Abstract
Proton pump inhibitors, PPIs, are widely prescribed and sold globally. Although initially intended for time-limited treatment of acute disorders, such as gastric ulcers and esophagitis, PPIs are now commonly used for prolonged durations and are considered safe for over the counter access. Recent studies have raised concern over associations between PPI use and acute kidney injury, chronic kidney disease, end-stage renal disease, and electrolyte abnormalities. The growing concern over potentially serious adverse drug reactions warrants an evaluation of post marketing surveillance data. In this study of over ten million FDA Adverse Event Reporting System records, we provided evidence of kidney injury and electrolyte imbalances in an alarming number of patients taking PPIs. Additionally, we assessed differences between specific PPIs and observed significant electrolyte and renal abnormalities for each individual drug with varying magnitudes.
Introduction
The World Health Organization includes proton pump inhibitors (PPIs) in the list of essential medicines and health products1. PPIs have demonstrated superior efficacy to histamine-2 receptor antagonists (H2RAs) in treatment of acid-related disorders and have replaced the H2RAs2,3. The current indications include treatment of gastroesophageal reflux disease, non-steroidal anti-inflammatory drug (NSAID) and Helicobacter pylori induced ulcers, duodenal ulcers, erosive esophagitis, and other pathological hypersecretory conditions, including Zollinger-Ellison syndrome4,5 (see Supplement-Appendix A for a more comprehensive indication list reported to FDA) and are now one of the most widely utilized medications6. The superior efficacy is credited to the mechanism of action. All currently marketed PPIs inhibit the hydrogen pump H +/K+ ATPase irreversibly, preventing the last and rate-limiting step in acid secretion by parietal cells in the stomach7,8. There are currently six Food and Drug Administration (FDA) approved PPIs: rabeprazole, lansoprazole, pantoprazole, esomeprazole, omeprazole, and dexlansoprazole. These were sequentially developed due to varying pharmacokinetic parameters, such as extended plasma half-life, routes of administration, and drug interactions9,10. The most common PPI adverse reactions (ADRs) are mild and include headache, nausea, stomach pain, diarrhea, vomiting, and flatulence. Serious allergic reactions include rash, facial swelling, throat tightness, and difficulty breathing11. Generally considered safe, PPIs are now commonly used for prophylaxis and sold over the counter in most of the industrialized countries, including the United States, with annual prescription and over the counter sales exceeding fourteen billion dollars anually12.
Recently, PPI use has come under scrutiny due to growing evidence of renal, cardiovascular, autoimmune and neurologic adverse effects. New data has revealed associations with myocardial infarction13, Clostridium difficile-associated diarrhea14, community acquired pneumonia15, bone fractures16, subacute cutaneous lupus erythematosus17,18, Alzheimer’s dementia19,20, and kidney injury21–29. Here we evaluated the frequencies of reported adverse events related to kidney injury and electrolyte disturbances in patients taking PPIs. We also compared the magnitude of the effects for individual PPIs.
Results
PPI “monotherapy”-related renal and electrolyte ADRs
Patients who used PPIs with no other reported concurrent medications had a significant increase in the frequency of the following renal adverse event reports compared to the H2RAs: chronic kidney disease (CKD) (OR 28.4, 95% CI [12.7, 63.5]), acute kidney injury (AKI) (4.2 [2.8, 6.3]), end-stage renal disease (ESRD) (35.5 [5.0, 250.0]), renal impairment of unspecified type (8.0 [5.0, 13.0]), and nephrolithiasis (2.8 [1.3, 6]) (Fig. 1b). The composite renal ADR frequency was 5.6% of the total PPI “monotherapy” ADRs reports and 0.7% for H2RA “monotherapy” reports (8.6 [6.6, 11]) (Fig. 1a).
Figure 1.
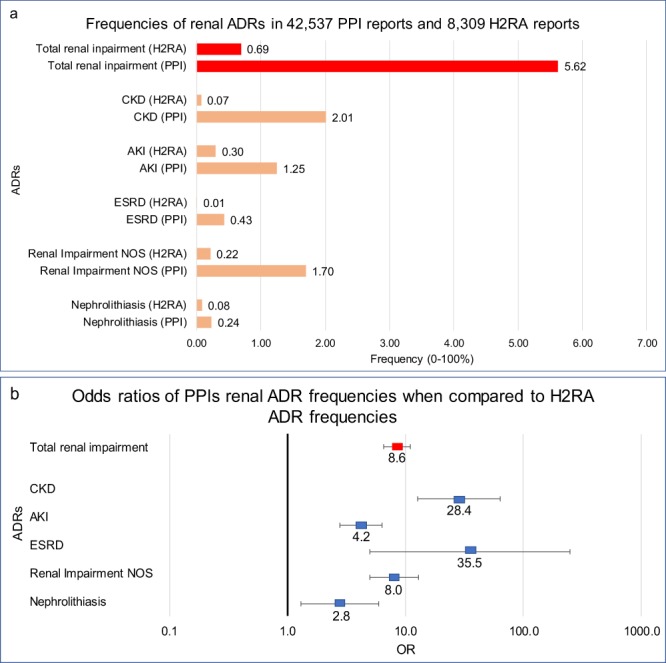
Frequencies and odds ratios (ORs) of renal adverse drug reactions (ADRs). (a) Frequencies of renal adverse events for patients in FAERS/AERS who took PPIs (n = 42,537) and H2RAs (n = 8,309). (b) Odds ratios were calculated comparing adverse event frequencies of PPI and H2RA patients. Abbreviations: CKD-Chronic Kidney Disease, AKI-Acute Kidney Injury, ESRD-End Stage Renal Disease, and NOS-Not otherwise specified. Ranges represent 95% confidence intervals (95% CI) (see Methods). X-axis is presented in log scale.
Interestingly, relative frequencies for electrolyte abnormalities followed the same trend (Fig. 2a) and were also increased for PPI users: hypomagnesemia (OR 78.5 [11, 560]), hypocalcemia (25.5 [6.4, 100]), hypokalemia (6.3 [2.6, 15]), and hyponatremia (2.2 [1.1, 4.6]) (Fig. 2b).
Figure 2.
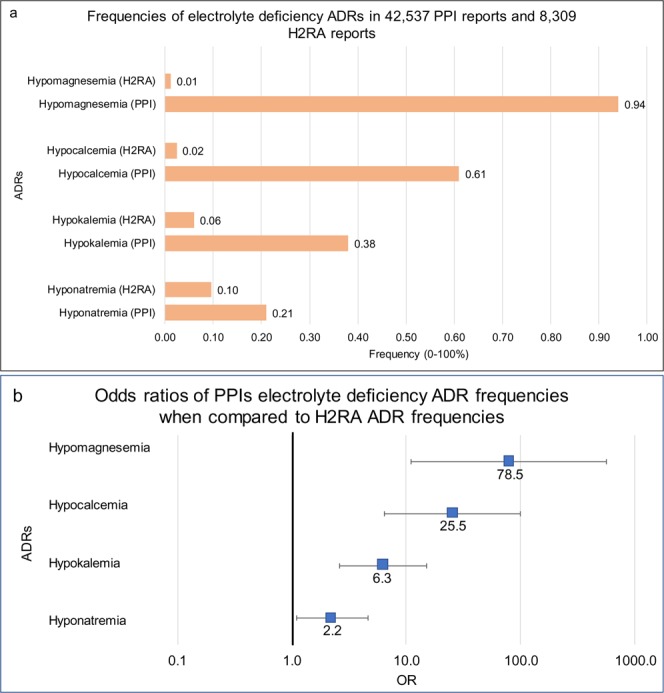
Frequencies and ORs of electrolyte related ADRs. (a) Frequencies of electrolyte related events for patients on PPIs (n = 42,537) and H2RAs (n = 8,309). (b) Odds ratios were calculated from adverse event frequencies. Ranges represent 95% confidence intervals (see Methods). X-axis is presented in log scale.
Renal impairment and Individual PPIs
Analysis of renal and electrolyte adverse effects for each individual PPI produced the following results shown in Fig. 3.
Figure 3.
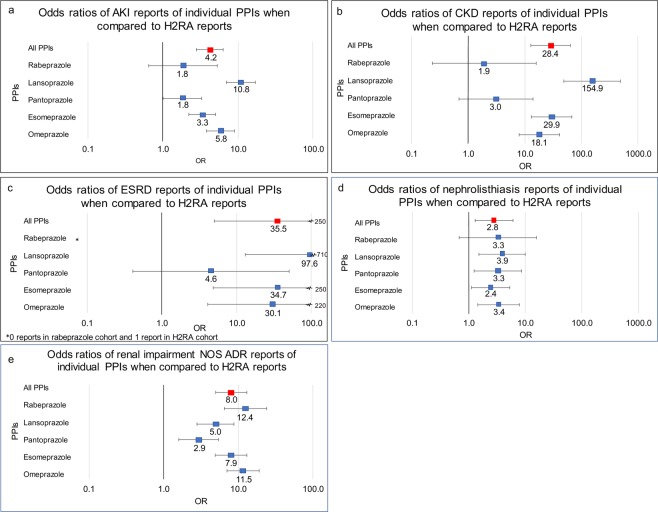
Renal ADR ORs of individual PPIs used as “monotherapy” (Rabeprazole n = 724, Lansoprazole n = 3,360, Pantoprazole n = 3,651, Esomeprazole n = 27,053, Omeprazole n = 7,749) when compared to H2RAs (n = 8,309). Odds ratios were calculated comparing (a) AKI (b) CKD, (c) ESRD (d) Nephrolithiasis and (e) renal impairment adverse event report frequencies of individual PPIs to all H2RAs (x-axis presented in log scale). NOS = not otherwise specified.
Acute Kidney Injury
Patients who received the following PPIs as “monotherapy” had a significant increase in the frequency of AKI reports: omeprazole (OR 5.8 [3.8, 8.9]), esomeprazole (3.3 [2.2, 5]), pantoprazole (1.8 [1.01, 3.3]), and lansoprazole (10.8 [7.0, 17]). Patients who received rabeprazole as “monotherapy” had an increase in AKI frequency, but it was not significant (1.8 [0.6, 5.3]) (Fig. 3a).
Chronic Kidney Disease
Patients who received the following PPIs as “monotherapy” had a significant increase in the frequency of CKD reports: omeprazole (OR 18.1 [7.9, 41]), esomeprazole (29.9 [13, 67]), and lansoprazole (154.9 [49, 490]). Patients who received rabeprazole and pantoprazole as “monotherapy” had an increase in CKD frequency, but the significance criteria were not met (1.9 [0.2,16]) and (3.0 [0.7, 14]) respectively (Fig. 3b).
End Stage Renal Disease
ESRD is of particular concern due to the limited prognosis in the absence of receiving dialysis or a kidney transplant. Very large OR values were determined for three widely used PPIs: omeprazole (OR 30.1 [4.1, 220]), esomeprazole (34.7 [4.8, 250]), and lansoprazole (97.6 [13, 710]) demonstrating a significant association with ESRD. The frequency of ESRD with pantoprazole “monotherapy” did not reach statistical significance (4.6 [0.4, 50]). Patients who received rabeprazole did not have any ESRD reports (Fig. 3c).
Nephrolithiasis
Within the PPI cohort, patients who received the following PPIs as “monotherapy” had a significant increase in the frequency of nephrolithiasis reports: omeprazole (OR 3.4 [1.4, 7.9]), esomeprazole (2.4 [1.1, 5.3]), pantoprazole (3.3 [1.2, 8.6]), and lansoprazole (3.9 [1.5, 10.1]). Patients who received rabeprazole as “monotherapy” according to FAERS reports had an increase in nephrolithiasis frequency but did not meet the significance criteria (3.3 [0.7, 15.8]) (Fig. 3d).
Renal Impairment
A large portion of renal impairment reports did not specify acuity of the injury, marked as renal impairment NOS (not otherwise specified). It was important to see if the observed renal side effects of PPIs persisted in this category of reports. In agreement with the preceding results, the OR values were significantly increased: omeprazole (OR 11.5 [7.1, 19]), esomeprazole (7.9 [4.9, 13]), pantoprazole (2.9 [1.6, 5.4]), lansoprazole (5.0 [2.8, 8.8]) and rabeprazole (12.4 [6.5, 24]) (Fig. 3e).
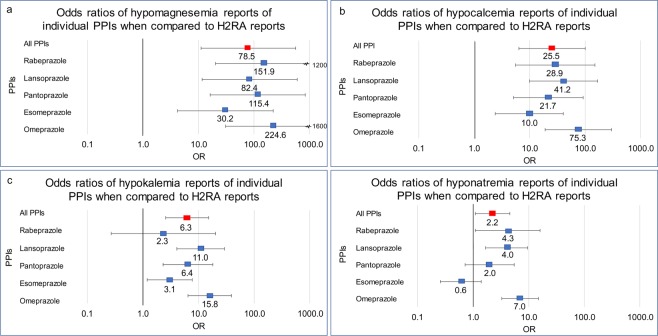
Electrolyte Disturbances: magnesium, calcium, potassium, sodium
All five PPIs were associated with a dramatic increase in hypomagnesemia reports (Fig. 4a, Table 1). Additionally, all the studied PPIs were associated with a significant increase in hypocalcemia reports (Fig. 4b, Table 1). Patients who received the following PPIs had an increase in the frequency of hypokalemia reports: omeprazole, esomeprazole, pantoprazole, and lansoprazole. Patients who received rabeprazole had an increase in hypokalemia frequency, but it was not statistically significant (Fig. 4c, Table 1).
Figure 4.
Electrolyte ADR odds ratios of individual PPIs used as “monotherapy” (Rabeprazole n = 724, Lansoprazole n = 3,360, Pantoprazole n = 3,651, Esomeprazole n = 27,053, Omeprazole n = 7,749) when compared to all H2RAs (n = 8,309). Odds ratios were calculated comparing (a) hypomagnesemia, (b) hypocalcemia, (c) hypokalemia, and (d) hyponatremia adverse event report frequencies of individual PPIs to all H2RAs.
Table 1.
Electrolyte ADR odds ratios and 95% CIs of individual PPIs used as “monotherapy” (Rabeprazole n = 724, Lansoprazole n = 3,360, Pantoprazole n = 3,651, Esomeprazole n = 27,053, Omeprazole n = 7,749) when compared to all H2RAs (n = 8,309).
| ADR | PPIs | OR | 95% CI lower | 95% CI upper |
|---|---|---|---|---|
| Hypomagnesemia | Omeprazole | 224.6 | 31.0 | 1600.0 |
| Esomeprazole | 30.2 | 4.2 | 220.0 | |
| Pantoprazole | 115.4 | 16.0 | 840.0 | |
| Lansoprazole | 82.4 | 11.0 | 600.0 | |
| Rabeprazole | 151.9 | 20.0 | 1200.0 | |
| Hypocalcemia | Omeprazole | 75.3 | 19.0 | 300.0 |
| Esomeprazole | 10.0 | 2.4 | 41.0 | |
| Pantoprazole | 21.7 | 5.1 | 93.0 | |
| Lansoprazole | 41.2 | 9.9 | 170.0 | |
| Rabeprazole | 28.9 | 5.6 | 150.0 | |
| Hypokalemia | Omeprazole | 15.8 | 6.3 | 39.0 |
| Esomeprazole | 3.1 | 1.2 | 7.7 | |
| Pantoprazole | 6.4 | 2.3 | 18.0 | |
| Lansoprazole | 11.0 | 4.1 | 29.0 | |
| Rabeprazole | 2.3 | 0.3 | 20.0 | |
| Hyponatremia | Omeprazole | 7.0 | 3.3 | 15.0 |
| Esomeprazole | 0.6 | 0.3 | 1.4 | |
| Pantoprazole | 2.0 | 0.7 | 5.5 | |
| Lansoprazole | 4.0 | 1.7 | 9.7 | |
| Rabeprazole | 4.3 | 1.1 | 16.0 |
Odds ratios were calculated comparing hypomagnesemia, hypocalcemia, hypokalemia, and hyponatremia adverse event report frequencies of individual PPIs to all H2RAs.
Hyponatremia was the least pronounced, yet still significant, electrolyte disturbance associated with omeprazole, lansoprazole, and rabeprazole. In contrast, reports of hyponatremia with esomeprazole or pantoprazole did not reach statistical significance. (Fig. 4d, Table 1).
Methods
FDA Adverse Event Reporting System
The FDA Adverse Event Reporting System (FAERS) supports FDA’s post marketing surveillance on drugs and biologic therapeutics submitted to FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Reporting is voluntary and is done by doctors, pharmacists, legal representatives, other healthcare providers and patients. The manufacturer is the only contributor that is legally required to forward the information to the FDA.
Over 10.3 million FAERS/AERS reports, from January 2004 to March 2018, were used for the analysis. Data sets are available online at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm.
Normalizing and combining the FAERS/AERS reports
Throughout the data set, the quarterly online reports were not homologous from year to year. Each quarterly set was downloaded in dollar-separated text format (.txt) and modified, standardized, and extended. Missing columns in data sets were added with no values and the column names were homogenized. The final data set version contained over 10.3 million reports. Most of the reports were submitted from the United States, and many were submitted from all around the world with their respective country specific demographic formats. Online drug databases were used to generate a dictionary with all international brand/generic drug names and to translate them into generic names.
Study outcomes
A fraction of the observed 20,317 outcomes in FAERS and AERS were grouped into the following generalized outcomes for a priori hypothesis: chronic kidney disease (CKD: defined in FAERS and AERS data sets as chronic kidney disease, and chronic renal failure), acute kidney injury (AKI: acute kidney injury, acute prerenal failure, renal failure acute), and end-stage renal disease (ESRD: end stage renal disease, end stage kidney disease), nephrolithiasis, renal impairment NOS (renal failure, renal impairment, renal disorder, renal injury), hypomagnesemia (hypomagnesemia, decreased blood magnesium), hypocalcemia (hypocalcemia, decreased blood calcium), hypokalemia (hypokalemia, decreased blood potassium) and hyponatremia (hyponatremia, decreased blood sodium).
Choice of cohorts
A total of 10,324,033 FAERS and AERS reports were collected. Reports where omeprazole, esomeprazole, rabeprazole, lansoprazole, pantoprazole, and dexlansoprazole were used, excluding reports with concurrent use of any H2RAs, were selected into the PPI cohort (n = 732,696). Reports where ranitidine, famotidine, cimetidine, and nizatidine were used, excluding reports with concurrent use of any PPI, were selected into the H2RA cohort (n = 162,189). Further, reports where PPIs and H2RAs were used as monotherapy were selected into the respective cohorts. The term “monotherapy” pertains to records where PPIs and H2RAs were the only reported medications. PPIs-only cohort comprised of 42,537 reports and H2RA-only cohort comprised of 8,309 reports.
Odds ratio analysis was performed by comparing the ADR frequencies of PPIs in relation to H2RA “monotherapy” frequencies. The PPI “monotherapy” cohort was further split into individual PPI cohorts which included omeprazole (n = 7,749) esomeprazole (n = 27,053), pantoprazole (n = 3,651), lansoprazole (n = 3,360), and rabeprazole (n = 724). There were no reports where dexlansoprazole was used as “monotherapy”. Reports with two or more PPIs used were excluded (Fig. 5). Demographic analysis was performed (Tables 2 and 3). Overall distributions strongly overlap and validate the cohort choice. Each individual PPI cohort frequency of renal and electrolyte ADRs was calculated and compared to the H2RA cohort to screen for potential ADR variability within individual PPIs in the cohort (Figs 3 and 4).
Figure 5.
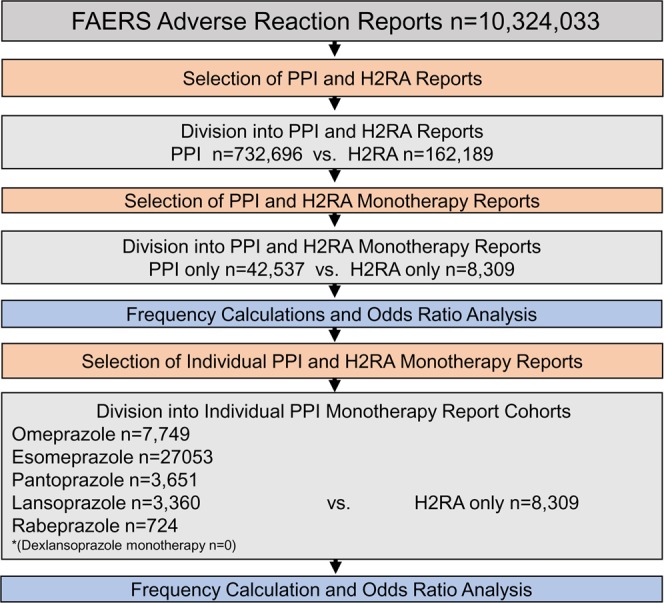
Legend: Inclusion exclusion and analysis cohort selection for adverse event rate comparison between PPIs and H2Ras as a class, as part of therapeutics, “monotherapy”, and between individual PPIs and all H2RAs.
Table 2.
Frequency of PPI and H2RA “monotherapy” reports in FAERS by country of origin.
| Country | No. of PPI reports | Frequency % | No. of H2RA reports | Frequency % | % Difference | >1% Difference |
|---|---|---|---|---|---|---|
| United States | 37,139 | 88.26 | 6,928 | 87.27 | 0.99 | |
| Great Britain | 1122 | 2.67 | 177 | 2.23 | 0.44 | |
| Japan | 472 | 1.12 | 382 | 4.81 | 3.69 | * |
| Germany | 357 | 0.85 | 24 | 0.30 | 0.55 | |
| France | 330 | 0.78 | 29 | 0.37 | 0.42 | |
| Canada | 309 | 0.73 | 22 | 0.28 | 0.46 | |
| Italy | 312 | 0.74 | 39 | 0.49 | 0.25 | |
| Brazil | 282 | 0.67 | 3 | 0.04 | 0.63 | |
| Turkey | 173 | 0.41 | 13 | 0.16 | 0.25 | |
| Australia | 151 | 0.36 | 7 | 0.09 | 0.27 | |
| China | 149 | 0.35 | 9 | 0.11 | 0.24 | |
| Denmark | 143 | 0.34 | 4 | 0.05 | 0.29 | |
| Spain | 138 | 0.33 | 13 | 0.16 | 0.16 | |
| Nederlands | 103 | 0.24 | 34 | 0.43 | 0.18 | |
| Sweden | 52 | 0.12 | 6 | 0.08 | 0.05 | |
| Singapore | 48 | 0.11 | 9 | 0.11 | 0.00 | |
| Belgium | 41 | 0.10 | 9 | 0.11 | 0.02 | |
| New Zealand | 42 | 0.10 | 3 | 0.04 | 0.06 | |
| Chile | 28 | 0.07 | 3 | 0.04 | 0.03 | |
| India | 15 | 0.04 | 15 | 0.19 | 0.15 | |
| Costa Rica | 30 | 0.07 | 0 | 0.00 | 0.07 | |
| Unknown | 198 | 0.47 | 45 | 0.57 | 0.10 |
Table 3.
Patient demographics in PPI and H2RA “monotherapy” cohorts.
| Sex | PPI reports (n = 42,537) | Frequency (%) | H2RA reports (n = 8,309) | Frequency (%) | P-value | % Difference |
|---|---|---|---|---|---|---|
| Female | 25,116 | 59.69 | 4,579 | 57.68 | <0.001 | 2.01 |
| Male | 12,000 | 28.52 | 2,710 | 34.14 | <0.001 | 5.62 |
| Unreported | 4,963 | 11.79 | 650 | 8.19 | <0.001 | 3.61 |
| Age difference | ||||||
| Mean age, years (SD) | 58.3 (15.9) | 55.6 (20.1) | <0.001 | 2.7 | ||
| Median age | 58.6 | 59.7 | <0.001 | 1.1 | ||
| Unreported (%) | 45.4 | 55.1 |
Statistical analysis
Descriptive Statistics
Frequencies for each studied side effect (Figs 1a and 2a) was calculated by the equation:
| 1 |
Comparative Statistics
ADR report rates were compared via the Odds Ratio (OR) analysis for Figs 1b, 2b, 3 and 4 using the following equations:
| 2 |
where
a: Number of cases in exposed group with an adverse event.
b: Number of cases in exposed group with no adverse event.
c: Number of cases in control group with the adverse event.
d: Number of cases in control group with no adverse event.
| 3 |
| 4 |
95% Confidence Interval;
| 5 |
Discussion
In this study, we quantified and confirmed the association between PPI exposure and the increased risk of AKI, CKD, ESRD, and electrolyte abnormalities by utilizing updated adverse event reports in the FAERS/AERS database. Most interestingly, the extended set of reports revealed an association between PPI exposure and unexpected significant risk for nephrolithiasis and renal impairment (Fig. 1). For the first time there were sufficient records for analysis of the effects of individual PPIs and observed varying degrees of electrolyte and renal abnormalities (Figs 3 and 4).
Our analysis of renal adverse effects was in general agreement with previous studies that have documented an increased risk of incident AKI, incident CKD, CKD progression and ESRD in large observational cohorts. Klesper and colleagues performed a nested case-control study, including 184,480 patients, and found an increased risk of AKI with PPI prescription (OR 1.72, 95% confidence interval [CI] [1.27, 2.32], p < 0.001)22. In a population-based cohort study of 290,593 patients over the age of 65, Antoniou and colleagues confirmed the association of PPI use with AKI (hazard ratio [HR] 2.52, 95% CI [2.27, 2.79])29. In the Atherosclerosis Risk in Communities (ARIC) study Lazarus et al. performed a population based prospective cohort study with 10,482 patients and found an increased risk of incident AKI and CKD when comparing PPI users to H2RA users (HR, 1.58; 95% CI [1.05, 2.04]) and (HR, 1.39; 95% CI [1.01, 1.91]), respectively)21. These population-based studies utilized ICD coding data to define the outcome of incident AKI and CKD. Xie and colleagues evaluated a prospective cohort including 193,591 patients from the Veteran’s Affairs database and documented not only an increased risk of incident CKD (HR, 1.28; 95% CI [1.23, 1.34]) but also an increased risk for ESRD when comparing PPI users to H2RA users (HR, 1.96; 95% CI [1.21, 3.18])23 and in a later study demonstrated that CKD progression and ESRD can occur without intervening AKI28. The difference in values between different studies may be due to multiple factors including definitions of renal injury and the diagnostic criteria as well as the time dependent analyses using hazard ratios. The FAERS and AERS data derived frequencies are additionally influenced by the severity related threshold of the report submission. In summary we documented an OR of 4.2 for AKI with the lower 95% CI boundary of 2.9, OR values as large as 28.4, with the 95% CI between 12.7 and 63.5 for CKD and 35.5 with 95% CI between 5.0 and 250.0 for ESRD. Our results indicate significant increase in nephrolithiasis reports with (OR 2.8 (95% CI [1.3, 6.0]). Nephrolithiasis finding is of particular interest since it has been associated with AKI, CKD, and ESRD progression but it only accounts for a small subset of renal injury cases30–32.
Hypomagnesemia was reported in the initial clinical trials and on the FDA package insert for every PPI as a rare side effect11. Accordingly, the frequency of hypomagnesemia reports for PPI patients is low, but the relative frequency was dramatically higher, almost eighty-fold, than for the H2RA control group. Secondly, detection bias may underestimate this adverse effect, since magnesium concentrations are not routinely measured compared to sodium, potassium and calcium. All five studied PPIs had comparable and increased ORs, with omeprazole showing the largest magnitude. Omeprazole, being the first marketed and the first over-the-counter PPI, has been used for the longest time, therefore patients were likely to have longer drug exposure. It may be prudent to monitor magnesium levels in patients with ongoing PPI therapy and other risk factors for hypomagnesemia.
Previous studies examining the effect of PPIs’ on calcium levels were not consistent. Multiple small-scale studies have shown that PPIs decrease gastrointestinal calcium absorption33–35 and this deleterious effect is attenuated by administering acidic liquids36. However, other studies have noted that the change in gastric pH does not correlate with calcium levels37,38. In our analysis of 42,537 PPI and 8,309 H2RA cases, we found a clear increase in hypocalcemia in patients taking PPIs compared to patients receiving H2RAs. Although the mechanism for hypocalcemia is not clearly defined, we can conclude that all PPIs are significantly associated with hypocalcemia.
We evaluated alterations in serum potassium concentrations. The previous evidence for hypokalemia with PPI use was limited, mainly consisting of case reports39–41. We found that PPI utilization resulted in moderately increased hypokalemia, when compared to the H2RA cohort. As noted earlier, PPI utilization was correlated with CKD, known to cause fluctuations in electrolytes. While CKD can be associated with both hypokalemia (e.g. in the case of tubular dysfunction) and hyperkalemia42, we only observed a significant increase in hypokalemia. However, a small number of cases of hyperkalemia were reported out of 42,537 reports in FAERS/AERS. In conclusion, hypokalemia was more common than hyperkalemia in our analysis of patients receiving PPIs. Each PPI was shown to have increased odds of hypokalemia, except for rabeprazole which was not significant (OR 2.3 CI [0.27, 20]).
Hyponatremia has been reported as a rare post marketing adverse reaction in FDA package inserts for pantoprazole, omeprazole, and esomeprazole11,43,44. In a retrospective study of 302 individuals receiving PPIs for longer than a year, Buon and colleagues found moderate hyponatremia in 18.7–46.3% of elderly patients45. It should be noted that although dysnatremia is associated with CKD46, we observed only a minor yet significant increase in hyponatremia reports. Hypernatremia was not a significant adverse effect in PPI users. Our analysis showed hyponatremia effect to be most pronounced for omeprazole, followed by lansoprazole and rabeprazole.
The observed increased risks of renal and electrolyte adverse effects of PPIs warrant more careful consideration in clinical practice. The risk-benefit ratio should be considered for the individual patient with respect to the adverse effects. When clinically indicated, PPIs should be used for the shortest duration necessary and chronic use is not recommended except for treatment of pathological hypersecretory conditions including Zollinger-Ellison syndrome and maintenance healing of erosive esophagitis11,43,44,47–54. It should be noted that the above-mentioned indications are FDA recommendations. Off-label and over-the-counter use of PPIs for the treatment of gastroesophageal reflux disorder (GERD) should be limited to four weeks11,43,44,52–54 but is often continued beyond the recommended limit. Continued use can result in rebound acid hypersecretion and hypergastrinemia after 4–8 weeks of therapy55,56 leading to chronic use.
Conclusion
In our study we observed various levels of increased risk of renal and electrolyte ADRs in FAERS reports of individual PPI drugs with respect to H2RA reports. Regardless of which PPI is initiated, it may be beneficial to follow dosing and duration recommendations established by the FDA11,43,44,52–54, American College of Gastroenterology47,48 and World Gastroenterology Organisation Global Guidelines49. It may be beneficial to monitor renal function and electrolytes including potassium, calcium, magnesium, and sodium. Although H2RAs have not been shown to be as effective as PPIs, they might be considered as alternatives for patients who are at high risk for developing renal and electrolytes imbalances.
Study Limitations
Since the FDA FAERS/AERS reporting is voluntary, only a subset of actual cases is represented, and ADR frequencies do not represent the population incidences. A recent study found that FAERS/AERS reporting can be biased by legal and scientific variables and newsworthiness50. Another study has shown that FAERS/AERS reporting was significantly underreported for statins51. Some limitation stems from the absence of comprehensive medical records. Although the indication section in the data set was used to exclude potential comorbidities, some concurrent medications and comorbidities may be missing from the records due to underreporting. This may have introduced noise into the cohort compositions, ADR frequencies and odds ratios. The mechanism of the adverse reaction cannot be derived from the FAERS/AERS records. The odds ratios represent frequency ratios of reported adverse effects between FAERS/AERS PPI and H2RA cohorts and are not based on population incidences. As with any association study, causality cannot be inferred from association. These cases were not clinically adjudicated for causality by experts.
Supplementary information
Acknowledgements
We thank Dr. Rabia Atayee for stimulating discussions and help with the project. We also thank Chris Edwards and Da Shi for contributions to processing the FAERS/AERS data files and supporting the computer environment.
Author Contributions
T.M. performed the experiments, R.A., L.A. and T.M. designed the study and, T.M., I.C., L.A. and R.A. drafted the manuscript and reviewed the final version. R.A. processed the data set.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39335-7.
References
- 1.20th WHO Model List of Essential Medicines. http://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1. (2017).
- 2.Clissold SP, Campoli-Richards DM. Omeprazole. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peptic ulcer disease and Zollinger-Ellison syndrome. Drugs. 1986;32:15–47. doi: 10.2165/00003495-198632010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Walan A, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989;320:69–75. doi: 10.1056/NEJM198901123200201. [DOI] [PubMed] [Google Scholar]
- 4.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 5.Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gawron AJ, et al. Brand name and generic proton pump inhibitor prescriptions in the United States: insights from the national ambulatory medical care survey (2006–2010) Gastroenterol Res Pract. 2015;2015:689531. doi: 10.1155/2015/689531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457:609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64:935–951. doi: 10.1007/s00228-008-0538-y. [DOI] [PubMed] [Google Scholar]
- 10.Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prilosec (omeprazole) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf (2012).
- 12.Shaheen NJ, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 13.Shah NH, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 15.Lambert AA, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. Plos One. 2015;10:e0128004. doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson C. Bone: proton-pump inhibitors and fractures. Nat Rev Endocrinol. 2012;8:625. doi: 10.1038/nrendo.2012.170. [DOI] [PubMed] [Google Scholar]
- 17.Reich A, Maj J. Subacute cutaneous lupus erythematosus due to proton pump inhibitor intake: case report and literature review. Arch Med Sci. 2012;8:743–747. doi: 10.5114/aoms.2012.30300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandholdt LH, Laurinaviciene R, Bygum A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2014;170:342–351. doi: 10.1111/bjd.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomm W, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol. 2016;73:410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 20.Haenisch B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus B, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med. 2016;176:238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol. 2013;14:150. doi: 10.1186/1471-2369-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, et al. Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol. 2016;27:3153–3163. doi: 10.1681/ASN.2015121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klatte DCF, et al. Association Between Proton Pump Inhibitor Use and Risk of Progression of Chronic Kidney Disease. Gastroenterology. 2017;153:702–710. doi: 10.1053/j.gastro.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 25.Toth-Manikowski S, Grams ME. Proton Pump Inhibitors and Kidney Disease - GI Upset for the Nephrologist? Kidney Int Rep. 2017;2:297–301. doi: 10.1016/j.ekir.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijarnpreecha K, et al. Associations of Proton-Pump Inhibitors and H2 Receptor Antagonists with Chronic Kidney Disease: A Meta-Analysis. Dig Dis Sci. 2017;62:2821–2827. doi: 10.1007/s10620-017-4725-5. [DOI] [PubMed] [Google Scholar]
- 27.Hung, S. C. et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. Fam Pract, 10.1093/fampra/cmx102 (2017). [DOI] [PubMed]
- 28.Xie Y, et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91:1482–1494. doi: 10.1016/j.kint.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Antoniou T, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3:E166–171. doi: 10.9778/cmajo.20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X, Lieske JC. Acute and chronic kidney injury in nephrolithiasis. Curr Opin Nephrol Hypertens. 2014;23:385–390. doi: 10.1097/01.mnh.0000447017.28852.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jungers P, Joly D, Barbey F, Choukroun G, Daudon M. ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis. 2004;44:799–805. doi: 10.1016/S0272-6386(04)01131-X. [DOI] [PubMed] [Google Scholar]
- 32.Alexander RT, et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graziani G, et al. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrol Dial Transplant. 1995;10:1376–1380. [PubMed] [Google Scholar]
- 34.Hardy P, et al. Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artif Organs. 1998;22:569–573. doi: 10.1046/j.1525-1594.1998.06200.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118:778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Hansen KE, et al. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res. 2010;25:2786–2795. doi: 10.1002/jbmr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bo-Linn GW, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640–647. doi: 10.1172/JCI111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serfaty-Lacrosniere C, et al. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr. 1995;14:364–368. doi: 10.1080/07315724.1995.10718522. [DOI] [PubMed] [Google Scholar]
- 39.Negri AL, Valle EE. Hypomagnesaemia/hypokalemia associated with the use of esomeprazole. Curr Drug Saf. 2011;6:204–206. doi: 10.2174/157488611797579320. [DOI] [PubMed] [Google Scholar]
- 40.Maeda Y, et al. Does a proton pump inhibitor cause hypokalemia? Intern Med. 2011;50:1045–1050. doi: 10.2169/internalmedicine.50.4877. [DOI] [PubMed] [Google Scholar]
- 41.Chandra, A. et al. Proton Pump Inhibitor Induced Hypokalemia. J Clin Stu2(5) (2017).
- 42.Gilligan S, Raphael KL. Hyperkalemia and Hypokalemia in CKD: Prevalence, Risk Factors, and Clinical Outcomes. Adv Chronic Kidney Dis. 2017;24:315–318. doi: 10.1053/j.ackd.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Nexium (esomeprazole magnesium) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022101s014021957s017021153s050lbl.pdf (2014).
- 44.Protonix (pantoprazole sodium) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020987s045lbl.pdf (2012).
- 45.Buon M, et al. Risk of proton pump inhibitor-induced mild hyponatremia in older adults. J Am Geriatr Soc. 2013;61:2052–2054. doi: 10.1111/jgs.12534. [DOI] [PubMed] [Google Scholar]
- 46.Kovesdy CP. Significance of hypo- and hypernatremia in chronic kidney disease. Nephrol Dial Transplant. 2012;27:891–898. doi: 10.1093/ndt/gfs038. [DOI] [PubMed] [Google Scholar]
- 47.Moayyedi PM, et al. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol. 2017;112:988–1013. doi: 10.1038/ajg.2017.154. [DOI] [PubMed] [Google Scholar]
- 48.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 49.Hunt R, et al. World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J Clin Gastroenterol. 2017;51:467–478. doi: 10.1097/MCG.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 50.Alatawi YM, Hansen RA. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) Expert Opin Drug Saf. 2017;16:761–767. doi: 10.1080/14740338.2017.1323867. [DOI] [PubMed] [Google Scholar]
- 51.Maciejewski, M. et al. Reverse translation of adverse event reports paves the way for de-risking preclinical off-targets. Elife6, 10.7554/eLife.25818 (2017). [DOI] [PMC free article] [PubMed]
- 52.Prevacid (lansoprazole) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020406s078-021428s025lbl.pdf (2012).
- 53.Dexilant (dexlansoprazole) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022287s014lbl.pdf (2012).
- 54.Aciphex (rabeprazole) Label – FDA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020973s035204736s005lbl.pdf (2014).
- 55.Reimer C, Søndergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137(80–87):87.e81. doi: 10.1053/j.gastro.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 56.Niklasson A, Lindström L, Simrén M, Lindberg G, Björnsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105:1531–1537. doi: 10.1038/ajg.2010.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.