Abstract
Bone morphogenetic proteins (BMPs) are the largest subfamily of the transforming growth factor-β superfamily, and they play important roles in the development of numerous organs, including the inner ear. The inner ear is a relatively small organ but has a highly complex structure and is involved in both hearing and balance. Here, we discuss BMPs and BMP signaling pathways and then focus on the role of BMP signal pathway regulation in the development of the inner ear and the implications this has for the treatment of human hearing loss and balance dysfunction.
Keywords: Bone morphogenetic protein (BMP) signaling, Development, Inner ear, Hearing, Balance
1. Introduction
Bone morphogenetic proteins (BMPs) are multifunctional cytokines that belong to the transforming growth factor-β (TGF-β) superfamily. The first BMP, discovered by Urist (1965), was originally identified as an osteoinductive growth factor with the capacity to induce endochondral bone formation. However, BMPs are now well known as multifunctional proteins that play important roles in various organs, including bone, cartilage, the nervous system, the kidney, blood vessels, and the ear (Hoffmann and Gross, 2001; Wordinger and Clark, 2007; Lowery and de Caestecker, 2010; Chen et al., 2012; Nakamura and Yanagita, 2012). They are involved in numerous biological activities, including iron metabolism, muscle development, stem cell and organ formation, vascular biology, and cancer development (Wordinger and Clark, 2007).
The inner ear, a tiny organ involved in both hearing and balance, can be divided into the bony labyrinth and the membranous labyrinth. It consists of three semicircular ducts, the saccule, the utricle, the endolymphatic duct, and the cochlear duct. This complex organ develops from the simple otic placode, which arises as a patch of thickened ectoderm adjacent to the hindbrain (Alvarez and Navascués, 1990; Sai and Ladher, 2015; Ekdale, 2016). The development of the inner ear has been studied for more than 100 years, but only recently have the numerous signaling pathways involved in this process been identified, including wingless-type MMTV integration site family (WNT) signaling, sonic hedgehog (SHH) signaling, notch signaling, and BMP signaling (Alsina et al., 2009).
2. BMP family and members in signaling pathways
2.1. BMPs
BMPs, of which around 20 members have been identified to date, are the largest subfamily of the TGF-β superfamily and have highly conserved structures. The one exception is BMP1, which does not belong to the TGF-β superfamily and has been reported to be a secreted N-glycosylated metalloprotease that cleaves the COOH-propeptides of procollagens І, II, and III, and to be crucial for cartilage and bone formation (Kessler et al., 1996). In addition, because BMPs were identified by multiple approaches, they might have synonyms, such as growth differentiation factors (GDFs), osteogenic proteins, Vg-related proteins, and osteogenin. In this review, only BMP and GDF are used to avoid confusion. According to their amino acid sequences and their different functions, BMPs are divided into four subfamilies (Fig. 1): (1) BMPs 2 and 4; (2) BMPs 5, 6, 7, 8a, and 8b; (3) BMPs 9 and 10; and (4) BMPs 12, 13, and 14 (Wordinger and Clark, 2007). Some BMPs are not included in the classification such as BMPs 3, 15, 16, 17, and 18 and GDF9. BMPs are reported to have various functions. BMP4 is the most studied BMP involved in inner ear development (Takemura et al., 1996; Blauwkamp et al., 2007; Omata et al., 2007).
Fig. 1.
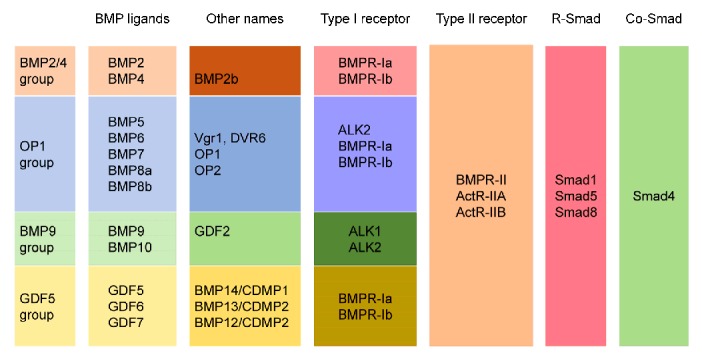
BMPs, BMP receptors, and activated Smads
Based on their function and structure, BMPs are divided into four subgroups: (1) BMPs 2 and 4; (2) BMPs 5, 6, 7, 8a, and 8b; (3) BMPs 9 and 10; and (4) BMPs 12, 13, and 14. In addition, the related BMP receptors, Smad proteins, and some other names for the BMP ligands are listed. BMP, bone morphogenetic protein; OP, osteogenic protein; GDF, growth differentiation factor; Vgr, vegetal related; DVR, decapentaplegic vegetal related; CDMP, cartilage-derived morphogenetic protein; BMPR, BMP receptor; ALK, activin receptor-like kinase; ActR-II, activin type II receptor
Except for BMP1, BMPs have conserved structures that are found in all members of the TGF-β superfamily, which are dimeric molecules synthesized as large precursors of about 400–500 amino acids. Their precursor proteins consist of one signal peptide at the N-terminus, one non-conserved pro-domain, and the mature sequence at the C-terminus. Cleavage of the precursor proteins by a subtilisin-like convertase yields the mature BMP monomer, which contains 100–140 amino acid residues. The monomer contains seven conserved cysteine residues, six of which form a cysteine knot. The seventh is involved in dimerization with another monomer by forming a covalent disulfide bond, thus forming the biologically active signaling molecule (Carreira et al., 2014a, 2014b; Katagiri and Watabe, 2016). However, some BMPs, including BMP3 and BMP15, lack the seventh cysteine and are biologically active as monomers. The other mature BMP molecules appear to exist either as homodimers consisting of two similar BMPs or as heterodimers consisting of two different BMPs.
2.2. BMP receptors
BMP signaling is transmitted through type І and type II transmembrane serine/threonine kinase receptors (Miyazono et al., 2005). These receptors share a similar structure comprising a relatively short extracellular domain, a single membrane-spanning domain, and an intracellular domain containing a serine-threonine kinase domain (Miyazono et al., 2010). In other TGF-β receptor systems, the type І receptors can bind to ligands only in the presence of the type II receptors to which the ligands are directly bound. However, BMPs can bind to type І receptors without type II receptors, but their binding affinities increase dramatically when both type І and type II receptors are present (Rosenzweig et al., 1995).
In vertebrates, seven type І receptors have been identified for the TGF-β superfamily, including activin receptor-like kinases (ALKs) 1–7. Three type II receptors have been identified for BMPs: the BMP type II receptor (BMPR-II), the activin type IIB receptor (ActR-IIB), and the activin type II receptor (ActR-II) (Miyazono et al., 2005). BMPR-II is specific for BMPs, while ActR-IIB and ActR-II also bind to other members of the TGF-β superfamily. BMPR-II has a unique C-terminal tail of 530 amino acids following the serine/threonine kinase domain.
2.3. Smad-dependent BMP pathways
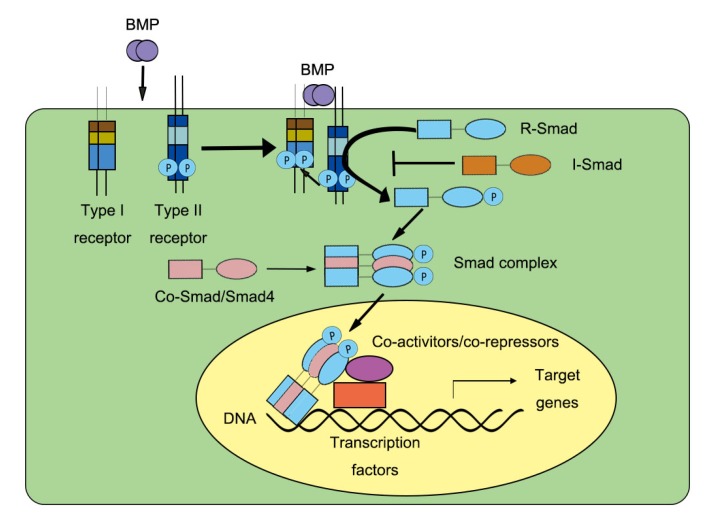
BMP binding activates type І receptors, and the signal is subsequently transmitted through both canonical Smad-dependent pathways (Fig. 2) and noncanonical Smad-independent pathways.
Fig. 2.
Smad-dependent BMP signaling
BMP ligands interact with the BMP receptor complex consisting of type І and type II receptors (BMPR-І and BMPR-ІI, respectively). After the interaction, BMPR-І phosphorylates BMPR-II and leads to the phosphorylation of R-Smads (Smad1/Smad5/Smad8). This activation allows the formation of Smad complexes consisting of R-Smads (Smad1/Smad5/Smad8) and Co-Smad (Smad4). The Smad complexes then translocate into the nucleus where they induce transcription of the target genes. R-Smad, receptor-regulated Smad; Co-Smad, common-mediator Smad; I-Smad, inhibitory Smad
Based on their functions, the eight Smad proteins are divided into three subgroups: receptor-regulated Smads (R-Smads), common-mediator Smads (Co-Smads), and inhibitory Smads (I-Smads) (Heldin et al., 1997). Smads 1, 5, and 8 are R-Smads and are activated by type І BMP receptors, while Smads 2 and 3 are involved in TGF-β signaling. Smad4 is the only Co-Smad known in mammals, and it is important for both BMP signaling and TGF-β signaling. Smads 6 and 7 are I-Smads that negatively regulate signaling by the R-Smads and Co-Smads (Miyazono et al., 2005).
The Smad protein structures are highly conserved and consist of Mad homology 1 (MH1) and MH2 domains. The MH2 domain interacts with type І receptors and is responsible for the transduction of the signaling, while the MH1 domain binds to specific DNA sequences and negatively regulates the function of the MH2 domain (Shi et al., 1998).
In the Smad-dependent BMP pathway, upon ligand binding, the constitutively active type II receptor phosphorylates the Gly-Ser domain of the type І receptor. The activated type І receptor recruits and phosphorylates R-Smads, which then form a complex with Co-Smads (Smad4). This Smad complex then translocates from the cytoplasm to the nucleus where it regulates the expression of target genes by directly binding to specific DNA sequences, interacting with certain DNA-binding proteins, and recruiting specific transcriptional coregulators (Miyazono et al., 2010; Macias et al., 2015).
2.4. Smad-independent BMP pathways
Smad-independent BMP pathways are also called noncanonical BMP pathways and include many branches of the mitogen-activated protein kinase (MAPK) pathways, the Rho-like GTPase signaling pathways, and the phosphatidylinositol-3-kinase (PI3K)/serine/threonine kinase (AKT) pathways (Derynck and Zhang, 2003; Zhang, 2009, 2017; Miyazono et al., 2010; Mu et al., 2012). Among these pathways, some regulate Smad activation, while others are unrelated to transcription.
2.5. Regulation of BMP signaling
BMP signaling is regulated by both intracellular and extracellular factors at various levels. Most of the mechanisms that control BMP pathways are inhibitory, including: (1) the action of extracellular BMP-binding proteins that inhibit BMP binding to its receptors; (2) the action of dominant-negative nonsignaling membrane pseudo-receptors; (3) the regulation of BMP signaling through the I-Smads; (4) the regulation of BMP signaling by the ubiquitin-mediated proteasomal system; and (5) other mechanisms (Gazzerro and Canalis, 2006; Brazil et al., 2015).
2.5.1 Extracellular mechanisms that regulate BMP signaling
Extracellular BMP antagonists are secreted peptides that bind to BMPs with high affinity, thus preventing them from binding to their receptors. These antagonists contain six cysteine residues that form a cysteine knot, similar to that of the TGF-β superfamily members. Based on the known spacing of the cysteine residues and their cysteine rings, the extracellular BMP antagonists have been categorized into three subgroups: (1) the differential screening-selected gene aberrative in neuroblastoma (DAN) family of proteins (eight-membered ring), (2) the twisted gastrulation protein, and (3) chordin and noggin (ten-membered ring) (Avsian-Kretchmer and Hsueh, 2004; Gazzerro and Canalis, 2006). The DAN family includes DAN, Coco, gremlin, cerberus, sclerostin, protein related to DAN and cerberus, and uterine sensitization-associated gene 1 protein (Kattamuri et al., 2012).
Previous studies suggest that these BMP antagonists play important roles in regulating cell functions and are essential for early development (McMahon et al., 1998). For example, homozygous deletion of the noggin gene in mice causes overactivity of BMPs and results in serious developmental abnormalities, including failure of neural tube formation and failure of axial skeleton and joint formation (Brunet et al., 1998; McMahon et al., 1998; Wijgerde et al., 2005). Previous reports also showed that BMP antagonists bind to different BMPs with different affinities. For example, noggin binds to BMPs 2, 4, 5, 6, and 7, and GDFs 5 and 6 with variable degrees of affinity (Zimmerman et al., 1996), while chordin binds specifically to BMPs 2, 4, and 7 (Piccolo et al., 1996).
Another extracellular mechanism to control BMP signaling is through the BMP and activin membrane-bound inhibitor (BAMBI), which is a non-signaling membrane pseudo-receptor with an extracellular domain similar to that of type І BMP receptors (BMPR-I). BAMBI can compete with the BMPR-Ia and BMPR-Ib receptors for BMP binding, thereby inhibiting the downstream signaling of BMPs.
2.5.2 Intracellular mechanisms that regulate BMP signaling
BMP signaling can also be regulated by antagonists within the target cell, including I-Smads, Smad-ubiquitination regulatory factors (Smurfs), and intracellular Smad-binding proteins. Among these intracellular mechanisms, the most widely studied are the I-Smads, which contain conserved MH2 domains, but divergent MH1 domains, and thus can compete with R-Smads by binding to the activated type І BMP receptors (von Bubnoff and Cho, 2001; Sieber et al., 2009; Li, 2015; Miyazawa and Miyazono, 2017). Smad6 can interact with activated Smad1 and prevent the formation of R-Smad/Co-Smad complexes (Hata et al., 1998). In the nucleus, Smad6 regulates BMP signaling by modifying the interactions of Smads 1, 5, and 8 with co-repressors. For example, Smad1 can induce transcription by dislodging transcriptional repressors, such as homeobox C8 (Hoxc-8), from DNA binding sites, while Smad6 can bind to Hoxc-8 and prevent the dislodging thereby inhibiting the expression of the target genes of BMP signaling (Bai and Cao, 2002; Gazzerro and Canalis, 2006).
The Smurf proteins are members of the ubiquitin enzyme family that participate in a cascade of ubiquitin transfer reactions which require three enzymes: ubiquitin-activating enzyme (E1), ubiquitin conjugating enzyme (E2), and ubiquitin ligases (E3). Both Smurfs 1 and 2 are Smad-specific E3 ubiquitin ligases (Gazzerro and Canalis, 2006; Das and Chang, 2012; Zhang et al., 2014). Smurf1 selectively interacts with R-Smads specific for BMP signaling, thus triggering their ubiquitination and degradation (Zhu et al., 1999). The Smurf2 protein controls both TGF-β and BMP signaling by selectively regulating the degradation of activated Smad2 and to some extent activated Smad1 and Smad3 (David et al., 2013). Besides interacting with R-Smads, the Smurf proteins also interact with I-Smads, which can act as adaptors to recruit Smurf proteins from the nucleus to the cytoplasm, and thus mediate receptor degradation and downregulation of BMP signaling (Izzi and Attisano, 2004).
3. BMP signaling in the development of the inner ear
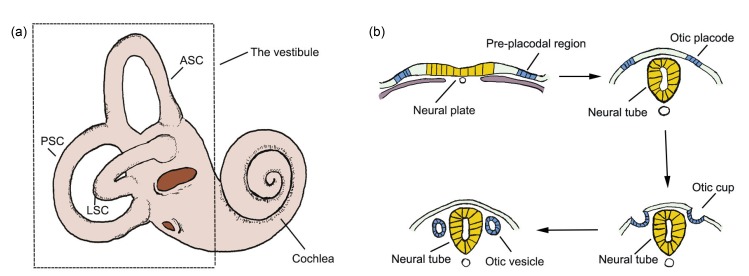
The vertebrate inner ear has a very complicated structure (Fig. 3a) and shows high sensitivity. It consists of the cochlea, which provides the sense of hearing, and the vestibule that provides the sense of balance (Kelley et al., 2005; Whitfield, 2015; Ekdale, 2016). The early development of the inner ear can be divided into three phases: the formation of the otic placode, which arises as thickened ectoderm adjacent to the caudal hindbrain, the morphogenesis of the otic placode into the otocyst, and the regional patterning of the otocyst to form the inner ear (Kelley et al., 2005).
Fig. 3.
Inner ear and the induction of the otocyst
(a) The inner ear consists of the cochlea, which is involved in hearing, and the vestibule, which is involved in balance. (b) The pre-placodal region is a zone of ectoderm that lies lateral to the neural plate. It gives rise to all sensory placodes in the head, including the otic placode. The placode invaginates into the otic cup, which later closes to become the otic vesicle/otocyst. ASC, anterior semicircular canal; LSC, lateral semicircular canal; PSC, posterior semicircular canal
3.1. Expression of BMPs during the induction of the placode and the formation of the otocyst
Specialized sensory organs in the heads of vertebrates arise as patches of thickenings in the embryonic ectoderm called cranial sensory placodes, including the otic placode that later forms the inner ear (Saint-Jeannet and Moody, 2014). These sensory placodes originate from a zone of ectoderm called the pre-placodal region (PPR), which lies lateral to the neural crest. The neural crest and the PPR originate from a zone of ectoderm that borders the neural plate. The bilateral otic placode is located at the hindbrain level of rhombomere 5 or rhombomere 4 in some species (Ruiz i Altaba and Jessell, 1991). The induction of the otic placode is the first stage of inner ear development (all staging relates to the chick and is according to Hamburger and Hamilton (1951)). After specification of the otic placode (stage 11), this placodal ectoderm rapidly invaginates to form a pit that later deepens into the otic cup (stages 12–16), and this finally closes to become the otic vesicle otocyst (stages 18–21) (Cole et al., 2000; Barald and Kelley, 2004; Ohyama et al., 2007).
Expression of the BMP4, BMP5, and BMP7 genes was observed in the chick prior the formation of the otocyst (Liem et al., 1995; Oh et al., 1996). BMP7 is the earliest BMP gene to be expressed in the presumptive otic placode and is expressed from stage 8. The otic placode becomes morphologically visible at stage 11 (Groves and Bronner-Fraser, 2000). In addition, incubation of 5–9 somite-stage chick embryos with the BMP receptor inhibitor dorsomorphin strongly downregulated expression of the Lmx1b gene—a marker of the posterior non-neurogenic otic epithelium (Abelló et al., 2010). This suggests that BMP7 has a possible influence on the development of the otic placode. BMP4 mRNA was detected in the medial and posterior placodes at stage 11 (Wu and Oh, 1996). In the otic cup, the expression of BMP7 is concentrated in the dorsal and posterior regions, which is similar to the pattern of expression of BMP4 and BMP5 but is much stronger and broader. The expression of BMP5 begins at stage 13 and disappears by stage 21 (embryonic day 3.5), while expression of BMP4 and BMP7 is more extensive and is still observed in the later stages (Oh et al., 1996). In zebrafish, bmp2b is the earliest bmp gene to be expressed in the ear, from 18 to 20 h post fertilisation (hpf), which is prior to otic placode formation. By 24 hpf, bmp2b and bmp4 were reported to be expressed in the zebrafish otic vesicle with patterns that are similar to that of BMP4 in chickens (Mowbray et al., 2001).
Previous studies have shown that a gradient of BMP signaling, which is high in the ventral and low in the dorsal region, is essential for the establishment of the dorsoventral axis and for the patterning of the ectoderm during gastrulation (Ramel and Hill, 2012). Inhibition of BMP signaling is required to the specification of the PPR after gastrulation (Glavic et al., 2004; Litsiou et al., 2005; Kwon et al., 2010; Reichert et al., 2013). However, even though the expression of BMPs during this period suggests a possible role of BMP signaling in otic placode induction and formation of the otocyst, compelling evidence for such a role is still lacking.
3.2. Expression of BMPs during otocyst patterning
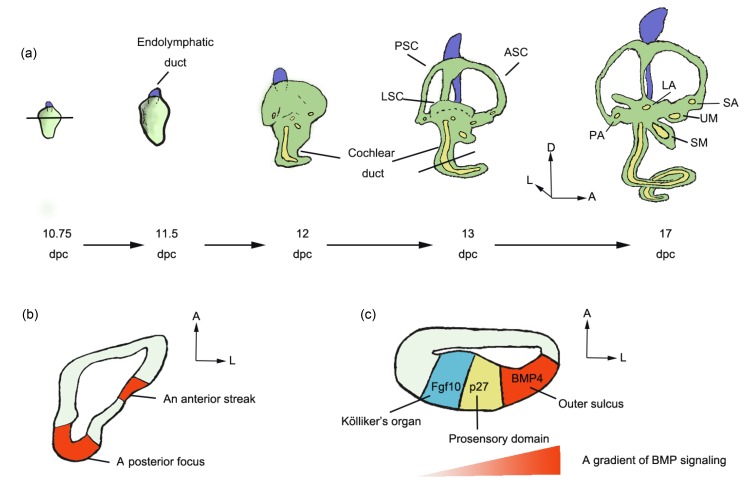
After the otocyst forms, it gradually establishes a ventral auditory chamber that gives rise to the cochlea, and a dorsal vestibular chamber that gives rise to the vestibular structures (Fig. 4a). Previous studies of the gene expression patterns of BMPs in the otocysts of different animals, including chickens, mice, zebrafish, and frogs, have shown that BMPs play critical roles in the morphogenesis of the inner ear (Oh et al., 1996; Takemura et al., 1996; Cole et al., 2000; Mowbray et al., 2001; Chang et al., 2002).
Fig. 4.
Specification of the inner ear
(a) The development of the mouse inner ear from 10.75 to 17 d past coitum (dpc). (b) In the mouse otocyst, BMP4 expression was detected in two regions at 10.75 dpc—an anterior streak and a posterior focus. (c) In the mouse cochlea, a gradient of BMP signaling was detected along the abneural–neural axis at 13.5 dpc. The BMP4 expression is high in the outer sulcus and low in Kölliker’s organ. ASC, anterior semicircular canal; LSC, lateral semicircular canal; PSC, posterior semicircular canal; LA, lateral ampulla; PA, posterior ampulla; SA, superior ampulla; SM, saccular macula; UM, utricular macula; A, anterior; D, dorsal; L, lateral
In the early developmental stages, BMP4 expression patterning in the chick otocyst, which is strongest in the anterior and posterior regions, is similar to the pattern in mice (Jones et al., 1991) and in Xenopus (Hemmati-Brivanlou and Thomsen, 1995). By the otocyst stage, BMP4 is expressed in specific domains of the otic epithelium that presage the appearance of all eight chicken sensory organs (the basilar papilla, three cristae, lagena, macula utriculi, macula sacculi, and macula neglecta) as well as in the otic mesenchyme (Oh et al., 1996; Wu and Oh, 1996). Similar expression of BMP4 in the frog inner ear was found in all sensory organs (Kil and Collazo, 2001). It was also reported that BMP4 is an early marker for the three cristae in the mouse inner ear (Morsli et al., 1998) and that it is expressed asymmetrically in the mouse cochlea (Ohyama et al., 2010). A study in zebrafish showed that bmp2b and bmp4 marked the developing cristae and that bmp4 was also expressed in the dorsal non-sensory region of the inner ear (Mowbray et al., 2001). Some other BMPs have been shown to be expressed in this process as well, including BMPs 2, 5, and 7 (Oh et al., 1996; Chang et al., 2002).
In addition to the expression of BMPs in the otocyst, some BMP regulators have also been observed in the otic mesenchyme and otocyst, and these might have an influence on otocyst patterning. Noggin is the most-studied BMP regulator. It was identified as an extracellular antagonist of BMP signaling that acts by binding to BMP2 and BMP4 with high affinity, thereby preventing these BMPs from activating their receptors. Noggin was reported to be expressed in tissues surrounding the otic cup at embryonic days 2.0–2.5 (E2.0–E2.5) and to be weakly expressed in the ventral tip of the cochlear duct at E4.0–E6.5 in the chick (Chang et al., 1999). Another study showed that noggin was expressed in the anterior and posterior periotic mesenchyme adjacent to the otic pit at early stages (stages 11–15), but disappeared by stages 17–20 in the chick embryo (Gerlach et al., 2000). Using RNA probes against noggin in mouse embryos, noggin was found to be expressed in the dorsal periotic mesenchyme at 9.5 d past coitum as well as in the cochlear duct and semicircular canals of the inner ear (Bok et al., 2007). Like noggin, DAN expression was observed in the development of both the mouse and chicken inner ears. However, in the chicken, DAN was found in both the otic epithelium and notochord, while in mice it was restricted to the otic mesenchyme (Gerlach-Bank et al., 2002). In addition, Smad6, an inhibitory SMAD, was expressed in the ventrolateral otocyst in chicken.
From all the studies described above, we conclude that BMPs, especially BMP4, as well as some BMP regulators, are expressed during the development of the inner ear of various species and are essential for this process. To further strengthen the evidence linking BMP signaling with otocyst patterning, exogenous BMP regulators and various knockout animals have been used. For a better understanding of this process, below we review separately the roles of BMP signaling in the patterning of the vestibule and the cochlea.
3.2.1 Roles of BMPs in the patterning of the vestibule
In the otocyst stages, BMP4 is expressed in all seven pre-sensory vestibular organs in chickens (Wu and Oh, 1996) and in all three presumptive cristae in mice (Morsli et al., 1998). For the non-sensory vestibular organs, BMP4 is expressed in the otic mesenchyme that later gives rise to the semicircular canals in both the mouse and chicken (Oh et al., 1996; Wu and Oh, 1996; Morsli et al., 1998). These studies show the expression of BMP4 and the possible influence it has on the development of the vestibule.
BMP signaling has been shown to influence the formation of the vestibular chamber as well as the subsequent morphogenesis of the sensory and non-sensory vestibular structures. Ohyama et al. (2010) have demonstrated the sufficient role of BMP signaling in the formation of the primordial canal outpouch that gives rise to the anterior and posterior semicircular canals. All components of the active BMP signaling pathway are present in or adjacent to the dorsolateral otocyst in the chicken. They drive the growth of cells involved in otocyst thinning and expansion, which results in the formation of the vestibular chamber from the dorsolateral otocyst. In addition, overexpression of BMP4 results in an enlarged primordial canal outpouch, whereas overexpression of a BMP antagonist inhibits this process. The analysis of mice with BMP4 haplo-insufficiency showed that BMP4 affects outgrowth of the lateral canal plate, resulting in defects in the lateral semicircular canal (Vervoort et al., 2010).
Previous studies have demonstrated that the formation of vestibular structures, especially the semicircular canals and sensory cristae, is sensitive to the BMP signaling pathway. Mice lacking the Bmp4 gene die as embryos (Winnier et al., 1995), but Bmp4 heterozygous null (Bmp4 +/−) mice survive, have semicircular canal dysfunction, show a poor response in the yaw axis, and exhibit circling behavior (Blauwkamp et al., 2007). Exogenous noggin, delivered to the chick inner ear during E2–E3 using an avian retrovirus or by implanting beads, affected the structural patterning of the cristae and led to the truncation of the semicircular canals (Chang et al., 1999; Gerlach et al., 2000). Co-implantation of BMP4 beads next to the noggin beads was reported to rescue these defects in the chick inner ear (Gerlach et al., 2000). Similarly, analysis of BMP4 otic-conditional-deleted mice and chickens—in which BMP signaling is knocked down specifically in the cristae—showed that BMP4 is essential for the formation of the three cristae and the three associated semicircular canals (Chang et al., 2008). In addition, Alk3-CKO; Alk6 +/− mutant mice, in which BMP signals were reduced, also showed malformation in the semicircular canals while the vestibular sensory structures appeared normal (Ohyama et al., 2010). Malformations of the semicircular canals were observed after implanting DAN-expressing cell pellets into the otocyst and the periotic mesenchyme. In addition, the endolymphatic ducts and sacs were partially or completely merged with the crus or grew toward or connected to the superior semicircular canal in the DAN-treated inner ear in chicken, while blocking DAN protein synthesis resulted in enlarged endolymphatic ducts and sacs, and smaller semicircular canals (Gerlach-Bank et al., 2004).
In addition, the BMP signaling pathway affects chondrogenesis of the otic capsule, which surrounds the developing otic vesicle and later develops into the bony labyrinth of the internal ear. Expression of BMP4 was detected in the otic mesenchyme, and addition of noggin or BMP4-specific antisense oligonucleotides to the mouse inner ear led to suppression of chondrogenesis in the otic capsule (Liu et al., 2003). Similarly, BMP4, as well as BMP2, has been suggested to play a role in the otic capsule chondrogenesis of chickens (Chang et al., 2002).
3.2.2 Roles of BMPs in the patterning of the cochlea
As mentioned above, BMP4 is expressed in the non-sensory cochlear structure from early stages in various species, including chickens, frogs (Kil and Collazo, 2001), and mice (Ohyama et al., 2010). However, in the chicken cochlea, BMP4 is detectable in the hair cells of the basilar papilla (Oh et al., 1996; Cole et al., 2000), while in the mouse cochlea it is observed only in Hensen’s and Claudius’ cells (supporting cells in the cochlea) (Takemura et al., 1996). Several studies have demonstrated the indispensable roles of BMP signaling in patterning both the sensory and non-sensory regions of the cochlea.
Mice lacking the Bmp4 gene die as embryos (Winnier et al., 1995), but Bmp4 heterozygous null (Bmp4 +/−) mice survive and show defects in both the structure and function of the cochlea (Blauwkamp et al., 2007). Bmp4 +/− mice have elevated hearing thresholds and have fewer neuronal processes in the organ of Corti. These findings suggest an indispensable role for BMP4 in cochlear development.
During the early stages of mouse cochlear development, the primordium is patterned into three distinct domains along the abneural–neural axis of the cochlea: Kölliker’s organ on the neuro side, the prosensory auditory domain, and the out sulcus on the abneural side. Ohyama et al. (2010) demonstrated that a BMP signal gradient is established in the mouse cochlea along the abneural–neural axis and that this gradient runs from high levels on the abneural side to low levels on the neural side. Alk3-CKO; Alk6 +/− mutant mice, in which BMP signaling is otic-conditionally deleted, showed expansion of the Kölliker’s organ region at the expense of the prosensory domain and the primordial out sulcus. In contrast, in cultured cochleae, a high dose of BMP4 led to expansion of the outer sulcus at the expense of Kölliker’s organ, while an intermediate dose induced the prosensory domain (Ohyama et al., 2010). BMP7 was also expressed throughout the mouse cochlea but was not localized to any particular region. These findings show the essential roles of an asymmetric gradient of BMP signaling in mouse cochlear patterning.
Similarly, expression profiling analysis demonstrated a gradient of BMP7 along the developing tonotopic axis of the basilar papilla in the chicken embryo. Disruption of that gradient in vivo or in vitro caused the loss of tonotopic organization and the failure of cochlear specification (Mann et al., 2014).
3.3. Functions of BMPs during cell type differentiation and functional maturation
During the late period of inner ear development, some BMPs are localized within particular cell types, which suggests possible roles of BMPs in cell type differentiation and functional maturation (Oh et al., 1996; Ohyama et al., 2010). In the chicken vestibule, BMP4 expression is eventually concentrated in supporting cells, and in the chicken cochlea it is localized in hair cells, whereas the expression of BMP7 is eventually concentrated in supporting cells of the cochlea (Oh et al., 1996).
An asymmetric gradient of BMP signaling in cell type determination in the mouse cochlea was described above, which is high in the out sulcus and low in Kölliker’s organ (Ohyama et al., 2010). Similarly, in the chicken embryo, a gradient of BMP7 along the developing tonotopic axis of the basilar papilla was observed, and changes in hair cell morphology and physiology were observed along this axis (Mann et al., 2014). Disruption of this gradient induced a loss of tonotopic organization and led to changes in hair cell morphology. In addition, exogenous BMP4 delivered to mouse cochlear explants induced an increase in the number of outer hair cells (Puligilla et al., 2007). These findings show the essential roles of the BMP signaling pathway in cell type differentiation and functional maturation.
Some recent studies have explored the effect of BMP signaling in the generation of hair cells in the inner ear, but the role of BMP signaling in this process is still not clear (Li et al., 2005; Pujades et al., 2006; Hwang et al., 2010). Pujades et al. (2006) demonstrated that BMP4 expression and BMP inhibition are critical for the proper generation of hair cells in the chick inner ear. Their study showed that high BMP4 activity inhibited cell fate specification, while suppression of BMP activity with noggin favored hair cell specification. Another study in the chicken otocyst culture system showed a different result. Li et al. (2005) found that the generation of hair cells and supporting cells was significantly reduced when BMP signaling was blocked using its antagonist noggin or soluble BMP receptors. An increase in hair cells was also observed in the presence of exogenous BMP4. Ohyama et al. (2010) gave a possible explanation for the differences between these studies, suggesting that high concentrations of BMP4 inhibit hair cell formation (Pujades et al., 2006) and low concentrations of BMP4 promote cell type differentiation (Li et al., 2005). A concentration-dependent effect of BMP4 in cell type differentiation has also been demonstrated in the mouse cochlea (Ohyama et al., 2010). In addition, Hwang et al. (2010) found that a BMP2 conditional knockout did not lead to abnormal hair cells in mice, while the lack of noggin caused increased rows of inner and outer hair cells in the organ of Corti, suggesting that noggin might regulate BMP4, but not BMP2, during the generation of hair cells in the cochlea.
Despite the numerous high-quality studies described above, the role of BMP signaling in the development of the inner ear is still not fully understood, but its critical importance has been clearly demonstrated. Most of these studies specifically demonstrated the importance of BMP4 and BMP inhibitors in this process, gave hints as to the complexity of BMP signaling within the inner ear, and provided evidence that the role played by BMP signaling might change in different developmental stages.
3.4. Expression of BMPs in the development of other organs
In addition to their essential functions in the development of the inner ear, BMPs are involved in the early formation and patterning of various other organs, including the eye, heart, and nervous system (Mehler et al., 1997; Krispin et al., 2010; Bond et al., 2012; Garside et al., 2013; Huang et al., 2015; Pandit et al., 2015). In the early stages of development, BMPs are involved in the specification of neuroectoderm from ectoderm (Ishimura et al., 2000), and several studies have suggested an essential role of BMP signaling in the formation and patterning of the neural tube (Liem et al., 1995; Lee et al., 1998; Lee and Jessell, 1999; Liu and Niswander, 2005). During the cell fate specification of the forebrain, a gradient of BMP signaling has also been detected in the neural crest. BMP signaling has been shown to have a promoting effect on neuronal cell differentiation and a suppressive role in oligodendroglial cell differentiation (Li et al., 1998; Mabie et al., 1999; Mehler et al., 2000; Moon et al., 2009).
4. Crosstalk between BMP signaling and other signaling pathways in the development of the inner ear
In addition to BMP signaling, other signaling pathways that are important in the development of the inner ear include SHH signaling and WNT signaling (Alsina et al., 2009; Wu and Kelley, 2012). The transformation from the simple epithelial otocyst to the structurally and functionally complex inner ear takes place through numerous morphogenetic events, and these signaling pathways coordinate the morphogenesis of the otocyst and determine its dorsoventral polarity (Whitfield, 2015). WNTs and BMPs are secreted from the dorsal tissue to the otocyst, while SHH is secreted from the ventral tissue to the otocyst (Ohta and Schoenwolf, 2018). In this part of the review, we focus on the interaction between BMP signaling and other signaling pathways in the process of polarizing and patterning the developing otocyst.
4.1. Crosstalk between BMP signaling and SHH signaling
In addition to the observation that BMPs are widely expressed in the developing otocyst (Wu and Oh, 1996), the SHH protein, which is a member of the hedgehog family of signaling proteins, is also critical for the development of numerous organs, including the inner ear (Ingham and McMahon, 2001). Studies in Shh-knockout mouse embryos reported the malformation of the cochlear ducts while the dorsal inner ear structures were morphologically normal, which indicates that SHH is required for the formation of ventral inner ear structures (Riccomagno et al., 2002). A recent study showed that BMPs regulate the expression of Dlx5 and Hmx3, which are transcription factors required for the formation of the primordial canal pouch. The expression of Dlx5 is regulated through the canonical Smad-dependent BMP pathway, while the expression of Hmx3 is regulated by a noncanonical BMP pathway related to cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) (Ohta and Schoenwolf, 2018). PKA is also essential for the regulation of SHH signaling. The SHH signaling pathway is regulated by gliotactin (GLI) transcription factors, which can be functionally divided into activators (GLIAs) and repressors (GLIRs). PKA can phosphorylate full-length GLI, promoting its transformation into GLIR (Ohta et al., 2016a). This suggests that the noncanonical BMP pathway related to PKA might negatively regulate the SHH signaling pathway. SHH signaling was reported to inhibit PKA activity and to downregulate the expression of Hmx3. Moreover, Otc2, another gene involved in the development of the inner ear, is upregulated by SHH signaling and downregulated by BMP signaling (Ohta et al., 2016b). These results suggest that SHH signaling might in turn downregulate BMP signaling in the developing inner ear. BMPs are expressed mainly in the dorsal otocyst, while SHH is expressed in the ventral otocyst, and thus the two signaling pathways might inhibit each other and polarize the otocyst.
4.2. Crosstalk between BMP signaling and WNT signaling
The interaction between BMP signaling and WNT signaling in the development of the inner ear is complex and is still poorly understood. Although both WNTs and BMPs are expressed in spatially overlapping manners, and both are secreted from the dorsal tissue to the otocyst, the effect of WNT–BMP crosstalk varies in different parts of the inner ear and can be either synergistic or antagonistic. It was demonstrated that both WNT and canonical BMP signaling pathways are required to maintain the regulation of Dlx5 expression, which occurs throughout the entire dorsal otocyst and is indispensable for the formation of the vestibular organ (Ohta and Schoenwolf, 2018). The BMPs are likely to regulate Dlx5 expression in the dorsolateral otocyst, while the WNTs are likely to regulate Dlx5 expression in the dorsomedial portion of the otocyst. In addition, deletion of Bmp4 in the otic epithelium led to the loss of Dlx5 expression in the dorsolateral otocyst, while Dlx5 expression was still observed in the endolymphatic duct (Chang et al., 2008). These findings suggest that BMP signaling and WNT signaling are complementary in the development of the vestibular organ and have synergistic effects. However, when it comes to the regulation of cochlear patterning, WNT and BMP seem to have antagonistic effects (Munnamalai and Fekete, 2016). In the mouse’s cochlea, after activation of WNT signaling by CHIR99021—an inhibitor of glycogen synthase kinase 3β—the number of inner hair cells increased. This number also increased when using dorsomorphin, a selective small molecule inhibitor of BMP signaling, suggesting that both BMP inhibition and WNT activation increase the number of inner hair cells in the cochlea. In addition, BMP4 expression was weak in the cochlea when it was treated with CHIR99021, which suggests that the activation of WNT antagonizes BMP signaling in the development of the cochlea.
5. Conclusions and future directions
BMPs are an important subfamily of the TGF-β superfamily and play critical roles in numerous cellular activities. The transformation of the otocyst into the inner ear is a complex process, during which the ventral part develops into the cochlea and mediates sound perception, while the dorsal part develops into the vestibular system and maintains the body’s balance (Martin and Swanson, 1993; Wu and Kelley, 2012). It is clear from this review that BMP signaling plays important roles in the development of the inner ear, especially in the formation of the semicircular canals. However, the role of BMP signaling in the development of the other parts of the inner ear is not so clear. The influence of BMP signaling on the generation of hair cells is also unclear, and results have been contradictory, as indicated above. Deeper studies are needed to determine the role of BMP signaling in this process. Such studies might contribute to new methods for the regeneration of hair cells in the cochlea, utricle, and saccule, and thus lead to new therapeutic treatments for diseases related to the loss of hair cells. A recent study showed that BMP4 has effects on postnatal cochlear spiral ganglion neurons (SGNs) in mice in addition to its influence on the inner ear during development (Waqas et al., 2017), which indicates that BMPs and BMP signaling influence hearing in many different respects. It has been demonstrated that BMP4 promotes the survival and growth of neurites in cultured SGNs in vitro, and further studies are needed to determine the in vivo effects of BMPs on SGNs, thereby contributing to our understanding of sensorineural hearing loss that results from damage or loss of SGNs.
In addition to BMP signaling, several other signaling pathways have been shown to take part in the development of the inner ear, including the SHH signaling and WNT signaling pathways. It has also become clear in recent years that these signaling pathways and growth factors do not regulate the morphogenesis of the inner ear independently. Instead, there is a significant crosstalk among these signaling pathways (Fritzsch et al., 2006). We have reviewed some of the interactions between BMPs and other signaling pathways in the developing otocyst. It is clear that the noncanonical BMP pathway is important in the crosstalk with other signaling pathways, and further studies of other noncanonical pathways in the developing inner ear are needed. In addition, only a small number of studies have focused on the crosstalk among signaling pathways in the developing inner ear, and further studies in this regard are also needed.
Footnotes
Project supported by the National Key Technologies R&D Program of China (Nos. 2017YFA0103900 and 2016YFC0905200), the National Natural Science Foundation of China (Nos. 81620108005, 8177040802, and 81622013), and the Shanghai Pujiang Talents Plan (No. 18PJ1401700), China
Contributors: Jiao-yao MA and Dan YOU wrote this review. Jiao-yao MA and Xiao-ling LU draw the figures. Wen-yan LI and Dan YOU collected and screened references. Shan SUN wrote an outline. Hua-wei LI checked and approved the final version.
Compliance with ethics guidelines: Jiao-yao MA, Dan YOU, Wen-yan LI, Xiao-ling LU, Shan SUN, and Hua-wei LI declare that they have no conflict of interest.
This review does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abelló G, Khatri S, Radosevic M, et al. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol. 2010;339(1):166–178. doi: 10.1016/j.ydbio.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Alsina B, Giraldez F, Pujades C. Patterning and cell fate in ear development. Int J Dev Biol. 2009;53(8-10):1503–1513. doi: 10.1387/ijdb.072422ba. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez IS, Navascués J. Shaping, invagination, and closure of the chick embryo otic vesicle: scanning electron microscopic and quantitative study. Anat Rec. 1990;228(3):315–326. doi: 10.1002/ar.1092280311. [DOI] [PubMed] [Google Scholar]
- 4.Avsian-Kretchmer O, Hsueh AJ. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrinol. 2004;18(1):1–12. doi: 10.1210/me.2003-0227. [DOI] [PubMed] [Google Scholar]
- 5.Bai S, Cao X. A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-β signaling. J Biol Chem. 2002;277(6):4176–4182. doi: 10.1074/jbc.M105105200. [DOI] [PubMed] [Google Scholar]
- 6.Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131(17):4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- 7.Blauwkamp MN, Beyer LA, Kabara L, et al. The role of bone morphogenetic protein 4 in inner ear development and function. Hear Res. 2007;225(1-2):71–79. doi: 10.1016/j.heares.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bok J, Brunet LJ, Howard O, et al. Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev Biol. 2007;311(1):69–78. doi: 10.1016/j.ydbio.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012;72(7):1068–1084. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brazil DP, Church RH, Surae S, et al. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25(5):249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Brunet LJ, McMahon JA, McMahon AP, et al. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280(5368):1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 12.Carreira AC, Lojudice FH, Halcsik E, et al. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res. 2014;93(4):335–345. doi: 10.1177/0022034513518561. [DOI] [PubMed] [Google Scholar]
- 13.Carreira AC, Alves GG, Zambuzzi WF, et al. Bone morphogenetic proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys. 2014;561(6):64–73. doi: 10.1016/j.abb.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Chang W, Nunes FD, de Jesus-Escobar JM, et al. Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev Biol. 1999;216(1):369–381. doi: 10.1006/dbio.1999.9457. [DOI] [PubMed] [Google Scholar]
- 15.Chang W, ten Dijke P, Wu DK. BMP pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev Biol. 2002;251(2):380–394. doi: 10.1006/dbio.2002.0822. [DOI] [PubMed] [Google Scholar]
- 16.Chang W, Lin Z, Kulessa H, et al. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4(4):e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole LK, le Roux I, Nunes F, et al. Sensory organ generation in the chicken inner ear: contributions of Bone morphogenetic protein 4, Serrate1, and Lunatic fringe . J Comp Neurol. 2000;424(3):509–520. doi: 10.1002/1096-9861(20000828)424:3<509::AID-CNE8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Chang C. Regulation of early Xenopus embryogenesis by Smad ubiquitination regulatory factor 2. Dev Dyn. 2012;241(8):1260–1273. doi: 10.1002/dvdy.23811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David D, Nair SA, Pillai MR. Smurf E3 ubiquitin ligases at the cross roads of oncogenesis and tumor suppression. Biochim Biophys Acta. 2013;1835(1):119–128. doi: 10.1016/j.bbcan.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 22.Ekdale EG. Form and function of the mammalian inner ear. J Anat. 2016;228(2):324–337. doi: 10.1111/joa.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28(12):1181–1193. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garside VC, Chang AC, Karsan A, et al. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell Mol Life Sci. 2013;70(16):2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7(1-2):51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 26.Gerlach LM, Hutson MR, Germiller JA, et al. Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development. 2000;127(1):45–54. doi: 10.1242/dev.127.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Gerlach-Bank LM, Ellis AD, Noonen B, et al. Cloning and expression analysis of the chick DAN gene, an antagonist of the BMP family of growth factors. Dev Dyn. 2002;224(1):109–115. doi: 10.1002/dvdy.10079. [DOI] [PubMed] [Google Scholar]
- 28.Gerlach-Bank LM, Cleveland AR, Barald KF. DAN directs endolymphatic sac and duct outgrowth in the avian inner ear. Dev Dyn. 2004;229(2):219–230. doi: 10.1002/dvdy.10414. [DOI] [PubMed] [Google Scholar]
- 29.Glavic A, Maris Honoré S, Gloria Feijóo C, et al. Role of BMP signaling and the homeoprotein iroquois in the specification of the cranial placodal field. Dev Biol. 2004;272(1):89–103. doi: 10.1016/j.ydbio.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127(16):3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- 31.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88(1):49–92. [PubMed] [Google Scholar]
- 32.Hata A, Lagna G, Massagué J, et al. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12(2):186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldin CH, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 34.Hemmati-Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995;17(1):78–89. doi: 10.1002/dvg.1020170109. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann A, Gross G. BMP signaling pathways in cartilage and bone formation. Crit Rev Eukaryot Gene Expr. 2001;11(1-3):23–45. [PubMed] [Google Scholar]
- 36.Huang J, Liu Y, Filas B, et al. Negative and positive auto-regulation of BMP expression in early eye development. Dev Biol. 2015;407(2):256–264. doi: 10.1016/j.ydbio.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang CH, Guo D, Harris MA, et al. Role of bone morphogenetic proteins on cochlear hair cell formation: analyses of Noggin and Bmp2 mutant mice. Dev Dyn. 2010;239(2):505–513. doi: 10.1002/dvdy.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 39.Ishimura A, Maeda R, Takeda M, et al. Involvement of BMP-4/msx-1 and FGF pathways in neural induction in the Xenopus embryo. Dev Growth Differ. 2000;42(4):307–316. doi: 10.1046/j.1440-169x.2000.00514.x. [DOI] [PubMed] [Google Scholar]
- 40.Izzi L, Attisano L. Regulation of the TGFβ signalling pathway by ubiquitin-mediated degradation. Oncogene. 2004;23(11):2071–2078. doi: 10.1038/sj.onc.1207412. [DOI] [PubMed] [Google Scholar]
- 41.Jones CM, Lyons KM, Hogan BL. Involvement of bone morphogenetic protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111(2):531–542. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- 42.Katagiri T, Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8(6):a021899. doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kattamuri C, Luedeke DM, Nolan K, et al. Members of the DAN family are BMP antagonists that form highly stable noncovalent dimers. J Mol Biol. 2012;424(5):313–327. doi: 10.1016/j.jmb.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley MW, Wu DK, Popper AN, et al. Development of the Inner Ear. Springer, New York, NY; 2005. [DOI] [Google Scholar]
- 45.Kessler E, Takahara K, Biniaminov L, et al. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271(5247):360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 46.Kil SH, Collazo A. Origins of inner ear sensory organs revealed by fate map and time-lapse analyses. Dev Biol. 2001;233(2):365–379. doi: 10.1006/dbio.2001.0211. [DOI] [PubMed] [Google Scholar]
- 47.Krispin S, Nitzan E, Kalcheim C. The dorsal neural tube: a dynamic setting for cell fate decisions. Dev Neurobiol. 2010;70(12):796–812. doi: 10.1002/dneu.20826. [DOI] [PubMed] [Google Scholar]
- 48.Kwon HJ, Bhat N, Sweet EM, et al. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6(9):e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22(1):261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 50.Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12(21):3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Corrales CE, Wang Z, et al. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol, 5:16. 2005 doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q. Inhibitory SMADs: potential regulators of ovarian function. Biol Reprod. 2015;92(2):50. doi: 10.1095/biolreprod.114.125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Cogswell CA, Loturco JJ. Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J Neurosci. 1998;18(21):8853–8862. doi: 10.1523/jneurosci.18-21-08853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liem KF, Jr, Tremml G, Roelink H, et al. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82(6):969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 55.Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132(18):4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- 56.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6(12):945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- 57.Liu W, Oh SH, Kang YK, et al. Bone morphogenetic protein 4 (BMP4): a regulator of capsule chondrogenesis in the developing mouse inner ear. Dev Dyn. 2003;226(3):427–438. doi: 10.1002/dvdy.10258. [DOI] [PubMed] [Google Scholar]
- 58.Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev. 2010;21(4):287–298. doi: 10.1016/j.cytogfr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci. 1999;19(16):7077–7088. doi: 10.1523/jneurosci.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macias MJ, Martin-Malpartida P, Massagué J. Structural determinants of Smad function in TGF-β signaling. Trends Biochem Sci. 2015;40(6):296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mann ZF, Thiede BR, Chang W, et al. A gradient of Bmp7 specifies the tonotopic axis in the developing inner ear. Nat Commun, 5:3839. 2014 doi: 10.1038/ncomms4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev Biol. 1993;159(2):549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- 63.McMahon JA, Takada S, Zimmerman LB, et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12(10):1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehler MF, Mabie PC, Zhang D, et al. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20(7):309–317. doi: 10.1016/S0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- 65.Mehler MF, Mabie PC, Zhu G, et al. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22(1-2):74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- 66.Miyazawa K, Miyazono K. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol, 9:a022095. 2017 doi: 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16(3):251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 69.Moon BS, Yoon JY, Kim MY, et al. Bone morphogenetic protein 4 stimulates neuronal differentiation of neuronal stem cells through the ERK pathway. Exp Mol Med. 2009;41(2):116–125. doi: 10.3858/emm.2009.41.2.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morsli H, Choo D, Ryan A, et al. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18(9):3327–3335. doi: 10.1523/jneurosci.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mowbray C, Hammerschmidt M, Whitfield TT. Expression of BMP signalling pathway members in the developing zebrafish inner ear and lateral line. Mech Dev. 2001;108(1-2):179–184. doi: 10.1016/S0925-4773(01)00479-8. [DOI] [PubMed] [Google Scholar]
- 72.Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347(1):11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 73.Munnamalai V, Fekete DM. Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development. 2016;143(21):4003–4015. doi: 10.1242/dev.139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura J, Yanagita M. BMP modulators in kidney disease. Discov Med. 2012;13(68):57–63. [PubMed] [Google Scholar]
- 75.Oh SH, Johnson R, Wu DK. Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. J Neurosci. 1996;16(20):6463–6475. doi: 10.1523/jneurosci.16-20-06463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohta S, Schoenwolf GC. Hearing crosstalk: the molecular conversation orchestrating inner ear dorsoventral patterning. WIREs Dev Biol. 2018;7(1):e302. doi: 10.1002/wdev.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohta S, Wang B, Mansour SL, et al. BMP regulates regional gene expression in the dorsal otocyst through canonical and non-canonical intracellular pathways. Development. 2016;143(12):2228–2237. doi: 10.1242/dev.137133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohta S, Wang B, Mansour SL, et al. SHH ventralizes the otocyst by maintaining basal PKA activity and regulating GLI3 signaling. Dev Biol. 2016;420(1):100–109. doi: 10.1016/j.ydbio.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51(6-7):463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- 80.Ohyama T, Basch ML, Mishina Y, et al. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30(45):15044–15051. doi: 10.1523/Jneurosci.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Omata Y, Nojima Y, Nakayama S, et al. Role of bone morphogenetic protein 4 in zebrafish semicircular canal development. Dev Growth Differ. 2007;49(9):711–719. doi: 10.1111/j.1440-169X.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- 82.Pandit T, Jidigam VK, Patthey C, et al. Neural retina identity is specified by lens-derived BMP signals. Development. 2015;142(10):1850–1859. doi: 10.1242/dev.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piccolo S, Sasai Y, Lu B, et al. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86(4):589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pujades C, Kamaid A, Alsina B, et al. BMP-signaling regulates the generation of hair-cells. Dev Biol. 2006;292(1):55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Puligilla C, Feng F, Ishikawa K, et al. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236(7):1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramel MC, Hill CS. Spatial regulation of bmp activity. FEBS Lett. 2012;586(14):1929–1941. doi: 10.1016/j.febslet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 87.Reichert S, Randall RA, Hill CS. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development. 2013;140(21):4435–4444. doi: 10.1242/dev.098707. [DOI] [PubMed] [Google Scholar]
- 88.Riccomagno MM, Martinu L, Mulheisen M, et al. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16(18):2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenzweig BL, Imamura T, Okadome T, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA. 1995;92(17):7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruiz i Altaba A, Jessell TM. Retinoic acid modifies the pattern of cell differentiation in the central nervous system of neurula stage Xenopus embryos. Development. 1991;112(4):945–958. doi: 10.1242/dev.112.4.945. [DOI] [PubMed] [Google Scholar]
- 91.Sai X, Ladher RK. Early steps in inner ear development: induction and morphogenesis of the otic placode. Front Pharmacol, 6:19. 2015 doi: 10.3389/fphar.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saint-Jeannet JP, Moody SA. Establishing the pre-placodal region and breaking it into placodes with distinct identities. Dev Biol. 2014;389(1):13–27. doi: 10.1016/j.ydbio.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi Y, Wang YF, Jayaraman L, et al. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell. 1998;94(5):585–594. doi: 10.1016/S0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 94.Sieber C, Kopf J, Hiepen C, et al. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20(5-6):343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 95.Takemura T, Sakagami M, Takebayashi K, et al. Localization of bone morphogenetic protein-4 messenger RNA in developing mouse cochlea. Hear Res. 1996;95(1-2):26–32. doi: 10.1016/0378-5955(95)00233-2. [DOI] [PubMed] [Google Scholar]
- 96.Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 97.Vervoort R, Ceulemans H, van Aerschot L, et al. Genetic modification of the inner ear lateral semicircular canal phenotype of the BMP4 haplo-insufficient mouse. Biochem Biophys Res Commun. 2010;394(3):780–785. doi: 10.1016/j.bbrc.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 98.von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239(1):1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 99.Waqas M, Sun S, Xuan C, et al. Bone morphogenetic protein 4 promotes the survival and preserves the structure of flow-sorted Bhlhb5+ cochlear spiral ganglion neurons in vitro . Sci Rep. 2017;7(1):3506. doi: 10.1038/s41598-017-03810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whitfield TT. Development of the inner ear. Curr Opin Genet Dev. 2015;32:112–118. doi: 10.1016/j.gde.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Wijgerde M, Karp S, McMahon J, et al. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286(1):149–157. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 102.Winnier G, Blessing M, Labosky PA, et al. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9(17):2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 103.Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood) 2007;232(8):979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- 104.Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16(20):6454–6462. doi: 10.1523/jneurosci.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4(8):a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J, Zhang X, Xie F, et al. The regulation of TGF-β/SMAD signaling by protein deubiquitination. Protein Cell. 2014;5(7):503–517. doi: 10.1007/s13238-014-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang YE. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol. 2017;9(2):a022129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu H, Kavsak P, Abdollah S, et al. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400(6745):687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 110.Zimmerman LB, de Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86(4):599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]