Abstract
Background
We report on the results of a Japanese postmarketing drug-use survey of suvorexant (Belsomra®) tablets.
Methods
A survey with a ≤ 6-month observation period after the start of administration was conducted, targeting insomnia patients who were treated with suvorexant for the first time in Japan. Information on the safety and efficacy of the drug product was collected. The evaluation period was July 21, 2015–August 12, 2017, and the target number of patients was 3428.
Results
The mean administration period for the safety analysis population of 3248 patients was 113 days. At 6 months after the start of treatment, 48.6% (1577/3248) of the patients had been continually receiving treatment, and 51.4% (1671/3248) of the patients discontinued/dropped out of treatment before 6 months. Among the patients who discontinued/dropped out of the treatment, more than 30% discontinued due to improvement. The mean treatment duration for those who had discontinued treatment for this reason was 62 days. The incidence rate of adverse drug reactions among those in the safety analysis population was 9.7%, and the common adverse drug reactions were somnolence (3.6%), insomnia (1.2%), dizziness (1.1%), and nightmare (0.8%), all of which are described in the product label. No additional noteworthy events were observed. In 2439 patients with a final overall global assessment of sleep judged by physicians, the ‘improved’ rate was 74.0%. Among 2424 patients who provided a final overall global assessment, the improvement rate was 73.2%, which was comparable with the improvement rate judged by physicians. Regarding clinical effects (based on patient diary data or physician’s assessment), reduction in median sleep latency and increase in median total sleep time (reduction from 60 to 50 min and increase from 300 to 360 min compared with baseline, respectively) were observed at 1 week after the start of treatment and onwards, and the effect was maintained after the start of treatment for 6 months. A similar effect was observed irrespective of age groups or reasons for using suvorexant.
Conclusion
This survey was an exploratory observational study without a control group; the interpretation of results may require the consideration of factors that may have caused bias in the results, such as demographic characteristics and effects of other drugs. However, the results suggest that suvorexant can be a useful drug in daily clinical practice for treating insomnia.
Key Points
| We report on the results of a Japanese postmarketing drug-use survey of suvorexant (Belsomra® tablets 10, 15, and 20 mg), a new type of anti-insomnia drug that functions as an orexin receptor antagonist. |
| Suvorexant was well-tolerated and improved clinical outcome in the real-world clinical setting; the incidence rate of adverse drug reactions in the safety analysis population was 9.7%, and suvorexant showed improvement of insomnia in > 70% of patients. |
| The results suggest that suvorexant can be a useful drug in daily clinical practice for treating insomnia. However, this was an exploratory observational study without a control group; therefore, interpretation of the data may require consideration of factors that could have caused bias in the results. |
Introduction
Insomnia is a common sleep disorder with a high incidence [1]. In general, γ-aminobutyric acid A (GABAA) receptor agonists, such as benzodiazepines and non-benzodiazepines, and melatonin receptor agonists are used for treatment depending on the symptoms of insomnia and demographic characteristics of the patients. On the other hand, suvorexant (Belsomra® tablets 10, 15, and 20 mg), is a new type of anti-insomnia drug that functions as an orexin receptor antagonist. Suvorexant possesses a different pharmacological activity than conventional anti-insomnia drugs, and inhibits the nuclei of the central nervous system (CNS) arousal system that are under the control of orexin neurons by selectively blocking the receptors (OX1R and OX2R) of orexin, which is a wake-promoting neurotransmitter.
Suvorexant was approved for use in Japan on September 26, 2014, at a dose of 20 mg for adults and 15 mg for elderly patients, after its efficacy and safety were demonstrated by clinical trials [2]. However, suvorexant could be administered to patients with more diverse demographic characteristics than those studied in the original clinical development program after being marketed. Thus, we started a drug-use survey for suvorexant on July 21, 2015, to collect or confirm information on the safety and efficacy of suvorexant for use in daily clinical practice in Japan. In an interim report, safety and efficacy were evaluated from 791 Japanese patients whose survey results were collected from commencement to August 12, 2016, and no additional noteworthy adverse events were observed [3]. In this article, we report the results of the final analysis of the surveys of 3428 Japanese patients collected up to the end date of August 12, 2017.
This survey was performed in compliance with the ministerial ordinance on ‘Good Post-marketing Study Practice’ (GPSP), which was authorized by the Ministry of Health, Labour and Welfare in Japan (Ordinance No. 171, dated December 20, 2004).
Patients and Methods
Survey Patients
A total of 3428 insomnia patients who received suvorexant for the first time were included in this survey. They were registered by a central registration method at medical institutions that signed a written contract for this survey. Patients were excluded from registration if they were registered beyond the survey period specified in the contract, were double registered, had a history of suvorexant treatment, were contraindicated per the Japanese package insert [4] (such as patients with a history of hypersensitivity to suvorexant, patients who were taking drugs that strongly inhibit cytochrome P450 (CYP) 3A), or took suvorexant to treat insomnia induced by temporary factors. Suvorexant 10 mg was additionally approved for use in Japan on September 13, 2016 and the 10 mg tablets were included in the current study after marketing on December 15, 2016.
Survey Methods
As indicated in the Japanese package insert [4], Belsomra® was orally administered once daily immediately before bedtime at a dose of 20 mg for adults and 15 mg for elderly patients.
As a general principle, the intended observation period was for 1 month after the initiation of treatment. When patients continued treatment with suvorexant, the observation period was extended until 6 months after initiation. When treatment with suvorexant was completed (discontinued because of improvement in insomnia) or discontinued before 6 months after initiation, a 30-day follow-up period was set after the completion or discontinuation for the collection of information on adverse events. The survey period was July 21, 2015 to August 12, 2017
Survey Items
Patient sex, age, reason for using suvorexant, duration of insomnia, medical history, complications, diagnosis of narcolepsy (including cataplexy), and narcolepsy-like symptoms before treatment with suvorexant, which are also commonly observed in the general population without narcolepsy [major symptoms include long-term intolerable drowsiness and dozing off in the daytime lasting for ≥ 3 months, emotional atonia (cataplexy: sudden loss of strength when patients felt deeply delighted, angry, or surprised), hypnagogic hallucinations (visual and auditory hallucinations experienced during sleep onset), or sleep paralysis (inability to move despite awareness during sleep/wake transitions)], were evaluated.
Information on previous therapy for insomnia, other concomitant drugs for the treatment of insomnia, and use of suvorexant (initial dose, duration, reason for discontinuation, etc.) were collected. Drugs listed in the Japanese insomnia treatment guidelines [1], were considered to be drugs for previous therapy or other concomitant drugs for the treatment of insomnia.
Information on safety was collected through a physician’s medical interview. Regardless of a possible causal relationship with suvorexant, information on all adverse events after the initiation of suvorexant was collected during the observation period with a physician’s assessment of severity and causality.
Information on efficacy, including global improvement and sleep effects (sleep latency and total sleep time), was collected. The overall global improvement assessment by physicians was evaluated at the end of the observation period (1 week, 1 month, 3 months, and 6 months after the initiation of treatment with suvorexant or on the day of completion/discontinuation). A physician in charge made a comprehensive assessment of improvement through a medical interview and categorized results as ‘improved,’ ‘unchanged,’ or ‘deteriorated’ by comparing them with results before initiation. The overall global improvement at 6 months after the initiation of suvorexant or on the day of completion/discontinuation was considered to be ‘the final overall global improvement.’ Overall global improvement judged by patients was evaluated at the same timepoints as the physician’s assessment. Patients assessed improvement by categorizing results as ‘improved,’ ‘unchanged,’ or ‘deteriorated’ compared with results before initiation, and the physician in charge recorded the results during the interview. Information on sleep effects (sleep latency and total sleep time) was collected at the following timepoints: pre-dose and 1 week, 1 month, 3 months, and 6 months after the initiation of treatment with suvorexant, or on the day of completion/discontinuation through a medical interview with/without a patient’s sleep diary conducted by the physician in charge.
Analysis Methods
For the safety assessment, patients were excluded from the analysis if they had an initial visit only (no subsequent visits), a registration violation was found after data collection (e.g., patients who were previously treated with suvorexant; had contraindications mentioned in the Japanese package insert [4]; took suvorexant to treat insomnia induced by temporary factors; and the patient information was different from that at the time of registration), or they did not take suvorexant. Data on adverse drug reactions (adverse events for which a causal relationship with suvorexant could not be ruled out) were evaluated using MedDRA®/J (Medical Dictionary for Regulatory Activities (MedDRA®) Japanese translation) version 20.0 Preferred Term (PT). If the same PT was observed more than once in the same patient, the event was counted only once.
Multivariate logistic regression was performed to evaluate demographic characteristics that might have influenced the occurrence of somnolence, which was a noteworthy event in the current survey. Factors that were expected to be medically valid were selected: sex, age, body mass index (BMI), duration of insomnia, co-morbid conditions that may be associated with insomnia (e.g., schizophrenia, depression, manic-depressive illness, anxiety disorder, and dementia), concomitant drugs, and initiation status of suvorexant (i.e., hypnotic naïve, switch, or add-on therapy).
For efficacy assessment, patients were excluded from the analysis if (1) the initial dose was different from that specified in the Japanese package insert [4] (20 mg once daily for adults and 15 mg once daily for elderly patients); (2) no data regarding overall global improvement and sleep effects were obtained; (3) physicians did not assess overall global improvement and sleep effects within 30 days after the end of the observation period; or (4) there was a discrepancy between a physician’s judgement and the reason for discontinuation.
Regarding overall global improvement in sleep, the improvement rate was calculated with the following formula:
In addition, multivariate logistic regression was performed to search for factors that could affect overall global improvement. In this analysis, ‘improved’ was categorized as the ‘improvement’ group, while ‘unchanged’ and ‘deteriorated’ were categorized as the ‘no improvement’ group. Factors that were expected to be medically valid were selected as variables, including sex, age, BMI, duration of insomnia, co-morbid conditions that may be associated with insomnia (e.g., schizophrenia, depression, manic-depressive illness, anxiety disorder, and dementia), concomitant medications, and initiation status of suvorexant.
Regarding sleep effects, the median of sleep latency and total sleep time were calculated from all available data at each timepoint of collection (pre-dose and 1 week, 1 month, 3 months, and 6 months after the initiation of treatment with suvorexant or on the day of completion/discontinuation).
For safety and efficacy assessments, attention was focused on patient populations with special demographic characteristics described in the Japanese regulatory agency (Pharmaceuticals and Medical Device Agency [PMDA]) review report [5] or the Japanese package insert [4], such as patients with narcolepsy (including cataplexy), respiratory dysfunction, psychiatric disorders, elderly patients, and patients using suvorexant with a concomitant drug for treating insomnia.
To understand the initiation status of suvorexant in daily clinical practice, patients were classified based on the use of other drugs for treating insomnia, as shown in Table 1. The continuation status of patients in each initiation status group (continued, discontinued because of improvement, discontinued due to inadequate effects, discontinued due to no efficacy, discontinued due to the occurrence of adverse events, dropped out due to no subsequent revisit after the second visit) was also summarized.
Table 1.
Patients classified based on use of other drugs for insomnia
| Classification | Condition |
|---|---|
| Naïve | Patients who received suvorexant without any history of use of drugs for insomnia (drugs used 50 days or longer before initiation of suvorexant are not considered as drugs used) |
| Switching | Patients who received suvorexant after discontinuation or dose reduction of existing drug(s) for insomnia within 14 days before initiation of suvorexant |
| Add-on | Patients who received suvorexant without changing the use of existing drug(s) for insomnia within 14 days before initiation of suvorexant |
| Others | Patients other than the above |
Results
Composition of Patients
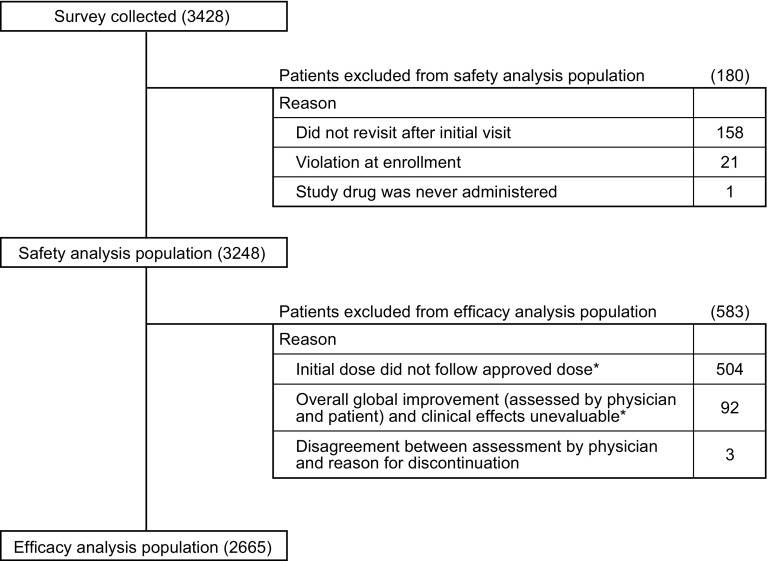
Data from 3428 patients were collected from 884 medical institutions within Japan. Among these patients, 180 were excluded for various reasons, including 158 patients who only had the initial visit and no subsequent visits. In total, 3248 patients were included in the safety analysis population, 2665 of whom were included in the efficacy analysis population; 583 patients were excluded from these analyses, including those who started treatment with suvorexant at an unapproved dose (N = 504) and those with no overall global improvement or sleep measures information (N = 92) (Fig. 1).
Fig. 1.
Composition of patients. *16 patients in the ‘Initial dose did not follow approved dose’ and ‘Overall global improvement (assessed by physician and patient) and clinical effects unevaluable’ categories overlapped
Patient Background
The composition of the 3248 patients in the safety analysis population is shown in Table 2. In total, 1977 patients (60.9%) were females, and the mean age was 62.1 years. Further, 1758 patients (54.1%) were ≥ 65 years (elderly) and 1028 (31.7%) were ≥ 75 years (latter-stage elderly). The most common reason for using suvorexant was difficulty in falling asleep (2429 patients; 74.8%). The duration of insomnia was less than 1 year in 1313 patients (40.4%), at least 1 year and less than 10 years in 1481 patients (45.6%), and at least 10 years in 454 patients (14.0%).
Table 2.
Demographic characteristics (safety analysis population)
| Item | Safety analysis set | |
|---|---|---|
| N | %a | |
| Overall | 3248 | |
| Sex | ||
| Male | 1271 | 39.1 |
| Female | 1977 | 60.9 |
| Age group 1 (years) | ||
| < 15 | 2 | 0.1 |
| ≥ 15 to < 65 | 1488 | 45.8 |
| ≥ 65 | 1758 | 54.1 |
| Age group 2 (years) | ||
| < 65 | 1490 | 45.9 |
| ≥ 65 to < 75 | 730 | 22.5 |
| ≥ 75 | 1028 | 31.7 |
| Age group 3 (years) | ||
| < 65 | 1490 | 45.9 |
| ≥ 65 to < 85 | 1490 | 45.9 |
| ≥ 85 | 268 | 8.3 |
| BMI (kg/m2) | ||
| < 25.0 | 1357 | 41.8 |
| ≥ 25.0 | 497 | 15.3 |
| Unknown | 1394 | 42.9 |
| Reason for use (multi-count) | ||
| Difficulty falling asleep | 2429 | 74.8 |
| Nocturnal awakening | 1596 | 49.1 |
| Early morning awakening | 349 | 10.7 |
| Others | 31 | 1.0 |
| Duration of insomnia (years) | ||
| < 1 | 1313 | 40.4 |
| ≥ 1 to < 10 | 1481 | 45.6 |
| ≥ 10 | 454 | 14.0 |
| Medical examinationb | ||
| Psychiatry | 917 | 28.2 |
| Internal medicine | 2004 | 61.7 |
| Others | 327 | 10.1 |
| Narcolepsy-like events | ||
| No | 3113 | 95.8 |
| Yes | 92 | 2.8 |
| Unknown | 43 | 1.3 |
| Diagnosis of narcolepsy (including cataplexy) | ||
| No | 3206 | 98.7 |
| Suspected | 4 | 0.1 |
| Yes | 1 | < 0.1 |
| Unknown | 37 | 1.1 |
| Respiratory dysfunction | ||
| No | 2866 | 88.2 |
| Yes | 121 | 3.7 |
| Unknown | 261 | 8.0 |
| Chronic obstructive pulmonary disease (COPD) | ||
| No | 3077 | 94.7 |
| Yes | 50 | 1.5 |
| Unknown | 121 | 3.7 |
| Obstructive sleep apnea (OSA) | ||
| No | 2919 | 89.9 |
| Yes | 73 | 2.2 |
| Unknown | 256 | 7.9 |
| Severe respiratory dysfunction | ||
| No | 2959 | 91.1 |
| Yes | 28 | 0.9 |
| Unknown | 261 | 8.0 |
| Hepatic function disorder | ||
| No | 2984 | 91.9 |
| Yes | 141 | 4.3 |
| Unknown | 123 | 3.8 |
| Severe hepatic function disorder | ||
| No | 3120 | 96.1 |
| Yes | 5 | 0.2 |
| Unknown | 123 | 3.8 |
| Structural brain disorder | ||
| No | 3142 | 96.7 |
| Yes | 106 | 3.3 |
| Psychiatric disorder | ||
| No | 2182 | 67.2 |
| Yes | 1007 | 31.0 |
| Unknown | 59 | 1.8 |
| Schizophrenia | ||
| No | 3095 | 95.3 |
| Yes | 92 | 2.8 |
| Unknown | 61 | 1.9 |
| Depression | ||
| No | 2680 | 82.5 |
| Yes | 508 | 15.6 |
| Unknown | 60 | 1.8 |
| Manic-depressive illness | ||
| No | 3103 | 95.5 |
| Yes | 84 | 2.6 |
| Unknown | 61 | 1.9 |
| Anxiety disorder | ||
| No | 2976 | 91.6 |
| Yes | 211 | 6.5 |
| Unknown | 61 | 1.9 |
| Dementia | ||
| No | 3043 | 93.7 |
| Yes | 144 | 4.4 |
| Unknown | 61 | 1.9 |
| Medical history | ||
| No | 2498 | 76.9 |
| Yes | 543 | 16.7 |
| Unknown | 207 | 6.4 |
| Prior medication | ||
| No | 1946 | 59.9 |
| Yes | 1277 | 39.3 |
| Unknown | 25 | 0.8 |
| Concomitant medication | ||
| No | 982 | 30.2 |
| Yes | 2241 | 69.0 |
| Unknown | 25 | 0.8 |
| Concomitant insomnia medication | ||
| No | 2335 | 71.9 |
| Yes | 888 | 27.3 |
| Unknown | 25 | 0.8 |
| Initiation status | ||
| Naïve | 1946 | 59.9 |
| Switch | 703 | 21.6 |
| Add-on | 536 | 16.5 |
| Others | 63 | 1.9 |
BMI body mass index
aTotal for each item will not be 100% due to rounding
bPsychiatric and psychosomatic medicines are given as ‘psychiatry’
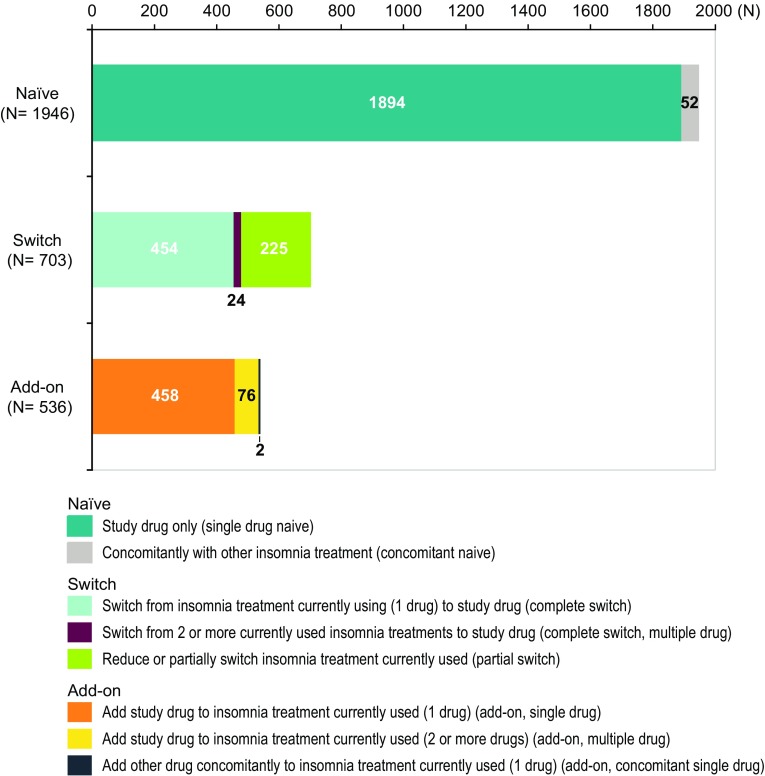
Among patients with special demographic characteristics of interest, there was one patient (0.03%) who was diagnosed with narcolepsy (including cataplexy) before receiving treatment with suvorexant, 141 patients with hepatic function disorder [4.3%, including five patients (0.2%) with severe hepatic function disorder (which was recorded as ‘severe’ in the survey form)], and 121 patients with respiratory dysfunction (3.7%, including 28 patients (0.9%) with severe respiratory dysfunction). Of the 121 patients with respiratory dysfunction, 73 patients had obstructive sleep apnea (OSA) (26 patients were severe) and 50 patients had chronic obstructive pulmonary disease (COPD) (four patients were severe). In total, 106 patients (3.3%) had structural brain disorder; 1007 (31.0%) had a psychiatric disorder, including 508 patients (15.6%) with depression, 211 (6.5%) with anxiety disorder, 92 (2.8%) with schizophrenia, and 84 (2.6%) with manic-depressive illness; and 144 (4.4%) had dementia. The previous clinical trials of suvorexant [6, 7] specified exclusion criteria and targeted patients with insomnia as the primary disease, while the current survey included patients with a broader background. There were 1277 patients (39.3%) who had received prior drugs for treating insomnia and 888 (27.3%) who received other concomitant drugs for treating insomnia. Regarding the initiation status of suvorexant, 1946 patients (59.9%) were treatment-naïve, 703 patients (21.6%) were switched from other insomnia treatments, and 536 patients (16.5%) were receiving add-on therapy. Although most treatment-naïve patients were treated with only suvorexant [97.3% (1894/1946 patients)], there were some patients who started treatment with suvorexant simultaneously with other insomnia treatments [2.7% (52/1946 patients)]. For those who switched treatment, most had discontinued their prior treatment before they started treatment with suvorexant (complete switch) [68.0% (478/703 patients; 454 patients switched to suvorexant from a single drug)] and some discontinued/reduced treatment or switched to another drug and started treatment with suvorexant (partial switch) [32.0% (225/703 patients)]. For patients who received suvorexant as add-on treatment, most patients added suvorexant to their single treatment (either adding on only suvorexant or adding on suvorexant plus one or more other drugs) [85.8% (460/536 patients)], but some patients [14.2% (76/536 patients)] added suvorexant to their multiple treatments (add-on to multiple drugs) (Fig. 2). The mean initial dose of suvorexant was 16.9 mg, and the mean treatment period was 113 days.
Fig. 2.
Initiation status of suvorexant (safety analysis population). Data for 63 patients in the “Others” category of Table 1 not shown
Safety
Among the 3248 patients in the safety analysis population, 377 adverse drug reactions (adverse events for which a causal relationship with suvorexant could not be ruled out based on the clinician’s assessment) were observed in 315 patients (9.7%) (Table 3). The most common adverse drug reactions were somnolence (117 patients; 3.6%), insomnia (40 patients; 1.2%), dizziness (35 patients; 1.1%), and nightmare (27 patients; 0.8%), none of which was considered serious. Most adverse drug reactions were resolved or resolving; 169 were resolved, 33 were resolving, ten were not resolved, and seven were unknown. In total, 8.0% (260/3428; Fig. 4) of patients discontinued suvorexant after an adverse event. A total of 13 serious adverse drug reactions were reported in eight patients (0.2%; two events of delirium, one each of agitation, dissociative disorder, irritability, suicidal ideation, coughing, dyspnea, pneumonia aspiration, falling, femoral neck fracture, subdural hematoma, and traumatic intracranial hemorrhage). All serious adverse drug reactions were resolved or resolving except in three patients [one with dissociative disorder (outcome: unknown), one with falling (not resolved), and one with traumatic intracranial hemorrhage and subdural hematoma (resolved with sequelae)]. After the end of this survey, the causal relationship for subdural hematoma and traumatic intracranial hemorrhage with suvorexant were ruled out by the reporting physician.
Table 3.
Incidence of adverse drug reactions and other events (safety analysis population)
| Items | Cumulative results of drug-use survey |
|---|---|
| Number of patients in the safety analysis population | 3248 |
| Number of patients with adverse drug reactions | 315 |
| Number of adverse drug reaction events | 377 |
| Rate of occurrence | 9.70% |
| Type of adverse drug reactions | Patients with adverse drug reactions | |
|---|---|---|
| N | % | |
| Mental disorder | 100 | 3.08 |
| Abnormal dreams | 7 | 0.22 |
| Agitation | 1 | 0.03 |
| Delirium | 2 | 0.06 |
| Depression | 1 | 0.03 |
| Disorientation | 1 | 0.03 |
| Dissociative disorder | 1 | 0.03 |
| Hallucination | 1 | 0.03 |
| Hallucination, visual | 2 | 0.06 |
| Hypnagogic hallucination | 1 | 0.03 |
| Initial insomnia | 9 | 0.28 |
| Insomnia | 40 | 1.23 |
| Irritability | 1 | 0.03 |
| Middle insomnia | 6 | 0.18 |
| Nervousness | 1 | 0.03 |
| Nightmare | 27 | 0.83 |
| REM sleep abnormality | 1 | 0.03 |
| Sleep talking | 1 | 0.03 |
| Suicidal ideation | 1 | 0.03 |
| Nervous system disorders | 185 | 5.70 |
| Disturbance in attention | 1 | 0.03 |
| Dizziness | 35 | 1.08 |
| Dyslalia | 1 | 0.03 |
| Head discomfort | 2 | 0.06 |
| Headache | 12 | 0.37 |
| Hypersomnia | 6 | 0.18 |
| Paresthesia | 1 | 0.03 |
| Sedation | 4 | 0.12 |
| Sleep paralysis | 5 | 0.15 |
| Sleep phase rhythm disturbance | 1 | 0.03 |
| Somnolence | 117 | 3.60 |
| Cognitive disorder | 1 | 0.03 |
| Restless legs syndrome | 1 | 0.03 |
| Poor quality sleep | 4 | 0.12 |
| Angiopathy | 2 | 0.06 |
| Hot flush | 2 | 0.06 |
| Respiratory, thoracic and mediastinal disorders | 4 | 0.12 |
| Cough | 1 | 0.03 |
| Dyspnea | 1 | 0.03 |
| Pneumonia aspiration | 1 | 0.03 |
| Rhinitis allergic | 1 | 0.03 |
| Sleep apnea syndrome | 1 | 0.03 |
| Gastrointestinal disorders | 15 | 0.46 |
| Abdominal discomfort | 1 | 0.03 |
| Diarrhea | 3 | 0.09 |
| Gastritis | 1 | 0.03 |
| Nausea | 9 | 0.28 |
| Salivary hypersecretion | 1 | 0.03 |
| Skin and subcutaneous tissue disorders | 8 | 0.25 |
| Cold sweat | 1 | 0.03 |
| Drug eruption | 1 | 0.03 |
| Eczema | 1 | 0.03 |
| Hyperhidrosis | 1 | 0.03 |
| Night sweats | 1 | 0.03 |
| Pruritus | 2 | 0.06 |
| Rash | 2 | 0.06 |
| Renal and urinary disorders | 1 | 0.03 |
| Pollakiuria | 1 | 0.03 |
| General disorders and administration site conditions | 42 | 1.29 |
| Asthenia | 1 | 0.03 |
| Discomfort | 1 | 0.03 |
| Face edema | 1 | 0.03 |
| Feeling abnormal | 11 | 0.34 |
| Feeling cold | 1 | 0.03 |
| Hangover | 7 | 0.22 |
| Malaise | 17 | 0.52 |
| Edema peripheral | 2 | 0.06 |
| Thirst | 3 | 0.09 |
| Investigation | 1 | 0.03 |
| Blood glucose increased | 1 | 0.03 |
| Injury, poisoning and procedural complications | 3 | 0.09 |
| Fall | 2 | 0.06 |
| Femoral neck fracture | 1 | 0.03 |
| Subdural hematomaa | 1 | 0.03 |
| Traumatic intracranial hemorrhagea | 1 | 0.03 |
aIn a patient who experienced subdural hematoma and traumatic intracranial hemorrhage, the causal relationship to the survey drug was denied by the additional information given by the physician after completion of the survey
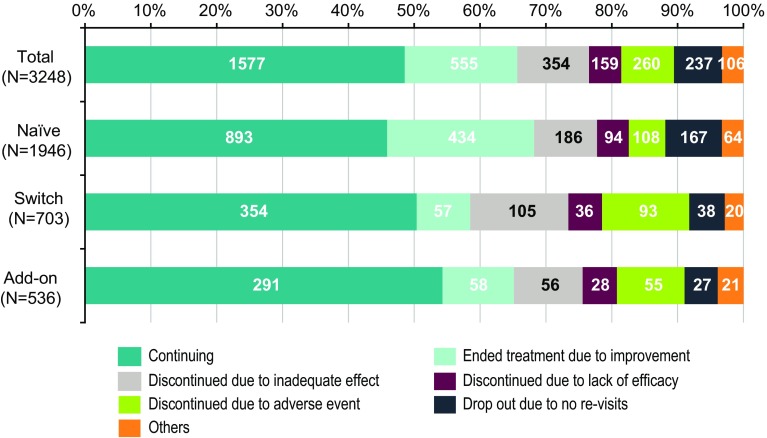
Fig. 4.
Continuation status of suvorexant (safety analysis population). “Total” includes 63 patients in the “Others” category of Table 1
Regarding adverse drug reactions that necessitated evaluation in the PDMA review report [5] or were stated in the Japanese package insert [4], two patients experienced adverse drug reactions that were reported to affect potentially hazardous machinery operation (e.g., driving) (one patient with disturbance in attention and one with somnolence), but no accidents or associated injuries occurred. Narcolepsy-like events occurred in five patients with sleep paralysis, two with falls, and one with hypnagogic hallucination. Parasomnia, abnormal sleep-related events, and sleepwalking occurred in 27 patients with nightmare, seven with abnormal dreams, one with REM sleep abnormality, and one with sleep talking. Suicidal ideation occurred in one patient. There were no adverse drug reactions related to suicidal behavior or dependency (i.e., alcohol or drug dependence). All outcomes were recovering or recovered except for unknown outcome for nightmare (one case) and abnormal dreams (two cases), and unrecovered outcome for sleep paralysis (one case), fall (one case), and nightmare (two cases).
Among the 180 patients who were excluded from the safety analysis population, one case of malaise occurred in one patient; the patient recovered.
Multivariate logistic regression analysis revealed that concomitant drug use and duration of insomnia were factors that affected the occurrence of somnolence, which was intensively investigated in this survey (Table 4). Patients who used concomitant drugs had a higher risk of somnolence than those who did not use them (odds ratio 3.007, 95% confidence interval CI 1.307–6.918; p = 0.010). On the other hand, patients with a duration of insomnia of ≥ 10 years had a lower risk of somnolence than those with a duration of insomnia of < 1 year (odds ratio 0.308, 95% CI 0.112–0.845; p = 0.022).
Table 4.
Analysis of factors potentially influencing an adverse drug reaction of somnolence (safety analysis population)
| Item | N | Adverse drug reaction of somnolence | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | % | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| Overall | 3248 | 117 | 3.6 | ||||||
| Sex | |||||||||
| Male | 1271 | 46 | 3.6 | 1 | 1 | ||||
| Female | 1977 | 71 | 3.6 | 0.992 | 0.680–1.447 | 0.967 | 0.857 | 0.512–1.434 | 0.558 |
| Age (years) | |||||||||
| < 65 | 1490 | 76 | 5.1 | 1 | 1 | ||||
| ≥ 65 to < 85 | 1490 | 34 | 2.3 | 0.434 | 0.288–0.655 | < 0.001 | 0.659 | 0.373–1.163 | 0.150 |
| ≥ 85 | 268 | 7 | 2.6 | 0.499 | 0.228–1.094 | 0.083 | 0.497 | 0.140–1.766 | 0.280 |
| BMI (kg/m2) | |||||||||
| < 25.0 | 1357 | 47 | 3.5 | 1 | 1 | ||||
| ≥ 25.0 | 497 | 18 | 3.6 | 1.047 | 0.602–1.821 | 0.870 | 0.921 | 0.517–1.639 | 0.779 |
| Duration of insomnia (years) | |||||||||
| < 1 | 1313 | 54 | 4.1 | 1 | 1 | ||||
| ≥ 1 to < 10 | 1481 | 53 | 3.6 | 0.865 | 0.588–1.274 | 0.463 | 0.598 | 0.338–1.056 | 0.076 |
| ≥ 10 | 454 | 10 | 2.2 | 0.525 | 0.265–1.040 | 0.065 | 0.308 | 0.112–0.845 | 0.022 |
| Schizophrenia | |||||||||
| No | 3095 | 113 | 3.7 | 1 | 1 | ||||
| Yes | 92 | 4 | 4.3 | 1.200 | 0.433–3.325 | 0.726 | 0.327 | 0.044–2.450 | 0.277 |
| Depression | |||||||||
| No | 2680 | 88 | 3.3 | 1 | 1 | ||||
| Yes | 508 | 29 | 5.7 | 1.783 | 1.159–2.744 | 0.008 | 1.127 | 0.583–2.182 | 0.722 |
| Manic-depressive illness | |||||||||
| No | 3103 | 110 | 3.5 | 1 | 1 | ||||
| Yes | 84 | 7 | 8.3 | 2.474 | 1.115–5.487 | 0.026 | 1.669 | 0.476–5.854 | 0.423 |
| Anxiety disorder | |||||||||
| No | 2976 | 106 | 3.6 | 1 | 1 | ||||
| Yes | 211 | 11 | 5.2 | 1.489 | 0.787–2.816 | 0.221 | 1.298 | 0.531–3.168 | 0.567 |
| Dementia | |||||||||
| No | 3043 | 113 | 3.7 | 1 | 1 | ||||
| Yes | 144 | 4 | 2.8 | 0.741 | 0.270–2.038 | 0.562 | 0.708 | 0.160–3.137 | 0.649 |
| Concomitant medication | |||||||||
| No | 982 | 19 | 1.9 | 1 | 1 | ||||
| Yes | 2241 | 98 | 4.4 | 2.318 | 1.410–3.810 | < 0.001 | 3.007 | 1.307–6.918 | 0.010 |
| Initiation status | |||||||||
| Naïve | 1946 | 59 | 3.0 | 1 | 1 | ||||
| Switch | 703 | 33 | 4.7 | 1.575 | 1.020–2.434 | 0.041 | 1.504 | 0.793–2.854 | 0.212 |
| Add-on | 536 | 21 | 3.9 | 1.304 | 0.785–2.166 | 0.305 | 1.147 | 0.553–2.380 | 0.713 |
BMI body mass index, CI confidence interval
Incidence rates of adverse drug reactions according to demographic characteristics are shown in Table 5, and patients with a background of special interest and the adverse drug reactions that occurred in those patients are indicated in Sects. 3.3.1–3.3.4.
Table 5.
Adverse drug reactions by demographic characteristic (safety analysis population)
| Items | N | Number of patients with events | Number of events | Rate of occurrence (%) |
|---|---|---|---|---|
| Overall | 3248 | 315 | 377 | 9.7 |
| Sex | ||||
| Male | 1271 | 129 | 147 | 10.1 |
| Female | 1977 | 186 | 230 | 9.4 |
| Age group 1 (years) | ||||
| < 15 | 2 | 0 | 0 | 0.0 |
| ≥ 15 to < 65 | 1488 | 168 | 206 | 11.3 |
| ≥ 65 | 1758 | 147 | 171 | 8.4 |
| Age group 2 (years) | ||||
| < 65 | 1490 | 168 | 206 | 11.3 |
| ≥ 65 to < 75 | 730 | 63 | 76 | 8.6 |
| ≥ 75 | 1028 | 84 | 95 | 8.2 |
| Age group 3 (years) | ||||
| < 65 | 1490 | 168 | 206 | 11.3 |
| ≥ 65 to < 85 | 1490 | 126 | 147 | 8.5 |
| ≥ 85 | 268 | 21 | 24 | 7.8 |
| BMI (kg/m2) | ||||
| < 25.0 | 1357 | 135 | 160 | 9.9 |
| ≥ 25.0 | 497 | 52 | 66 | 10.5 |
| Unknown | 1394 | 128 | 151 | 9.2 |
| Reason for use (multi-count) | ||||
| Difficulty falling asleep | 2429 | 217 | 264 | 8.9 |
| Nocturnal awakening | 1596 | 176 | 215 | 11.0 |
| Early morning awakening | 349 | 43 | 52 | 12.3 |
| Others | 31 | 8 | 10 | 25.8 |
| Duration of insomnia (years) | ||||
| < 1 | 1313 | 116 | 139 | 8.8 |
| ≥ 1 to < 10 | 1481 | 149 | 181 | 10.1 |
| ≥ 10 | 454 | 50 | 57 | 11.0 |
| Medical examinationa | ||||
| Psychiatry | 917 | 127 | 157 | 13.8 |
| Internal medicine | 2004 | 150 | 171 | 7.5 |
| Others | 327 | 38 | 49 | 11.6 |
| Narcolepsy-like events | ||||
| No | 3113 | 303 | 361 | 9.7 |
| Yes | 92 | 9 | 11 | 9.8 |
| Unknown | 43 | 3 | 5 | 7.0 |
| Diagnosis of narcolepsy (including cataplexy) | ||||
| No | 3206 | 311 | 373 | 9.7 |
| Suspected | 4 | 1 | 1 | 25.0 |
| Yes | 1 | 0 | 0 | 0.0 |
| Unknown | 37 | 3 | 3 | 8.1 |
| Respiratory dysfunction | ||||
| No | 2866 | 274 | 332 | 9.6 |
| Yes | 121 | 10 | 10 | 8.3 |
| Unknown | 261 | 31 | 35 | 11.9 |
| Chronic obstructive pulmonary disease (COPD) | ||||
| No | 3077 | 301 | 361 | 9.8 |
| Yes | 50 | 2 | 2 | 4.0 |
| Unknown | 121 | 12 | 14 | 9.9 |
| Obstructive sleep apnea (OSA) | ||||
| No | 2919 | 275 | 333 | 9.4 |
| Yes | 73 | 8 | 8 | 11.0 |
| Unknown | 256 | 32 | 36 | 12.5 |
| Severe respiratory dysfunction | ||||
| No | 2959 | 282 | 340 | 9.5 |
| Yes | 28 | 2 | 2 | 7.1 |
| Unknown | 261 | 31 | 35 | 11.9 |
| Hepatic function disorder | ||||
| No | 2984 | 276 | 331 | 9.2 |
| Yes | 141 | 22 | 25 | 15.6 |
| Unknown | 123 | 17 | 21 | 13.8 |
| Severe hepatic function disorder | ||||
| No | 3120 | 297 | 355 | 9.5 |
| Yes | 5 | 1 | 1 | 20.0 |
| Unknown | 123 | 17 | 21 | 13.8 |
| Structural brain disorder | ||||
| No | 3142 | 307 | 367 | 9.8 |
| Yes | 106 | 8 | 10 | 7.5 |
| Psychiatric disorder | ||||
| No | 2182 | 166 | 190 | 7.6 |
| Yes | 1007 | 144 | 181 | 14.3 |
| Unknown | 59 | 5 | 6 | 8.5 |
| Schizophrenia | ||||
| No | 3095 | 296 | 350 | 9.6 |
| Yes | 92 | 13 | 20 | 14.1 |
| Unknown | 61 | 6 | 7 | 9.8 |
| Depression | ||||
| No | 2680 | 239 | 282 | 8.9 |
| Yes | 508 | 70 | 88 | 13.8 |
| Unknown | 60 | 6 | 7 | 10.0 |
| Manic-depressive illness | ||||
| No | 3103 | 298 | 356 | 9.6 |
| Yes | 84 | 11 | 14 | 13.1 |
| Unknown | 61 | 6 | 7 | 9.8 |
| Anxiety disorder | ||||
| No | 2976 | 282 | 332 | 9.5 |
| Yes | 211 | 27 | 38 | 12.8 |
| Unknown | 61 | 6 | 7 | 9.8 |
| Dementia | ||||
| No | 3043 | 300 | 358 | 9.9 |
| Yes | 144 | 9 | 12 | 6.3 |
| Unknown | 61 | 6 | 7 | 9.8 |
| Medical history | ||||
| No | 2498 | 224 | 268 | 9.0 |
| Yes | 543 | 69 | 82 | 12.7 |
| Unknown | 207 | 22 | 27 | 10.6 |
| Prior medication | ||||
| No | 1946 | 132 | 157 | 6.8 |
| Yes | 1277 | 182 | 219 | 14.3 |
| Unknown | 25 | 1 | 1 | 4.0 |
| Concomitant medication | ||||
| No | 982 | 47 | 57 | 4.8 |
| Yes | 2241 | 267 | 319 | 11.9 |
| Unknown | 25 | 1 | 1 | 4.0 |
| Concomitant insomnia medication | ||||
| No | 2335 | 192 | 225 | 8.2 |
| Yes | 888 | 122 | 151 | 13.7 |
| Unknown | 25 | 1 | 1 | 4.0 |
| Initiation status | ||||
| Naïve | 1946 | 132 | 157 | 6.8 |
| Switch | 703 | 110 | 137 | 15.6 |
| Add-on | 536 | 68 | 76 | 12.7 |
| Others | 63 | 5 | 7 | 7.9 |
BMI body mass index
aPsychiatric and psychosomatic medicines are given as ‘psychiatry’
Patients with Narcolepsy Including Cataplexy
No adverse drug reaction was observed in one patient with narcolepsy diagnosed before the initiation of suvorexant. Among four patients with narcolepsy suspected before the initiation of suvorexant, an adverse drug reaction of poor quality sleep (non-severe, recovered) occurred in one patient (0.03%).
Elderly Patients
Among patients aged ≥ 65 years, adverse drug reactions that occurred in ≥ 5 patients were somnolence (41 patients; 2.3%), dizziness (24 patients; 1.4%), insomnia (23 patients; 1.3%), nightmare (11 patients; 0.6%), headache (6 patients; 0.3%), and nausea and feeling abnormal (5 patients each; 0.3%). The incidence rate of adverse drug reactions in elderly patients [8.4% (147/1758 patients)] was lower than that in non-elderly patients under the age of 65 years [11.3% (168/1490 patients)]. The incidence rate of adverse drug reactions in latter-stage elderly patients aged ≥ 75 years was 8.2% (84/1028 patients), which was lower than the incidence rates in patients under the age of 65 years and those aged between 65 and 74 years (11.3% (168/1490 patients) and 8.6% (63/730 patients), respectively).
Patients with Respiratory Dysfunction
Adverse drug reactions occurred in 8.3% (10/121 patients) of patients with respiratory dysfunction: five patients with somnolence and one each with insomnia, dizziness, pruritus, feeling abnormal, and hangover. All events were not serious, and the patients recovered. No adverse drug reaction related to respiratory depression was observed.
Adverse drug reactions occurred in 7.1% (2/28 patients) of patients with severe respiratory dysfunction. In severe OSA patients, there was one incidence each of insomnia and pruritus that were not serious. The patients recovered from both events.
Patients with Psychiatric Disorders
The incidence of adverse drug reactions in patients with psychiatric disorder(s) was 14.3% (144/1007 patients), higher than the 7.6% incidence in patients without psychiatric disorder(s) (166/2182 patients). Adverse drug reactions that occurred in five or more patients with psychiatric disorder(s) were somnolence (56 patients), insomnia (20 patients), dizziness (18 patients), malaise (11 patients), and nightmare (ten patients). The outcomes were as follows: 88, recovered; 17, recovering; four, not recovered; and six, outcome unknown. For each psychiatric disorder, the rate of patients experiencing adverse drug reactions with/without co-morbid schizophrenia was 14.1% (13/92 patients)/9.6% (296/3095 patients), with/without co-morbid depression was 13.8% (70/508 patients)/8.9% (239/2680 patients), with/without manic-depressive illness was 13.1% (11/84 patients)/9.6% (298/3103 patients), and with/without anxiety disorder was 12.8% (27/211 patients)/9.5% (282/2976 patients).
Of the 144 patients with a psychiatric disorder who experienced adverse drug reactions, 86.1% (124/144 patients) were also taking CNS-active medications versus 13.9% (20/144 patients) not taking these medications.
Efficacy
Overall Global Improvement
In the 2439 patients who had a final overall global physician assessment, the proportion of patients judged by the physician to be ‘improved’ (improvement rate) was 74.0% (1806/2439 patients). The proportions of patients judged to be ‘unchanged’ and ‘deteriorated’ were 22.2% (542/2439 patients) and 3.7% (91/2439 patients), respectively (Table 6). In the 2424 evaluable patients who had a final overall global self-assessment, the proportion who judged themselves to be ‘improved’ (improvement rate) was 73.2% (1775/2424 patients), similar to the results assessed by the physician.
Table 6.
Final overall global improvement by demographic characteristic (physician’s assessment) (efficacy analysis population)
| Items | Number of patients by characteristic | Improved patients | Unchanged patients | Deteriorated patients | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Overall | 2439 | 1806 | 74.0 | 542 | 22.2 | 91 | 3.7 |
| Sex | |||||||
| Male | 943 | 686 | 72.7 | 218 | 23.1 | 39 | 4.1 |
| Female | 1496 | 1120 | 74.9 | 324 | 21.7 | 52 | 3.5 |
| Age group 1 (years) | |||||||
| < 65 | 997 | 731 | 73.3 | 226 | 22.7 | 40 | 4.0 |
| ≥ 65 | 1442 | 1075 | 74.5 | 316 | 21.9 | 51 | 3.5 |
| Age group 2 (years) | |||||||
| < 65 | 997 | 731 | 73.3 | 226 | 22.7 | 40 | 4.0 |
| ≥ 65 to < 75 | 565 | 417 | 73.8 | 128 | 22.7 | 20 | 3.5 |
| ≥ 75 | 877 | 658 | 75.0 | 188 | 21.4 | 31 | 3.5 |
| Age group 3 (years) | |||||||
| < 65 | 997 | 731 | 73.3 | 226 | 22.7 | 40 | 4.0 |
| ≥ 65 to < 85 | 1213 | 898 | 74.0 | 273 | 22.5 | 42 | 3.5 |
| ≥ 85 | 229 | 177 | 77.3 | 43 | 18.8 | 9 | 3.9 |
| BMI (kg/m2) | |||||||
| < 25.0 | 1046 | 782 | 74.8 | 233 | 22.3 | 31 | 3.0 |
| ≥ 25.0 | 377 | 276 | 73.2 | 82 | 21.8 | 19 | 5.0 |
| Unknown | 1016 | 748 | 73.6 | 227 | 22.3 | 41 | 4.0 |
| Reason for use (multi-count) | |||||||
| Difficulty falling asleep | 1826 | 1356 | 74.3 | 410 | 22.5 | 60 | 3.3 |
| Nocturnal awakening | 1222 | 908 | 74.3 | 262 | 21.4 | 52 | 4.3 |
| Early morning awakening | 261 | 190 | 72.8 | 59 | 22.6 | 12 | 4.6 |
| Others | 22 | 16 | 72.7 | 5 | 22.7 | 1 | 4.5 |
| Duration of insomnia (years) | |||||||
| < 1 | 968 | 756 | 78.1 | 189 | 19.5 | 23 | 2.4 |
| ≥ 1 to < 10 | 1124 | 814 | 72.4 | 262 | 23.3 | 48 | 4.3 |
| ≥ 10 | 347 | 236 | 68.0 | 91 | 26.2 | 20 | 5.8 |
| Medical examinationa | |||||||
| Psychiatry | 689 | 492 | 71.4 | 167 | 24.2 | 30 | 4.4 |
| Internal medicine | 1515 | 1139 | 75.2 | 320 | 21.1 | 56 | 3.7 |
| Others | 235 | 175 | 74.5 | 55 | 23.4 | 5 | 2.1 |
| Narcolepsy-like events | |||||||
| No | 2344 | 1746 | 74.5 | 513 | 21.9 | 85 | 3.6 |
| Yes | 63 | 43 | 68.3 | 16 | 25.4 | 4 | 6.3 |
| Unknown | 32 | 17 | 53.1 | 13 | 40.6 | 2 | 6.3 |
| Diagnosis of narcolepsy (including cataplexy) | |||||||
| No | 2404 | 1784 | 74.2 | 531 | 22.1 | 89 | 3.7 |
| Suspected | 2 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 |
| Yes | 1 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 |
| Unknown | 32 | 21 | 65.6 | 9 | 28.1 | 2 | 6.3 |
| Respiratory dysfunction | |||||||
| No | 2151 | 1611 | 74.9 | 461 | 21.4 | 79 | 3.7 |
| Yes | 102 | 78 | 76.5 | 22 | 21.6 | 2 | 2.0 |
| Unknown | 186 | 117 | 62.9 | 59 | 31.7 | 10 | 5.4 |
| Chronic obstructive pulmonary disease (COPD) | |||||||
| No | 2306 | 1711 | 74.2 | 508 | 22.0 | 87 | 3.8 |
| Yes | 44 | 37 | 84.1 | 7 | 15.9 | 0 | 0.0 |
| Unknown | 89 | 58 | 65.2 | 27 | 30.3 | 4 | 4.5 |
| Obstructive sleep apnea (OSA) | |||||||
| No | 2197 | 1647 | 75.0 | 471 | 21.4 | 79 | 3.6 |
| Yes | 60 | 43 | 71.7 | 15 | 25.0 | 2 | 3.3 |
| Unknown | 182 | 116 | 63.7 | 56 | 30.8 | 10 | 5.5 |
| Severe respiratory dysfunction | |||||||
| No | 2230 | 1670 | 74.9 | 479 | 21.5 | 81 | 3.6 |
| Yes | 23 | 19 | 82.6 | 4 | 17.4 | 0 | 0.0 |
| Unknown | 186 | 117 | 62.9 | 59 | 31.7 | 10 | 5.4 |
| Hepatic function disorder | |||||||
| No | 2254 | 1680 | 74.5 | 491 | 21.8 | 83 | 3.7 |
| Yes | 106 | 75 | 70.8 | 27 | 25.5 | 4 | 3.8 |
| Unknown | 79 | 51 | 64.6 | 24 | 30.4 | 4 | 5.1 |
| Severe hepatic function disorder | |||||||
| No | 2356 | 1752 | 74.4 | 517 | 21.9 | 87 | 3.7 |
| Yes | 4 | 3 | 75.0 | 1 | 25.0 | 0 | 0.0 |
| Unknown | 79 | 51 | 64.6 | 24 | 30.4 | 4 | 5.1 |
| Structural brain disorder | |||||||
| No | 2357 | 1740 | 73.8 | 529 | 22.4 | 88 | 3.7 |
| Yes | 82 | 66 | 80.5 | 13 | 15.9 | 3 | 3.7 |
| Psychiatric disorder | |||||||
| No | 1642 | 1246 | 75.9 | 343 | 20.9 | 53 | 3.2 |
| Yes | 756 | 534 | 70.6 | 185 | 24.5 | 37 | 4.9 |
| Unknown | 41 | 26 | 63.4 | 14 | 34.1 | 1 | 2.4 |
| Schizophrenia | |||||||
| No | 2321 | 1728 | 74.5 | 507 | 21.8 | 86 | 3.7 |
| Yes | 76 | 52 | 68.4 | 21 | 27.6 | 3 | 3.9 |
| Unknown | 42 | 26 | 61.9 | 14 | 33.3 | 2 | 4.8 |
| Depression | |||||||
| No | 2012 | 1500 | 74.6 | 441 | 21.9 | 71 | 3.5 |
| Yes | 386 | 280 | 72.5 | 87 | 22.5 | 19 | 4.9 |
| Unknown | 41 | 26 | 63.4 | 14 | 34.1 | 1 | 2.4 |
| Manic-depressive illness | |||||||
| No | 2333 | 1742 | 74.7 | 505 | 21.6 | 86 | 3.7 |
| Yes | 64 | 38 | 59.4 | 23 | 35.9 | 3 | 4.7 |
| Unknown | 42 | 26 | 61.9 | 14 | 33.3 | 2 | 4.8 |
| Anxiety disorder | |||||||
| No | 2243 | 1669 | 74.4 | 492 | 21.9 | 82 | 3.7 |
| Yes | 154 | 111 | 72.1 | 36 | 23.4 | 7 | 4.5 |
| Unknown | 42 | 26 | 61.9 | 14 | 33.3 | 2 | 4.8 |
| Dementia | |||||||
| No | 2279 | 1692 | 74.2 | 505 | 22.2 | 82 | 3.6 |
| Yes | 118 | 88 | 74.6 | 23 | 19.5 | 7 | 5.9 |
| Unknown | 42 | 26 | 61.9 | 14 | 33.3 | 2 | 4.8 |
| Medical history | |||||||
| No | 1875 | 1398 | 74.6 | 413 | 22.0 | 64 | 3.4 |
| Yes | 403 | 286 | 71.0 | 98 | 24.3 | 19 | 4.7 |
| Unknown | 161 | 122 | 75.8 | 31 | 19.3 | 8 | 5.0 |
| Prior medication | |||||||
| No | 1446 | 1136 | 78.6 | 281 | 19.4 | 29 | 2.0 |
| Yes | 976 | 659 | 67.5 | 256 | 26.2 | 61 | 6.3 |
| Unknown | 17 | 11 | 64.7 | 5 | 29.4 | 1 | 5.9 |
| Concomitant medication | |||||||
| No | 727 | 572 | 78.7 | 136 | 18.7 | 19 | 2.6 |
| Yes | 1695 | 1223 | 72.2 | 401 | 23.7 | 71 | 4.2 |
| Unknown | 17 | 11 | 64.7 | 5 | 29.4 | 1 | 5.9 |
| Concomitant insomnia medication | |||||||
| No | 1756 | 1336 | 76.1 | 364 | 20.7 | 56 | 3.2 |
| Yes | 666 | 459 | 68.9 | 173 | 26.0 | 34 | 5.1 |
| Unknown | 17 | 11 | 64.7 | 5 | 29.4 | 1 | 5.9 |
| Initiation status | |||||||
| Naïve | 1446 | 1136 | 78.6 | 281 | 19.4 | 29 | 2.0 |
| Switch | 538 | 344 | 63.9 | 151 | 28.1 | 43 | 8.0 |
| Add-on | 410 | 292 | 71.2 | 101 | 24.6 | 17 | 4.1 |
| Others | 45 | 34 | 75.6 | 9 | 20.0 | 2 | 4.4 |
Totals will not be 100% due to rounding
BMI body mass index
aPsychiatric and psychosomatic medicines are given as ‘psychiatry’
Even if the population who were excluded because the initial dose, as stated in the Japanese package insert [4] (adult: 20 mg once daily; elderly: 15 mg once daily), was not administered were included in the analysis, the proportion of patients judged by the physician to be ‘improved’ (improvement rate) was similar to that already described.
The physician-assessed global improvement rates in patient groups of special interest were as follows:
Elderly patients: the improvement rate was 73.3% in those < 65 years. Among elderly patients, improvement rates were 74.5% (≥ 65 years), 75.0% (≥ 75 years), and 77.3% (≥ 85 years).
Insomnia symptoms as the reason for using suvorexant: the improvement rates for those patients with difficulty falling asleep, nocturnal awakening, or early morning awakening were 74.3%, 74.3%, and 72.8%, respectively.
Duration of insomnia and use of prior drugs for insomnia as well as other concomitant drugs for insomnia: in terms of the duration of insomnia, the improvement rates were 78.1%, 72.4%, and 68.0% for patients whose duration of insomnia was < 1 year, ≥ 1 to < 10 years, and ≥ 10 years, respectively. The improvement rates were 67.5% with prior drug use for insomnia and 78.6% without any prior drug use for insomnia. In terms of whether other concomitant drugs were used for insomnia, the improvement rates were 68.9% with the use of other concomitant drugs and 76.1% without the use of other concomitant drugs.
Patients with psychiatric disorders: in terms of having or not having a psychiatric disorder, the improvement rates were 70.6% with a psychiatric disorder and 75.9% without a psychiatric disorder. Regarding the types of psychiatric disorder, the improvement rates in patients with/without schizophrenia were 68.4%/74.5%, with/without depression were 72.5%/74.6%, with/without manic-depressive illness were 59.4%/74.7%, and with/without anxiety disorder were 72.1%/74.4%.
Duration of insomnia, use of concomitant medication, and initiation status of suvorexant were identified as factors that affect physician-judged overall global improvement based on the multivariate logistic regression analysis results (Table 7). The probability of efficacy in patients with a duration of insomnia of less than 1 year was higher than that of patients with a duration of insomnia of ≥ 1 to < 10 years and of ≥ 10 years. On the other hand, the probability of efficacy was lower in patients who used concomitant medications and who switched drugs. Similar results to those described for the physician’s judgement were observed in the analysis for overall global improvement judged by the patients.
Table 7.
Analysis of factors potentially influencing overall global improvement (physician’s assessment) (efficacy analysis population)
| Item | N | Overall global improvement | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| Improved patients | Improvement rate (%) | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| Overall | 2439 | 1806 | 74.0 | ||||||
| Sex | |||||||||
| Male | 943 | 686 | 72.7 | 1 | 1 | ||||
| Female | 1496 | 1120 | 74.9 | 1.116 | 0.928–1.343 | 0.245 | 1.126 | 0.876–1.447 | 0.355 |
| Age (years) | |||||||||
| < 65 | 997 | 731 | 73.3 | 1 | 1 | ||||
| ≥ 65 to < 85 | 1213 | 898 | 74.0 | 1.037 | 0.858–1.255 | 0.705 | 1.163 | 0.876–1.543 | 0.296 |
| ≥ 85 | 229 | 177 | 77.3 | 1.239 | 0.882–1.739 | 0.217 | 1.172 | 0.720–1.906 | 0.524 |
| BMI (kg/m2) | |||||||||
| < 25.0 | 1046 | 782 | 74.8 | 1 | 1 | ||||
| ≥ 25.0 | 377 | 276 | 73.2 | 0.923 | 0.706–1.205 | 0.554 | 0.973 | 0.736–1.285 | 0.846 |
| Duration of insomnia (years) | |||||||||
| < 1 | 968 | 756 | 78.1 | 1 | 1 | ||||
| ≥ 1 to < 10 | 1124 | 814 | 72.4 | 0.736 | 0.602–0.900 | 0.003 | 0.741 | 0.553–0.994 | 0.045 |
| ≥ 10 | 347 | 236 | 68.0 | 0.596 | 0.454–0.783 | < 0.001 | 0.600 | 0.404–0.890 | 0.011 |
| Schizophrenia | |||||||||
| No | 2321 | 1728 | 74.5 | 1 | 1 | ||||
| Yes | 76 | 52 | 68.4 | 0.743 | 0.454–1.217 | 0.238 | 0.781 | 0.417–1.464 | 0.441 |
| Depression | |||||||||
| No | 2012 | 1500 | 74.6 | 1 | 1 | ||||
| Yes | 386 | 280 | 72.5 | 0.902 | 0.706–1.152 | 0.407 | 1.051 | 0.730–1.512 | 0.790 |
| Manic-depressive illness | |||||||||
| No | 2333 | 1742 | 74.7 | 1 | 1 | ||||
| Yes | 64 | 38 | 59.4 | 0.495 | 0.298–0.823 | 0.007 | 0.736 | 0.364–1.490 | 0.395 |
| Anxiety disorder | |||||||||
| No | 2243 | 1669 | 74.4 | 1 | 1 | ||||
| Yes | 154 | 111 | 72.1 | 0.888 | 0.617–1.278 | 0.522 | 1.405 | 0.848–2.329 | 0.187 |
| Dementia | |||||||||
| No | 2279 | 1692 | 74.2 | 1 | 1 | ||||
| Yes | 118 | 88 | 74.6 | 1.018 | 0.665–1.556 | 0.936 | 1.077 | 0.610–1.904 | 0.797 |
| Concomitant medication | |||||||||
| No | 727 | 572 | 78.7 | 1 | 1 | ||||
| Yes | 1695 | 1223 | 72.2 | 0.702 | 0.571–0.864 | < 0.001 | 0.716 | 0.520–0.987 | 0.041 |
| Initiation status | |||||||||
| Naïve | 1446 | 1136 | 78.6 | 1 | 1 | ||||
| Switch | 538 | 344 | 63.9 | 0.484 | 0.390–0.601 | < 0.001 | 0.675 | 0.496–0.920 | 0.013 |
| Add-on | 410 | 292 | 71.2 | 0.675 | 0.527–0.865 | 0.002 | 0.863 | 0.603–1.238 | 0.424 |
BMI body mass index, CI confidence interval
Sleep Measures
Changes in median sleep latency and total sleep time by age group and reason for using suvorexant are shown in Fig. 3. (Data from physician interviews with/without a patient’s sleep diary were used for the analysis.)
Fig. 3.
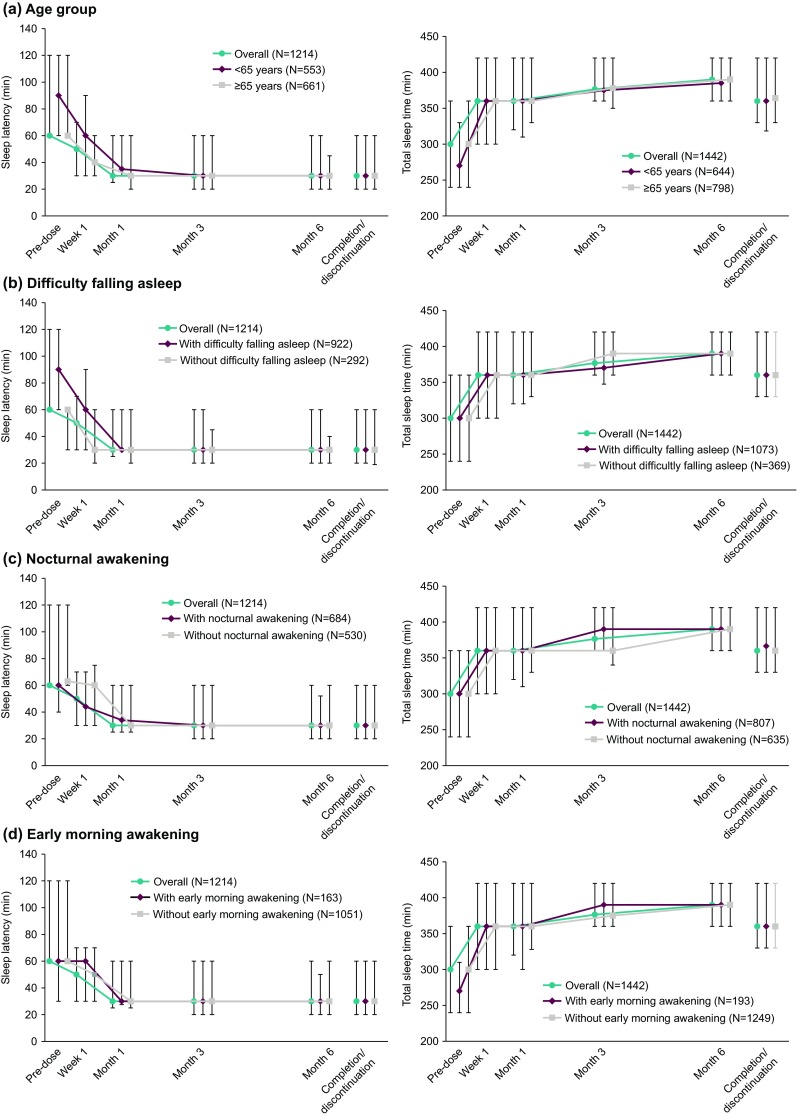
Change in sleep latency and total sleep time (efficacy analysis population) by a age group; b difficulty falling asleep; c nocturnal awakening; and d early morning awakening. Data are presented as median and interquartile range
The median sleep latency was 60 min at pre-dose, 50 min at Week 1, 30 min at Month 1, and this reduction in sleep latency was then maintained up to Month 6. In terms of age groups, reduced sleep latency was observed from Week 1 and was maintained from Month 1 to Month 6 in patients < 65 years and those ≥ 65 years.
The median total sleep time per night changed from 300 min at pre-dose to 360 min at Week 1 and was subsequently maintained at no less than 360 min up to Month 6. In terms of age groups, the median sleep time started lengthening at Week 1, and did not return to the pre-dose value up to Month 6 in patients aged < 65 and ≥ 65 years.
Regarding reasons for using suvorexant, reduction in the median sleep latency was observed at all timepoints in all groups (i.e., patients with difficulty in falling asleep, those with difficulty in falling asleep and early morning awakening, those with difficulty in falling asleep and nocturnal awakening, and those with difficulty in falling asleep, early morning awakening, and nocturnal awakening). Reduced sleep latency was maintained in patients across all reasons for using suvorexant up to Month 6, with no clear differences in changes between the reasons for use. Similarly, lengthening of total sleep time was observed in patients across all reasons for using suvorexant at all observation timepoints and was maintained up to Month 6.
Continuation Status of Suvorexant in Each Initiation Status Group
The continuation status of suvorexant in each initiation status group is shown in Fig. 4. At 6 months after the start of treatment, 48.6% (1577/3248) of the patients had been continually receiving treatment and 51.4% (1671/3248) had discontinued/dropped out of treatment before 6 months.
Among patients who discontinued or dropped out from treatment, the percentage of patients who discontinued treatment because of improvement was the highest [17.1% (555/3248 patients)]. With respect to the initiation status of suvorexant, the percentage of patients who completed treatment because of improvement was the highest in naïve patients [22.3% (434/1946 patients)]. On the other hand, among switched patients, many discontinued treatment because of inadequate effects [14.9% (105/703 patients)] and adverse event occurrence [13.2% (93/703 patients)].
Among the reasons for discontinuation, the mean duration of treatment was as follows: 61.7 days for improvement in insomnia, 45.2 days for inadequate effects, 30.5 days for no efficacy, 32.2 days for the occurrence of adverse events, 52.3 days for dropout due to no subsequent revisit after the second visit, and 56.9 days for other reasons.
Regarding the initiation of treatment in patients who discontinued treatment due to improvement, the mean duration of treatment was 57.0 days for naïve patients, 79.2 days for switched patients, and 77.2 days for add-on patients.
Moreover, for patients who received add-on treatment, 12.1% (65/536 patients) ended, reduced the number of, or reduced the dose of the prior medications.
Discussion
We conducted this survey to evaluate the safety and efficacy of suvorexant use in daily clinical practice in Japan. In this report, data from 3428 patients were collected from 884 medical institutions. Among the safety analysis population, more than half of patients were elderly (31.7% were ≥ 75 years and 8.3% were ≥ 85 years). In addition, this population included patients with a variety of demographic characteristics, such as those with psychiatric disorders and dementia, who were excluded from the clinical trials. These observations suggest that this survey population reflects the conditions of patients in a real-world clinical practice setting. Among the safety analysis population, 59.6% of patients had insomnia for at least 1 year and 14.0% had insomnia for at least 10 years. Although 59.9% of patients were treatment-naïve, many patients received prior medications for insomnia or received other concomitant medications for insomnia.
Regarding safety, the incidence rate of adverse drug reactions was 9.7% (serious adverse drug reactions: 0.2%) and the common adverse drug reactions were somnolence, insomnia, dizziness, and nightmare, all of which are included the product label [4]. In terms of outcomes, > 90% of patients were recovered or recovering. In total, 8.0% of patients in the survey discontinued the use of suvorexant due to adverse events, although some patients continued the use of suvorexant after adverse drug reactions and their adverse drug reactions and were resolved and resolving. The relatively high proportion of patients who discontinued suvorexant after adverse events (8.0%) compared with the overall incidence rate of adverse drug reactions (9.7%) may indicate than in a survey setting patients tend to report adverse drug reactions that are significant for them. In the suvorexant clinical trials, more patients reported adverse drug reactions (46.5%) but relatively few discontinued due to them (3.0%) [6]. While insomnia is not specified as an adverse drug reaction in the Japanese package insert, insomnia is the indication for use of suvorexant, and these reports may well reflect insufficient efficacy in some patients. Regarding somnolence, which was intensively investigated in the current survey and is the most commonly occurring adverse drug reaction, multivariate logistic regression analysis was conducted to identify the demographic characteristics that affect the occurrence of such reactions. As a result, the use of concomitant medications and the duration of insomnia were identified as factors. We did not analyze the effects of different types of concomitant medications, but, given that 27% of patients were using insomnia treatments other than suvorexant, it is possible that concomitant administration of these and other CNS-suppressant drugs (such as antidepressants) influenced the occurrence of somnolence. Additionally, it is possible that many refractory insomnia patients were included in the survey population with a longer duration of insomnia; this could have lowered the occurrence of somnolence in these patients.
Regarding adverse drug reactions that necessitated evaluation in the PDMA review report [5] or were stated in the Japanese package insert [4], two patients experienced adverse drug reactions that were reported to affect potentially hazardous machinery operation (e.g., driving); narcolepsy-like events occurred in five patients with sleep paralysis, two with falls, and one with hypnagogic hallucination; parasomnia, abnormal sleep-related events, and sleepwalking occurred in 27 patients with nightmare, seven with abnormal dreams, one with REM sleep abnormality, and one with sleep talking; and suicidal ideation occurred in one patient. More than 80% of the outcomes of those adverse drug reactions were recovering or recovered. No adverse drug reactions related to suicidal behavior and dependency were observed.
The influence of demographic characteristics was assessed for narcolepsy (including cataplexy) patients, elderly patients, patients with respiratory dysfunction, and patients with psychiatric disorder.
There were no adverse drug reactions in the only patient who was diagnosed with narcolepsy prior to suvorexant treatment. On the other hand, a non-serious adverse drug reaction occurred in one of four patients who were suspected of having narcolepsy. The Japanese package insert of suvorexant states that suvorexant should be administered with caution in patients with narcolepsy or cataplexy [4]. However, the occurrence or aggravation of narcolepsy as a result of administration of suvorexant was not reported in the patients in the current survey.
The incidence rate of adverse drug reactions in latter-stage elderly patients aged ≥ 75 years was 8.2%, which was lower than the incidence rates in patients under the age of 65 years and those aged between 65 and 74 years (11.3% and 8.6%, respectively). Although no safety concern regarding elderly patients was identified in the clinical trial, the Japanese package insert states that suvorexant should be administered with caution in elderly patients due to the fact that elderly individuals generally have deteriorated physiological functions [4]. However, elderly patients did not exhibit a high incidence rate of adverse drug reactions in this survey.
In phase I clinical trials of suvorexant, no obvious safety issue on respiratory function was observed in patients with mild to moderate COPD or OSA [8, 9], and no adverse drug reaction related to respiratory depression was observed in this survey, even though some patients with severe COPD or OSA were included.
The incidence rate of adverse drug reactions in patients with psychiatric disorder(s) was almost two times the rate reported in patients without psychiatric disorder(s) (14.3% vs. 7.6%, respectively). Among patients with a psychiatric disorder and experiencing an adverse drug reaction, the majority (86.1%) were taking other CNS-active medications, as compared to those with a psychiatric disorder and not taking these medications (13.9%). Note that the Japanese package insert states that suvorexant should be used with careful administration in patients concomitantly taking CNS depressants [4].
Multivariate logistic regression results did not detect diagnoses of schizophrenia, depression, manic-depressive illness, and anxiety disorder as risk factors for somnolence, which occurred at the highest rate in patients.
Based on these results, we conclude that the occurrence of adverse drug reactions in daily clinical practice was similar to that observed in clinical trials and that no additional noteworthy findings in terms of the safety of suvorexant have been observed thus far.
In the efficacy assessment, the ‘improved’ rate based on the judgement by the physician and patient were 74.0% and 73.2%, respectively, which was not clinically or significantly different. We also evaluated subgroups of special interest, including elderly patients, reason for using suvorexant, duration of insomnia, use of prior drugs and concomitant drugs for insomnia, and patients with psychiatric disorders.
In terms of age group, the improvement rates were 74.5% (aged ≥ 65 years), 75.0% (aged ≥ 75 years), and 77.3% (aged ≥ 85 years). These values were not significantly different from the improvement rate of the entire population (74.0%).
In terms of reason for using suvorexant, the improvement rates were not significantly different among patients with difficulty in falling asleep, those with nocturnal awakening, and those with early morning awakening. The consistent improvement rate was observed irrespective of the symptoms of insomnia.
Patients with longer durations of insomnia, those using prior drugs for insomnia, and those using other concomitant drugs for insomnia showed a tendency to have lower improvement rates. We speculate that these populations included patients with refractory insomnia, leading to the low improvement rate.
Regarding patients with/without psychiatric disorder(s), the improvement rate was lower in patients with psychiatric disorder(s) (70.6%) than those without psychiatric disorder(s) (75.9%). As a potential reason for this observation, it is speculated that many refractory insomnia patients were included in patients with psychiatric disorder(s); in fact, the proportions of patients with ≥ 10 years’ duration of insomnia, those using prior drugs, and those using concomitant drugs for insomnia were higher in patients with psychiatric disorder(s) than those without psychiatric disorder(s) (20.9% (158/756 patients) vs. 11.3% (186/1642 patients), 58.1% (439/756 patients) vs. 32.2% (528/1642 patients), and 44.2% (334/756 patients) vs. 19.8% (325/1642 patients), respectively).
Multivariate logistic regression was performed, considering confounding factors that could affect overall global improvement. The probability of efficacy was higher in patients with a duration of insomnia of < 1 year than in those with a longer duration (≥ 1 to < 10 years or ≥ 10 years). In addition, the probability of efficacy was lower in patients who used concomitant medications and who switched treatment. On the other hand, specific psychiatric disorders (schizophrenia, depression, manic-depressive illness, and anxiety disorder) and dementia were not detected as factors that affect overall global improvement.
Patients who had been recently diagnosed with insomnia have the tendency to improve following treatment with suvorexant. On the other hand, the improvement rate was lower in patients with a longer duration of insomnia or those treated with concomitant medications, probably because many of these patients had refractory insomnia with a prolonged symptom. Regarding switched patients, they discontinued or reduced the amount of prior medication before the initiation of suvorexant; therefore, the total number of anti-insomnia drugs after initiation of suvorexant was likely the same or decreased. For naïve patients and those receiving add-on treatment, the total number of anti-insomnia drugs was increased. Such a change in the number of drugs could have led to a difference in overall global improvement between switched patients, add-on patients, and naïve patients.
Regarding clinical effects, reduction in median sleep latency and lengthening of median total sleep time were observed in both non-elderly and elderly patients after the start of treatment. Similar results were observed in the analysis focusing on reasons for using suvorexant. Efficacy was confirmed regardless of age and reason for use, showing consistency in the results of assessment by the physician and the patients.
Regarding the continuation status of suvorexant in each initiation status group, the proportion of patients who discontinued or dropped out from treatment before 6 months was 51.4%. We confirmed that 33.2% (555/1671 patients) of these patients discontinued treatment because of improvement. With respect to initiation status of suvorexant, the percentage of patients who discontinued treatment because of improvement was particularly high for naïve patients (41.2% of naïve patients that discontinued or dropped out from treatment).
According to Japanese treatment guidelines [1], patients should reduce the amount of hypnotics or cease treatment as immediately as possible after remission from insomnia. In this survey, we observed patients who completed insomnia treatment and those who were able to reduce the amount of concomitant drugs for insomnia. This suggests that treatment with suvorexant may be helpful for reduction or cessation of such drugs.
The authors acknowledge several limitations regarding the study. Notably, this survey was an exploratory observational study without a control group. In addition, it is possible there were many confounding factors such as variations in the demographic characteristics, baseline characteristics of insomnia, existence of co-morbidities, and use of concomitant drugs. Thus, the interpretation of our results may require consideration.
Conclusions
In this postmarketing survey study of suvorexant use in Japan, no new safety concerns were identified. In addition, an improvement rate of 74.0% was achieved by a population with more diverse demographic characteristics than that assessed in previous clinical trials [6, 7]. Reduction in sleep latency and lengthening of total sleep time were also observed after 1 week of treatment, and the effect was maintained for 6 months. Thus, these data support the use of suvorexant in daily clinical practice for treating insomnia.
Acknowledgements
The authors are grateful to all doctors and staff of medical institutions in Japan for providing us with the data. The authors also acknowledge Christopher Lines, Kathryn M. Connor, and W. Joseph Herring (Merck & Co., Inc., Kenilworth, NJ, USA) and Shoki Okuda (MSD K.K., Tokyo, Japan) for their review and editorial input into this manuscript. This manuscript was originally peer reviewed and published in Japanese in the Japanese Journal of Sleep Medicine (2018;12:209–27). The manuscript has been reproduced here in English with the permission of the publisher, Life Science Co. Ltd.
Funding
The study was funded by MSD K.K., Tokyo, Japan.
Compliance with Ethical Standards
Conflict of interest
Yuko Asai, Hideki Sano, Makoto Miyazaki, Mika Iwakura, Yoshikazu Maeda, and Mitsuyoshi Hara are employees of MSD K.K., Tokyo, Japan.
Reference
- 1.MHLW (Ministry of Health, Labour and Welfare) Comprehensive Research Project on Scientific Research/Countermeasures Concerning Persons with disabilities “Study group on clinical guidelines for proper use and dosage decrease/discontinuation of hypnotics” and The Japanese Society of Sleep Research/Working group for formulating guidelines on hypnotics use version, Clinical Guidelines for Proper Use and Withdrawal of Hypnotics; 2013 (in Japanese). http://www.jssr.jp/data/pdf/suiminyaku-guideline.pdf. Accessed 23 Nov 2017.
- 2.MSD K.K. Belsomra tablets interview form. Revised November 2016. 6th ed. Tokyo: MSD K.K.; 2016 (in Japanese). http://www.info.pmda.go.jp/go/interview/1/170050_1190023F1024_1_007_1F. Accessed 28 Nov 2018.
- 3.Asai Y, Miyazaki M, Iwakura M, Hara M. The interim report about the drug use—results survey of orexinergic receptor antagonist, Belsomra, in patients with insomnia. Jpn J Sleep Med. 2017;11(2):249–263. [Google Scholar]
- 4.MSD K.K. Belsomra tablets package insert. Revised November 2016. 5th ed. Tokyo: MSD K.K.; 2016 (in Japanese). http://www.info.pmda.go.jp/downfiles/ph/PDF/170050_1190023F1024_1_11.pdf. Accessed 28 Nov 2018.
- 5.Pharmaceuticals and Medical Devices Agency. Belsomra tablets 15 mg and 20 mg Review Report. Tokyo: MSD K.K.; 14 Sep 2014 (in Japanese). http://www.pmda.go.jp/drugs/2014/P201400117/170050000_22600AMX01302_A100_4.pdf. Accessed 28 Nov 2018.
- 6.Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, et al. Suvorexant in patients with insomnia: Results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136–148. doi: 10.1016/j.biopsych.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Uchimura N, Hisada S, Takahashi K, Tsumori K, Shimamoto T, Herring WJ. Evaluation of efficacy and safety of suvorexant, an orexin receptor antagonist, in patients with insomnia as a primary disease—comparison of Japanese and overall population/non Japanese multiregional clinical trial III randomized, double-blind, placebo-controlled trial on efficacy. Jpn J Sleep Med. 2015;9(3):395–411. [Google Scholar]
- 8.Sun H, Palcza J, Rosenberg R, Kryger M, Siringhaus T, Rowe J, et al. Effects of suvorexant, an orexin receptor antagonist, on breathing during sleep in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(3):416–426. doi: 10.1016/j.rmed.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Palcza J, Card D, Gipson A, Rosenberg R, Kryger M, et al. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med. 2016;12(1):9–17. doi: 10.5664/jcsm.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]