Abstract
Introduction:
Current evidence indicates mitochondrial dysfunction in humans with obesity. Acute exercise appears to enhance mitochondrial function in muscle of non-obese humans, but its effects on mitochondrial function in muscle of humans with obesity are not known. We sought to determine whether acute aerobic exercise stimulates mitochondrial function in subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria in humans with obesity.
Methods:
We assessed maximal ATP production rate (MAPR) and citrate synthase (CS) activity in isolated SS and IMF mitochondria from subjects with BMI < 27 kg/m2 (median age 25 years, (interquartile range (IQR)) 22–39 years) and subjects with BMI > 32 kg/m2 (median age 29 years, IQR 20–39 years) before and 3 hours after a 45-min cycling exercise at an intensity corresponding to 65% heart rate reserve. SS and IMF mitochondria were isolated from muscle biopsies using differential centrifugation. MAPR and CS activity were determined using luciferase- and spectrophotometric enzyme-based assays, respectively.
Results:
Exercise increased MAPR in IMF mitochondria in both non-obese subjects and subjects with obesity (P < 0.05), but CS specific activity did not change in either group (P > 0.05). Exercise increased MAPR supported by complex II in SS mitochondria, in both groups (P < 0.05), but MAPR supported by complex I or palmitate did not increase by exercise in the subjects with obesity (P > 0.05). CS specific activity increased in SS mitochondria in response to exercise only in non-obese subjects (P < 0.05).
Conclusions:
In non-obese humans, acute aerobic exercise increases MAPR in both SS and IMF mitochondria. In humans with obesity, the exercise increases MAPR in IMF mitochondria, but this response is less evident in SS mitochondria.
Keywords: subsarcolemmal mitochondria, intermyofibrillar mitochondria, citrate synthase, adiposity
INTRODUCTION
Obesity is an underlying cause for chronic diseases, and its adverse effects on metabolism can be exacerbated by physical inactivity (1). Physical exercise can help to prevent or manage metabolic disorders, and these effects may be mediated by acute effects of exercise on improving the function of skeletal muscle mitochondria. Impaired responses with respect to the function of skeletal muscle mitochondria are considered to sustain a pathophysiological state linked to metabolic diseases (2). Mitochondria in skeletal muscle have a primary role in supporting the energy needs of the tissue via the production of adenosine triphosphate (ATP). Acute exercise perturbates skeletal muscle metabolism, including the activity of mitochondrial enzymes (3). Skeletal muscle mitochondrial function appears to improve immediately after acute exercise in healthy, non-obese humans (4, 5), along with an increase in the activity of citrate synthase (CS) (3), a key enzyme in mitochondrial substrate metabolism.
Mitochondria in skeletal muscle are organized into two major subpopulations, one found beneath the sarcolemma, comprising subsarcolemmal (SS) mitochondria, and the other located between myofibrils, comprising intermyofibrillar (IMF) mitochondria. These mitochondrial subpopulations display distinct biochemical and functional (6), as well as proteomic (7), characteristics. It may be intuitive that exercise-mediated contractile activity affects primarily the function of IMF mitochondria because of their proximity to the contractile apparatus. However, current evidence indicates that exercise training improves the activity of mitochondrial enzymes more in SS, than IMF, mitochondria (8).
Mitochondrial function appears lower in muscle of humans with obesity/insulin resistance, as documented by lower muscle CS activity (9), fatty acid oxidation (10), and ATP production (11). However, other studies have reported comparable mitochondrial function between humans with obesity/insulin resistance and healthy controls (12, 13). Regardless of the impact of obesity/insulin resistance on mitochondrial function in the basal state, the function of the overall muscle mitochondrial pool, appears to improve after exercise training in individuals with obesity (10). However, no evidence exists with respect to the effects of exercise specifically on the SS versus IMF mitochondria in humans with obesity. Studying SS and IMF mitochondria independently allows for more specific exploration of the mechanisms that mediate alterations in energy metabolism in muscle of humans with obesity in the basal state as well as following exercise.
Given the evidence indicating improvements in overall muscle mitochondrial function (4, 5) and CS activity (3) after acute exercise, a main objective of this study was to test the hypothesis that ATP production rate increases following aerobic exercise in both SS and IMF mitochondria in non-obese subjects as well as subjects with obesity.
METHODS
Subjects
The study procedures were approved by the Institutional Review Board at Mayo Clinic and all experiments were conducted at the Clinical Studies Infusion Unit (CSIU) at Mayo Clinic in Scottsdale, Arizona.
Statistical power analysis was based on previous data describing an increase in maximal ATP production rate (MAPR) in mitochondria after acute infusion of plasma amino acids (14), and for an estimated effect size (ES) of 1.3. We considered this effect to be physiologically relevant and hypothesized that a corresponding ES associated with acute exercise will be at least of the same magnitude. This is because physical exercise, and when compared to perturbation of the plasma milieu by amino acids, places direct energetic stress on muscle mitochondria. For an ES of 1.3, an α = 0.05, and a 1 – β = 0.8, a sample size of n = 7 per group was determined. Therefore, we recruited 14 participants that did not take part in regular physical activity more than two days per week (i.e., sedentary individuals). Seven had body mass index (BMI) < 27 kg/m2 (i.e., non-obese subjects), while the other seven had BMI > 32 kg/m2 (i.e., subjects with obesity). The purpose and the experimental design of the study, as well as the risks related to the study procedures, were explained to the study participants prior to obtaining written consent. Subjects invited into the study were healthy based on screening that involved medical history, routine physical examination, and standard blood and urine analyses. Screening procedures included also a 2-hour oral glucose tolerance test (OGTT) to estimate insulin sensitivity using the Matsuda Insulin Sensitivity Index (ISI) (15). The ISI was calculated from the plasma insulin and glucose responses to the OGTT (15). Subjects were excluded from the study if there was evidence of diabetes (i.e., either fasting plasma glucose ≥ 126 mg/dl or 2-h plasma glucose ≥ 200 mg/dl).
Subjects that qualified for the study returned to the CSIU on a separate day from the screening visit to have body composition determined using whole-body bioelectrical impedance analysis (BIA 310e, Biodynamics Corp., Shoreline, WA). Waist-to-hip ratio was calculated from circumference measurements at the level of the umbilicus and the widest portion of the buttocks, respectively (16). Maximal oxygen uptake (VO2max) was measured by employing an incremental (work rate: 20 watts/min; pedaling rate: 65 revolutions/min) cycle ergometer (Lode Corival, Lode B.V., Groningen, Netherlands) test to volitional exhaustion. During the VO2max test, expired gases were continuously monitored using a metabolic cart (MedGraphics Metabolic Cart, Saint Paul, MN). The VO2max test also included a 12-lead electrocardiogram monitoring, as well as monitoring of blood pressure and blood oxygen saturation, the latter using finger pulse oximetry. All subjects were verbally encouraged to cycle until exhaustion (17), and they reached a respiratory exchange ratio > 1.1 at the end of the VO2max test (18).
Experimental Design
Subjects returned to the CSIU on a separate day to collect muscle biopsies as well as blood samples before and after an acute bout of aerobic exercise. Subjects were instructed to fast overnight and abstain from performing any form of exercise beyond their normal daily physical activities for three days prior to the main experiment. Compliance with these instructions was verified prior to initiation of the experimental procedures at ~ 7 AM. An intravenous (IV) line was place in a dorsal hand vein to collect arterialized blood samples and kept patent for the duration of the experiments using infusion of normal saline.
Subjects rested in bed for 3 hours prior to collection of a muscle biopsy sample (i.e., Basal study period). A single session of aerobic exercise was initiated soon after the muscle biopsy. Subjects rested in bed for 3 hours immediately following the completion of the exercise session and before the collection of a post-exercise muscle biopsy sample (i.e., Exercise study period). Blood samples were collected from the IV line for the determinations of plasma glucose and insulin concentrations at the beginning of the Basal study period and at 20 min intervals during the last 60 min of the Basal and Exercise study periods. Plasma glucose and insulin concentrations were used to calculate a quantitative insulin sensitivity check index (QUICKI) (19).
The exercise session consisted of cycling on a recumbent cycle ergometer for 45 min at an intensity corresponding to 65% heart rate reserve (HRR). A target exercise heart rate for each subject was calculated based on the maximal heart rate achieved during the VO2max test (20). The heart rate was continuously monitored during the exercise session and the workload was adjusted as necessary to maintain the subject’s heart rate during exercise at the target heart rate (±5 bpm).
Muscle biopsy samples (~100 mg) were collected from the vastus lateralis muscle. After removing blood, and visible fat and connective tissues, the samples were immediately placed into ice-cold Chappell-Perry solution (Solution I; mM): 100 KCl, 40 Tris-HCl, 10 Tris-Base, 5 MgCl2, 1 EDTA, 1 ATP, pH 7.5). For technical reasons, a post-exercise muscle biopsy was not collected from one non-obese subject and one subject with obesity and, therefore, data from these biopsies were not included in the analyses comparing the effects of exercise.
Analytical Procedures
Isolation of skeletal muscle mitochondria
Isolation of SS and IMF mitochondria from the muscle samples was performed using differential centrifugation, and by following procedures we have described in detailed previously (14). Briefly, muscles were first minced with scissors in Solution I (above) followed by gentle homogenization of the samples by hand in a glass-to-glass Potter-Elvehjem homogenizer. They were then centrifuged at 800 × g to obtain the supernatant containing SS mitochondria. The pellet containing IMF mitochondria was incubated with the protease enzyme Nagarse (P-8038; Sigma-Aldrich; St. Louis, MO) to liberate IMF mitochondria from the myofibrillar proteins. After 7 min, Solution I was added to the samples to stop the enzymatic digestion. Resuspended samples were centrifuged at 800 × g to obtain the supernatant containing the IMF mitochondria. The supernatants containing the SS and IMF mitochondria were centrifuged at 14,000 × g and the resulting pellets were resuspended in solution containing (mM) 100 KCl, 40 Tris-HCl, 10 Tris-Base, 5 MgCl2, 1 EDTA, 0.2 ATP, at pH 7.5. Following two more consecutive centrifugations at 7000 × g and 4000 × g the final mitochondrial pellets containing the SS and IMF mitochondria were each resuspended in identical volumes of mannitol-sucrose buffer solution containing (mM) 220 Mannitol, 70 Sucrose, 10 Tris-HCl, 1 EGTA, pH 7.40.
Citrate synthase activity and protein concentrations
CS activity was determined spectrophotometrically at 37°C by the method of Srere (21), and following procedures we have previously used (22). Briefly, after mitochondria were lysed in 0.1 mmol Tris that contained Triton X-100 (0.1 %), mitochondrial lysate was added to acetyl coenzyme A and 5,5’-Dithiobis(2-nitrobenzoic acid). The production of the formed 2-nitro-5-benzoic acid was determined spectrophotometrically by measuring absorbance at 412 nm.
Protein concentrations in the mitochondrial isolates were determined by the method of Lowry (23).
Mitochondrial ATP production assay
MAPR was assessed in freshly isolated SS and IMF mitochondria using a luciferin–luciferase bioluminescence assay. ATP production rates were monitored at 28oC for 10 min in 0.2 ml reaction volumes in a 96-well plate using an automated luminometer (FlexStation® 3 Multi-Mode Microplate Reader; Molecular Devices; San Jose, CA). The reaction mixture, adapted from Wanders (24), included (mM): 100 KCl, 50 3-(N-Morpholino)propanesulfonic acid, 10 D-glucose, 10 MgCl2, 1.0 EGTA, 0.2% BSA, 0.5 D-luciferin (Life Technologies; Eugene, OR), 100 dithiothreitol, and 10 KH2PO4. Additionally, the reaction mixture included 1.25 μg/ml firefly luciferase (Life Technologies; Eugene, OR). MAPR was measured in the presence of saturating amounts of the following substrates (mM): 1 malate, 1 pyruvate, and 10 glutamate (MPG); 10 succinate plus 1 rotenone (SUCC+R); 1 malate plus 1 palmitoylcarnitine (M+PC). ATP production rate resulting from the metabolism of these substrates was evaluated in the presence of saturating amounts of ADP (total of 25 mM) to induce MAPR. The MPG substrate combination was utilized to measure ATP production as it relates to electron transfer to complex I of the electron transport chain (ETC). The SUCC + R combination measures electron transfer to complex II, while inhibiting reversible electron flow through complex I. M+PC substrate combination was used to assess mitochondrial lipid metabolism. Using PC allows fatty acid uptake into mitochondria bypassing the need for carnitine palmitoyltransferase I (CPT-I), a rate-limiting enzyme in the entry of fatty acids into the mitochondria. All reactions were initiated with the addition of isolated SS or IMF mitochondria.
Quantitation of mitochondrial ATP production
Reaction mixture, substrates, and aliquots from the isolated SS or IMF mitochondrial fractions (0.05 μg protein) were added in duplicate into wells of a 96-well plate. Manual mixing was achieved using a multichannel pipette, and the plate was loaded into the FlexStation® Microplate Reader. Relative luminescence unit (RLU) values were converted to ATP concentrations using a linear equation derived from an ATP concentration standard curve run on the same 96-well plate. Six ATP concentration standards ranging from 25 to 1000 pmol were used to generate the ATP concentration standard curve. ATP concentration was plotted as a function of time, and the rate of ATP synthesis was determined as the linear slope through the collected data points. MAPR was expressed relative to the protein in the isolated SS and IMF mitochondrial fractions.
Plasma hormone and glucose concentrations
Plasma glucose concentrations were measured with an automated glucose analyzer (STAT 2300; Yellow Springs Instruments). Plasma insulin concentrations were measured using a commercially available kit (ALPCO Diagnostics, Salem, NH).
Statistical Analyses
Differences between groups were compared using the non-parametric Mann-Whitney test. The Wilcoxon matched-pairs signed rank test was used to test for differences within groups in response to the exercise (i.e., post- exercise versus pre-exercise responses). Correlation between variables was analyzed by calculating the Spearman’s rank-order correlation coefficient (rs). Statistical significance was set at P < 0.05. Data are presented as median (25 – 75% interquartile range). Analyses were performed using the GraphPad Prism 7 statistical software (GraphPad Software, San Diego, CA).
RESULTS
Subject Characteristics
Anthropometric, physiological, and blood chemistry characteristics for the subjects participated in the study are shown in Table 1. Per study design, subject groups differed in BMI. Subjects differed also with respect to several other characteristics (i.e., adiposity, waist-to-hip ratio, plasma insulin concentrations, insulin sensitivity) in a way that the two subject groups represented typical populations of non-obese individuals and individuals with obesity.
Table 1 –
Subject anthropometric, physiological, and blood chemistry characteristics
| Non-obese | Obese | |
|---|---|---|
| F/M | 3/4 | 3/4 |
| Age (years) | 25 (22 – 39) | 29 (20 – 39) |
| Weight (kg) | 72.9 (63 – 83.7) | 98.2 (85.1 – 103.0)* |
| BMI (kg/m2) | 24.8 (23.2 – 26.7) | 34.7 (32.5 – 37.6)* |
| Body fat mass (%) | 20.2 (16.8 – 29.8) | 34.9 (30.0 – 38.2)* |
| FFM (kg) | 55.3 (46.6 – 66.8) | 63.9 (53.2 – 70.9) |
| VO2max (ml·min−1) | 2442 (1874 – 3003) | 1966 (1722 – 2663) |
| VO2max (ml·kgFFM−1·min−1) | 39.9 (36.6 – 46.0) | 32.9 (28.9 – 40.3)* |
| Heart rate max (beats·min−1) | 163 (147 – 189) | 166 (161 – 180) |
| Waist-to-hip ratio | 0.84 (0.77 – 0.87) | 0.93 (0.89 – 0.97)* |
| Fasting plasma glucose (mg·dL−1) | 81.6 (75.8 – 92.1) | 81.8 (79.6 – 88.7) |
| Fasting plasma insulin (μIU·mL−1) | 3.8 (3.6 – 5.8) | 13.1 (6.9 – 22.7)* |
| QUICKI | 0.40 (0.37 – 0.40) | 0.33 (0.31 – 0.36)* |
| Matsuda-ISI | 9.3 (4.1 – 13.5) | 3.8 (2.4 – 6.2)* |
| HbA1c (%) | 5.2 (5.0 – 5.4) | 5.4 (5.2 – 5.5) |
| Plasma triglycerides (mg·dL−1) | 65 (45 – 193) | 120 (85 – 146) |
| Total plasma cholesterol (mg·dL−1) | 153 (129 – 171) | 186 (162 – 215)* |
| Plasma HDL-Cholesterol (mg·dL−1) | 54 (51 – 71) | 44 (41 – 69) |
| Plasma LDL-Cholesterol (mg·dL−1) | 66 (63 – 88) | 115 (90 – 145) |
Values are median (25–75% interquartile range); BMI, body mass index; FFM, fat-free mass; VO2max, maximal oxygen uptake; QUICKI, quantitative insulin sensitivity check index; Matsuda-ISI, Matsuda insulin-sensitivity index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein
P < 0.05 vs Non-obese.
Exercise Session
All subjects completed the 45-min cycling exercise session. Heart rate responses during exercise, expressed as %HRR, were not different between the subjects with obesity [68 (66 – 71)] and the non-obese subjects [69 (67 – 71)] (P > 0.05).
Mitochondrial Protein Yield
Protein yield in the SS and IMF mitochondria did not differ between groups or within groups before and after the exercise session (Table 2).
Table 2 –
Protein yield in the subsarcolemmal and intermyofibrillar mitochondrial fractions in the Basal state and at 3 hours following aerobic exercise (3 hrs post-exercise)
| Basal | 3 hrs post-exercise | |
|---|---|---|
| Protein (mg·g wet muscle−1) | ||
| Subsarcolemmal | ||
| Non-obese | 2.6 (2.3 – 2.9) | 2.3 (2.0 – 2.6) |
| Obese | 2.6 (1.6 – 3.4) | 2.0 (1.6 – 3.8) |
| Intermyofibrillar | ||
| Non-obese | 1.7 (1.2 – 5.6) | 2.7 (1.5 – 3.5) |
| Obese | 2.3 (1.8 – 3.5) | 2.1 (1.0 – 3.1) |
Values are median (25–75% interquartile range); no statistically significant differences were detected within or between groups.
Citrate Synthase Activity
Whole-muscle CS activity (μmol/min/g wet muscle) was not different between subjects with obesity (n = 7) [7.9 (4.6 – 11.5)] and non-obese controls (n = 7) [7.4 (6.1 – 8.5)] at the basal state (P > 0.05). Whole-muscle CS activity (μmol/min/g wet muscle) increased significantly at 3 hrs after the exercise in the non-obese subjects (n = 6) [9.6 (8.6 – 10.9); P < 0.05] but not in the subjects with obesity (n = 6) [9.5 (6.4 – 11.6); P > 0.05].
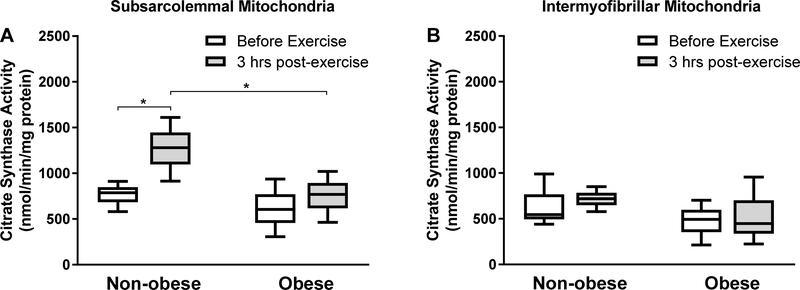
CS specific activity (nmol/min/mg protein) did not differ at basal between subjects with obesity and non-obese controls (P > 0.05) in either SS [691 (324 – 925) vs 803 (586 – 901)] or IMF [631 (219 – 701) vs 570 (441 – 871)] mitochondria. CS specific activity responses to exercise in the SS and IMF mitochondrial fractions are shown in Figure 1. In IMF mitochondria, no differences were found in the response of CS specific activity to exercise in either the subjects with obesity (ES, 0.2) or the non-obese subjects (ES, 0.1) (for both P > 0.05; Figure 1B). In SS mitochondria, however, CS specific activity increased in response to exercise in the non-obese subjects (ES, 1.4), but not in the subjects with obesity (ES, 0.4), and in a way that the CS specific activity in the SS mitochondria in the non-obese subjects was higher than that in the subjects with obesity at 3 hours after the exercise session (P < 0.05; Figure 1A).
Figure 1.
Citrate synthase (CS) specific activity. CS specific activity in subsarcolemmal (A) and intermyofibrillar (B) mitochondria isolated from non-obese subjects (n = 6) and subjects with obesity (n = 6) before exercise and at 3 hours following aerobic exercise (3 hrs post-exercise). Data are presented as median (25–75% interquartile range). *P < 0.05.
Maximal ATP Production Rate
In IMF mitochondria, basal-state MAPR was not different (P > 0.05) between subjects with obesity (n = 7) and non-obese controls (n = 7) in the presence of MPG [338 (176 – 490) vs 197 (138 – 275)], SUCC+R [126 (89 – 203) vs 110 (60 – 179)], or M+PC [104 (70 – 225) vs 131 (61 – 187)] substrates. Similarly, in SS mitochondria, basal-state MAPR was not different (P > 0.05) between subjects with obesity (n = 7) and non-obese controls (n =7) in the presence of the MPG [388 (236 – 415) vs 248 (215 – 290)], SUCC+R [154 (122 – 181) vs 140 (96 – 174)], or M+PC [144 (86 – 208) vs 108 (95 – 168)] substrates.
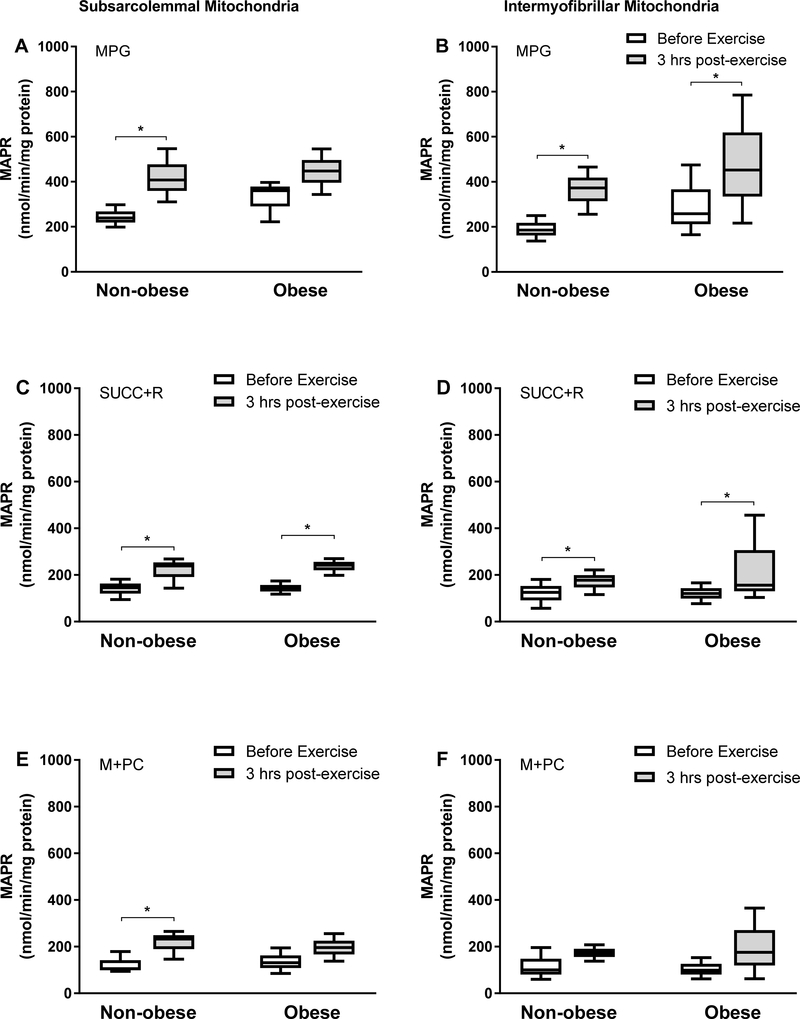
MAPR responses to the exercise session in the two mitochondrial subpopulations are shown in Figure 2. In SS mitochondria, MAPR was significantly higher (P < 0.05) at 3 hrs following the exercise session in the non-obese subjects, and in the presence of either MPG (ES, 1.9; Figure 2A), SUCC+R (ES, 1.3; Figure 2C), or M+PC (ES, 1.4; Figure 2E) substrates. However, in the same mitochondrial fraction, MAPR was significantly higher (P < 0.05) after exercise in the subjects with obesity only in the presence of SUCC+R (ES, 1.9), but not MPG (ES, 1.0) or M+PC (ES, 0.9) (for both P > 0.05; Figure 2). With respect to the IMF mitochondria, both groups showed significant increase in MAPR after the exercise session with MPG (non-obese ES, 1.4; obese, 0.7; Figure 2B) and SUCC+R (non-obese ES, 0.8; obese, 0.8; Figure 2D) substrates. However, MAPR did not increase in either group in the presence of M+PC (non-obese ES, 0.8; obese, 0.8; Figure 2F).
Figure 2.
Maximal ATP production rate (MAPR). MAPR in subsarcolemmal (A, C, E) and intermyofibrillar (B, D, F) mitochondria isolated from non-obese subjects (n = 6) and subjects with obesity (n = 6) before exercise and at 3 hours following aerobic exercise (3 hrs post-exercise). Data are presented as median (25–75% interquartile range). MAPR was measured in the presence of Malate (1 mM) + Pyruvate (1 mM) + Glutamate (10 mM) (MPG), Succinate (10 mM) + Rotenone (1 mM) (SUCC+R), or Malate (1 mM) + Palmitoylcarnitine (1 mM) (M+PC) as substrates. *P < 0.05.
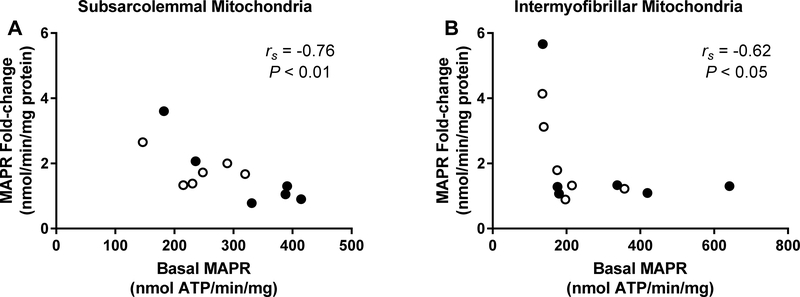
Changes induced by the exercise session in MAPR correlated inversely with the MAPR measured in the basal state. These correlations were significant for both SS and IMF mitochondria when MPG was used as substrate (Figure 3). Although SUCC+R and M+PC induced less robust MAPR, the inverse correlation between the changes in MAPR and basal-state MAPR was significant also when SUCC+R was used as substrate in SS mitochondria (P < 0.01) and tended to be significant when the M+PC was used as substrate in both SS and IMF mitochondria (for both P = 0.06).
Figure 3.
Scatter plot and Spearman’s rs correlation (rs) between maximal ATP production rate (MAPR) in the basal state and change in MAPR from basal state in response to aerobic exercise in subsarcolemmal (A) and intermyofibrillar (B) mitochondria in non-obese subjects (open circles; n = 6) and subjects with obesity (dark circles; n = 6). MAPR was measured in the presence of Malate (1 mM) + Pyruvate (1 mM) + Glutamate (10 mM).
Concentrations of Plasma Glucose and Insulin and Insulin Sensitivity
Responses for plasma glucose and insulin concentrations at the blood sampling time points during the course of the experiments are depicted in Supplemental Figure 1 (see Figure, Supplemental Digital Content 1, Plasma glucose and insulin concentrations). As it was rather expected, these responses were more variable between subjects with obesity than the more homogeneous group of non-obese subjects. Plasma glucose and insulin concentrations in the basal state and at 3 hours following the exercise session are summarized in Table 3. At basal state, plasma glucose concentration did not differ between groups (P > 0.05), but subjects with obesity had higher plasma insulin concentration (P < 0.05). In response to the exercise session, plasma glucose concentration decreased only in the non-obese subjects, but plasma insulin concentration decreased in both non-obese subjects as well as the subjects with obesity (P < 0.05). However, plasma insulin concentration in the subjects with obesity remained higher than that in the non-obese subjects at 3 hours after the exercise (P < 0.05).
Table 3 –
Plasma insulin and glucose concentrations as well as calculated insulin sensitivity (i.e., QUICKI) in the Basal state and at 3 hours following aerobic exercise (3 hrs post-exercise)
| Basal | 3 hrs post-exercise | |
|---|---|---|
| Insulin (μIU·mL−1) | ||
| Non-obese | 3.8 (3.5 – 6.0) | 3.2 (2.1 – 4.6)* |
| Obese | 10.3 (6.3 – 18.4)† | 7.2 (5.3 – 17.6)*† |
| Glucose (mg·dL−1) | ||
| Non-obese | 83.3 (76.4 – 92.3) | 79.5 (71.5 – 83.6)* |
| Obese | 83.0 (79.1 – 89.5) | 81.1 (75.0 – 83.4) |
| QUICKI | ||
| Non-obese | 0.40 (0.36 – 0.41) | 0.43 (0.39 – 0.45)* |
| Obese | 0.34 (0.32 – 0.37)† | 0.36 (0.32 – 0.38)*† |
Values are median (25–75% interquartile range); QUICKI, quantitative insulin sensitivity check index
P < 0.05 versus Basal
P <0.05 versus Non-obese.
Insulin sensitivity as estimated by the QUICKI mirrored, albeit inversely, the responses of plasma insulin concentration, and it increased in both subject groups in response to exercise (P < 0.05; Table 3). However, and similar to the basal-state responses, insulin sensitivity remained lower in the subjects with obesity when compared to that in the non-obese subjects at 3 hours after the exercise (P < 0.05; Table 3).
DISCUSSION
Herein, we show differential effects of acute exercise on the function of mitochondria localized in distinct subcellular regions in skeletal muscle of humans with obesity. Specifically, we show that an acute session of aerobic exercise increases MAPR throughout the mitochondrial reticulum in skeletal muscle of non-obese humans, but this effect is comparably smaller in humans with obesity for mitochondria located beneath the plasma membrane. Acute exercise had also comparably smaller effects on increasing the specific activity of CS in the same mitochondrial subpopulation (i.e., SS mitochondria) in humans with obesity.
To our knowledge, few studies (25, 26), including studies in subjects with obesity (14), have assessed ATP production at a mitochondrial subpopulation-specific level in human muscle. Moreover, no data exist about MAPR in separate mitochondrial subpopulations in response to exercise. The threshold associated with an effect size describing a physiologically/clinically relevant increase in MAPR in skeletal muscle mitochondria can be a matter of scientific debate. As described in the Methods section, we sought to determine changes in MAPR in response to exercise corresponding to an effect size of at least 1.3. Although there was still an effect of acute exercise on increasing MAPR in SS mitochondria in subjects with obesity, this effect was smaller than that in the non-obese subjects in the presence of MPG (i.e., 1.0) or M+PC (i.e., 0.9). These data indicate that the effects of exercise on increasing MAPR supported specifically by protein complex I and fatty acids in SS mitochondria in individuals with obesity are comparably smaller than those in non-obese individuals. “Compromised mitochondrial plasticity” has been previously suggested to exist in the metabolic state associated with insulin resistance, and it has been described as reduced ability of muscle mitochondria to modify their function in response to a metabolic stimulus (27). Our findings indicate that with respect to the exercise stimulus, reduced ability of muscle mitochondria to modify their function appears more evident in humans with obesity/insulin resistance in mitochondria located in the subsarcolemmal region of muscle.
Previous studies have described improved mitochondrial function (i.e., respiration) following exercise in the presence of malate+pyruvate (i.e., complex I-supported mitochondrial function) (4) or PC (i.e., fatty acid-supported mitochondrial function) (5) in non-obese subjects either in muscle fibers (4) or isolated mitochondria (5) representative of the overall muscle mitochondrial pool. Our results in the non-obese subjects are in line with these findings. The reason(s) behind the comparably smaller effects of exercise on MAPR specifically in SS mitochondria in the subjects with obesity cannot be elucidated based on the present experimental design. Because we used palmitoylcarnitine as substrate, metabolism of fatty acids in our study is independent of the transport of fatty acids into the mitochondria. However, comparably smaller effects of exercise on mitochondrial ATP production in the presence of M+PC in subjects with obesity may involve mechanisms beyond β-oxidation, such as lower electron-transferring flavoprotein activity and/or complex I activity.
In the presence of succinate (i.e., complex II-supported mitochondrial function) subjects with obesity displayed exercise-induced effects on MAPR in SS mitochondria that were comparable to those in non-obese subjects. This finding differs from the comparably smaller effects on MAPR observed in the subjects with obesity in SS mitochondria with the MPG and M+PC substrates. Substrate-dependent differences in the response of MAPR between groups may relate to intrinsic differences in the proteins forming the complexes of the ETC. For example, protein complex II is the only mitochondrial protein complex formed by proteins encoded exclusively by nuclear, and not mitochondrial, DNA (28). Interestingly, in humans with peripheral vascular disease acute exercise improves mitochondrial function supported by complex II, but not complex I (29), and similar to our findings in the subjects with obesity. Regardless of the biological mechanism(s) involved, our study shows that exercise-induced increase in MAPR in SS mitochondria supported specifically by protein complex II of the ETC is not affected by the metabolic environment associated with obesity.
Similar to previous reports (3, 30), we found that acute exercise increased whole-muscle CS activity in the non-obese subjects. These changes in CS activity at the whole-muscle level can result from changes in the content of CS and/or changes in the specific activity of CS (i.e., CS activity for given protein content) in muscle. With respect to the latter, we found that in the non-obese subjects exercise increased also the specific activity of CS in the SS mitochondria. This suggests exercise-mediated modifications of the CS protein, rather than changes in the content of CS, and when considering also the low turnover rate of CS in muscle (31). Similarly, increases in MAPR after the exercise session in our study resulted likely from changes in the function of mitochondrial proteins involved in oxidative phosphorylation, rather than changes in the content of mitochondrial proteins given the low turnover rate of overall mitochondrial protein in muscle (32). The comparably smaller effect of exercise to upregulate the specific activity of CS in obesity may be secondary to the metabolic environment of obesity and an associated hyperacetylation of mitochondrial proteins (33). Hyperacetylation of CS is linked to lower CS activity (34), and the presence of such modifications on CS in humans with obesity may minimize the acute effects of exercise on enhancing CS activity. Our findings that the CS specific activity response to exercise was blunted in subjects with obesity specifically in the SS mitochondria underline the importance of understanding mitochondrial enzyme activities, as well as overall ATP production, in muscle from humans with obesity in a subcellular location-specific manner, rather than in whole-muscle homogenates.
Mitochondrial function has been linked to insulin sensitivity (35), and available evidence indicates that individuals that respond to exercise training with increased ATP synthesis show enhanced insulin sensitivity (36). We did not assess insulin sensitivity directly using the hyperinsulinemic-euglycemic clamp, as such experimental manipulation alters the mitochondrial function (37). Yet, when compared to the hyperinsulinemic-euglycemic clamp, QUICKI, measured under steady-state plasma glucose and insulin concentrations, provides reliable estimates of insulin sensitivity in peripheral tissues (19). In the present study insulin sensitivity improved following exercise in both groups concomitant with improvements in MAPR in IMF mitochondria. On the other hand, studies in rodents with obesity/insulin resistance show that improvements in insulin sensitivity are observed as result of enhanced activity of CS and enzymes involved in β-oxidation specifically in SS mitochondria (38). Assuming a cause-effect relationship exists between mitochondrial function and insulin sensitivity, comparable exercise-induced improvements in insulin sensitivity between groups in the present study should have been mediated primarily by comparable enhancements in the function of IMF mitochondria. Nevertheless, our study design is limited with respect to its ability to address the considerable controversy that exists regarding the precise role of muscle mitochondria in regulating insulin sensitivity.
It is possible that lack of statistical significance in the responses to exercise in SS mitochondria in the subjects with obesity is due to Type 2 error. However, P values are confounded because of their reliance on sample size (39), whereas in our study we sought to determine statistically significant differences based on a pre-determined effect size, which was employed to calculate a sample size. A limitation of the present study is that measurements were performed in isolated mitochondria and, according to available evidence, isolation of muscle mitochondria appears to alter the structure and function of mitochondria (40). However, isolation of mitochondria from muscle is necessary in order to study mitochondrial subpopulations that are known to exhibit biochemical, functional, and proteomic differences (6, 7). Importantly, data in our study were contrasted only in mitochondria that were isolated using exactly the same procedures and, therefore, our findings reflect differences in mitochondria function associated with the experimental manipulation rather than the mitochondrial isolation procedure. We have previously found that the enzyme nagarse, which is important to isolate functional IMF mitochondria, modifies (i.e., enhances) the function of isolated mitochondria. For that reason, we did not contrast data isolated with and without nagarse (i.e., SS vs IMF mitochondria). Consequently, our comparisons in mitochondrial function are limited to the responses within a given mitochondrial subpopulation.
In conclusion, we show that aerobic exercise acutely modifies the function of skeletal muscle mitochondria resulting in enhanced mitochondrial ATP production and substrate metabolism. However, humans with obesity show comparably smaller effects on increasing mitochondrial ATP production as well as carbohydrate and lipid oxidation in response to the same exercise specifically in subsarcolemmal mitochondria.
Supplementary Material
Acknowledgements
We acknowledge the guidance and technical expertise of Dr. Wayne Willis related to the isolation of skeletal muscle mitochondria and performance of the mitochondrial function assays. We also acknowledge the expertise provided by Matthew R. Buras from the Mayo Clinic in Arizona Biostatistics Core related to the statistical aspects of the study. We thank the staff at the Clinical Studies Infusion Unit at Mayo Clinic in Scottsdale, Arizona with respect to their help in subject recruitment and conduct of the studies. We also thank the subjects for their participation and commitment to the study procedures.
The study was supported by NIH/NIDDK grant DK094062 (CSK).
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
The results of the present investigation do not constitute endorsement by ACSM. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Supplemental Digital Content
SDC 1— Supplemental Figure 1 - Insulin and glucose.pdf
REFERENCES
- 1.Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32(8):1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12(4):537–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R441–7. [DOI] [PubMed] [Google Scholar]
- 4.Tonkonogi M, Walsh B, Tiivel T, Saks V, Sahlin K. Mitochondrial function in human skeletal muscle is not impaired by high intensity exercise. Pflugers Arch. 1999;437(4):562–8. [DOI] [PubMed] [Google Scholar]
- 5.Fernstrom M, Bakkman L, Tonkonogi M, et al. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24-h ultraendurance exercise. J Appl Physiol (1985). 2007;102(5):1844–9. [DOI] [PubMed] [Google Scholar]
- 6.Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol. 1993;264(2 Pt 1):C383–9. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira R, Vitorino R, Alves RM, et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 2010;10(17):3142–54. [DOI] [PubMed] [Google Scholar]
- 8.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado MV, Ferreira DM, Castro RE, et al. Liver and muscle in morbid obesity: the interplay of fatty liver and insulin resistance. PLoS One. 2012;7(2):e31738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berggren JR, Boyle KE, Chapman WH, Houmard JA. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab. 2008;294(4):E726–32. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D, Defronzo RA. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia. 2009;52(4):574–82. [DOI] [PubMed] [Google Scholar]
- 12.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50(4):790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen S, Ara I, Rabol R, et al. Are substrate use during exercise and mitochondrial respiratory capacity decreased in arm and leg muscle in type 2 diabetes? Diabetologia. 2009;52(7):1400–8. [DOI] [PubMed] [Google Scholar]
- 14.Kras KA, Hoffman N, Roust LR, Patel SH, Carroll CC, Katsanos CS. Plasma Amino Acids Stimulate Uncoupled Respiration of Muscle Subsarcolemmal Mitochondria in Lean but Not Obese Humans. J Clin Endocrinol Metab. 2017;102(12):4515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO). Waist circumference and waist–hip ratio: report of a World Health Organization expert consultation, Geneva, 8–11 December 2008. Geneva, Switzerland, 2011. [Google Scholar]
- 17.Wood RE, Hills AP, Hunter GR, King NA, Byrne NM. Vo2max in overweight and obese adults: do they meet the threshold criteria? Med Sci Sports Exerc. 2010;42(3):470–7. [DOI] [PubMed] [Google Scholar]
- 18.Howley ET, Bassett DR, Jr., Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–301. [PubMed] [Google Scholar]
- 19.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10. [DOI] [PubMed] [Google Scholar]
- 20.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. [DOI] [PubMed] [Google Scholar]
- 21.Srere PA. Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 22.Kras KA, Willis WT, Barker N, Czyzyk T, Langlais PR, Katsanos CS. Subsarcolemmal mitochondria isolated with the proteolytic enzyme nagarse exhibit greater protein specific activities and functional coupling. Biochem Biophys Rep. 2016;6:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 24.Wanders RJ, Groen AK, Van Roermund CW, Tager JM. Factors determining the relative contribution of the adenine-nucleotide translocator and the ADP-regenerating system to the control of oxidative phosphorylation in isolated rat-liver mitochondria. Eur J Biochem. 1984;142(2):417–24. [DOI] [PubMed] [Google Scholar]
- 25.Elander A, Sjostrom M, Lundgren F, Schersten T, Bylund-Fellenius AC. Biochemical and morphometric properties of mitochondrial populations in human muscle fibres. Clin Sci (Lond). 1985;69(2):153–64. [DOI] [PubMed] [Google Scholar]
- 26.Fischer JC, Ruitenbeek W, Stadhouders AM, et al. Investigation of mitochondrial metabolism in small human skeletal muscle biopsy specimens. Improvement of preparation procedure. Clin Chim Acta. 1985;145(1):89–99. [DOI] [PubMed] [Google Scholar]
- 27.Jelenik T, Roden M. Mitochondrial plasticity in obesity and diabetes mellitus. Antioxid Redox Signal. 2013;19(3):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker JE. Determination of the structures of respiratory enzyme complexes from mammalian mitochondria. Biochim Biophys Acta. 1995;1271(1):221–7. [DOI] [PubMed] [Google Scholar]
- 29.van Schaardenburgh M, Wohlwend M, Rognmo O, Mattsson EJ. Mitochondrial Respiration after One Session of Calf Raise Exercise in Patients with Peripheral Vascular Disease and Healthy Older Adults. PLoS One. 2016;11(10):e0165038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonkonogi M, Harris B, Sahlin K. Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand. 1997;161(3):435–6. [DOI] [PubMed] [Google Scholar]
- 31.Booth FW, Holloszy JO. Cytochrome c turnover in rat skeletal muscles. J Biol Chem. 1977;252(2):416–9. [PubMed] [Google Scholar]
- 32.Tran L, Kras KA, Hoffman N, et al. Lower Fasted-State but Greater Increase in Muscle Protein Synthesis in Response to Elevated Plasma Amino Acids in Obesity. Obesity (Silver Spring). 2018;26(7):1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao AW, Canto C, Houtkooper RH. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO molecular medicine. 2014;6(5):580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui XX, Li X, Dong SY, Guo YJ, Liu T, Wu YC. SIRT3 deacetylated and increased citrate synthase activity in PD model. Biochem Biophys Res Commun. 2017;484(4):767–73. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocrine connections. 2015;4(1):R1–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kacerovsky-Bielesz G, Chmelik M, Ling C, et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes. 2009;58(6):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren JL, Bulur S, Ovalle F, Windham ST, Gower BA, Fisher G. Effects of acute hyperinsulinemia on skeletal muscle mitochondrial function, reactive oxygen species production, and metabolism in premenopausal women. Metabolism. 2017;77:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen LL, Zhang HH, Zheng J, et al. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial beta-oxidation. Metabolism. 2011;60(11):1598–609. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan GM, Feinn R. Using Effect Size-or Why the P Value Is Not Enough. Journal of graduate medical education. 2012;4(3):279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picard M, Taivassalo T, Ritchie D, et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6(3):e18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.