST, Sanacora G, Bloch MH (2017): Hippocampal volume changes following electroconvulsive therapy: A systematic review and meta-analysis. Biol Psychiatry Cogn Neurosci Neuroimaging 2:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Svensson M, Grahm M, Ekstrand J, Hoglund P, Johansson M, Tingstrom A (2016): Effect of electroconvulsive seizures on cognitive flexibility. Hippocampus 26:899–910. [DOI] [PubMed] [Google Scholar]
- 87.Nordanskog P, Larsson MR, Larsson EM, Johanson A (2014): Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr Scand 129:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rocha RB, Dondossola ER, Grande AJ, Colonetti T, Ceretta LB, Passos IC, et al. (2016): Increased BDNF levels after electroconvulsive therapy in patients with major depressive disorder: A meta-analysis study. J Psychiatr Res 83:47–53. [DOI] [PubMed] [Google Scholar]
- 89.Mulders PC, van Eijndhoven PF, Pluijmen J, Schene AH, Tendolkar I, Beckmann CF (2016): Default mode network coherence in treatment-resistant major depressive disorder during electroconvulsive therapy. J Affect Disord 205:130–137. [DOI] [PubMed] [Google Scholar]
- 90.Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. (2001): Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: A randomized controlled trial. JAMA 285:1299–1307. [DOI] [PubMed] [Google Scholar]
- 91.Jelovac A, Kolshus E, McLoughlin DM (2013): Relapse following successful electroconvulsive therapy for major depression: A meta-analysis. Neuropsychopharmacology 38:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, et al. (2008): Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 1:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McClintock SM, Brandon AR, Husain MM, Jarrett RB (2011): A systematic review of the combined use of electroconvulsive therapy and psychotherapy for depression. J ECT 27:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCall WV, Dunn A, Rosenquist PB, Hughes D (2002): Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: Antidepressant and memory effects. J ECT 18: 126–129. [DOI] [PubMed] [Google Scholar]
- 95.McCall WV, Reboussin DM, Weiner RD, Sackeim HA (2000): Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: Acute antidepressant and cognitive effects. Arch Gen Psychiatry 57:438–444. [DOI] [PubMed] [Google Scholar]
- 96.Ng C, Schweitzer I, Alexopoulos P, Celi E, Wong L, Tuckwell V, et al. (2000): Efficacy and cognitive effects of right unilateral electroconvulsive therapy. J ECT 16:370–379. [DOI] [PubMed] [Google Scholar]
- 97.Semkovska M, McLoughlin DM (2010): Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta-analysis. Biol Psychiatry 68:568–577. [DOI] [PubMed] [Google Scholar]
- 98.Brakemeier EL, Merkl A, Wilbertz G, Quante A, Regen F, Buhrsch N, et al. (2014): Cognitive-behavioral therapy as continuation treatment to sustain response after electroconvulsive therapy in depression: A randomized controlled trial. Biol Psychiatry 76:194–202. [DOI] [PubMed] [Google Scholar]
- 99.Fenton L, Fasula M, Ostroff R, Sanacora G (2006): Can cognitive behavioral therapy reduce relapse rates of depression after ECT? a preliminary study. J ECT 22:196–198. [DOI] [PubMed] [Google Scholar]
- 100.Wilkinson ST, Ostroff RB, Sanacora G (2017): Computer-assisted cognitive behavior therapy to prevent relapse following electroconvulsive therapy. J ECT 33:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzgerald PB, Fountain S, Daskalakis ZJ (2006): A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117:2584–2596. [DOI] [PubMed] [Google Scholar]
- 102.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. (2010): Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch Gen Psychiatry 67:507–516. [DOI] [PubMed] [Google Scholar]
- 103.Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. (2015): Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry 14:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. (2007): Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol Psychiatry 62:1208–1216. [DOI] [PubMed] [Google Scholar]
- 105.Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. (2009): Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: Clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology 34:522–534. [DOI] [PubMed] [Google Scholar]
- 106.Berlim MT, Van den Eynde F, Jeff Daskalakis Z (2013): Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: A meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 38:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. (2017): Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry 74:143–152. [DOI] [PubMed] [Google Scholar]
- 108.Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, et al. (2014): A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: Durability of benefit over a 1-year follow-up period. J Clin Psychiatry 75:1394–1401. [DOI] [PubMed] [Google Scholar]
- 109.Moreines JL, McClintock SM, Holtzheimer PE (2011): Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: A review. Brain Stimul 4:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luber B, Lisanby SH (2014): Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 85(Pt 3):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guse B, Falkai P, Wobrock T (2010): Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: A systematic review. J Neural Transm 117:105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janicak PG, Dokucu ME (2015): Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat 11:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Raedt R, Vanderhasselt MA, Baeken C (2015): Neurostimulation as an intervention for treatment resistant depression: From research on mechanisms towards targeted neurocognitive strategies. Clin Psychol Rev 41:61–69. [DOI] [PubMed] [Google Scholar]
- 114.Lantrip C, Gunning FM, Flashman L, Roth RM, Holtzheimer PE (2017): Effects of transcranial magnetic stimulation on the cognitive control of emotion: Potential antidepressant mechanisms. J ECT 33:73–80. [DOI] [PubMed] [Google Scholar]
- 115.Takano B, Drzezga A, Peller M, Sax I, Schwaiger M, Lee L, et al. (2004): Short-term modulation of regional excitability and blood flow in human motor cortex following rapid-rate transcranial magnetic stimulation. Neuroimage 23:849–859. [DOI] [PubMed] [Google Scholar]
- 116.Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, et al. (2005): Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol 3:e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G (2007): TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One 2:e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ridding MC, Rothwell JC (2007): Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 8:559–567. [DOI] [PubMed] [Google Scholar]
- 119.Hayashi T, Ohnishi T, Okabe S, Teramoto N, Nonaka Y, Watabe H, et al. (2004): Long-term effect of motor cortical repetitive transcranial magnetic stimulation [correction]. Ann Neurol 56:77–85. [DOI] [PubMed] [Google Scholar]
- 120.Gersner R, Kravetz E, Feil J, Pell G, Zangen A (2011): Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J Neurosci 31:7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Donse L, Padberg F, Sack AT, Rush AJ, Arns M (2018): Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul 11:337–345. [DOI] [PubMed] [Google Scholar]
- 122.Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM, McCann U (2009): Repetitive TMS combined with exposure therapy for PTSD: A preliminary study. J Anxiety Disord 23:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. (2013): Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder–A pilot study. Brain Stimul 6:377–383. [DOI] [PubMed] [Google Scholar]
- 124.Kozel FA, Motes MA, Didehbani N, DeLaRosa B, Bass C, Schraufnagel CD, et al. (2018): Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. J Affect Disord 229:506–514. [DOI] [PubMed] [Google Scholar]
- 125.Paulus W (2011): Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil 21:602–617. [DOI] [PubMed] [Google Scholar]
- 126.Das S, Holland P, Frens MA, Donchin O (2016): Impact of transcranial direct current stimulation (tDCS) on neuronal functions. Front Neurosci 10:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liebetanz D, Nitsche MA, Tergau F, Paulus W (2002): Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125:2238–2247. [DOI] [PubMed] [Google Scholar]
- 128.Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W (2004): Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology 29:1573–1578. [DOI] [PubMed] [Google Scholar]
- 129.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. (2016): Transcranial direct current stimulation for acute major depressive episodes: Meta-analysis of individual patient data. Br J Psychiatry 208:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loo CK, Husain MM, McDonald WM, Aaronson S, O’Reardon JP, Alonzo A, et al. (2018): International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul 11:125–133. [DOI] [PubMed] [Google Scholar]
- 131.Horvath JC, Forte JD, Carter O (2015): Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimul 8:535–550. [DOI] [PubMed] [Google Scholar]
- 132.Koster EHW, Hoorelbeke K, Onraedt T, Owens M, Derakshan N (2017): Cognitive control interventions for depression: A systematic review of findings from training studies. Clin Psychol Rev 53:79–92. [DOI] [PubMed] [Google Scholar]
- 133.Segrave RA, Arnold S, Hoy K, Fitzgerald PB (2014): Concurrent cognitive control training augments the antidepressant efficacy of tDCS: A pilot study. Brain Stimul 7:325–331. [DOI] [PubMed] [Google Scholar]
- 134.Brunoni AR, Boggio PS, De Raedt R, Bensenor IM, Lotufo PA, Namur V, et al. (2014): Cognitive control therapy and transcranial direct current stimulation for depression: A randomized, double-blinded, controlled trial. J Affect Disord 162:43–49. [DOI] [PubMed] [Google Scholar]
- 135.Bajbouj M, Aust S, Spies J, Herrera-Melendez AL, Mayer SV, Peters M, et al. (2017): PsychotherapyPlus: Augmentation of cognitive behavioral therapy (CBT) with prefrontal transcranial direct current stimulation (tDCS) in major depressive disorder-study design and methodology of a multicenter double-blind randomized placebo-controlled trial [published online ahead of print Dec 6]. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- 136.Clarke PJ, Browning M, Hammond G, Notebaert L, MacLeod C (2014): The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: Evidence from transcranial direct current stimulation. Biol Psychiatry 76:946–952. [DOI] [PubMed] [Google Scholar]
- 137.Saunders N, Downham R, Turman B, Kropotov J, Clark R, Yumash R, et al. (2015): Working memory training with tDCS improves behavioral and neurophysiological symptoms in pilot group with post-traumatic stress disorder (PTSD) and with poor working memory. Neurocase 21:271–278. [DOI] [PubMed] [Google Scholar]
- 138.Murrough JW, Abdallah CG, Mathew SJ (2017): Targeting glutamate signalling in depression: Progress and prospects. Nat Rev Drug Discov 16:472–486. [DOI] [PubMed] [Google Scholar]
- 139.Song D, Yu DSF, Li PWC, Lei Y (2018): The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int J Nurs Stud 79:155–164. [DOI] [PubMed] [Google Scholar]