Abstract
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common types of leukemia. In the present case, an evidence-guided treatment plan of supplements and lifestyle changes were used to support the patient.
Case Presentation
A 56-y-old female presented to her primary care physician for a routine physical in 2001. Complete blood cell results suggest pathology among white blood cells. Flow cytometry was used to confirm the presence of CLL. Other than an episode of splenomegaly in 2005 and mild lymphadenopathy, the patient has remained asymptomatic since diagnosis in 2001. In late 2001, the patient began a physician-assisted regimen of alternative dietary supplements and lifestyle changes.
Conclusion
Nutritional supplementation along with lifestyle changes appears to have supported the maintenance of stable and indolent CLL in this patient. It is important for physicians to be prepared to engage with their patients on use of supplements and lifestyle changes in managing their disease.
Chronic lymphocytic leukemia (CLL) is one of the most common types of leukemia among adults in the United States and is still considered incurable.1,2 It affects B and T lymphocytes as well as natural killer cells, but the majority of CLL cases diagnosed are of the B-cell phenotype.3 CLL results from the uncontrolled clonal growth of small B lymphocytes in a manner that often leads to the crowding out of healthy cells. The disease affects bone marrow and peripheral blood, which can lead to pathology in the lymph nodes, liver, and spleen.4 The initial symptoms of CLL vary but may include loss of energy, weight loss, enlarged lymph nodes, and splenomegaly.4 Despite this, many patients remain asymptomatic for a number of years. Physicians typically monitor patients with CLL for signs of infection, autoimmunity, and bone marrow failure, which are common long-term complications.5
CLL is often found after a routine complete blood count (CBC) that exhibits an abnormally high white blood cell (WBC) count. This elevation in WBC counts often occurs long before the patient experiences any illness from the disease. A number of prognostic markers are used in tracking the progression of CLL, including lymphocyte doubling time, level of immunoglobulin variable region of the heavy chain variation, CD-38 expression, Zap-70 expression, β-2-microglobulin levels, and serum CD-23 levels.6,7 The staging of CLL progression is typically determined using the Rai and Binet classification systems.8,9,10 Both staging systems depend on the following factors: spleen and liver size, platelet counts, hemoglobin levels, and the number of affected lymph nodes.9,10
Our goal is to inform clinicians on the value of integrating life style and alternative modalities into care of cancer patients. This case report was prepared in accordance with the CAse REport (CARE) guidelines.11 A timeline of the patient’s medical history and course of care is presented in Figure 1.
Figure 1.
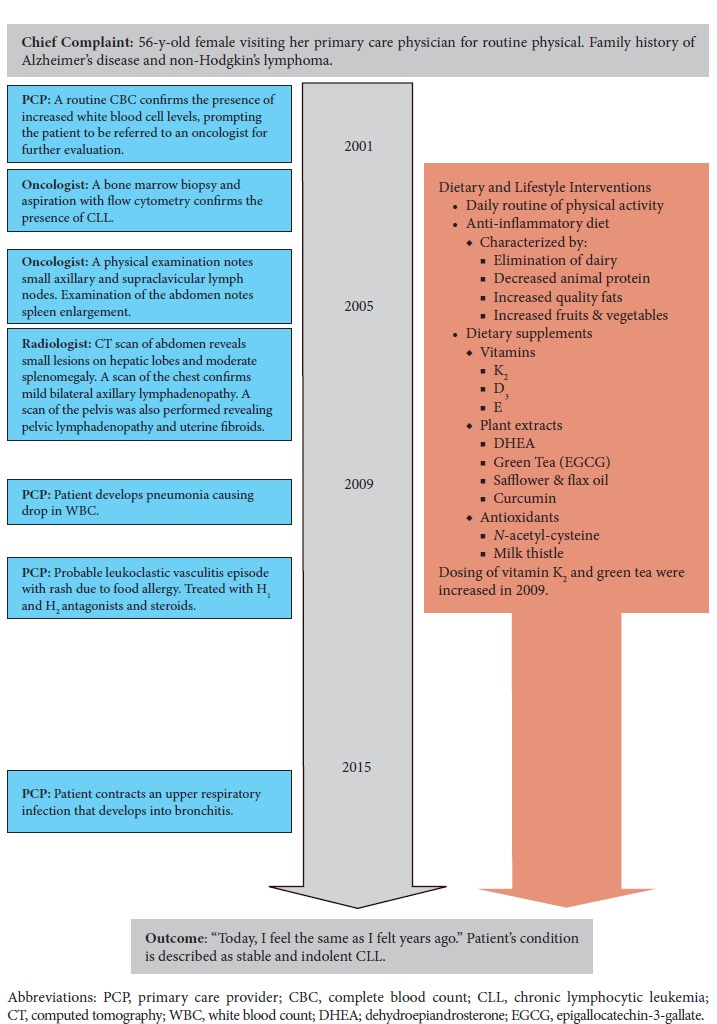
Timeline of Patient’s Medical History and Course of Care
Patient Information
The patient was a 56-year-old female visiting her primary care physician for a routine physical in 2001. The initial CBC gave the following results: hemoglobin, 13.7; hematocrit, 42; WBC count, 53.7; and platelets, 204. The patient was then referred to an oncologist in the area for a definitive diagnosis of CLL. The patient was self-referred to the George Washington Center for Integrative Medicine (Washington, DC, USA) following her diagnosis in September 2001. Prior to her diagnosis in 2001, she had been relatively healthy with no major illnesses or surgeries to report. Other than her brother being diagnosed with non-Hodgkin’s lymphoma, she had no family history related to the disease.
Clinical Findings
The physical exam performed by her oncologist was unremarkable at the time of diagnosis.
Diagnostic Assessment
A flow cytometry report showed the presence of a monoclonal B-cell population, which variably expressed CD19, CD20, CD11C, CD23, and aberrant CD5. The report also found a positive but dim population of kappa molecules. FISH was also performed, which showed normal CCND1-IgH, ataxia-telangiectasia mutated, chromosome 12, 13q, and TP53. The blood smear sample shows smudge cells as well as CLL cells.
Based on the workup, her CLL was characterized as stable Rai stage II and Binet stage A. Binet clinical stage A is characterized by no anemia (Hb ≥ 10.0 g/dL) or thrombocytopenia (platelets ≥ 100 × 109/L) and less than 3 areas of lymphoid involvement.10 Binet stage A patients have a median survival of more than 10 years.10 Rai stage II CLL is characterized by lymphocytosis with either hepatomegaly or splenomegaly with or without lymphadenopathy.9
Since diagnosis in 2001, the patient has remained asymptomatic for more than 15 years. Lab results demonstrate a gradual increase in her WBC during this period, doubling in comparison with her count at diagnosis 3 years later in 2004. She did experience an episode of splenomegaly in 2005. A computed tomography scan of the abdomen was performed confirming the presence of moderate splenomegaly. The scan also revealed small lesions on the anterior left and posterior right hepatic lobes. Overall, this trend of increasing WBC has remained with in normal limits. Conversely, her platelet counts show a decreasing trend that appears to have stabilized.
The patient’s maximum WBC reached 175 000; however, it stabilized and later began to slowly decrease, possibly plateauing in the 120 000 to 130 000 range after high-dose epigallocatechin-3-gallate (EGCG) was added to her diet and supplements regimen.
Therapeutic Interventions and Follow-up
Given the lack of effective treatment for early stage CLL in asymptomatic patients, a “watch and wait” approach to treatment was taken.4,5 Meaning, the physician observed the patient’s condition with physical exams and lab tests withholding the use of drugs or other therapies. The decision to wait and observe was made weighing the risks and side effects of chemotherapy with the patient’s need for intervention based on disease-staging measures.
During this period, the patient also began a physician-assisted regimen of alternative dietary supplements. The complete list of supplements included the following: vitamin K2; mixed omega-3/omega-6 oil; vitamin D3; meriva-500 (curcumin); combination of milk thistle and broccoli extract; N-acetyl-cysteine, methylation support product combining methyl-B12, methylfolate, riboflavin, vitamin B6, and trimethylglycin; high-potency multivitamin with activated B vitamins, mixed tocopherols, and carotenoids; low-dose dehydroepiandrosterone; and high-dose EGCG green tea extract (equivalent of approximately 1800 mg of EGCG per day).
As part of her health regimen, the patient also adopted an anti-inflammatory diet. Anti-inflammatory diets are characterized by eliminating dairy; increasing the consumption of quality fats, fruits, vegetables; and decreased animal protein. She also began walking daily to maintain a level of physical activity. The patient’s last visit was in June 2016 for her regular check-up, and no new findings were reported. She agreed with the approach and agreed to continue to adhere to the regimen recommended by her physician.
Discussion
We have presented a case of a woman whose CLL has been well managed for more than 15 years without the use of chemotherapy or other forms of conventional treatment. There are a number of individuals whose CLL does not progress to the point of requiring chemotherapy. Given that there are currently no conventional treatments for early stage CLL, patients should feel comfortable exploring the body of literature on natural medicines available. It is not uncommon for patients with leukemia to seek out other forms of therapy not prescribed by their oncologists.27 For this reason, it is important for oncologists to be knowledgeable of popular therapies. We hope to highlight the value of a concerted effort between patients and physicians in devising a health regimen with thoughtful and evidenced nutritional supplementation. The management of this patient’s CLL can be explained by the explained in the context of the supplements she was prescribed, including omega-3, EGCG, meriva-500, and vitamin D3.
The blend of organic safflower and flax seed oil with a 4:1 ratio of omega-6 to omega-3. Omega-3 polyunsaturated fatty acids are essential fatty acids that are believed to downregulate nuclear factor kappa B (NF-κB), a key mediator of inflammatory processes in the body.28 Chronic inflammation as a result of the upregulation proinflammatory molecules such as NF-κB are believed to provide a cellular environment favorable for malignant cell growth.23 The activation of NF-κB has been associated with more aggressive tumor growth and resistance to both chemotherapy and radiotherapy.23 So, the use of the BodyBio Balance oil, which contains omega-3 may have aided in dimming the population of κ-positive lymphocytes observed on the flow cytometry report in 2015.
An in vitro study demonstrated that vitamin D analogs caused preferential apoptosis in primary CLL cells through a p53-independent mechanism.17 It is also suggested that vitamin D insufficiency is a risk factor for the disease, and high vitamin D levels are predictive of a longer time to first treatment in CLL.17,29
A clinical trial found EGCG to be effective against CLL.31 Preclinical research on EGCG, the active ingredient in green tea, suggests that it may interfere with vascular endothelial growth factor (VEGF) receptors in these cells.21,22 CLL cells are characterized by their resistance to apoptosis, which is believed to be maintained by the secretion and binding of VEGF. There is also preclinical evidence indicating that curcumin may potentiate the effects of EGCG on CLL.26,32,33 The patient consumed 1000 mg of curcumin phytosome daily. Furthermore, results from a clinical trial on Rai stage 0/1 CLL patients suggests these patients may benefit from curcumin therapy.33
Conclusion
The patient’s oncologist never discouraged her to take supplements. However, when the patient approached the oncologist for guidance on diet and supplements, the oncologist did not provide any recommendations, and, moreover expressed that diet and supplements are not going to alter her illness course. Although the patient learned how to navigate her care between 2 different providers, she often felt uneasy that her oncologist did not want to engage into any discussion about integrative approaches. Although this case points toward a possible way of slowing down the CLL progression, it also underscores the dire need for field of oncology to embrace lifestyle strategies of managing indolent cancers by either adding these methods to the treatment toolbox or at least aggressively engaging into collaboration with integrative medicine providers, who often care for such patients. Fortunately, many academic centers and large medical systems have begun integrating lifestyle and alternative modalities into care of cancer patients. The authors do hope that in the future, integrative oncology strategies will be available to every cancer patient.
Figure 2.
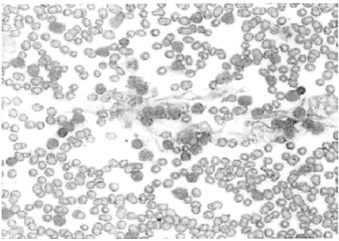
Peripheral Blood Smear Image With Smudge Cells and a Chronic Lymphocytic Leukemia Cell Population
Figure 3.
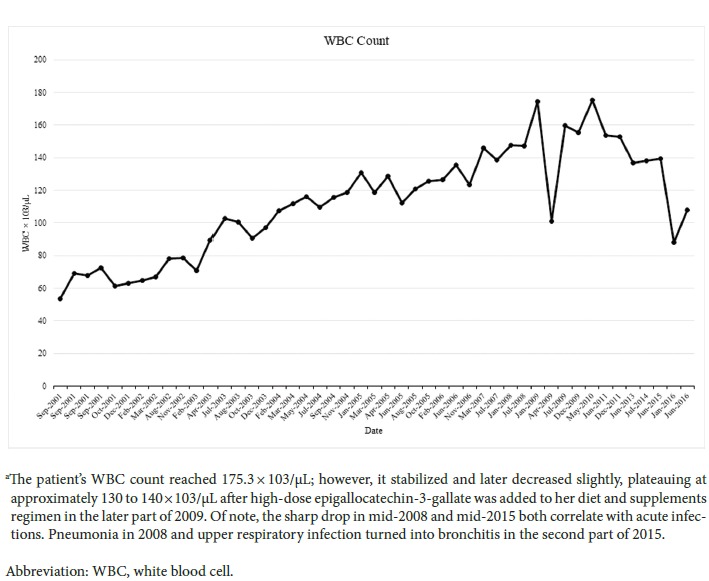
Fluctuations in WBC Count by Month Between 2001 and 2016a
Table 1.
| Stage | Description | Median Survival (mo) | Median Survival (y) | |
|---|---|---|---|---|
| Rai9 | ||||
| Low risk | 0 | Lymphocytosis only | 140 | >10 |
| Intermediate risk | I | Lymphocytosis and lymphadenopathy | 100 | 6 |
| II | Lymphocytosis in blood and marrow with splenomegaly and/or hepatomegaly, with or without lymphadenopathy | 70 | ||
| High risk | III | Lymphocytosis with anemia (hemoglobin <11 g/dL or hematocrit <33%) | 20 | 2 |
| IV | Lymphocytosis with thrombocytopenia (platelet count <100 000/mm3) | 20 | ||
| Binet10 | ||||
| A | Enlargement of <3 lymphoid areas; no anemia or thrombocytopenia | 140 | >7 | |
| B | Enlargement of ≥3 lymphoid areas; no anemia or thrombocytopenia | 60 | <5 | |
| C | Anemia (hemoglobin <10g/dL) and/or thrombocytopenia (platelet count <100 000/mm3) | 24 | <2 | |
Table 2.
Dietary Supplements Utilized in Case
| Product Description | Daily Dosage | Indications for Use | |
|---|---|---|---|
| Vitamin Supplements | |||
| Vitamin K2 | Vitamin K3 induces apoptosis leukemia cells.15 | ||
| Vitamin D3 | A fat soluble vitamin | Induced apoptosis in primary CLL cells in vitro and is also known to be important in calcium and bone homeostasis.16,17 | |
| Vitamin E | Mixed tocopherols and carotenoids | Tocopherols have been found to slow the growth of various cancer types.18 | |
| Plant Extract Supplements | |||
| Methylation support | Methyl B12, methylfolate, riboflavin, vitamin B6, and trimethylglycin (methyl-guard plus) | Vitamin B12 and folate deficiency have been associated with anemia in CLL symptomatology.19 | |
| DHEA | Dehydroepiandrosterone made from yam or soy extract | 25 mg | Research suggests that DHEA supplements may help increase bone density in older adults.20 |
| Green tea extract | High-dose epigallocatechin-3-gallate | 1800 mg | Increases apoptosis among CLL cells.21,22 |
| Safflower and flax seed oil | 4:1 ratio of omega 6:omega 3 (BodyBio Oil) | Inhibition of nuclear factor kappa B activation.23 | |
| Curcumin | Curcumin phytosome (Meriva-500) | 1000 mg | Curcumin is immune supportive and also has anti-inflammatory effects on the body.24 |
| Antioxidant Supplements | |||
| N-acetyl-cysteine | Antioxidant amino acid | N-acetyl-cysteine has potent antioxidant effects and is believed to assist in the detoxification process as well as prevent CLL cell-mediated T-cell dysfunction.25 | |
| Milk thistle | The flavonolignan silybin is the major constituent of silymarin, a complex extracted from milk thistle fruit26 | Milk thistle is believed to conserve tissue glutathione, which is thought to be liver-protective and have anticancer potential.26 | |
Abbreviations: CLL, chronic lymphocytic leukemia; DHEA, dehydroepiandrosterone.
Biographies
Gregory Haskin is a current student at Drexel Medical center and a graduate of the Complementary and Alternative Medicine Program in the Department of Physiology and Biophysics, Georgetown University, in Washington, DC.
Mikhail Kogan, MD, is the medical director of the George Washington Center for Integrative Medicine in Washington, DC.
Footnotes
Author Disclosure Statement
This case report was prepared according to the CARE guidelines. Written consent was obtained from the patient for submission of this case report.
References
- 1.American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 2.Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371(9617):1017-1029. [DOI] [PubMed] [Google Scholar]
- 3.Boelens J, Lust S, Vanhoecke B, Offner F. Chronic lymphocytic leukaemia. Anticancer Res. 2009;29:605-615. [PubMed] [Google Scholar]
- 4.Zelenetz AD, Gordon LI, Wierda WG, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1.2015: Clinical practice guidelines in oncology. J Nat Comprehensive Cancer Net. 2015;13(3):326-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M. ESMO Guidelines Working Group: Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(suppl 6):vi50-vi54. [DOI] [PubMed] [Google Scholar]
- 6.Kalil N, Cheson BD. Chronic lymphocytic leukaemia. Oncologist. 1999;4;352-369. [PubMed] [Google Scholar]
- 7.Ferrarini M, Cutrona G, Neri A, Morabito F. Prognostic factors in CLL. Leukemia Supp. 2012;1(suppl 2):S29-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroers R, Griesinger F, Trumper L, et al. Combined analysis of ZAP-70 and CD38 expression as a predictor of disease progression in B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:750-758. [DOI] [PubMed] [Google Scholar]
- 9.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46(2):219-234.1139039 [Google Scholar]
- 10.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198-206. [DOI] [PubMed] [Google Scholar]
- 11.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, CARE Group The CARE Guidelines: Consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2(5):38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallek M, Cheson BD, Catovsky D. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghia P, Hallek M. Management of chronic lymphocytic leukemia. Haematologica. 2014;99(6):965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malek S. Molecular biomarkers in chronic lymphocytic leukemia. Adv Exp Med Biol. 2013;792:193-214. [DOI] [PubMed] [Google Scholar]
- 15.Lin C, Kang J, Zheng R. Vitamin K3 triggers human leukemia cell death through hydrogen peroxide generation and histone hyperacetylation. Pharmazie. 2005;60(10):765-771. [PubMed] [Google Scholar]
- 16.Pepper C, Fegan C. CLL: A supplementary question?. Blood. 2011;117(5):1439-1440. [DOI] [PubMed] [Google Scholar]
- 17.Molica S, Digiesi G, Antenucci A, et al. Vitamin D insufficiency predicts time to first treatment (TFT) in early chronic lymphocytic leukemia (chronic lymphocytic leukemia). Leuk Res. 2012;36:443-447. [DOI] [PubMed] [Google Scholar]
- 18.Hardman WE. Diet components can suppress inflammation and reduce cancer risk. Nutr Res Pract. 2014;8(3):233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauro FR, Gentile M, Foa R. Erythropoietin and chronic lymphocytic leukemia. Rev Clin Exp Hematol. 2002;suppl 1:21-31. [PubMed] [Google Scholar]
- 20.Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol. 2000;53:561-568. [DOI] [PubMed] [Google Scholar]
- 21.Beliveau R, Gingras D. Green tea: Prevention and treatment of cancer by nutraceuticals. Lancet. 2004;364:1021-1022. [DOI] [PubMed] [Google Scholar]
- 22.Forester SC, Lambert JD. Antioxidant effects of green tea. Mol Nutr Food Res. 2011;55(6):844-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Sethi Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochem Biophys Acta. 2010;1805(2):167-180. [DOI] [PubMed] [Google Scholar]
- 24.Rayburn ER, Ezell SJ, Zhang R. Anti-Inflammatory agents for cancer therapy. Mol Cell Pharmacol. 2009;1(1):29-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia L, Gribben JG. Dangerous power: Mitochondria in CLL cells. Blood Apr. 2014;123(17):2596-2597. [DOI] [PubMed] [Google Scholar]
- 26.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14:226-246. [PubMed] [Google Scholar]
- 27.Wesa K, Cassileth BR. Is there a role for complementary therapy in the management of leukemia? Expert Rev Anticancer Ther. 2009;9(9):1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahrmann JF, Ballester OF, Ballester G, et al. Inhibition of nuclear factor kappa B activation in early-stage chronic lymphocytic leukemia by omega-3 fatty acids. Cancer Invest. 2013;31:24-38. [DOI] [PubMed] [Google Scholar]
- 29.Shanafelt TD, Drake MT, Maurer MJ, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011;117(5):1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemanne D, Block KI, Kressel BR, Sukhatme VP, White JD. A case of complete and durable molecular remission of chronic lymphocytic leukemia following treatment with epigallocatechin-3-gallate, an extract of green tea. Cureus. 2015;7(12):e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanafelt TD, Call TG, Zent CS, et al. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with epigallocatechin-3-gallate. Clin Cancer Res. 2009;15:1250-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golombick T, Diamond T, Manoharan A, Ramakrishna R. The effect of curcumin (as Meriva) on absolute lymphocyte count (ALC), NK cells and T cell populations in patients with stage 0/1 chronic lymphocytic leukemia. J Cancer Ther. 2015;6:566-571. [Google Scholar]


