Abstract
Volumetric reductions in the hippocampus and medial prefrontal cortex (mPFC) are among the most well documented neural abnormalities in major depressive disorder (MDD). Hippocampal and mPFC structural reductions have been specifically tied to MDD illness progression markers, including greater number of major depressive episodes (MDE), longer illness duration, and non-remission/treatment resistance. Chronic stress plays a critical role in the development of hippocampal and mPFC deficits, with some studies suggesting that these deficits occur irrespective of MDE occurrence. However, preclinical and human research also points to other stress-mediated neurotoxic processes, including enhanced inflammation and neurotransmitter disturbances, which may require the presence of a MDE and contribute to further brain structural decline as the illness advances. Specifically, hypothalamic-pituitary-adrenal axis dysfunction, enhanced inflammation and oxidative stress, and neurotransmitter abnormalities (e.g., serotonin, glutamate, gamma-Aminobutyric acid) likely interact to facilitate illness progression in MDD. Congruent with stress-sensitization models of MDD, with each consecutive MDE, it may take lower levels of stress to trigger these neurotoxic pathways leading to more pronounced brain volumetric reductions. Given that stress and MDD have overlapping and distinct influences on neurobiological pathways implicated in hippocampal and mPFC structural decline, further work is needed to clarify which precise mechanisms ultimately contribute to MDD development and maintenance.
Keywords: hippocampus, medial prefrontal cortex, stress, depression, illness progression
Major depressive disorder (MDD) is frequently a chronic, progressive illness. Approximately 60% of individuals with MDD will experience recurrent episodes and each successive episode carries a 10–20% risk of failing to remit with current therapeutic approaches (1). While several neural pathways have been linked to the development and recurrence of depression, the hippocampus and medial prefrontal cortex (mPFC) have been repeatedly implicated in the pathophysiology and progression of this illness (2,3).
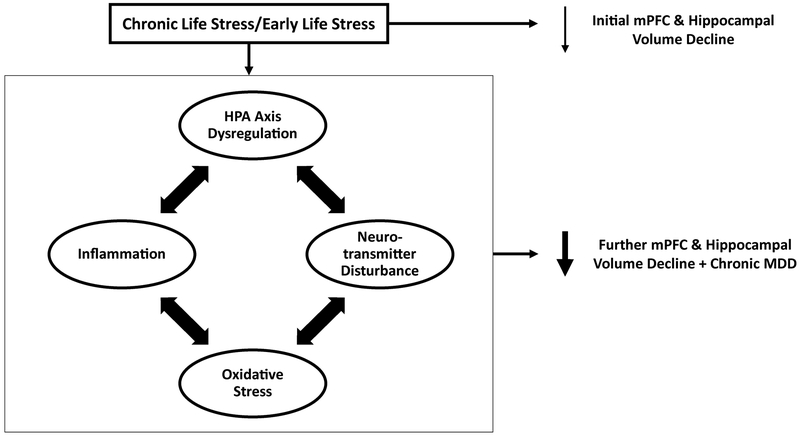
In this review, we examine clinical and preclinical data pointing to the pivotal role of stress in the development of hippocampal and mPFC abnormalities in depression and a chronic (often treatment-refractory) course of the disorder. Based on existing evidence, we propose a model (Figure 1), by which chronic/severe life stress can trigger the initial development of mPFC and hippocampal volume reductions. However, these reductions are neither necessary nor sufficient for inducing a major depressive episode (MDE). On the other hand, stress also instigates other neurotoxic processes (hypothalamic pituitary adrenal (HPA) axis dysregulation, inflammation, oxidative stress, neurotransmitter disturbances) that interact and may drive the development of a chronic type of MDD marked by further reductions in hippocampal and mPFC volumes. Expanding on recent conceptualizations, we highlight how each of these stress-linked neurotoxic processes has been related to hippocampal and mPFC structural aberrations and the development of persistent courses of depression.
Figure 1. The Role of Chronic Stress and Neurotoxic Processes on Brain Volumetric Reductions and MDD Illness Progression.
Chronic life stress can trigger the initial development of medial prefrontal cortex (mPFC) and hippocampal volume reductions. However, these reductions are neither necessary nor sufficient to produce a major depressive episode. On the other hand, stress also initiates a set of neurotoxic processes (hypothalamic pituitary adrenal (HPA) axis dysregulation, inflammation, neurotransmitter disturbances) that interact and may drive the development of a more chronic type of MDD marked by further reductions in hippocampal and mPFC volume reductions.
Hippocampus and mPFC volume reductions: Cause versus consequence of MDD?
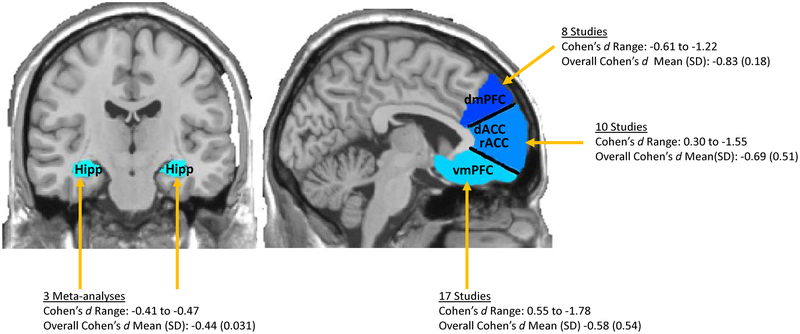
MDD is phenomenologically, etiologically, and pathophysiologically highly heterogeneous. Consequently, identifying reliable biological or imaging markers has been significantly more challenging than anticipated. One exception has been structural imaging of the hippocampus and mPFC; several meta-analyses have demonstrated that, relative to healthy controls (HCs), individuals with MDD show reduced hippocampus and mPFC volumes, including dorsal and ventral mPFC portions (dmPFC/vmPFC) extending into the medial orbital frontal cortex (mOFC) as well as dorsal and rostral portions of the anterior cingulate cortex (rACC/dACC) (2,4–14). These meta-analyses have reported moderate effect sizes for reduced hippocampal volume in MDD (Cohen’s d range = −0.41 to −0.47; 6,8,9). While most studies have examined the whole hippocampus due to spatial resolution limitations, those that have parcellated the hippocampus into different subfields have found evidence for reduced cornu ammonis 1–3, dentate gyrus/cornu ammonis 4, and subiculum volumes as well as both anterior and posterior subdivisions in MDD (e.g., 15,16). With respect to mPFC volume reductions, small to large effect sizes have been reported (Figure 2). Given that extant meta-analyses have also included portions of the PFC outside of the mPFC, we focused on calculating effect sizes for individual studies that targeted aspects of the mPFC, including the vmPFC/mOFC, the rACC/dACC, and the dmPFC (Figure 2 and Supplementary Table 1). Additionally, these estimates are likely impacted by antidepressant use and presence of other psychiatric comorbidities, given that they were not exclusionary in most studies. Notably, longitudinal studies have shown that common antidepressants lead to increases in hippocampus and mPFC volumes (17). Moreover, individuals with MDD and an anxiety disorder were found to have more pronounced mPFC reductions than those with just one disorder (18). Despite these effects, the volume reduction findings remain significant even when accounting for antidepressant use and common psychiatric comorbidities (5,7,8).
Figure 2. Estimated Effect Sizes of Hippocampal and mPFC Volume Reductions in MDD.
The medial prefrontal cortex (mPFC) includes ventral portions of the mPFC (ventral medial prefrontal cortex; vmPFC, medial orbitofrontal cortex; mOFC) and dorsal portions of the mPFC (dorsal medial prefrontal cortex; dmPFC) as well as rostral anterior cingulate cortex; rACC and dorsal anterior cingulate cortex; dACC). In this figure, we provide a range, mean (standard deviation) of effect sizes calculated from individual studies for each of the mPFC subdivisions. We also provide a range, mean (standard deviation) of effect sizes for reduced hippocampal volumes in MDD calculated from three prior meta-analyses. Negative effect size values indicate that those with MDD have reduced mPFC and hippocampal volumes compared to healthy controls.
While the presence of hippocampal and mPFC volume reductions in first-onset MDD is mixed (2,7), they have been consistently associated with markers of a progressive course of MDD characterized by more recurrent episodes/relapses (3,19–25), longer illness duration (24,26–30), and non-remission/treatment resistance (28,31–38). Importantly, a meta-analysis found that hippocampus volume reduction was only found in patients who had been depressed for at least two years and had more than one episode (2). More critically, longitudinal work has shown that hippocampal and mPFC volume reductions become more pronounced when depressive symptoms do not remit (35,38). These findings suggest that hippocampal and mPFC volume reductions may be a selective marker for the propensity towards and/or the consequence of episodic recurrence. However, contrary to this, several other studies have found that these structural changes may reflect a pre-existing vulnerability to a MDE, particularly for individuals with certain genetic profiles and/or early life stress (ELS) histories (39–45).
Interestingly, a preclinical longitudinal study involving a chronic mild stress model of depression attempted to directly address the cause/consequence debate (46). This study found that both resilient rats and susceptible rats exhibiting a depressive phenotype were characterized by reduced hippocampal cell proliferation. Additionally, amongst the susceptible rats, the development of depressive behaviors occurred prior to reductions in hippocampal cell proliferation (46). These results contradict both causal and consequence hypotheses of hippocampal abnormalities in MDD. Instead, this study suggests that hippocampal changes result from stress and are likely independent of MDE development. Consistent with this hypothesis, other preclinical work has demonstrated similar stress-related microstructural changes in the hippocampus in both resilient mice and mice exhibiting depressive behavior (47). Additionally, longitudinal human imaging work has highlighted that greater baseline life stress was associated with the development of smaller hippocampal volumes three months later in a sample of HCs (48). A possible explanation for these seemingly contradictory findings may be that chronic stress drives initial hippocampal and mPFC abnormalities, but other risk factors need to be present (e.g., genetic, abnormal physiological response to stress) for development of a MDE. Moreover, possibly via the upregulation of stress-induced neurotoxic processes (e.g., inflammation, oxidative stress), further hippocampal decline occurs among individuals who develop chronic MDD. While the presence of hippocampal and mPFC damage alone may not be sufficient for inducing a MDE, recent work has shown that the induction of long-term potentiation within the hippocampus is sufficient to generate antidepressant effects (49). Thus, abnormalities within these structures may nevertheless contribute to the maintenance or exacerbation of depressive symptoms (see Table 1 for a cause versus consequence debate synopsis).
Table 1.
A summary of the cause versus consequence debate of hippocampal and medial prefrontal cortex (mPFC) volume reductions in major depressive disorder (MDD). A body of human research has supported the hypothesis that hippocampal and mPFC volume reductions occur prior to the onset of depression, and ultimately cause a first major depressive episode (MDE). However, independent evidence has shown that hippocampal and mPFC deficits are only found amongst those with a history of major depressive episodes and greater illness progression. Further, a third hypothesis has emerged from preclinical and human research, demonstrating that hippocampal and mPFC deficits are produced by stress and occur irrespective of whether depressive symptomology emerges.
|
Cause mPFC and hippocampal volume reductions cause initial MDD onset |
Consequence mPFC and hippocampal volume reductions occur as a result of having multiple major depressive episodes |
Independence mPFC and hippocampal volume reductions are a result of stress and independent of MDD development |
Stress and hippocampal/mPFC volume reduction in MDD
Two classes of stress-related models, the stress sensitization and stress autonomy models, have been proposed to explain how relationships between stress and depression change as a function of illness course (50). Both models posit that while first MDEs are highly likely to follow periods of severe stress, the strength of this relationship declines as a function of illness course. However, while the stress sensitization model hypothesizes that with recurrent episodes of depression, more minor stressors are capable of triggering MDEs, the stress autonomy model posits that successive MDEs develop independently of stressful life events. In support of both models, prospective studies have found that the likelihood of a severe stressful life event occurring in the three months prior to an MDE onset was reduced amongst those with a prior history of depression compared to those experiencing their first MDE (51,52). However, more consistent with the stress sensitization model, these longitudinal studies also found that the likelihood of a MDE onset increased when nonsevere life stressors were present in the three months prior to the episode amongst those with a prior history of depression (51,52).
Additionally, cross-sectional research examining the role of stressful life events in hippocampal and mPFC anomalies in MDD is supportive of both stress models. A human study found that a greater number of stressful life events in the three-months prior to an initial MDE was linked to increased hippocampal volume reductions in males with a first MDE (53). However, a recent study demonstrated that individuals with a history of multiple MDEs exhibited smaller hippocampal and mPFC volumes, yet reported less perceived life stress, compared to those with fewer episodes (3). This suggests that while high levels of stress may have an important role in initiating hippocampal vulnerabilities amongst individuals with a first MDE, this relationship may be weaker amongst those with recurrent MDEs. However, given the cross-sectional nature of this study, it is unclear whether the hippocampal declines were driven by lower stress levels (supportive of a stress sensitization model) or are occurring independent of stress (supportive of a stress autonomy model). Future prospective work is needed to clarify whether hippocampal and mPFC volume decline related to MDD illness progression are linked to stress dependent or independent mechanisms.
While stress-related models of MDD have historically focused on relationships between recent life stressors and MDE onset, researchers have expanded these models to incorporate ELS. Consistent with the stress sensitization model, longitudinal research examining relationships between ELS, recent life stressors, and development of MDEs has shown that individuals with an ELS history are more prone to developing an MDE under less amounts of recent life stress than those without an ELS history (54,55). Further bolstering connections between ELS and illness progression, a meta-analysis found that a history of childhood maltreatment was associated with a greater probability of developing recurrent and persistent cycles of depression as well as treatment-resistance (56). Imaging studies have also demonstrated that an ELS history was associated with hippocampal and mPFC volumetric decline (57–65). Some of these studies have noted that the relationship may be independent of the presence of MDD (61,64,65). However, the link between ELS and the development of chronic courses of depression marked by greater hippocampal and mPFC damage may be mediated by the persistent manifestation of neurotoxic processes that are triggered by even minor stressors. Congruent with this idea, one study found that individuals with an ELS history, both with and without an MDD diagnosis, exhibited increased adrenocorticotropic hormone (ACTH) levels in response to a moderate laboratory stressor compared to HCs and individuals with MDD without ELS. Individuals with both an MDD diagnosis and an ELS history showed the highest stress-related ACTH response (66). Fitting stress sensitization models of MDD, there is also evidence that ELS enhances inflammatory responses to minor daily life stressors in adulthood (67). However, further longitudinal work linking ELS, recent life stress, neural deficits, and the development of recurrent depressive cycles is warranted to further clarity these relationships.
The role of HPA axis dysregulation in stress-mediated hippocampal and mPFC deficits
Animal models have established that prolonged stress can lead to HPA axis hyperreactivity and depressive behaviors along with hippocampal and mPFC abnormalities (68,69). Specifically, excessively high as well as blunted glucocorticoid receptor expression has been linked to reduced neurogenesis within the dentate gyrus (69). Higher circulating levels of glucocorticoids have also been shown to cause neuronal atrophy and dendritic retraction within the mPFC (70).
In accordance with the animal literature, a meta-analysis of clinical studies noted that individuals with MDD showed higher basal cortisol levels, particularly in the afternoon when cortisol levels should be dropping (71). However, this meta-analysis highlighted substantial variability in HPA axis functioning in MDD (71). Longitudinal studies have found that both enhanced and blunted cortisol levels predicted MDE recurrence amongst those with a prior history of MDD (72–74). Similarly, cortisol responses to a laboratory psychosocial stressor in MDD are mixed, with some showing cortisol hyperreactivity, while others hyporeactivity (75,76).
One of the factors complicating the interpretation of abnormal cortisol levels in MDD is the potential presence of glucocorticoid resistance (77). A lower cortisol output may reflect its bioavailability or it may be an indicator of reduced responsiveness to the presence of glucocorticoids (77). Additionally, even in the presence of cortisol hypersecretion, elevated cortisol may represent an attempt to counteract inflammatory responses in the presence of high glucocorticoid levels (77). Thus, a measure of glucocorticoid expression is also needed to clarify the pathophysiology of HPA axis aberrations leading to MDD-related neural abnormalities. Another confounding factor is antidepressant use, which has been found to reduce HPA axis activity (78). Thus, further research is needed to tease apart which mechanisms may contribute to different aspects of HPA dysregulation in depression.
Relatedly, HPA axis dysregulation has also been associated with hippocampal and mPFC structural abnormalities. Specifically, higher baseline cortisol levels as well as higher cortisol/dehydroepiandrosterone (DHEA) ratios have been linked to smaller hippocampal and mPFC volumes amongst HC and MDD samples (79,80), further highlighting the importance of stress (rather than the presence of MDD per se) in facilitating initial structural damage. Critically, animal models have shown that DHEA reduces the adverse effects of cortisol on the central nervous system (81), so it is likely important to consider other stress-related hormones when assessing cortisol levels.
However, in addition to these positive findings, several imaging studies in MDD have failed to find associations between cortisol and structural abnormalities (82–87). This may partly reflect evidence supporting the impact of chronic stress and persistent types of MDD on mPFC and hippocampal morphology. Importantly, these studies did not report on levels of life stress prior to neuroimaging assessment. Moreover, none of these studies examined whether relationships between structural deficits and cortisol levels vary as a function of clinical MDD illness progression markers (e.g., number of MDEs, illness duration) of MDD. Additionally, given that these studies are cross-sectional, and brain structural changes may occur long after stress exposure, prospective studies are needed to link distinct cortisol trajectories with different life stress profiles, structural deficits, and illness course. This would clarify potential HPA axis-related mechanisms leading to hippocampal and mPFC decline in MDD.
Stress-mediated inflammatory pathways to hippocampal and mPFC deficits in MDD
Another possible stress-related mechanism linking structural changes in the hippocampus and mPFC to MDD illness progression is inflammation (88). Particularly relevant to this review, animal models have demonstrated that chronic unpredictable stress promotes the production of pro-inflammatory cytokines in the hippocampus (89) and mPFC (90,91). Moreover, the stimulating effects of peripheral pro-inflammatory cytokines on brain microglia can result in reduced hippocampal neurogenesis (92). Thus, stress-mediated enhancement of central and peripheral pro-inflammatory cytokines may contribute to subsequent structural alterations. With respect to connections with MDD, a preclinical chronic stress study demonstrated that mice exhibiting anhedonic behavior (but not stress-resilient mice) showed enhanced inflammation in the mPFC (90). This suggests that some stress-related mPFC and hippocampal inflammatory processes may require the presence of depressive symptomology in addition to stress.
Accordingly, recent imaging studies in depressed individuals have reported enhanced neuroinflammation in the hippocampus and mPFC compared to HCs (93,94). Additionally, higher levels of peripheral pro-inflammatory cytokines, greater expression of inflammation-related genes, and reduced expression of neuroprotective genes, have all been associated with greater hippocampal volume decline and mPFC thinning in MDD (95–97). Enhanced peripheral inflammation has also been related to MDD recurrence/relapse, number of episodes, illness duration, and treatment non-response (98–100). However, responders to antidepressant treatment showed reduced peripheral inflammation over the course of treatment compared to nonresponders (101).
Oxidative stress as a mechanism contributing to MDD-related brain structural deficits
Closely tied to inflammatory mechanisms, preclinical studies have also demonstrated that chronic stress initiates production of oxidative stress and impairment of antioxidant defense mechanisms in the hippocampus and mPFC (102). This can result in hippocampal and mPFC cellular damage and reduced hippocampal neurogenesis (102), likely contributing to hippocampal and mPFC atrophy. With respect to MDD, increased oxidative stress, lower antioxidant levels, and imbalanced oxidant:antioxidant levels have been well documented within the hippocampus (103,104) and mPFC (104–106), resulting in associated markers of DNA/RNA damage in these regions. While few studies have examined relationships between oxidative stress and brain structural deficits in MDD, one found that lower antioxidant levels were associated with more pronounced hippocampal volume reductions in MDD (107). Consistent with neural markers of progressive illness, greater oxidative stress, lower antioxidant, and greater oxidative stress-related DNA/RNA damage have been linked to clinical markers of MDD illness progression, including greater chronicity, more MDEs, and treatment resistance (98,108–113). Conversely, response to antidepressant treatment has been shown to normalize the oxidant: antioxidant imbalance seen in MDD (108). Findings to date suggest a psychosocial stress-induced oxidative stress pathway that contributes to hippocampus and mPFC structural deficits in MDD.
Serotonin dysfunction contribute to brain structural decline and MDD illness progression
Neurotransmitter disturbances, such as serotonin dysfunction, are likely prominent pathways to illness progression in MDD. Preclinical chronic mild stress models of depression show reduced serotonin concentration, expression, release, and neurotransmission in both the hippocampus and mPFC (114–116). Interestingly, a study comparing stress-resilient mice versus those exhibiting anhedonic behavior noted that stress-elevated expression of 5HT2A receptors within the mPFC occurred in both groups of mice, whereas additional 5-HT transporter elevations occurred exclusively in the depression-susceptible mice (90). This suggests that some stress-related serotonergic abnormalities are independent of the development of depressive behavior, whereas others were specific to anhedonia development.
While the animal literature has demonstrated clear pathways to serotonin dysregulation within specific brain regions that are linked to depressive behaviors, human imaging findings in MDD have been more mixed (117–119). There has been evidence of decreased 5HT1A mRNA levels within the hippocampus of individuals with MDD compared to HCs (117). Conversely, two recent meta-analyses failed to find significant alterations in serotonin transporter binding/availability in the hippocampus and mPFC in MDD (118,119). Discrepancies may be due to clinical heterogeneity, including illness stage, particularly since greater serotonin dysfunction has been described amongst individuals with MDD who have experienced a greater number of past MDEs (120).
Few studies have examined associations between hippocampal/mPFC volume reductions and serotonin system dysfunction. Recent longitudinal studies have linked smaller hippocampal volumes to MDD onset among individuals with ELS and carrying the s allele of the serotonin transporter gene, which is associated with reduced serotonin uptake (44,45). One of these studies noted that greater parental display of positive behaviors was protective against hippocampal volume reductions amongst those carrying the s allele. However, these connections have yet to be explored in conjunction with MDD illness progression markers, which is an important area for future research given the connection between greater serotonin dysfunction and number of MDEs. Additionally, more studies are needed to clarify which serotonergic disturbances are consequences of stress that do not necessarily lead to MDD, and which result in MDD development.
Glutamatergic contributions to MDD-related brain structural decline
The glutamate system is also highly impacted by stress. Chronic stress disrupts glutamate release, glutamate clearance from the synapse, and glutamate transmitter expression, but the direction of the effects is region-specific (121). Some studies have reported enhanced chronic stress-related glutamate release and glutamate receptor expression, but reduced glutamate clearance/metabolism in the hippocampus (122,123). A study that directly compared stress-resilient to stress-susceptible mice displaying depressive behaviors indicated that enhanced hippocampal glutamate expression was unique to depression-susceptible mice (122). A different pattern has been described in the mPFC, with some reporting reduced chronic stress-related glutamate receptor expression, which has been speculated as a potential protective mechanism against excessive glutamate signaling and excitoxicity (e.g., 124–125). However, these studies did not examine potential differences between stress-resilient versus MDD susceptible mice. In contrast to these results, reduced glutamate clearance (and thus enhanced accumulation of glutamate in the mPFC) has been shown to produce anhedonic behaviors (126). Accordingly, increased chronic-stress related glutamate expression in the hippocampus and mPFC may be unique to those who develop the depressive phenotype.
With respect to in vivo human imaging work in MDD, magnetic resonance spectroscopy (MRS) studies have provided evidence of glutamate dysfunction in MDD (e.g., 127,128). However, the glutamate measures are primarily intracellular, so it is difficult to interpret relationships with glutamate neurotransmission. Additionally, many of these studies have reported on combined glutamate and glutamine (Glx) metabolite levels or metabolite ratios, further complicating interpretations. Generally, studies have found reduced Glx/glutamate levels in the mPFC and hippocampus in MDD, with those having a more chronic course showing greater declines compared to individuals experiencing a first episode (127,128). While difficult to interpret, reduced glutamate levels may reflect cellular abnormalities associated with decreased glutamate availability resulting from hyperactive glutamate neurotransmission (129). While yet to be applied to MDD samples, a human imaging study noted associations between increased extracellular glutamate levels and hippocampal volume reductions (130).
There is also emerging evidence of impaired glutamate-glutamine cycling in the mPFC and hippocampus amongst those with MDD. Glutamate is produced in neurons from glutamine by glutamase. Astrocytes uptake glutamate once it is released from the synaptic terminal and convert glutamate back to glutamine via glutamine synthase. A preclinical study showed that inhibiting glutamine synthase and glutamine transport in the mPFC led to the development of depressive behavior (131). Additionally, another preclinical study showed that enhanced stimulation of glutamate cycling in the mPFC via three different classes of antidepressants was associated with the onset of rapid antidepressant action (132). Several human postmortem studies have also shown reductions in the expression of several components critical to astrocyte function, largely in the mPFC, but also some evidence in the hippocampus, suggesting impaired glutamate-glutamine cycling (133). Together, these findings indicate that glutamate dysregulation likely has a strong role in neural and clinical markers of MDD illness progression MDD.
GABAergic disturbances associated with brain structural decline and chronic MDD
GABA is also likely centrally involved in hippocampal and mPFC abnormalities in MDD (134). With respect to preclinical models of depression, chronic stress has been shown to decrease GABA expression in the hippocampus and mPFC (135,136). Serotonin is known to regulate the GABAergic system (137). Therefore, when the serotonin system is dysregulated, this can contribute to reduced GABAergic inhibitory control, resulting in enhanced glutamate and HPA axis reactivity, and ultimately reduced hippocampal neurogenesis (124,138).
Several human MRS studies have reported lower mPFC GABA levels in MDD (139–141). Reduced mPFC GABA levels in MDD have been associated with smaller hippocampal volumes (139) and treatment resistance (142,143). However, antidepressants known to target the monoaminergic system have also shown to counteract GABAergic abnormalities (134). This suggests that GABA may have a key role in multiple pathways leading to hippocampal and mPFC structural deficits in MDD and treatment resistance.
A common pathway leading to MDD illness progression-related hippocampal and mPFC volume reductions
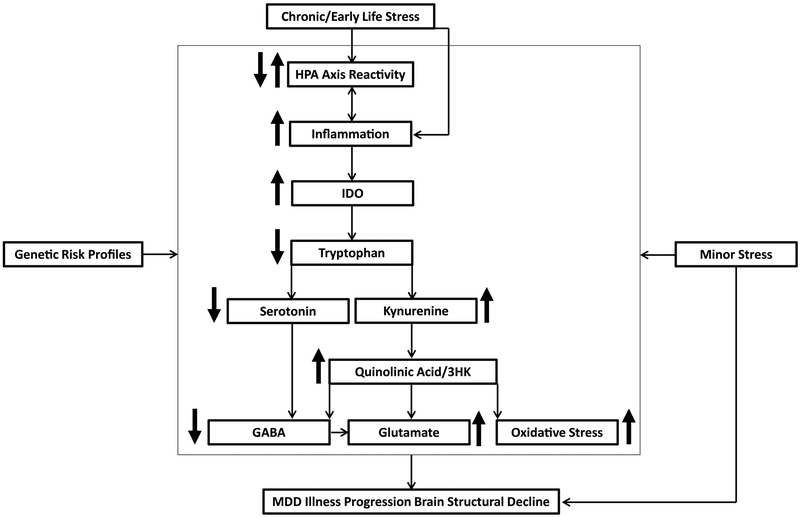
Based on the above evidence, it is likely that these neurotoxic processes interact to form multiple pathways leading to hippocampal and mPFC volume decline and MDD illness progression. One possible common pathway involves chronic stress triggering HPA axis dysfunction and enhanced production of cell-mediated immune cytokines, which activate indoleamine 2,3-dioxygenase (IDO;68). IDO is an enzyme that catabolizes tryptophan leading to serotonin depletion and production of kynurenine. Kynurenine is then converted to neuroprotective metabolites (kynurenic acid (KynA)) or neurotoxic metabolites (3-hydroxykynurenine (3HK), and quinolinic acid (QA)) (68). The production of neurotoxic 3HK and QA leads to increased glutamate and oxidative stress as well as reduced GABAergic inhibitory control. Given the connection between serotonin and GABA, reduced serotonin also contributes to decreased GABA expression. Together, this pathway may trigger hippocampal and mPFC cellular damage and volume reductions (138,144,145). In line with stress sensitization models, with each successive MDE, it may take smaller amounts of stress to trigger these pathways and produce further brain structural decline. Early life stress and genetic risk profiles may program this vulnerability early on by enhancing psychological and biological reactivity to minor stressful life events (Figure 3). In support of this pathway, a study found that a lower KynA/3HK ratio correlated with reduced hippocampal volumes in MDD and partially mediated the relationship between MDD and mPFC cortical thinning (146,147). Additionally, preclinical evidence suggests that neurotoxic dorsal hippocampal kynurenine metabolism may drive depressive behavior when inflammation is enhanced (148).
Figure 3. A Stress-Mediated Neurotoxic Pathway Leading to Chronic MDD Hippocampal and mPFC Volume Reduction.
Chronic stress sets off a cascade of neurotoxic processes. The presence of chronic stress triggers dysregulation of the hypothalamic pituitary adrenal (HPA) axis, which can either be enhanced or blunted due to glucocorticoid resistance. This HPA axis dysregulation triggers the immune system, leading to enhanced inflammation (stress can also have a direct effect on inflammation not mediated by the HPA axis). In turn, enhanced inflammation can produce further dysregulation of the HPA axis. Pro-inflammatory cytokines then activate indoleamine 2,3-dioxygenase (IDO), an enzyme that catabolizes tryptophan, which leads to serotonin depletion and the production of kynurenine. Kynurenine can be converted to neurotoxic 3HK and quinolinic acid, which can increase glutamate release and oxidative stress, as well as reduce GABA inhibitory control. Dysregulated serotonin levels lead to further reductions in GABA inhibitory control. This reduction in GABA inhibitory control can lead to further glutamate release. With successive major depressive episodes, even minor levels of stress can trigger this pathway, leading to further hippocampal and mPFC volume decline. Genetic risk profiles can also increase vulnerability to chronic stress triggering of these neurotoxic pathways to illness progression and brain structural decline.
Conclusions
The proposed model provides numerous directions for future research. Much of the preclinical literature has focused on the impact of chronic stress on the hippocampus and mPFC, without directly comparing animals that did not exhibit depressive behaviors (resilient phenotype) versus those exhibiting depressive behaviors (susceptible phenotype). Those studies that directly compared the two groups have provided some promising leads on identifying which neurotoxic processes affecting hippocampal and mPFC structure are unique to the development of depression. This work can inform which neurotoxic components to target in future prospective studies of MDD and brain structural decline. Prospective studies focusing on “at risk” samples by virtue of living in highly stressful environments, would address which stress-related neurotoxic processes affecting hippocampal and mPFC structure are relevant to MDD onset and which processes are implicated at different stages of MDD illness. Additionally, these studies would clarify temporal relationships between neurotoxic processes, hippocampal and mPFC structural aberrations, and MDD illness progression. This would allow for a more precise identification of stress-related mechanisms signifying biomarkers of MDD, which could lead to more effective treatment targets.
Supplementary Material
Acknowledgements
E.LB. was supported by a Kaplen Fellowship (Harvard Medical School), the Adam J. Corneel Young Investigator Award (McLean Hospital), and a Klingenstein Third Generation Foundation Postdoctoral Fellowship, M.T.T was supported by R00 MH102355 and R01 MH108605 grants from the National Institute of Mental Health (NIMH), and D.A.P. was supported by R37 MH068376, R01 MH101521, 1R01 MH108602, and R01 MH095809. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
E.L.B has no conflicts of interest to disclose. In the past three years, M.T.T. has served as a paid consultant to NeuroCog Trials, Avanir Pharmaceuticals, and Blackthorn Therapeutics. Over the past 3 years, D.A.P. has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science, and Takeda Pharmaceuticals, for activities unrelated to the current review. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
References
- 1.Monroe SM, Harkness K (2011): Recurrence in major depression: A conceptual analysis. Psychol Rev 118:655–674. [DOI] [PubMed] [Google Scholar]
- 2.McKinnon MC, Yucel K, Nazarov A, MacQueen GM (2009): A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Treadway MT, Waskom ML, Dillon DG ,Holmes AJ, Park MTM, Chakravarty MM, et al. (2015): Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 77: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Videbech P, Ravnkilde B (2004): Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966. [DOI] [PubMed] [Google Scholar]
- 5.Campbell S, Marriott M, Nahmias C, MacQueen GM (2004): Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry 161:598–607. [DOI] [PubMed] [Google Scholar]
- 6.Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS (2009): Brain volume abnormalities in major depressive disorder:A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 30:3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole J, Costafreda SG, McGuffin P, Fu CHY (2011): Hippocampal atrophy in first episode depression: A meta-analysis of magnetic resonance imaging studies. J Affect Disord 134:483–487. [DOI] [PubMed] [Google Scholar]
- 8.Kempton MJ (2011):Structural Neuroimaging Studies in Major Depressive Disorder. Arch Gen Psychiatry 68:675–690. [DOI] [PubMed] [Google Scholar]
- 9.Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM (2012): Magnetic resonance imaging studies in unipolar depression: Systematic review and meta-regression analyses. Eur Neuropsychopharmacol 22:1–16. [DOI] [PubMed] [Google Scholar]
- 10.Bora E, Fornito A, Pantelis C, Yücel M (2012): Gray matter abnormalities in major depressive disorder: A meta-analysis of voxel based morphometry studies. J Affect Disord 138: 9–18. [DOI] [PubMed] [Google Scholar]
- 11.Du MY, Wu QZ, Yue Q, Li J, Liao Y, Kuang WH et al. (2012):Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry 36:11–16. [DOI] [PubMed] [Google Scholar]
- 12.Lai CH (2013): Gray matter volume in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Psychiatry Res Neuroimaging 211: 37–46. [DOI] [PubMed] [Google Scholar]
- 13.Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, et al. (2016): Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp 37:1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise T, Radua J, Via E., Cardoner N, Abe O, Adams TM, et al. (2017). Common and distinct patterns of grey-matter volume alteration on major depression and bipolar disorder: Evidence from voxel-based meta-analysis. Mol Psychiatry 22:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han K- M, Won E, Sim Y, Tae W-S (2016): Hippocampal subfield analysis in medication-naieve female patients with major depressive disorder. J Affect Disord 194: 21–29. [DOI] [PubMed] [Google Scholar]
- 16.Malykhin NV, Carter R, Seres P, Coupland NJ (2010): Structural changes in the hippocampus in major depressive disorder: Contributions of disease and treatment. J Psychiatry Neurosci 35: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frodl T, Jager M, Smajstrlova I, Born C, Bottlender R, Palladino T, et al. , (2008): Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: A 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci 33: 423–430. [PMC free article] [PubMed] [Google Scholar]
- 18.van Tol M-J, van der Wee NJA, van den Heuvel OA, Nielen MMA, Demenescu LR, Aleman A, et al. , (2010): Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry 67: 1002–10011. [DOI] [PubMed] [Google Scholar]
- 19.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. (2003): Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 100:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronmüller KT, Pantel J, Köhler S, Victor D, Giesel F, Magnotta VA, et al. (2008): Hippocampal volume and 2-year outcome in depression. Br J Psychiatry 192:472–473. [DOI] [PubMed] [Google Scholar]
- 21.Yucel K, McKinnon MC, Chahal R, Taylor VH, MacDonald K, Joffe R, et al. (2008): Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology 33:3157–3163. [DOI] [PubMed] [Google Scholar]
- 22.Stratmann M, Konrad C, Kugel H, Krug A, Schoning S, Ohrmann P, et al. (2014): Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS One 9 (7):e102692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisse LEM, Biessels GJ, Stegenga BT, Kooistra M, van der Veen PH, Zwanenburg JJM, et al. (2015): Major depressive episodes over the course of 7 years and hippocampal subfield volumes at 7 tesla MRI: The PREDICT-MR study. J Affect Disord 175:1–7. [DOI] [PubMed] [Google Scholar]
- 24.Bludau S, Bzdok D, Gruber O, Kohn N, Riedl V, Sorg C, et al. (2016): Medial prefrontal aberrations in major depressive disorder revealed by cytoarchitectonically informed voxel-based morphometry. Am J Psychiatry 173:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathias SR, Knowles EEM, Kent JW, McKay DR, Curran JE, de Almeida MAA, et al. (2016): Recurrent major depression and right hippocampal volume: A bivariate linkage and association study. Hum Brain Mapp 37:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996): Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999): Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19:5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheline YI, Gado MH, Kraemer HC (2003): Untreated depression and hippocampal volume loss. Am J Psychiatry 160:1516–1518. [DOI] [PubMed] [Google Scholar]
- 29.Serra-Blasco M, Portella MJ, Gómez-Ansón B, de Diego-Adelino X, Vives-Gilabert Y, Puigdemont D, et al. (2013): Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br J Psychiatry 202:434–440. [DOI] [PubMed] [Google Scholar]
- 30.Travis S, Coupland NJ, Silversone PH, Huang Y, Fujiwara E, Carter R, et al. (2015): Dentate gyrus volume and memory performance in major depressive disorder. J Affect Disord 172:159–164. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh MH, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC (2002): Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry 17:519–525. [DOI] [PubMed] [Google Scholar]
- 32.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, et al. (2004): Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry 65 492–499. [DOI] [PubMed] [Google Scholar]
- 33.MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R (2008): Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry 64:880–883. [DOI] [PubMed] [Google Scholar]
- 34.Hoogenboom W, Perlis R (2012): Feasibility of studying brain morphology in major depressive disorder with structural magnetic resonance imaging and clinical data from the electronic medical record: Psychiatry Res 211:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC (2014): Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry 22:1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P (2015): A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int J Neuropsychopharmacol. 18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra-Blasco M, de Diego-Adeliño J, Vives-Gilabert Y, Trujols J, Puigdemont D, Carceller-Sindreu M, et al. (2016): Naturalistic course of major depressive disorder predicted by clinical and structural neuroimaging data: a 5-Year Follow-Up. Depress Anxiety 33:1055–1064. [DOI] [PubMed] [Google Scholar]
- 38.Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, et al. (2008): Depression-related variation in brain morphology over 3 Years. Arch Gen Psychiatry 65:1156–1165. [DOI] [PubMed] [Google Scholar]
- 39..Chen MC, Hamilton JP, Gotlib IH (2010): Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry 67:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao U, Chen L, Bidesi AS, Shad MU, Thomas MA, Hammen CL (2011): Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry 67: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amico F, Meisenzahl E, Koutsouleris N, Reiser M, Moller HJ, Frodl T (2011): Structural MRI correlates for vulnerability and resilience to major depressive disorder. J Psychiatry Neurosci 36:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carballedo A, Lisiecka D, Fagan A, Saleh K, Ferguson Y, Connolly G, et al. (2012): Early life adversity is associated with brain changes in subjects at family risk for depression. World J Biol Psychiatry 13:569–578. [DOI] [PubMed] [Google Scholar]
- 43.Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, et al. (2012): Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci 32:18087–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Little K, Olsson CA, Whittle S, Youssef GJ, Byrne ML, Simmons JG, et al. (2014): Association between serotonin transporter genotype, brain structure and adolescent-onset major depressive disorder: A longitudinal prospective study. Transl Psychiatry 4: e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little K, Olsson CA, Youssef GJ, Whittle S, Simmons JG, Yucel M, et al. (2015): Linking the serotonin transporter gene, family environments, hippocampal volume and depression onset: A prospective imaging gene × environment analysis. J Abnorm Psychol 124:834–849. [DOI] [PubMed] [Google Scholar]
- 46.Jayatissa MN, Hennigsen K, Nikolajsen G, West MJ, Wiborg O (2010): A reduced number of hippocampal granule cells does not associate with an anhedonia-like phenotype in a rat chronic mild stress model of depression. Stress, 13: 95–105. [DOI] [PubMed] [Google Scholar]
- 47.Delgado y Palacios R, Campo A, Henningsen K, Verhoye M, Poot D, Dijkstra J, et al. (2011): Magnetic resonance imaging and spectroscopy reveal differential hippocampal changes in anhedonic and resilient subtypes of the chronic mild stress rat model. Biol Psychiatry 70: 449–457. [DOI] [PubMed] [Google Scholar]
- 48.Papagni SA, Benetti S, Arulanantham S, Mccrory E, McGuire P, Mechelli A (2011): Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress, 14, 227–232. [DOI] [PubMed] [Google Scholar]
- 49.Kanzari A, Bourier-Lucas C, Freyssin A, Abrous DN, Haddjeri N, Lucas G (2018): Inducing a long-term potentiation in the dendate gyrus is sufficient to produce rapid antidepressant effects. Mol Psychiatry 23:587–596. [DOI] [PubMed] [Google Scholar]
- 50.Monroe SM, Harkness KL (2005): Life Stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychol Rev 112: 417–445. [DOI] [PubMed] [Google Scholar]
- 51.Ormel J, Oldehinkel AJ, Brilman EI (2001): The interplay of neuroticism , difficulties, and life events in the etiology of major and subsyndromal, first and recurrent depressive episodes in later life. Am J Psychiatry 158:885–891. [DOI] [PubMed] [Google Scholar]
- 52.Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S (2011):Severe and nonsevere events in first onsets versus recurrences of depression. Evidence for stress sensitization. J Abnorm Psychol 120:142–154. [DOI] [PubMed] [Google Scholar]
- 53.Kronmüller KT, Pantel J, Götz B, Kohler S, Victor D, Mudt C, et al. (2008): Life events and hippocampal volume in first-episode major depression. J Affect Disord. 110:241–247. [DOI] [PubMed] [Google Scholar]
- 54.Hammen C, Henry R, Daley SE (2000): Depression and sensitization to stressors among young women as a function of childhood adversity. J Consult Clin Psychol 68:782–787. [PubMed] [Google Scholar]
- 55.Oldehinkel AJ, Ormel J, Verhulst FC, Nederhof E (2014): Childhood adversities and adolescent depression: A matter of both risk and resilience. Dev Psychopathol 26:1067–1075. [DOI] [PubMed] [Google Scholar]
- 56.Nanni V, Uher R, Danese A (2012):Childhood maltreatment preditcs unnfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry 169: 141–151. [DOI] [PubMed] [Google Scholar]
- 57.Vythilingham M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. (2002): Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC (2009): Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One 4 (3):e4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frodl T, Reinhold E, Koutsouleris N, Donohoe G., Bondy B, Reiser M, et al. (2010): Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 35:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM (2010): Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res.44:799–807. [DOI] [PubMed] [Google Scholar]
- 61.Opel N, Redlich R, Zwanzger P, Grotegerd D, Arolt V, Heindel W, et al. (2014): Hippocampal atrophy in major depression: A function of childhood maltreatment rather than diagnosis. Neuropsychopharmacology 39:2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerritsen L, Van Velzen L, Schmaal L, van der Graaf Y, van der Wee N, van Tol M- J, et al. (2015): Childhood maltreatment modifies the relationship of depression with hippocampal volume. Psychol Med 45:3517–3526. [DOI] [PubMed] [Google Scholar]
- 63.Colle R, Segawa T, Chupin M, Tran Dong MNT, Hardy P, Falissard B, et al. (2017): Early life adversity is associated with a smaller hippocampus in male but not female depressed in-patients: A case-control study. BMC Psychiatry 17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaney A, Carballedo A, Amico F, Fagan A, Skokauskas N, Meaney J., et al. (2014): Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J Psychiatry Neurosci 39: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teicher MH, Anderson CM, Polcari A (2012): Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109: E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. (2000). Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284: 592–597. [DOI] [PubMed] [Google Scholar]
- 67.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK (2012): Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moylan S, Maes M, Wray NR, Berk M (2013): The neuroprogressive nature of major depressive disorder: Pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18: 595–606. [DOI] [PubMed] [Google Scholar]
- 69.Saaltink DJ, Vreugdenhil E Stress, glucocorticoid receptors, and adult neurogenesis: A balance between excitation and inhibition? (2014): Cell Mol Life Sci 71:2499–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willner P, Scheel-Krüger J, Belzung C (2013): The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 37:2331–2371. [DOI] [PubMed] [Google Scholar]
- 71.Stetler C, Miller GE (2011): Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom Med 73:114–126. [DOI] [PubMed] [Google Scholar]
- 72.Bockting CLH, Lok A, Visser I, Assies J, Koeter MW, Schene AH (2012): Lower cortisol levels predict recurrence in remitted patients with recurrent depression: A 5.5 year prospective study. Psychiatry Res 200: 281–287. [DOI] [PubMed] [Google Scholar]
- 73.Lok A, Mocking RJT, Ruhé HG, Visser I, Koeter MWJ, Assies J, et al. (2012): Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology. 37: 892–902. [DOI] [PubMed] [Google Scholar]
- 74.Vreeburg SA, Hoogendijk WJG, DeRijk RH, van Dyck R, Smit JH, Zitman FG, et al. (2013). Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology. 38:1494–1502. [DOI] [PubMed] [Google Scholar]
- 75.Zorn JV., Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH (2017): Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 77: 25–36. [DOI] [PubMed] [Google Scholar]
- 76.Morris MC, Rao U (2014): Cortisol response to psychosocial stress during a depressive episode and remission. Stress 17:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raison CL, Miller AH (2003): When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160: 1554–1565. [DOI] [PubMed] [Google Scholar]
- 78.McKay MS, Zakzanis KK (2010): The impact of treatment on HPA axis activity in unipolar major depression. J Psychiatr Res 44: 183–192. [DOI] [PubMed] [Google Scholar]
- 79.Travis SG, Coupland NJ, Hegadoren K, Silverstone PH, Huang Y, Carter R, et al. (2016): Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J Affect Disord 201: 34–41. [DOI] [PubMed] [Google Scholar]
- 80.Jin RO, Mason S, Mellon SH, Epel ES, Reus VI, Mahan L, et al. (2016): Cortisol/DHEA ratio and hippocampal volume: A pilot study in major depression and healthy controls. Psychoneuroendocrinology 72: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH (2009): Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30:65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaymak SU, Demir B, Şentürk S, Tatar I, Aldur MM, Uluǧ B (2010): Hippocampus, glucocorticoids and neurocognitive functions in patients with first-episode major depressive disorders. Eur Arch Psychiatry Clin Neurosci 260: 217–223. [DOI] [PubMed] [Google Scholar]
- 83.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N (2004): A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 161: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 84.Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. (2004): Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol Psychiatry 56: 101–112. [DOI] [PubMed] [Google Scholar]
- 85.Colla M, Kronenberg G, Deuschle M, Meichel K, Hagen T, Bohrer M, et al. (2007): Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res 41: 553–560. [DOI] [PubMed] [Google Scholar]
- 86.Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJG, Penninx BWJH, Geerlings MI (2011): Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes-the SMART Medea study. Biol Psychiatry 70: 373–380. [DOI] [PubMed] [Google Scholar]
- 87.Schuhmacher A, Mössner R, Jessen F, Scheef L, Block W, Belloche AC, et al. (2012): Association of amygdala volumes with cortisol secretion in unipolar depressed patients. Psychiatry Res - Neuroimaging 202: 96–103. [DOI] [PubMed] [Google Scholar]
- 88.Slavich GM, Irwin MR (2014): From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull 140: 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. (2016): Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry 80:12–22. [DOI] [PubMed] [Google Scholar]
- 90.Couch Y, Anthony DC, Dolgov O, Revischin A, Festoff B, Santos AI et al. (2013). Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav Immun 29:136–146. [DOI] [PubMed] [Google Scholar]
- 91.Pan Y, Chen XY, Zhang QY, Kong LD (2014): Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41:90–100. [DOI] [PubMed] [Google Scholar]
- 92.Chesnokova V, Pechnick RN, Wawrowsky K (2016): Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun 58: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. (2015): Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, et al. (2018). Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol Psychiatry 83:61–69. [DOI] [PubMed] [Google Scholar]
- 95.Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, et al. (2012): Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: High IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry 2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Velzen LS, Schmaal L, Milaneschi Y, van Tol MJ, van der Wee NJA, Veltman DJ, et al. (2017): Immunometabolic dysregulation is associated with reduced cortical thickness of the anterior cingulate cortex. Brain Behav Immun 60: 361–368. [DOI] [PubMed] [Google Scholar]
- 97.Savitz J, Frank MB, Victor T, Bebak M, Bellgowan PS, McKinney BA, et al. (2013):Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun 31: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sowa-Kućma M, Styczeń K, Siwek M, Misztak P, Nowak RJ, Dudek D, et al. (2018): Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: Effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog Neuro-Psychopharmacology Biol Psychiatry. 81:372–383. [DOI] [PubMed] [Google Scholar]
- 99.Dunjic-Kostic B, Ivkovic M, Radonjic NV, Petronijevic ND, Pantovic M, Damjanovic A, et al. (2013): Melancholic and atypical major depression - Connection between cytokines, psychopathology and treatment. Prog Neuro-Psychopharmacology Biol Psychiatry 43: 1–6. [DOI] [PubMed] [Google Scholar]
- 100.Eller T, Vasar V, Shlik J, Maron E (2008): Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry 32: 445–450. [DOI] [PubMed] [Google Scholar]
- 101.Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. (2017): Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Filipović D, Todorović N, Bernardi RE, Gass P (2017): Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive- and anxiety-like symptoms. Brain Struct Funct 222:1–20. [DOI] [PubMed] [Google Scholar]
- 103.Che Y, Wang JF, Shao L, Young LT (2010): Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J Psychiatry Neurosci 5:296–302. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meyer JH, Wilson AA, Sagrati S, Miler L., Rusjan P, Bloomfield PM, et al. (2009): Brain monoamine oxidase a binding in major depressive disorder. Arch Gen Psychiatry 66:1304–1312. [DOI] [PubMed] [Google Scholar]
- 105.Michel TM, Frangou S, Thiemeyer D, Camara S, Jecel J, Nara K, et al. (2007): Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder-a postmortem study. Psychiatry Res 151: 145–150. [DOI] [PubMed] [Google Scholar]
- 106.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011). Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol.14:123–130. [DOI] [PubMed] [Google Scholar]
- 107.Lindqvist D, Mueller S, Mellon SH, Su Y, Epel ES, Reus VI, et al. (2014). Peripheral antioxidant markers are associated with total hippocampal and CA3/dendate gyrus volume in MDD and healthy controls-preliminary findings. Psychiatry Research: Neuroimaging 224: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baek S-E, Lee G-J, Rhee C-K, Rho D-Y, Kim D-H, Huh S, et al. (2016). Decreased total antioxidant activity in major depressive disorder patients non-responsive to antidepressant treatment. Psychiatry Investig 13:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Śliwiński T (2015): Elevated level of DNA damage and impaired repair of oxidative DNA damage in patients with recurrent depressive disorder. Med Sci Monit. 21:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Czarny P, Kwiatkowski D, Toma M, Kubiak J, Silwinska A, Talarowska M, et al. (2017): Impact of single nucleotide polymorphisms of base excision repair genes on DNA damage and efficiency of DNA repair in recurrent depression disorder. Mol Neurobiol 54:4150–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jorgensen A, Krogh J, Miskowiak K, Bolwig TG, Kessing LV, Fink-Jensen A, et al. (2013): Systemic oxidatively generated DNA/RNA damage in clinical depression: Associations to symptom severity and response to electroconvulsive therapy. J Affect Disord 149: 355–362. [DOI] [PubMed] [Google Scholar]
- 112.Stefanescu C, Ciobica A (2012): The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord 143: 34–38. [DOI] [PubMed] [Google Scholar]
- 113.Talarowska M, Orzechowska A, Szemraj J, Su K- P, Maes M, Gałecki P (2014): Manganese superoxide dismutase gene expression and cognitive functions in recurrent depressive disorder. Neuropsychobiology.70:23–28. [DOI] [PubMed] [Google Scholar]
- 114.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z (2005): Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res 161:45–59. [DOI] [PubMed] [Google Scholar]
- 115.Luo DD, An SC, Zhang X (2008): Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull 77:8–12. [DOI] [PubMed] [Google Scholar]
- 116.Harro J, Tõnissaar M, Eller M, Kask A, Oreland L (2001): Chronic variable stress and partial 5-HT denervation by parachloroamphetamine treatment in the rat: Effects on behavior and monoamine neurochemistry. Brain Res 899: 227–239. [DOI] [PubMed] [Google Scholar]
- 117.López-Figueroa AL, Norton CS, López-Figueroa MO, Armellini-Dodel D, Burke S, Akil H et al. (2004): Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry 55:225–233. [DOI] [PubMed] [Google Scholar]
- 118.Gryglewski G, Lanzenberger R, Kranz GS, Cumming P (2014): Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab 34: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kambeitz JP, Howes OD (2015): The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. J Affect Disord 186: 358–366. [DOI] [PubMed] [Google Scholar]
- 120.Maes M, Ringel K, Kubera M, Berk M, Rybakowski J (2012): Increased autoimmune activity against 5-HT: A key component of depression that is associated with inflammation and activation of cell-mediated immunity, and with severity and staging of depression. J Affect Disord 136: 386–392. [DOI] [PubMed] [Google Scholar]
- 121.Grimm S, Luborzewski A, Schubert F, Merkyl A, Kronenberg G, Colla M, et al. (2012): Region-specific glutamate changes in patients with unipolar depression. J Psychiatr Res 46:1059–1065. [DOI] [PubMed] [Google Scholar]
- 122.Sun H, Su R, Zhang X, Wen J, Yao D, Gao X, et al. (2017): Hippocampal GR- and CB1-mediated mGluR5 differentially produces susceptibility and resilience to acute and chronic mild stress in rats. Neuroscience. 357: 295–302. [DOI] [PubMed] [Google Scholar]
- 123.De Vasconcellos-Bittencourt APS, Vendite DA, Nassif M, Crema LM, Frozza R, Thomazi AP, et al. (2011): Chronic stress and lithium treatments alter hippocampal glutamate uptake and release in the rat and potentiate necrotic cellular death after oxygen and glucose deprivation. Neurochem Res 36:793–800. [DOI] [PubMed] [Google Scholar]
- 124.Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA (2017): Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience 346: 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012): Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73: 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.John CS, Smith KL, Van’T Veer A, Gompf HS, Carlezon WA, Cohen BM, et al. (2012): Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology.37: 2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Diego-Adeliño J, Portella MJ, Gómez-Ansón B, Lopez-Moruelo O, Serra-Blasco M. Yolanda V, et al. (2013): Hippocampal abnormalities of glutamate/glutamine, N-acetylaspartate and choline in patients with depression are related to past illness burden. J Psychiatry Neurosci 38:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Portella MJ, de Diego-Adeliño J, Gómez-Ansón B, Morgan-Ferrando R, Vives Y, Puigdemont D, et al. (2011): Ventromedial prefrontal spectroscopic abnormalities over the course of depression: A comparison among first episode, remitted recurrent and chronic patients. J Psychiatr Res 45:427–434. [DOI] [PubMed] [Google Scholar]
- 129.Croarkin PE, Nakonezny PA, Husain MM, Melton T, Buyukdura JS, Kennard BD, et al. (2013): Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder. JAMA Psychiatry 70:291–299. [DOI] [PubMed] [Google Scholar]
- 130.Cavus I, Pan JW, Hetherington HP, Walid A- S, Hitten PS, Vives KP, et al. (2008): Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia.49: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 131.Lee Y, Son H, Kim G, Kim S, Lee DH, Roh GS, et al. (2013): Glutamate deficiency in the prefrontal cortex increases depressive-like behaviors in male mice. J Psychiatry Neurosci, 38, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chowdry GM, Zhang J, Thomas M, Banas M, Ma X, Pittman B, et al. (2017): Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant effects. Molecular Psychiatry, 22, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sancora G, Banasr M (2013): From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biological Psychiatry, 73: 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luscher B, Shen Q, Sahir N (2011): The GABAergic deficit hypothesis of major depressive disorder Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hemanth Kumar BS, Mishra SK, Rana P, Singh S, Khushu S (2012): Neurodegenerative evidences during early onset of depression in CMS rats as detected by proton magnetic resonance spectroscopy at 7T. Behav Brain Res 232: 53–59. [DOI] [PubMed] [Google Scholar]
- 136.Ma K, Xu A, Cui S, Sun MR, Xue YC, Wang JH (2016): Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl Psychiatry 6: e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feng J, Cai X, Zhao J, Yan Z (2001): Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci 21: 6502–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rubio-Casillas A, Fernández-Guasti A (2016): The dose makes the poison: From glutamate-mediated neurogenesis to neuronal atrophy and depression. Rev Neurosci. 27:599–622. [DOI] [PubMed] [Google Scholar]
- 139.Abdallah CG, Jackowski A, Sato JR, Mao X, Kang G, Cheema R, et al. (2015): Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. 25:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. (2012): Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: Relationship to anhedonia. Arch Gen Psychiatry 69:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007): Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- 142.Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ (2010): Evidence of Cortical Inhibitory Deficits in Major Depressive Disorder. Biol Psychiatry 67:458–464. [DOI] [PubMed] [Google Scholar]
- 143.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. (2009): Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Capuron L, Miller AH.(2011): Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol Ther 130: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lugo-Huitron R, Muniz PU, Pineda P, Pedrazza-Chaverri J, Rios C, Perex-de la Cruz V (2013): Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid Med Cell Longev 104024: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowen PSF, et al. (2015): Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, et al. (2016): Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 53: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC (2016): Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 6:e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.