Abstract
To understand the effects of Lactobacillus rhamnosus GG (ATCC 53103) on intestinal barrier function in pre-weaning piglets under normal conditions, twenty-four newborn littermate piglets were randomly divided into two groups. Piglets in the control group were orally administered with 2 mL 0.1 g/mL sterilized skim milk while the treatment group was administered the same volume of sterilized skim milk with the addition of viable L. rhamnosus at the 1st, 3rd, and 5th days after birth. The feeding trial was conducted for 25 d. Results showed that piglets in the L. rhamnosus group exhibited increased weaning weight and average daily weight gain, whereas diarrhea incidence was decreased. The bacterial abundance and composition of cecal contents, especially Firmicutes, Bacteroidetes, and Fusobacteria, were altered by probiotic treatment. In addition, L. rhamnosus increased the jejunal permeability and promoted the immunologic barrier through regulating antimicrobial peptides, cytokines, and chemokines via Toll-like receptors. Our findings indicate that oral administration of L. rhamnosus GG to newborn piglets is beneficial for intestinal health of pre-weaning piglets by improving the biological, physical, and immunologic barriers of intestinal mucosa.
Keywords: Lactobacillus rhamnosus, Gut microbiota, Intestinal physical barrier, Intestinal immunological barrier, Piglet
1. Introduction
Diarrhea, an important cause of morbidity and mortality in livestock, is one of the most common diseases of suckling piglets worldwide (Toledo et al., 2012). In October 2010, the severe outbreak of porcine epizootic diarrhea in southern China caused the death of more than 1 000 000 piglets (Sun et al., 2012). Diarrheal disease was also reported in European countries (Hanke et al., 2015; Theuns et al., 2015) and spread rapidly to approximately 50% of the US swine breeding herds from July 2013 to July 2014 (Goede and Morrison, 2015). To fight against diarrhea, antibiotics have been applied widely in neonatal piglets to enhance gut function and prevent diarrhea (Hermann-Bank et al., 2015; Ngamwongsatit et al., 2016). However, the promiscuous use of antibiotics could influence the balance of gut bacteria and result in the emergence and spread of resistant bacteria (Kemper, 2008). Since bacterial colonization of the mammalian gastrointestinal tract begins at birth (Hermann-Bank et al., 2015), it is necessary to establish a healthy intestinal microbiota of newborn suckling piglets. Gut microbiota constitutes the intestinal biological barrier (Natividad and Verdu, 2013; Kelly et al., 2015), prevents pathogen colonization, and converts indigestible substances into digestible components that benefit the host and help in the maturation of the gastrointestinal tract as well as of the immune system (Guarner and Malagelada, 2003; Bauer et al., 2006; Ley et al., 2008; Koenig et al., 2011). As well as the biological barrier, the physical barrier and immune mediators are also important in defending against pathogens (McCracken and Lorenz, 2001). In the presence of an intact epithelial cell layer, the paracellular pathway between cells must be sealed. This function is achieved by a physical barrier, especially tight junctions, which are multi-protein complexes to limit solute flux along the paracellular pathway. The tight junction is, therefore, the rate-limiting step in transepithelial transport and the principal determinant of mucosal permeability (Turner, 2009). Additionally, the cytokines and chemokines secreted by enterocytes and the antimicrobial peptides secreted by Paneth cells could regulate bacterial interactions with the mucosal surface, so as to maintain the intestinal immune homeostasis (Rescigno, 2011).
Probiotics are nonpathogenic bacteria that exert a beneficial influence on host health, physiology, or both (Rajput et al., 2013), and further improve intestinal structure, aid in the development of immunity to defend against pathogens, and subsequently improve growth performance (Lei et al., 2013). In animal production, several selected probiotics have been applied to improve animal health and performance, such as Lactobacillus, Streptococcus, Saccharomyces, Aspergillus, and Bacillus species (Tannock, 2001). As a prominent probiotic member, Lactobacillus could augment intestinal health by modulating the gut microbiota of mice with metabolic syndrome (Wang et al., 2015), attenuating the negative effects of alcohol on mouse tight junction expression (Chen et al., 2016), and activating the gut mucosal immune system of mice (Galdeano and Perdigón, 2006). Although there are some reports on probiotics’ effects on pig intestinal barrier functions (Zhang et al., 2010; Li et al., 2012; Deng et al., 2013; Hou et al., 2015), few studies have administered Lactobacillus rhamnosus to newborn piglets in the first few days after birth to determine its protective effects on intestinal functions without challenging with pathogens. Therefore, this study was conducted to determine the effects of orally administered L. rhamnosus on the intestinal barrier function in pre-weaning piglets under normal conditions.
2. Materials and methods
2.1. Bacterial preparation
The L. rhamnosus GG (ATCC 53103) in freeze-dried powder form was purchased from the China Center of Industrial Culture Collection (CICC), and cultured in de Man-Rogosa-Sharpe (MRS) medium (Oxoid, Basingstoke, UK) in anaerobic condition at 37 °C until reaching the logarithmic phase. Then the bacterial strain was separated by centrifugation (15 min at 3000g) and washed twice with sterile phosphate-buffered saline (pH 7.4). Thereafter, the bacteria were re-suspended in 0.1 g/mL sterile skim milk made from cows’ milk powder to prepare the required concentration (5×108 CFU/mL).
2.2. Animals and treatments
Six sows (Duroc×Landrace×Yorkshire) were participants in this experiment. Four newborn piglets with similar initial weights were selected from each sow’s offspring. The twenty-four newborn piglets were then randomly divided into two groups. Each group had three litters with four pigs per litter (half male and half female). The basal diet was supplemented with minerals and vitamins to meet or exceed the requirements for pigs (National Research Council, 1998). All pigs were fed ad libitum. The experiment was approved by and performed in accordance with the guidelines of the Zhejiang University Animal Care and Use Committee, Hangzhou, China. Piglets in the control group were orally administered with 2 mL of sterile skim milk, while the treatment group (L. rhamnosus group) received the same volume of sterile skim milk suspended with viable L. rhamnosus (5×108 CFU/mL) by gavage at the 1st, 3rd, and 5th days after birth. From Day 12, piglets were provided with free access to water and a supplemented pre-starter feed. Body weights were recorded at the beginning and the weaning day (the 25th day) in this experiment. Diarrhea was observed every day. Composition and nutrient levels in the diets of sows are listed in TablesS1 and S2, respectively. Composition and nutrient levels in the diets of the pre-starter feed were listed in Tables S3 and S4, respectively. No antibiotic was added throughout the trial. The schematic flow diagram of the study design can be found in Fig. S1.
2.3. Sample collection
On Day 25, six piglets (half male and half female) were randomly picked from each group to collect intestine samples. Briefly, ketamine (11 mg/kg) and xylazine (1.5 mg/kg) were injected to minimize stress. Thereafter, chemical euthanasia was performed using an overdose of intravenous pentobarbital via a catheterized ear vein (Li et al., 2012). The mid-jejunum segments were carefully dissected and rinsed with sterilized saline. Mucosae were gently scraped off and cecal contents were carefully squeezed out, then placed in liquid nitrogen immediately, and stored at −80 °C till further analysis.
2.4. ELISA
The mucosa samples were diluted 1:2 (v/v) in sterile saline solution and homogenized with a glass homogenizer. Then the homogenates were centrifuged at 3000g for 15 min at 4 °C and supernatants were collected for the measurement of the concentrations of interleukin-6 (IL-6), IL-8, IL-10, IL-12, transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) by porcine enzyme-linked immunosorbent assay kit (ELISA kit; R&D Systems, Inc., Minneapolis, USA) as per the manufacturer’s instructions. Data are presented as pg/g wet weight (Li et al., 2012).
2.5. Diamine oxidase assay
The mucosa samples were homogenized with ice-cold physiologic saline (1:10, w/v) and centrifuged at 2000g for 10 min (Centrifuge, Eppendorf, Germany). Supernatant was collected for determination of the diamine oxidase (DAO) activity. The kit for DAO was obtained from Nanjing Bioengineering Institute, Nanjing, China, and the DAO value was determined by spectrophotometry according to the manufacturer’s instructions.
2.6. RNA extraction and qRT-PCR
Total RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) were performed according to Wang et al. (2017). The primer sequences used for qRT-PCR are listed in Table 1. The 2−∆∆ C T method was used to estimate mRNA abundance. Relative gene expression levels were normalized by the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 1.
Gene names, primer sequences, and product sizes
| Gene symbol | Gene name | GenBank accession No. | Primer sequence | Product size (bp) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001206359.1 | F: 5'-ATGGTGAAGGTCGGAGTGAAC-3' R: 5'-CTCGCTCCTGGAAGATGGT-3' | 235 |
| MUC2 | Mucin 2 | XM_002347185.2 | F: 5'-ACGACTTTGACGGACACTGCT-3' R: 5'-AGGGGACGTTCTCGGTGAT-3' | 99 |
| pBD-1 | Porcine β-defensin-1 | NM_213838.1 | F: 5'-CGCCTCCTCCTTGTATTCCTC-3' R: 5'-GGTGCCGATCTGTTTCATCTT-3' | 144 |
| PMAP-37 | Porcine myeloid antimicrobial peptide 37 | NM_213863.1 | F: 5'-CACCTGCAATGAGGGTGTCA-3' R: 5'-GTCGCAACCGTGGTCTTCG-3' | 68 |
| LYZ | Lysozyme C-2 precursor | NM_214392 | F: 5'-CCGCTACTGGTGTAATGATGG-3' R: 5'-ATGCTTTAACGCCTAGTGGAT-3' | 143 |
| OCLN | Occludin | NM_001163647 | F: 5'-AATGCTTTCTCAGCCAGCGTAT-3' R: 5'-GCAAGCGTGGAGGCAACA-3' | 153 |
| ZO-1 | Zonula occludens-1 | XM_003121673.1 | F: 5'-GCCTCCTGAGTTTGATAGTGG-3' R: 5'-CTCGGCAGACCTTGAAATAGA-3' | 287 |
| MCP-1 | Monocyte chemoattractant protein-1 | NM_214214.1 | F: 5'-AGAAGAGTCACCAGCAGCAAG-3' R: 5'-TAGGGCAAGTTAGAAGGAAATG-3' | 206 |
| TNF-α | Tumor necrosis factor-α | NM_214022.1 | F: 5'-CATCGCCGTCTCCTACCA-3' R: 5'-CCCAGATTCAGCAAAGTCCA-3' | 199 |
| IFN-γ | Interferon-γ | NM_213948.1 | F: 5'-GAGCCAAATTGTCTCCTTCTAC-3' R: 5'-CGAAGTCATTCAGTTTCCCAG-3' | 140 |
| TGF-β1 | Transforming growth factor-β1 | NM_214015.1 | F: 5'-GGACCTTATCCTGAATGCCTT-3' R: 5'-TAGGTTACCACTGAGCCACAAT-3' | 133 |
| IL-8 | Interleukin-8 | NM_213867.1 | F: 5'-ATGCCAGTGCATAAATACGC-3' R: 5'-TTGGGAGCCACGGAGAAT-3' | 251 |
| TLR2 | Toll-like receptor 2 | NM_213761.1 | F: 5'-GGTCCGATGCTGGTCTTTAT-3' R: 5'-GCAAGTCACCCTTTATGTTATTCA-3' | 83 |
| TLR9 | Toll-like receptor 9 | NM_213958.1 | F: 5'-CCCACGACAGCCGAATAG-3' R: 5'-GGAACAGGGAGCAGAGCA-3' | 122 |
| TLR6 | Toll-like receptor 6 | NM_213760.1 | F: 5'-TCTGCTCAAGGACTTCCGTGT-3' R: 5'-CAGCCCAGTGACTCCGATG-3' | 79 |
| TLR8 | Toll-like receptor 8 | NM_214187.1 | F: 5'-GGATACCATTGCGGCGATAA-3' R: 5'-CCAGGGCAGCCAACATAACT-3' | 71 |
F: forward; R: reverse
2.7. DNA extraction
Genomic DNA was extracted from every cecal content sample using a TIANamp Stool DNA kit according to the manufacturer’s protocols (Tiangen Biotech, Beijing, China).
2.8. 454 pyrosequencing
The 454 pyrosequencing was performed according to Zhang et al. (2014). Amplification of the bacterial V3 region of the 16S rRNA gene was conducted using a primer set (Casserly and Erijman, 2003), and the 5' terminus of each forward and reverse primer contained an 8-bp barcode sequence to tag specific samples (Table 2).
Table 2.
Barcode sequences and primer sequences
| Group | Primer | Barcode sequence | Primer sequence (5'→3') |
| Control | V3-60F | AGATACTG | AGATACTGCCTACGGGAGGCAGCAG |
| V3-60R | AGATACTGATTACCGCGGCTGCT | ||
| V3-61F | AGAGTATG | AGAGTATGCCTACGGGAGGCAGCAG | |
| V3-61R | AGAGTATGATTACCGCGGCTGCT | ||
| V3-63F | AGAGTGAT | AGAGTGATCCTACGGGAGGCAGCAG | |
| V3-63R | AGAGTGATATTACCGCGGCTGCT | ||
| L. rhamnosus | V3-55F | AGATATGT | AGATATAGCCTACGGGAGGCAGCAG |
| V3-55R | AGATATAGATTACCGCGGCTGCT | ||
| V3-58F | AGATAGAT | AGATAGTGCCTACGGGAGGCAGCAG | |
| V3-58R | AGATAGTGATTACCGCGGCTGCT | ||
| V3-62F | AGATACAT | AGAGTAGTCCTACGGGAGGCAGCAG | |
| V3-62R | AGAGTAGTATTACCGCGGCTGCT |
2.9. Statistical analysis
Data are presented as means with their standard deviations (SDs). They were analyzed with SPSS 16.0 for Windows, using independent samples t-test. Differences were considered statistically significant at P<0.05 or P<0.01. Different superscripts denote significant difference between groups.
3. Results
3.1. L. rhamnosus improved growth performance
Compared with the control group, L. rhamnosus significantly increased the final body weight and daily weight gain, while decreasing the diarrhea rate from 6.41% to 4.44% (Table 3).
Table 3.
Body weight gain and diarrhea rate of suckling piglets
| Group | Body weight at birth (kg) | Final body weight (kg) | Daily weight gain (kg) | Diarrhea rate (%) |
| Control | 1.43±0.03 | 6.67±0.13b | 0.22±0.01b | 6.41±0.72a |
| L. rhamnosus | 1.38±0.04 | 7.62±0.13a | 0.25±0.01a | 4.44±0.34b |
The data followed by the different small letters in the same column are significantly different (P<0.05). Diarrhea rate (%)=(number of piglets with diarrhea in seven days/total number of piglets in the group)×100%. Data are expressed as mean±SD (n=12)
3.2. L. rhamnosus regulated bacterial diversity and composition in cecal contents
The phylogenetic differences within the intestinal microbiota were assessed by principal component analysis (PCoA) (Fig. 1). L. rhamnosus treatment had a distinct microbiota composition that clustered separately from control diet-fed piglets. The α-diversity (richness and evenness) of the communities was measured by Shannon, chao1, PD_whole_tree, observed_species, and goods_coverage’s indices (Table 4). However, no significant differences were observed between the two groups. To assess specific changes in the gut microbiota, we compared the relative abundance of the phylum identified from sequencing (Fig. 2). Results showed that Firmicutes, Bacteroidetes, and Fusobacteria phyla were dominant in the control group, comprising 33.56%, 35.89%, and 15.20% of the sequences, respectively; however, in L. rhamnosus group, Firmicutes percentage was dominant and the Bacteroidetes proportion was decreased. Noticeably, Fusobacteria almost disappeared in the L. rhamnosus group (Fig. 2).
Fig. 1.
Principal component analysis for the piglets’ gut microbiota
Table 4.
Changes in α-diversity of gut microbiota communities
| Group | Shannon | Chao1 | PD_whole_tree | Observed_species | Goods_coverage |
| Control | 5.15±0.71 | 251.13±105.56 | 11.02±3.05 | 178.67±62.74 | 0.98±0.00 |
| L. rhamnosus | 5.17±1.18 | 195.65±149.13 | 9.36±2.17 | 127.00±66.47 | 0.97±0.02 |
Data are expressed as mean±SD (n=3)
Fig. 2.

Composition of gut microbiota at phylum level
The abundance is presented in terms of a percentage of the total effective bacterial sequences in the sample, which were classified using the RDP Classifier at a confidence threshold of 50%
3.3. L. rhamnosus regulated mRNA expression of genes related to physical barrier function
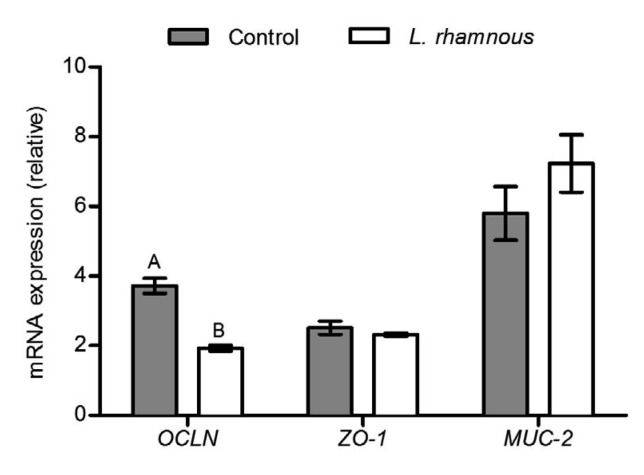
The results of expression of genes related to tight junctions in jejunal mucosa showed that the transcript level of occludin (OCLN) was significantly down-regulated by 48.12% with L. rhamnosus treatment, while no significant difference was found in the zonula occludens-1 (ZO-1) mRNA expression between the two groups (Fig. 3). L. rhamnosus increased mucin-2 (MUC-2) gene expression slightly (Fig. 3), but DAO activity was significantly decreased (Fig. 4).
Fig. 3.
Relative expression levels of OCLN, ZO-1, and MUC-2 in jejunal mucosa of piglets
The different capital letters on the bar mean that the values are very significantly different (P<0.01). Data are expressed as mean±SD (n=6)
Fig. 4.
DAO activity in jejunal mucosa of piglets
The different capital letters on the bar mean that the values are very significantly different (P<0.01). Data are expressed as mean±SD (n=6)
3.4. L. rhamnosus improved intestinal immune barrier function
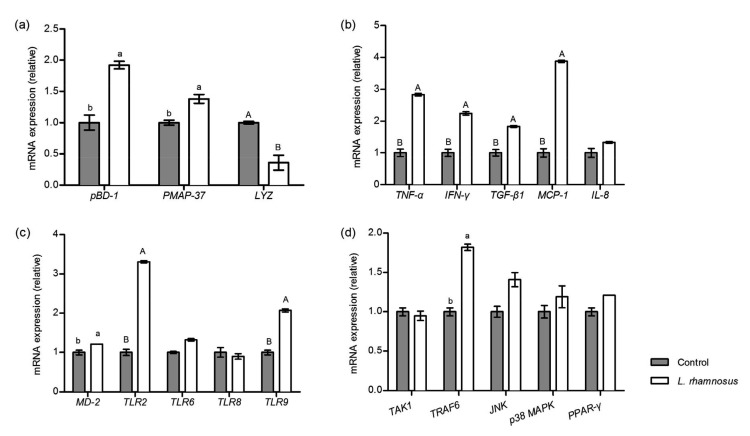
Oral administration of L. rhamnosus significantly up-regulated the mRNA levels of porcine β-defensin-1 (pBD-1) and porcine myeloid antimicrobial peptide 37 (PMAP-37), but decreased the expression of lysozyme C-2 precursor (LYZ) in jejunal mucosa of the suckling piglets (Fig. 5a). Results in Fig. 5b illustrate that L. rhamnosus markedly up-regulated the mRNA levels of both pro-inflammatory cytokines (TNF-α, IFN-γ) and the anti-inflammatory cytokine (TGF-β1). In the L. rhamnosus treatment group, chemokine MCP-1 mRNA expression was almost quadrupled compared with that in the control group (1.00 vs. 3.88). Despite this, most of the pro-inflammatory cytokine concentrations in jejunal mucosa were significantly decreased in the L. rhamnosus group, including IL-1β, IL-6, IL-12, IL-8, and TNF-α, whereas the IFN-γ concentration was increased (Table 5), which was in line with the qRT-PCR result (Fig. 5b). Additionally, production of anti-inflammatory cytokines, such as IL-10 and TGF-β1, was markedly increased with oral administration of L. rhamnosus (Table 5).
Fig. 5.
Effect of L. rhamnosus on the immune barrier function of piglets
(a) Relative expression of pBD-1, PMAP-37, and LYZ in jejunal mucosa; (b) Relative expression of cytokine and chemotactic factor genes in jejunal mucosa; (c) Relative expression of TLR genes in jejunal mucosa; (d) Relative expression of innate immune signaling relative genes in jejunal mucosa. The different small letters or different capital letters on the bar mean that the values are significantly different (P<0.05) or very significantly different (P<0.01), respectively. Data are expressed as mean±SD (n=6)
Table 5.
Production of cytokines and chemotactic factor in jejunal mucosa of suckling piglets
| Group | IL-1β (pg/g) | IL-6 (pg/g) | IL-12 (pg/g) | TNF-α (pg/g) |
| Control | 3244.33±90.22A | 164.43±9.30A | 263.24±16.01A | 96.95±11.76A |
| L. rhamnosus | 2133.55±46.21B | 89.78±5.22B | 161.14±4.39B | 31.62±3.63B |
|
| ||||
|
| ||||
| Group | IFN-γ (pg/g) | IL-8 (pg/g) | IL-10 (pg/g) | TGF-β1 (pg/g) |
|
| ||||
| Control | 4721.51±268.08B | 25 102.68±468.97A | 199.16±14.92B | 149.19±3.78B |
| L. rhamnosus | 6733.47±254.09A | 14 776.40±512.04B | 242.55±9.01A | 200.97±7.44A |
The data followed by different capital letters in the same column are very significantly different (P<0.01). Data are expressed as mean±SD (n=6)
3.5. L. rhamnosus regulated transcript levels of key genes involved in innate immune signaling pathways in jejunal mucosa
In the L. rhamnosus group, Toll-like receptor 2 (TLR2), TLR9, and myeloid differentiation-2 (MD-2) mRNA expression levels were dramatically up-regulated. However, no significant difference was observed in TLR6 or TLR8 expression between the two groups (Fig. 5c). To determine whether L. rhamnosus played a role in innate immune signaling pathways, we detected mRNA expression of TGF-β activated kinase 1 (TAK1), TNF receptor associated factor 6 (TRAF6), c-Jun N-terminal kinase (JNK), p38 and peroxisome proliferator activated receptor γ (PPARγ) (Fig. 5d), but only TRAF6 expression was significantly increased by L. rhamnosus.
4. Discussion
Immediately after birth, the gastrointestinal tract of neonates is involved in a process of microbiota colonization and succession (Mackie et al., 1999; Gaskins et al., 2008). Increasing evidence has suggested that early exposure with desirable microbiota could alter the pattern of microbial succession as well as immunological maturation (Rakoff-Nahoum and Medzhitov, 2008; Hou et al., 2015). The use of probiotics in piglet nutrition has been increasingly discussed in recent years (Taras et al., 2006; Ayala et al., 2015; Patil et al., 2015). Many studies have found beneficial effects of probiotics supplementation on the growth performance and gut health of suckling piglets (Shim et al., 2005; Zeyner and Boldt, 2006; Hayakawa et al., 2016). However, little is known about the piglets’ intestinal functions following L. rhamnosus treatment in the first few days after birth under normal conditions. Thus, the objective of this experiment is to determine whether L. rhamnosus administration to newborn piglets in the first few days after birth can enhance the intestinal barrier functions, including the intestinal immune function, intestinal permeability, and gut microbial composition of pre-weaning piglets.
As is known, L. rhamnosus can improve the daily weight gain of piglets (Bocourt et al., 2004a, 2004b) and ameliorate diarrhea (Guarino et al., 2009). In the present study, L. rhamnosus also effectively increased the growth performance of pre-weaning piglets and alleviated the diarrhea, which implied that L. rhamnosus might improve the function of the intestinal barrier to defend against enteric pathogens (Nalle and Turner, 2015). Recently, accumulating evidence has suggested that gut microbiota plays a key role in gut health (Gareau et al., 2010; Flint et al., 2012). Intestinal bacterial communities are comprised of more than 1000 different species (Lozupone et al., 2012) and constitute an exceptionally diverse microbial ecosystem, which is critical for numerous physiologic processes (Stappenbeck et al., 2002; Hooper, 2004; Mazmanian et al., 2005). Genera of the phylum Firmicutes, which comprise the dominant microflora in the gut (Eckburg et al., 2005), including Bacillus, Enterococcus, Lactobacillus, and Lactococcus, play an important role in human and animal health (Haakensen et al., 2008). Although Bacteroidetes species also boost animal growth and health (Salyers, 1984), enterotoxigenic B. fragilis, a human colonic commensal, has been demonstrated to increase colonic thickness, inflammation and visible colonic tumors (Wu et al., 2009). Zhu et al. (2014) also found that Bacteroidetes species were more abundant in rats with colorectal cancer. Fusobacteria species have long been recognized in a wide spectrum of human infections (Klinge et al., 2002; Gmür et al., 2006; Allen-Vercoe et al., 2011). In the analysis of bacterial composition in cecal contents in this study, we observed that at the phylum level, the most remarkable result was the increase in Firmicutes and the decrease in Bacteroidetes as well as Fusobacteria with L. rhamnosus treatment. Similarly, the study of Angelakis and Raoult (2010) also noticed that Lactobacillus inoculation improved the weight gain via increasing the Firmicutes/Bacteroidetes ratio in the gut flora of newborn chicks and ducks. Hence, we speculate that L. rhamnosus could exert beneficial effects on piglets’ health via modulating the bacterial composition, especially the abundance of Firmicutes, Bacteroidetes, and Fusobacteria.
Microflora colonization can also be influenced by intestinal MUC-2, which is a major gel-forming mucin and is in direct contact with gut bacteria (Zhang et al., 2015). It was reported that dietary Lactobacillus supplementation increased MUC-2 concentration (Mao et al., 2016). Here, MUC-2 expression was also up-regulated by L. rhamnosus, but not to a statistically significant extent. DAO is an intracellular enzyme in the mucosal villous epithelial cells of humans and all mammals; the highest activity is detected in the jejunum and ileum (Meng et al., 2016). Following damage, necrosis, and exfoliation of the intestinal mucosal cells, DAO translocates into blood, which implies the destruction of the intestinal mucosal barrier and changes in intestinal permeability (Swank and Deitch, 1996; Sun et al., 2011). Compared with the control group, oral administration of L. rhamnosus significantly decreased DAO in jejunal mucosa. In addition, unlike the results in other papers (Mennigen et al., 2009; Liu et al., 2013), the tight junction OCLN in the present study was down-regulated when exposed to L. rhamnosus. As far as we are concerned, when the gut epithelium is damaged, the expression of tight junction proteins can also be increased in order to repair the damage; when there is no damage or in healthier conditions, they may remain at baseline or lower expression levels.
As the biggest immune organ, the intestine can secrete some bioactive substances to defend against foreign antigens, toxins, and macromolecules. Paneth cells in the intestinal villi are secretory cells that are specialized in the production of antimicrobial peptides (Rescigno, 2011). In this study, L. rhamnosus increased the mRNA expression of antimicrobial peptides pBD-1 and PMAP-37. On the other hand, LYZ expression decreased dramatically. LYZ hydrolyzes N-acetylmuramic acid and N-acetylglucosamine, which are constituents of the peptidoglycan layer of bacterial cell walls (Balcázar et al., 2007). Gram-negative bacteria, such as Escherichia coli, are more resistant to LYZ than Gram-positive bacteria, such as Lactobacillus, because their outer membranes hinder the access of lysozyme to peptidoglycan (Callewaert and Michiels, 2010). Therefore, we conjecture that due to the elevated antibacterial ability caused by pBD-1 and PMAP-37, there is no need for intestinal mucosa to express more LYZ to exert its bactericidal capacity and protect intestinal symbiotic bacteria in suckling piglets.
Innate immunological responses have evolved specifically to defend the intestinal mucosal interface by limiting direct bacterial contact with the epithelial surface. Enterocytes are known to serve as immuno-effector cells and are capable of secreting cytokines and chemokines to regulate inflammation (Kagnoff and Eckmann, 1997). Different strains of Lactobacillus species can elicit a wide range of cytokine responses in immune cells (Meijerink et al., 2010, 2012). In our findings, except for IFN-γ, levels of all the tested pro-inflammatory cytokines and chemokine were decreased, whereas levels of IL-10 and TGF-β1, the well-known anti-inflammatory cytokines, were increased in the L. rhamnosus group. The result of IFN-γ secretion was in line with previous studies in which Lactobacillus augmented the number of IFN-γ producing cells and increased the synthesis of IFN-γ in the small intestine of mice (Wen et al., 2014, 2015), as IFN-γ plays a key role in the maturation of immune cells and regulates their cellular proliferation in the intestine (Rumbo et al., 2004). As for qRT-PCR results, the IFN-γ and TGF-β transcript levels were in accordance with the ELISA results. Although mRNA expression is predictive for protein expression, in the present study, the TNFα and IL-8 transcription levels were not consistent with the protein secretions. However, in previous studies, the same inconsistency also existed (Brundel et al., 2001; Guo et al., 2008), implying that there may be some post-transcriptional regulation (Brundel et al., 2001). In addition, MCP-1 transcript level was markedly elevated by L. rhamnosus. Similar to our results, probiotic L. rhamnosus GG and E. coli Nissle 1917 have also been reported to enhance MCP-1 expression in other studies (Lan et al., 2005; Ukena et al., 2005). MCP-1 is a proinflammatory chemokine produced by many cells, including epithelial, endothelial, and smooth muscle cells (Cushing et al., 1990; Standiford et al., 1991). These cells are important for antiviral immune responses in the peripheral circulation and in tissue (Deshmane et al., 2009). Moreover, MCP-1 shows chemotactic activity for monocytes, basophils, natural killer cells, and T lymphocytes (Ukena et al., 2005). Taken together, in this study, L. rhamnosus could regulate immune responses and increase the antibacterial ability in the gut of piglets through altering the production of cytokines and chemokines.
Activation of TLRs provides information about the bacterial census in the intestine and activates expression of secreted antimicrobial proteins to maintain mucosal surface-associated bacterial populations at homeostatic levels (Duerkop et al., 2009). Several studies have showed that strain-specific Lactobacillus up-regulated TLR2 and TLR9 transcript levels in vitro (Cammarota et al., 2009; Vizoso Pinto et al., 2009). Dogi et al. (2010) also observed that Lactobacillus increased the numbers of IFN-γ and TNF-α positive cells via TLR9 in mice Peyer’s patches. In this research, we obtained similar results in that L. rhamnosus significantly elevated the expression of MD-2, TLR-2, and TLR-9. Noticeably, TRAF6, which is critical for TLR-mediated activation of dendritic cells (Han et al., 2015), was also induced by L. rhamnosus. Thus, we conclude that L. rhamnosus can regulate the jejunal mucosal immunologic barrier through TLRs in suckling piglets.
5. Conclusions
In conclusion, under normal conditions, oral administration of L. rhamnosus GG to newborn piglets is beneficial for the intestinal health of pre-weaning piglets by improving the biological, physical, and immunologic barriers of intestinal mucosa.
List of electronic supplementary materials
Composition of sows’ diet
Nutrient levels of sows’ diet
Composition of pre-starter feed
Nutrient levels of pre-starter feed
Experimental protocol design
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31472128), the Special Research Fund for the PhD Program of University, China (No. 20110101110101), and the Key Project of Science and Technology of Zhejiang Province, China (No. 2006C12086)
Contributors: Yang WANG and Li GONG performed the experiments and wrote the manuscript. Yan-ping WU and Zhi-wen CUI analyzed the data. Yong-qiang WANG and Yi HUANG edited the manuscript. Xiao-ping ZHANG and Wei-fen LI designed the study.
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1800022) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Yang WANG, Li GONG, Yan-ping WU, Zhi-wen CUI, Yong-qiang WANG, Yi HUANG, Xiao-ping ZHANG, and Wei-fen LI declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294–298. doi: 10.4161/gmic.2.5.18603. [DOI] [PubMed] [Google Scholar]
- 2.Angelakis E, Raoult D. The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS ONE. 2010;5(5):e10463. doi: 10.1371/journal.pone.0010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala L, Bocourt R, Castro M, et al. Effect of the probiotic additive Bacillus subtilis and their endospores on milk production and immune response of lactating sows. Cuban J Agric Sci. 2015;49(1):71–74. [Google Scholar]
- 4.Balcázar JL, de Blas I, Ruiz-Zarzuela I, et al. Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout (Salmo trutta) Br J Nutr. 2007;97(3):522–527. doi: 10.1017/S0007114507432986. [DOI] [PubMed] [Google Scholar]
- 5.Bauer E, Williams BA, Smidt H, et al. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7(2):35–51. [PubMed] [Google Scholar]
- 6.Bocourt R, Savon L, Diaz J, et al. Effect of the probiotic activity of Lactobacillus rhamnosus on productive and health indicators of piglets. Cuban J Agric Sci. 2004;38(1):75–79. [Google Scholar]
- 7.Bocourt R, Savon L, Diaz J. Effect of the probiotic activity of Lactobacillus rhamnosus on physiological indicators of suckling pigs. Cuban J Agric Sci. 2004;38(4):403–408. [Google Scholar]
- 8.Brundel BJJM, van Gelder IC, Henning RH, et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001;37(3):926–932. doi: 10.1016/S0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 9.Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci. 2010;35(1):127–160. doi: 10.1007/s12038-010-0015-5. [DOI] [PubMed] [Google Scholar]
- 10.Cammarota M, de Rosa M, Stellavato A, et al. In vitro evaluation of Lactobacillus plantarum DSMZ 12028 as a probiotic: emphasis on innate immunity. Int J Food Microbiol. 2009;135(2):90–98. doi: 10.1016/j.ijfoodmicro.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Casserly C, Erijman L. Molecular monitoring of microbial diversity in an UASB reactor. Int Biodeterior Biodegrad. 2003;52(1):7–12. doi: 10.1016/S0964-8305(02)00094-X. [DOI] [Google Scholar]
- 12.Chen RC, Xu LM, Du SJ, et al. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett. 2016;241:103–110. doi: 10.1016/j.toxlet.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Cushing SD, Berliner JA, Valente AJ, et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng J, Li YF, Zhang JH, et al. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res Vet Sci. 2013;94(1):62–68. doi: 10.1016/j.rvsc.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytok Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogi CA, Weill F, Perdigón G. Immune response of non-pathogenic Gram(+) and Gram(−) bacteria in inductive sites of the intestinal mucosa: study of the pathway of signaling involved. Immunobiology. 2010;215(1):60–69. doi: 10.1016/j.imbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31(3):368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flint HJ, Scott KP, Louis P, et al. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9(10):577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 20.Galdeano CM, Perdigón G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. 2006;13(2):219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaskins HR, Croix JA, Nakamura N, et al. Impact of the intestinal microbiota on the development of mucosal defense. Clin Infect Dis. 2008;46(S2):S80–S86. doi: 10.1086/523336. [DOI] [PubMed] [Google Scholar]
- 23.Gmür R, Munson MA, Wade WG. Genotypic and phenotypic characterization of Fusobacteria from Chinese and European patients with inflammatory periodontal diseases. Syst Appl Microbiol. 2006;29(2):120–130. doi: 10.1016/j.syapm.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Goede D, Morrison R. Production impact study update. Swine Health Monitoring Project 08/01/2014. University of Minnesota. http://www.cvm.umn.edu/sdec/SwineDiseases/pedv/SHMP_14/index.htm [Accessed on July 5, 2015].2015. [Google Scholar]
- 25.Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol. 2009;25(1):18–23. doi: 10.1097/MOG.0b013e32831b4455. [DOI] [PubMed] [Google Scholar]
- 26.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Xiao P, Lei S, et al. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin. 2008;40(5):426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 28.Haakensen M, Dobson CM, Deneer H, et al. Real-time PCR detection of bacteria belonging to the Firmicutes Phylum. Int J Food Microbiol. 2008;125(3):236–241. doi: 10.1016/j.ijfoodmicro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Han D, Walsh M, Choi Y, et al. TRAF6 expression in dendritic cells is essential for tolerance to dietary antigens (MUC8P.723) J Immunol. 2015;194(1S):204–3. [Google Scholar]
- 30.Hanke D, Jenckel M, Petrov A, et al. Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerg Infect Dis. 2015;21(3):493–496. doi: 10.3201/eid2103.141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayakawa T, Masuda T, Kurosawa D, et al. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim Sci J. 2016;87(12):1501–1510. doi: 10.1111/asj.12565. [DOI] [PubMed] [Google Scholar]
- 32.Hermann-Bank ML, Skovgaard K, Stockmarr A, et al. Characterization of the bacterial gut microbiota of piglets suffering from new neonatal porcine diarrhoea. BMC Vet Res, 11:139. 2015 doi: 10.1186/S12917-015-0419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12(3):129–134. doi: 10.1016/J.Tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Hou CL, Liu H, Zhang J, et al. Intestinal microbiota succession and immunomodulatory consequences after introduction of Lactobacillus reuteri I5007 in neonatal piglets. PLoS ONE. 2015;10(3):e0119505. doi: 10.1371/journal.pone.0119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100(1):6–10. doi: 10.1172/Jci119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci, 9:392. 2015 doi: 10.3389/Fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic. 2008;8(1):1–13. doi: 10.1016/j.ecolind.2007.06.002. [DOI] [Google Scholar]
- 38.Klinge L, Vester U, Schaper J, et al. Severe Fusobacteria infections (Lemierre syndrome) in two boys. Eur J Pediatr. 2002;161(11):616–618. doi: 10.1007/s00431-002-1026-5. [DOI] [PubMed] [Google Scholar]
- 39.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108(S1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan JG, Cruickshank SM, Singh JC, et al. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J Gastroenterol. 2005;11(22):3375–3384. doi: 10.3748/wjg.v11.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei K, Li YL, Yu DY, et al. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult Sci. 2013;92(9):2389–2395. doi: 10.3382/ps.2012-02686. [DOI] [PubMed] [Google Scholar]
- 42.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li WF, Huang Y, Li YL, et al. Effect of oral administration of Enterococcus faecium Ef1 on innate immunity of sucking piglets. Pak Vet J. 2012;33(1):9–13. [Google Scholar]
- 44.Liu FN, Li GH, Wen K, et al. Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. J Pediatr Gastroenterol Nutr. 2013;57(6):750–758. doi: 10.1097/MPG.0b013e3182a356e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 47.Mao XB, Gu CS, Hu HY, et al. Dietary Lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PLoS ONE. 2016;11(1):e0146312. doi: 10.1371/journal.pone.0146312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 49.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3(1):1–11. doi: 10.1046/J.1462-5822.2001.00090.X. [DOI] [PubMed] [Google Scholar]
- 50.Meijerink M, van Hemert S, Taverne N, et al. Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS ONE. 2010;5(5):e10632. doi: 10.1371/journal.pone.0010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meijerink M, Wells JM, Taverne N, et al. Immunomodulatory effects of potential probiotics in a mouse peanut sensitization model. FEMS Immunol Med Microbiol. 2012;65(3):488–496. doi: 10.1111/j.1574-695X.2012.00981.x. [DOI] [PubMed] [Google Scholar]
- 52.Meng Y, Zhang Y, Liu M, et al. Evaluating intestinal permeability by measuring plasma endotoxin and diamine oxidase in children with acute lymphoblastic leukemia treated with high-dose methotrexate. Anticancer Agents Med Chem. 2016;16(3):387–392. doi: 10.2174/1871520615666150812125955. [DOI] [PubMed] [Google Scholar]
- 53.Mennigen R, Nolte K, Rijcken E, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 54.Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8(4):720–730. doi: 10.1038/mi.2015.40. [DOI] [PubMed] [Google Scholar]
- 55.National Research Council. The National Academies Press, Washington, DC, USA; 1998. Nutrient Requirements of Swine, 10th Ed. [DOI] [Google Scholar]
- 56.Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69(1):42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Ngamwongsatit B, Tanomsridachchai W, Suthienkul O, et al. Multidrug resistance in Clostridium perfringens isolated from diarrheal neonatal piglets in Thailand. Anaerobe. 2016;38:88–93. doi: 10.1016/j.anaerobe.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Patil AK, Kumar S, Verma AK, et al. Probiotics as feed additives in weaned pigs: a review. Livest Res Int. 2015;3:31–39. [Google Scholar]
- 59.Rajput IR, Li LY, Xin X, et al. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult Sci. 2013;92(4):956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- 60.Rakoff-Nahoum S, Medzhitov R. Innate immune recognition of the indigenous microbial flora. Mucosal Immunol. 2008;1(1):S10–S14. doi: 10.1038/mi.2008.49. [DOI] [PubMed] [Google Scholar]
- 61.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Rumbo M, Anderle P, Didierlaurent A, et al. How the gut links innate and adaptive immunity. Ann N Y Acad Sci. 2004;1029:16–21. doi: 10.1196/annals.1309.003. [DOI] [PubMed] [Google Scholar]
- 63.Salyers AA. Bacteroides of the human lower intestinal tract. Annu Rev Microbiol. 1984;38:293–313. doi: 10.1146/Annurev.Mi.38.100184.001453. [DOI] [PubMed] [Google Scholar]
- 64.Shim SB, Verstegen MWA, Kim IH, et al. Effects of feeding antibiotic-free creep feed supplemented with oligofructose, probiotics or synbiotics to suckling piglets increases the preweaning weight gain and composition of intestinal microbiota. Arch Anim Nutr. 2005;59(6):419–427. doi: 10.1080/17450390500353234. [DOI] [PubMed] [Google Scholar]
- 65.Standiford TJ, Kunkel SL, Phan SH, et al. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266(15):9912–9918. [PubMed] [Google Scholar]
- 66.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99(24):15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun RQ, Cai RJ, Chen YQ, et al. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg Infect Dis. 2012;18(1):161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun YJ, Cao HJ, Jin Q, et al. Effects of penehyclidine hydrochloride on rat intestinal barrier function during cardiopulmonary bypass. World J Gastroenterol. 2011;17(16):2137–2142. doi: 10.3748/wjg.v17.i16.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20(4):411–417. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 70.Tannock GW. Molecular assessment of intestinal microflora. Am J Clin Nutr. 2001;73(2):410S–414S. doi: 10.1093/ajcn/73.2.410s. [DOI] [PubMed] [Google Scholar]
- 71.Taras D, Vahjen W, Macha M, et al. Performance, diarrhea incidence, and occurrence of Escherichia coli virulence genes during long-term administration of a probiotic Enterococcus faecium strain to sows and piglets. J Anim Sci. 2006;84(3):608–617. doi: 10.2527/2006.843608x. [DOI] [PubMed] [Google Scholar]
- 72.Theuns S, Conceição-Neto N, Christiaens I, et al. Complete genome sequence of a porcine epidemic diarrhea virus from a novel outbreak in Belgium, January 2015. Genome Announc. 2015;3(3):e00506–15. doi: 10.1128/genomeA.00506-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toledo A, Gómez D, Cruz C, et al. Prevalence of virulence genes in Escherichia coli strains isolated from piglets in the suckling and weaning period in Mexico. J Med Microbiol. 2012;61(1):148–156. doi: 10.1099/jmm.0.031302-0. [DOI] [PubMed] [Google Scholar]
- 74.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 75.Ukena SN, Westendorf AM, Hansen W, et al. The host response to the probiotic Escherichia coli strain Nissle 1917: specific up-regulation of the proinflammatory chemokine MCP-1. BMC Med Genet, 6:43. 2005 doi: 10.1186/1471-2350-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vizoso Pinto MG, Gómez MR, Seifert S, et al. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro . Int J Food Microbiol. 2009;133(1-2):86–93. doi: 10.1016/j.ijfoodmicro.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Wang JJ, Tang H, Zhang CH, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Wu YP, Wang YB. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl Microbiol Biotechnol. 2017;101(7):3015–3026. doi: 10.1007/s00253-016-8032-4. [DOI] [PubMed] [Google Scholar]
- 79.Wen K, Tin C, Wang HF, et al. Probiotic Lactobacillus rhamnosus GG enhanced Th1 cellular immunity but did not affect antibody responses in a human gut microbiota transplanted neonatal gnotobiotic pig model. PLoS ONE. 2014;9(4):e94504. doi: 10.1371/journal.pone.0094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen K, Liu FN, Li GH, et al. Lactobacillus rhamnosus GG dosage affects the adjuvanticity and protection against rotavirus diarrhea in gnotobiotic pigs. J Pediatr Gastroenterol Nutr. 2015;60(6):834–843. doi: 10.1097/MPG.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 81.Wu SG, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeyner A, Boldt E. Effects of a probiotic Enterococcus faecium strain supplemented from birth to weaning on diarrhoea patterns and performance of piglets. J Anim Physiol Anim Nutr. 2006;90(1-2):25–31. doi: 10.1111/j.1439-0396.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, Xu YQ, Liu HY, et al. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: effects on diarrhoea incidence, faecal microflora and immune responses. Vet Microbiol. 2010;141(1-2):142–148. doi: 10.1016/j.vetmic.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Q, Eicher SD, Applegate TJ. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult Sci. 2015;94(2):172–180. doi: 10.3382/ps/peu064. [DOI] [PubMed] [Google Scholar]
- 85.Zhang XP, Shu MA, Wang YB, et al. Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J Microbiol Biotechnol. 2014;30(9):2523–2531. doi: 10.1007/s11274-014-1677-1. [DOI] [PubMed] [Google Scholar]
- 86.Zhu QC, Jin ZM, Wu W, et al. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS ONE. 2014;9(3):e90849. doi: 10.1371/journal.pone.0090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Composition of sows’ diet
Nutrient levels of sows’ diet
Composition of pre-starter feed
Nutrient levels of pre-starter feed
Experimental protocol design