Abstract
Elizabethkingia bruuniana is a novel species of the Elizabethkingia genus. There is scant information on this microorganism. Here, we report the whole-genome features and antimicrobial susceptibility patterns of E. bruuniana strain EM798-26. Elizabethkingia strain EM798-26 was initially identified as E. miricola. This isolate contained a circular genome of 4,393,011 bp. The whole-genome sequence-based phylogeny revealed that Elizabethkingia strain EM798-26 was in the same group of the type strain E. bruuniana G0146T. Both in silico DNA-DNA hybridization and average nucleotide identity analysis clearly demonstrated that Elizabethkingia strain EM798-26 was a species of E. bruuniana. The pan-genome analysis identified 2,875 gene families in the core genome and 5,199 gene families in the pan genome of eight publicly available E. bruuniana genome sequences. The unique genes accounted for 0.2–12.1% of the pan genome in each E. bruuniana. A total of 59 potential virulence factor homologs were predicted in the whole-genome of E. bruuniana strain EM798–26. This isolate was nonsusceptible to multiple antibiotics, but susceptible to aminoglycosides, minocycline, and levofloxacin. The whole-genome sequence analysis of E. bruuniana EM798-26 revealed 29 homologs of antibiotic resistance-related genes. This study presents the genomic features of E. bruuniana. Knowledge of the genomic characteristics provides valuable insights into a novel species.
Introduction
Elizabethkingia is a genus of aerobic, gram-negative, nonmotile, non-spore-forming, and non-fermenting bacilli1. These microorganisms are extensively distributed in soil, water, and plants, but they do not normally exist in human microflora1–3. Among the members of this genus, the type species, E. meningoseptica, is the most well-known species that causes human infections since its first identification by Elizabeth O. King in 19594. The second species of the genus, E. miricola, was isolated in 2003 from condensation water on the space station Mir5. The third species, E. anophelis, was first recognized from the midgut of the mosquito Anopheles gambiae in 20116. Three new species, namely, E. bruuniana, E. ursingii, and E. occulta, were proposed to be novel members of the Elizabethkingia genus in 20171. As of now, six species are included in the Elizabethkingia genus. A noteworthy rise in the lethal infections associated with this genus has been identified worldwide recently7–12.
We previously published the complete genome sequence of the E. miricola strain EM798-26 isolated from the blood of a cancer patient (GenBank accession number CP023746)13. This isolate was initially identified as E. miricola using 16S ribosomal RNA (rRNA) gene sequencing, which showed a 99.9% identity to E. miricola ATCC 33958 and 99.6% identity to E. miricola BM10. After the proposal of the three novel Elizabethkingia species, we revisited the taxonomy of the E. miricola strain EM798-26 using in silico DNA-DNA hybridization (DDH) and average nucleotide identity (ANI) analysis based on whole-genome sequences. In this study, we reported the emendation of the strain EM798-26 as a later subjective synonym of E. bruuniana. We then investigated the genomic features and phylogenetic diversity of E. bruuniana isolates available in the National Center for Biotechnology Information (NCBI) genome sequence repository. We finally described the antimicrobial susceptibility patterns of E. bruuniana strain EM798-26.
Materials and Methods
Ethics and experimental biosafety statements
This study was approved by the Institutional Review Board of E-Da Hospital (EMRP-106-105). The need for patient’s informed consent was waived by the Institutional Review Board of E-Da Hospital as the retrospective analysis of anonymously clinical data posed no more than minimal risk of harm to subjects and involved no procedures for which written consent was normally required outside of the research context. The experiments in this study were approved by the Institutional Biosafety Committee of E-Da Hospital. All experiments were performed in accordance with relevant guidelines and regulations.
Isolate of Elizabethkingia strain EM798-26
Elizabethkingia strain EM798-26 was isolated from the blood of an 81-year-old male patient with diffuse large B-cell lymphoma. This patient was admitted due to neutropenic fever after chemotherapy for lymphoma. The blood culture was performed using BacT/ALERT 3D Microbial Identification System (bioMérieux, Marcy l’Etoile, France). This isolate was initially identified as E. meningoseptica using VITEK matrix-assisted laser desorption ionization–time of flight mass spectrometry (bioMérieux) by the clinical microbiology laboratory, and then it was stored as glycerol stocks at −80 °C until use. For experiments, the thawed isolate of strain EM798-26 was subcultured on tryptic soy agar with 5% sheep blood (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The total DNA of fresh colonies was extracted using a Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA).
16S rRNA sequencing and phylogenetic analysis
The primers and protocols for amplification and sequencing of 16S rRNA gene are listed in Table114,15. To evaluate the phylogenetic diversity between Elizabethkingia and other genera, the 16S rRNA gene sequences of the type species and common species of different gram-negative genera were compared (Supplementary Table S1). The sequences were aligned using the ClustalW function with default options in the MEGA software16. Genetic relationships were calculated using the neighbor-joining method based on Kimura 2-parameter distances in the MEGA software16. Phylogenetic trees were re-constructed in the Dendroscope software17.
Table 1.
Primers of PCR and sequencing for 16S rRNA in this study.
| Primer | Sequence (5′ to 3′) | Amplicon size (bp) | References |
|---|---|---|---|
| PCR of 16S rRNA | |||
| 8 f | CACGGATCCAGACTTTGAT(C/T)(A/C)TGGCTCAG | 1498 | 14 |
| 1512r | GTGAAGCTTACGG(C/T)TAGCTTGTTACGACTT | ||
| Sequencing of 16S rRNA | |||
| 8 f | CACGGATCCAGACTTTGAT(C/T)(A/C)TGGCTCAG | — | 15 |
| 534r | ATTACCGCGGCTGCTGG | ||
| 534 f | CCAGCAGCCGCGGTAAT | ||
| 968 f | AACGCGAAGAACCTTAC | ||
| 1512r | GTGAAGCTTACGG(C/T)TAGCTTGTTACGACTT | ||
Whole-genome sequencing
The whole genome of the strain EM798-26 was sequenced using Illumina HiSeq2000 (Illumina, San Diego, CA, USA) and PacBio (Pacific Biosciences, Menlo Park, CA, USA) sequencing platforms as our previous report13. The genome was then hybrid assembled, and structural errors were corrected by optical mapping (Bionano Genomics, San Diego, CA, USA).
Whole-genome phylogenetic analysis and species identification
To determine the phylogenetic origin with respect to other Elizabethkingia strains, the whole-genome sequences of 14 publicly published “E. miricola” strains and each type strain of E. meningoseptica KC1913T (=ATCC 13253 T), E. miricola GTC 862 T (=KCTC 12492 T = W3-B1), E. anophelis R26T, E. bruuniana G0146T, E. ursingii G4122T, and E. occulta G4070T were compared (Supplementary Table S2). The whole-genome sequence-based phylogenetic tree was constructed using the online pipeline Reference Sequence Alignment Based Phylogeny Builder (REALPHY)18. To confirm the species of Elizabethkingia, we calculated the in silico DDH and ANI values using Genome-to-Genome Distance Calculator (GGDC)19 and OrthoANI20, respectively. An ANI cutoff value of 95% and a DDH cutoff value of 70% was used as species delimitation criteria19,21. The heat maps were generated using CIMminer (https://discover.nci.nih.gov/cimminer/).
Genome annotation and analysis
The assembled genome was submitted to the NCBI Prokaryotic Genome Annotation Pipeline22 and the Rapid Annotations based on Subsystem Technology (RAST) Prokaryotic Genome Annotation Server (http://rast.nmpdr.org/)23,24 for genome annotations. The pan genome and core genome were analyzed using the Bacterial Pan Genome Analysis (BPGA) pipeline25. The graphical map of the circular genome was generated using the CGView Server (http://stothard.afns.ualberta.ca/cgview_server/)26. The virulence factors of the strain EM798-26 were predicted using the Virulence Factor Database (VFDB, http://www.mgc.ac.cn/VFs/)27. Antibiotic resistance genes were searched using the Antibiotic Resistance Genes Database BLAST Server (ARDB, https://ardb.cbcb.umd.edu/)28. An expectation value <1e-5 and ≥30% identity of the homologs were used as a threshold of the BLASTP searches29.
Antimicrobial susceptibility testing
The minimum inhibitory concentration (MIC) was examined using the broth microdilution method (Thermo Fisher Scientific/Trek Diagnostics Systems, Oakwood Village, OH, USA). The susceptibilities were determined according to the interpretive standards for “other non-Enterobacteriaceae” as suggested by the Clinical and Laboratory Standards Institute (CLSI) guideline30. The MIC of tigecycline was interpreted according to the Enterobacteriaceae susceptibility breakpoints of the USA FDA (susceptible MIC, ≤2 mg/L; intermediate MIC, 4 mg/L; resistant MIC, ≥8 mg/L)31.
Results and Discussion
Phylogenetic relationships between Elizabethkingia and other genera
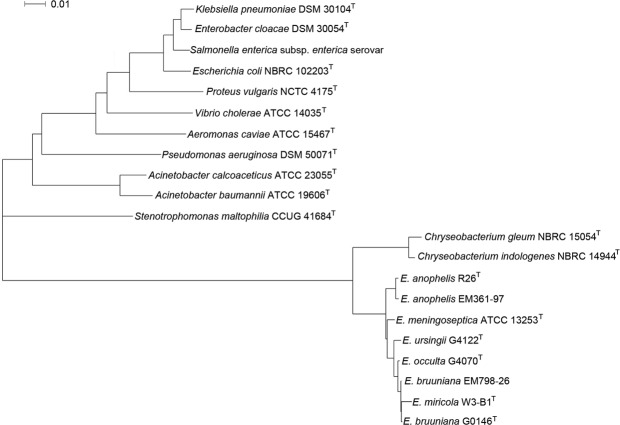
The phylogenetic analysis of 16S rRNA sequences between the Elizabethkingia species and the respective reference sequences of other gram-negative genera is shown in Fig. 1. On the basis of 16S rRNA gene sequencing, E. meningoseptica ATCC 13253 T, E. anophelis R26T, E. anophelis EM361-97 (our previously published genome9), E. miricola W3-B1T, E. bruuniana G0146T, E. bruuniana EM798-26, E. ursingii G4122T, and E. occulta G4070T were in the same branch of phylogenetic tree.
Figure 1.
Phylogenetic analysis of 16S rRNA gene sequencing using the neighbor-joining method based on Kimura 2-parameter distances. The tree was constructed from the 16S rRNA gene sequences of E. bruuniana EM798-26 and the respective reference sequences from GenBank. The scale length indicates 0.01 nucleotide substitutions per nucleotide site.
Chryseobacterium species were closest to Elizabethkingia species, and shared a recent common ancestor with Elizabethkingia. Both genera belong to the Flavobacteriaceae family. The phylogenetic branch of Stenotrophomonas maltophilia, Acinetobacter baumannii, and Pseudomonas aeruginosa were near to the branch of Elizabethkingia and Chryseobacterium. All these genera are glucose non-fermenting gram-negative bacilli. In contrast, the microorganisms of Enterobacteriaceae family, including Klebsiella pneumoniae, Enterobacter cloacae, Salmonella enterica, Escherichia coli, and Proteus vulgaris were farthest away from Elizabethkingia in the phylogenetic tree based on 16S rRNA gene sequences.
Genome description of strain EM798-26
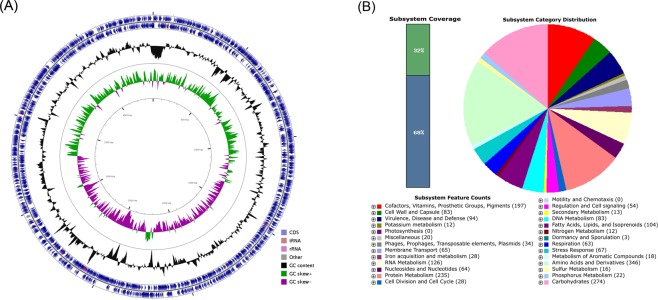
The total length of the assembled genome was 4,393,011 bp, with a mean G + C content of 35.73%. The genome coverage rate was 220.0× . The statistics of the assembly and annotation are shown in Table 2. The genome contained 3,877 protein-coding genes and 80 pseudogenes. The number of RNA genes was 72, including 15 rRNAs, 54 transfer RNAs (tRNAs), and three noncoding RNAs (ncRNAs) (Fig. 2A). These 3,877 genes could be classified into 27 categories and 360 subsystems (Fig. 2B). Of these subsystems, “amino acids and derivatives” was the largest and accounted for 346 genes, followed by “carbohydrates” (274 genes), “protein metabolism” (235 genes), and “cofactors, vitamins, prosthetic groups, pigments” (197 genes). In the category of “virulence, disease and defense”, 85 genes were related to “resistance to antibiotics and toxic compounds”, including “resistance to vancomycin” (1 gene), “multidrug resistance, tripartite systems found in gram-negative bacteria” (9 genes), “resistance to fluoroquinolones” (4 genes), “β-lactamase” (17 genes), and “multidrug resistance efflux pumps” (16 genes). The high number of antimicrobial resistance homologs suggests that Elizabethkingia strain EM798-26 might be a multidrug-resistant strain.
Table 2.
Assembly and annotation statistics.
| Content | Number |
|---|---|
| Genome size (bp) | 4,393,011 |
| Gene number | 4,117 |
| Gene length (bp) | 3,902,358 |
| GC content (%) | 35.73 |
| GC content in gene region (%) | 36.68 |
| Gene length/genome (%) | 88.83 |
| Gene average length (bp) | 948 |
| Intergenic region length (bp) | 490,655 |
| GC content in intergenic region (%) | 28.17 |
| Intergenic region length/genome (%) | 11.17 |
| N50 (bp) | 483,147 |
| L50 (bp) | 4 |
| Tandem repeat number | 118 |
| Tandem repeat length (bp) | 11,030 |
| Tandem repeat size (bp) | 3–736 |
| Tandem repeat length/genome (%) | 0.2511 |
| Genes (total) | 4,029 |
| CDS (total) | 3,957 |
| Genes (coding) | 3,877 |
| Genes (RNA) | 72 |
| ribosomal RNA (rRNA) | 15 |
| transfer RNA (tRNA) | 54 |
| noncoding RNA (ncRNA) | 3 |
| Pseudogenes | 80 |
Figure 2.
Genomic features of Elizabethkingia bruuniana EM798-26. (A) The genome of strain EM798-26 contained 3,877 protein-coding genes and 80 pseudogenes. There were 72 RNA genes, including 15 ribosomal RNAs (rRNAs), 54 transfer RNAs (tRNAs), and three noncoding RNAs (ncRNAs). The outer two circles demonstrate the coding sequence (CDS), tRNA, and rRNA. The third circle shows the GC content (black). The fourth circle represents the GC skew curve (positive GC skew, green; negative GC skew, violet). (B) The genome of E. bruuniana EM798-26 annotated using the Rapid Annotation System Technology (RAST) Server. The genome could be classified into 27 categories and 360 subsystems. The green part in the bar chart at the leftmost position corresponds to the percentage of proteins included. The pie chart and count of the subsystem features in the right panel demonstrate the percentage distribution and category of the subsystems.
Whole-genome sequence-based identification of Elizabethkingia species
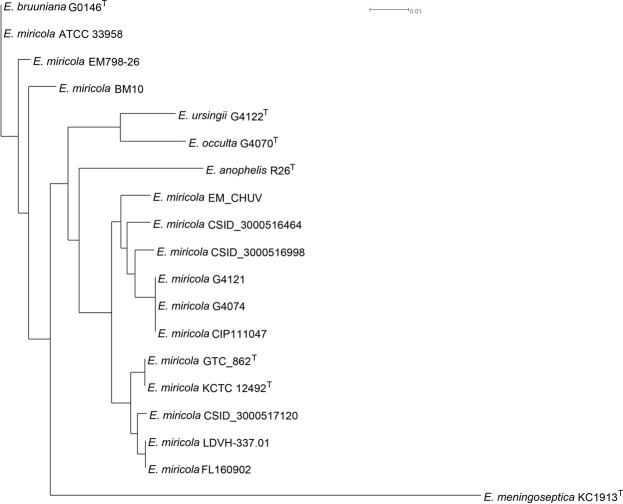
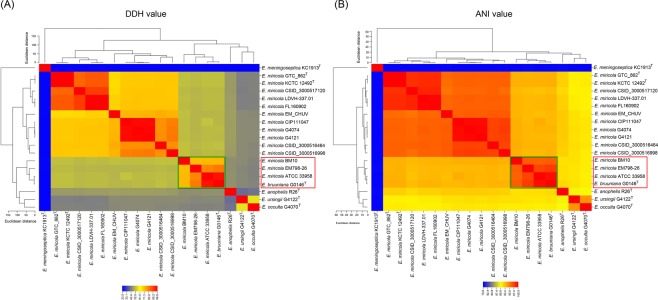
The whole-genome sequence-based phylogenetic tree was constructed and demonstrated that strains EM798-26, ATCC 33958, and BM10 were in the same genomic group with the type strain E. bruuniana G0146T (Fig. 3). Both in silico DDH (Fig. 4A) and ANI analysis (Fig. 4B) clearly revealed that strains EM798-26, ATCC 33958, BM10, and E. bruuniana G0146T belonged to the same species.
Figure 3.
The whole-genome sequence-based phylogenetic tree constructed using the Reference Sequence Alignment Based Phylogeny Builder (REALPHY).
Figure 4.
Species identification based on whole-genome sequencing. (A) in silico DNA-DNA hybridization (DDH) using the Genome-to-Genome Distance Calculator (GGDC). (B) Average nucleotide identity (ANI) analysis using OrthoANI.
Before the proposal of the three new species in the Elizabethkingia genus, substantial sequence variability in the whole-genome sequences of the E. miricola strains has been identified and taxonomic re-classification of some strains has been suggested32,33. Based on the whole-genome sequence study, Nicholson et al. proposed that strains in genomospecies 3 as E. bruuniana sp. nov. and those in genomospecies 4 as E. ursingii sp. nov. and E. occulta sp. nov.1. At the time of proposing the novel species of Elizabethkingia, Nicholson et al. also re-classified E. miricola ATCC 33958 and E. miricola BM10 into the species of E. bruuniana based on the results of the whole-genome DDH, optical maps, and matrix-assisted laser desorption ionization-time of flight mass spectrometry1. Similar to the strains ATCC 33958 and BM10, our study clearly demonstrated that strain EM798-26 should also be re-assigned to the species of E. bruuniana, but not E. miricola.
Pan-genome comparisons
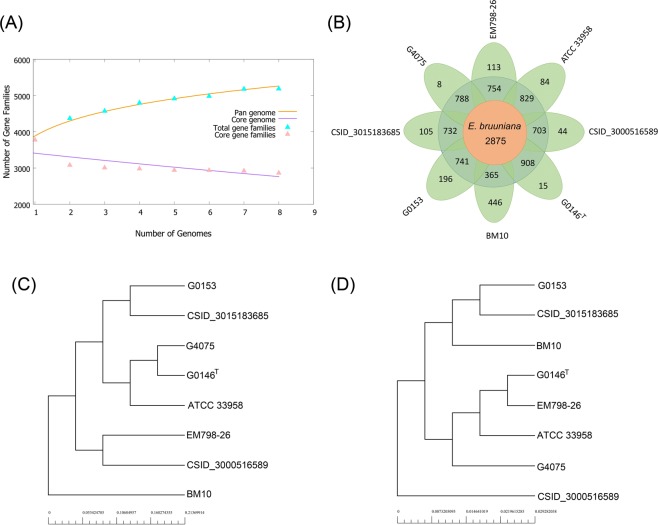
Pan-genome analysis has been applied in the evaluation of the genome diversity, genome dynamics, species evolution, pathogenesis, and other features of microorganisms34. To better understand the phylogenetic relationship and bacterial evolution, we performed pan-genome analysis of eight publicly available whole-genome sequences of E. bruuniana isolates (Fig. 5; Supplementary Table S2). The evolution of the pan and core genome is presented in Fig. 5A. As the addition of each new genome sequence of E. bruuniana, the number of gene families in the pan genome increased from 3,884 to 5,199, and that of gene families in the core genome decreased from 3,511 to 2,875 (Fig. 5A,B). The core genome accounted for on average 55.3% of the pan genome. The gene families of the pan genome represent the housing capacity of the genetic determinants and those of the core genome are usually related to bacterial replication, translation, and maintenance of cellular homeostasis34,35. In our study, the unique genes of each E. bruuniana strain exhibited a wide distribution, ranging from 8 (0.2%) to 446 (12.1%). These unique genes are under relaxed mutation pressure34,36 and usually have an association with the pathogenicity and virulence of the microorganisms34. Phylogeny based on the pan genome demonstrated that E. bruuniana EM798-26 was closer to E. bruuniana CSID_3000516589 (Fig. 5C). In contrast, the tree based on the core genome showed that strain EM798-26 was at a position near the type strain E. bruuniana G0146T (Fig. 5D). These findings suggest the diverse genetic evolution of the pan and core genomes in different E. bruuniana strains.
Figure 5.
Pan-genome analysis of eight E. bruuniana isolates in the repertoire of GenBank. (A) Pan-genome and core genome plot shows the progression of the pan (orange line) and core (purple line) genomes as more genomes are added for analysis. The pan genome is still open, as the new additional genome significantly increases the total repertoire of genes. Extrapolation of the curve indicates that the gene families in pan genome increased from 3,884 to 5,199, and those in core genome decreased from 3,511 to 2,875. (B) Flower plot shows the numbers of core genes (inner circle), accessory genes (middle circle), and unique genes (outer circle). (C) Phylogenetic tree based on the pan genome. (D) Phylogenetic tree based on the core genome.
Potential virulence factors
A total of 59 potential virulence factor homologs were predicted by VFDB with the criteria of ≥30% identity and <1e-5 expectation value (Supplementary Table S3). These genes conferred biofilm formation, capsule polysaccharide synthesis, inhibition of the alternative complement pathway and complement-mediated opsonophagocytosis, iron siderophore synthesis, superoxide dismutase expression, prevention of phagocytosis, prevention of antibody-mediated opsonization, and other functions. These virulence factor homologs in the E. bruuniana strain EM798-26 are also commonly found in other Elizabethkingia species, such as E. anophelis9,29. However, these potential virulence factors need more experiments to prove their pathogenicity.
Antimicrobial susceptibility testing and antimicrobial resistance-associated genes
The antimicrobial susceptibility testing of E. bruuniana strain EM798-26 is shown in Table 3. This isolate was non-susceptible to all tested β-lactams, β-lactam-lactamase inhibitors, and carbapenems, but susceptible to gentamicin, amikacin, minocycline, and levofloxacin.
Table 3.
Antimicrobial susceptibility of E. bruuniana strain EM798-26 and E. anophelis strain EM361-97.
| Antimicrobial agent | E. bruuniana strain EM798-26 | E. anophelis strain EM361-97 | ||
|---|---|---|---|---|
| MIC (mg/L) | Interpretation | MIC (mg/L) | Interpretation | |
| Piperacillin | >64 | R | 32 | I |
| Piperacillin-tazobactam | 32/4 | I | 16/4 | S |
| Ticarcillin-clavulanic acid | >64/2 | R | >64/2 | R |
| Ceftazidime | >16 | R | >16 | R |
| Cefepime | >32 | R | 32 | R |
| Ceftriaxone | >32 | R | >32 | R |
| Aztreonam | >16 | R | >16 | R |
| Imipenem | >8 | R | >8 | R |
| Meropenem | >8 | R | >8 | R |
| Gentamicin | 4 | S | >8 | R |
| Tobramycin | >8 | R | >8 | R |
| Amikacin | 16 | S | >32 | R |
| Tetracycline | >8 | R | >8 | R |
| Minocycline | <1 | S | <1 | S |
| Tigecycline | 2 | S | 2 | S |
| Ciprofloxacin | 2 | I | >2 | R |
| Levofloxacin | <1 | S | >8 | R |
| Trimethoprim-sulfamethoxazole | >4/76 | R | >4/76 | R |
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; I, intermediate; R, resistant.
We compared the antimicrobial susceptibility patterns between E. bruuniana strain EM798-26 and E. anophelis strain EM361-97 which was published in our previous study9. Both strains demonstrated resistance to multiple antibiotics, but they exhibited susceptibility to minocycline (MIC < 1 mg/L) and tigecycline (MIC = 2 mg/L). However, E. bruuniana strain EM798-26 was susceptible to levofloxacin, but E. anophelis strain EM361-97 was resistant to this antimicrobial agent.
Previous studies showed that Elizabethkingia isolates were usually resistant to many antimicrobial agents. For example, E. anophelis and E. meningoseptica isolated from Hong Kong8, the USA11, and South Korea37 demonstrated high resistance to most β-lactams, including ceftazidime, ceftriaxone, and imipenem, but variable susceptibility to piperacillin-tazobactam, cefepime, ciprofloxacin, and levofloxacin. However, there have been no previous studies describing the antimicrobial susceptibility of E. bruuniana. Our study first demonstrated the antimicrobial susceptibility testing patterns of E. bruuniana. As there was only one isolate in our study, further large-scale studies are necessary to investigate the antimicrobial susceptibility pattern of E. bruuniana.
We investigated the antimicrobial resistance-associated genes using the Antibiotic Resistance Genes Database BLAST Server with a threshold of ≥30% identity and <1e-5 expectation value. The whole-genome sequence analysis of E. bruuniana EM798-26 revealed 29 homologs of antibiotic resistance-related genes (Supplementary Table S4). These antibiotic resistance genes included β-lactamases, multidrug resistance efflux pumps (aminoglycoside and macrolide), NADP-requiring oxidoreductase, dihydrofolate reductase, undecaprenyl pyrophosphate phosphatase, sulfonamide-resistant dihydropteroate synthase, VanA, B, E, and G vancomycin resistance operon genes, the adenosine triphosphate-binding cassette (ABC) superfamily, the resistance-nodulation-division (RND) family, the major facilitator superfamily (MFS), and the small multidrug resistance (SMR) family. The whole-genome analysis suggested that E. bruuniana EM798-26 could be a strain resistant to multiple antibiotics. The manifestation of multidrug resistance is compatible with the antimicrobial susceptibility testing of this isolate.
Conclusions
This study presents the species identification, genomic features, and antimicrobial susceptibility patterns of E. bruuniana. There is no similar study to describe the genomic features and antimicrobial susceptibility patterns of E. bruuniana in the literature. Knowledge on the phylogenetic relationship, genomic traits, and antimicrobial susceptibility patterns provides valuable information on this novel species.
Supplementary information
Acknowledgements
This work was supported by grants EDPJ106075 from E-Da Hospital and MOST 106-2314-B-214-009-MY2 from the Ministry of Science and Technology, Taiwan.
Author Contributions
All authors provided significant contributions, and all authors are in agreement regarding the content of the manuscript. Conception/design: Jiun-Nong Lin and Hsi-Hsun Lin; provision of study materials: Chung-Hsu Lai; collection and assembly of data: Jiun-Nong Lin, Chung-Hsu Lai, Chih-Hui Yang, and Yi-Han Huang; data analysis and interpretation: all authors; manuscript writing: Jiun-Nong Lin and Chih-Hui Yang; and final approval of the manuscript: all authors.
Data Availability
The species, strains, and GenBank accession numbers of microorganisms for 16S rRNA gene analysis are listed in Supplementary Table S1. The names of organisms, strains, biosample numbers, bioproject numbers, assembly numbers, isolated origins, and release dates of bacteria used in this study are shown in Supplementary Table S2. All data are available in the NCBI genome sequence repository.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38998-6.
References
- 1.Nicholson AC, et al. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. nov. Antonie Van Leeuwenhoek. 2017;111:55–72. doi: 10.1007/s10482-017-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Sys. Evol Microbiol. 2005;55:1287–1293. doi: 10.1099/ijs.0.63541-0. [DOI] [PubMed] [Google Scholar]
- 3.Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: an important emerging pathogen causing healthcare-associated infections. J Hosp Infect. 2014;86:244–249. doi: 10.1016/j.jhin.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 4.King EO. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959;31:241–247. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, et al. Chryseobacterium miricola sp. nov., a novel species isolated from condensation water of space station Mir. Syst Appl Microbiol. 2003;26:523–528. doi: 10.1078/072320203770865828. [DOI] [PubMed] [Google Scholar]
- 6.Kämpfer P, et al. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int J Syst Evol Microbiol. 2011;61:2670–2675. doi: 10.1099/ijs.0.026393-0. [DOI] [PubMed] [Google Scholar]
- 7.Teo J, et al. First case of E anophelis outbreak in an intensive-care unit. Lancet. 2013;382:855–856. doi: 10.1016/S0140-6736(13)61858-9. [DOI] [PubMed] [Google Scholar]
- 8.Lau SK, et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci Rep. 2016;6:26045. doi: 10.1038/srep26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JN, Lai CH, Yang CH, Huang YH, Lin HH. Genomic features, phylogenetic relationships, and comparative genomics of Elizabethkingia anophelis strain EM361-97 isolated in Taiwan. Sci Rep. 2017;7:14317. doi: 10.1038/s41598-017-14841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JN, Lai CH, Yang CH, Huang YH, Lin HH. Clinical manifestations, molecular characteristics, antimicrobial susceptibility patterns and contributions of target gene mutation to fluoroquinolone resistance in Elizabethkingia anophelis. J Antimicrob Chemother. 2018;73:2497–2502. doi: 10.1093/jac/dky197. [DOI] [PubMed] [Google Scholar]
- 11.Perrin A, et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat Commun. 2017;8:15483. doi: 10.1038/ncomms15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navon L, et al. Notes from the Field: Investigation of Elizabethkingia anophelis Cluster - Illinois, 2014–2016. MMWR. 2016;65:1380–1381. doi: 10.15585/mmwr.mm6548a6. [DOI] [PubMed] [Google Scholar]
- 13.Lin, J. N., Lai, C. H., Yang, C. H., Huang, Y. H. & Lin, H. H. Complete genome sequence of Elizabethkingia miricola strain EM798-26 isolated from the blood of a cancer patient. Genome Announc. 6, (2018). [DOI] [PMC free article] [PubMed]
- 14.Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans AD. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiol. 1997;143(Pt 9):2983–2989. doi: 10.1099/00221287-143-9-2983. [DOI] [PubMed] [Google Scholar]
- 15.Hantsis-Zacharov E, Shakéd T, Senderovich Y, Halpern M. Chryseobacterium oranimense sp. nov., a psychrotolerant, proteolytic and lipolytic bacterium isolated from raw cow’s milk. Int J Syst Evol Microbiol. 2008;58:2635–2639. doi: 10.1099/ijs.0.65819-0. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- 18.Bertels F, Silander OK, Pachkov M, Rainey PB, van Nimwegen E. Automated reconstruction of whole-genome phylogenies from short-sequence reads. Mol Biol Evol. 2014;31:1077–1088. doi: 10.1093/molbev/msu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I, Kim YO, Park SC, Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 21.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatusova T, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz RK, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overbeek R, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42:D206–214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhari NM, Gupta VK, Dutta C. BPGA- an ultra-fast pan-genome analysis pipeline. Sci Rep. 2016;6:24373. doi: 10.1038/srep24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant JR, Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36:W181–184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Zheng D, Liu B, Yang J, Jin Q. VFDB 2016: hierarchical and refined dataset for big data analysis–10 years on. Nucleic Acids Res. 2016;44:D694–697. doi: 10.1093/nar/gkv1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Pop M. ARDB–Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009;37:D443–447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teo J, et al. Comparative genomic analysis of malaria mosquito vector-associated novel pathogen Elizabethkingia anophelis. Genome Biol Evol. 2014;6:1158–1165. doi: 10.1093/gbe/evu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, M100-S26 (Clinical and Laboratory Standards Institute, 2016).
- 31.Kelesidis T, Karageorgopoulos DE, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J Antimicrob Chemother. 2008;62:895–904. doi: 10.1093/jac/dkn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doijad S, Ghosh H, Glaeser S, Kämpfer P, Chakraborty T. Taxonomic reassessment of the genus Elizabethkingia using whole-genome sequencing: Elizabethkingia endophytica Kämpfer et al. 2015 is a later subjective synonym of Elizabethkingia anophelis Kämpfer et al. 2011. Int J Syst Evol Microbiol. 2016;66:4555–4559. doi: 10.1099/ijsem.0.000821. [DOI] [PubMed] [Google Scholar]
- 33.Eriksen HB, Gumpert H, Faurholt CH, Westh H. Determination of Elizabethkingia diversity by MALDI-TOF mass spectrometry and whole-genome sequencing. Emerg Infect Dis. 2017;23:320–323. doi: 10.3201/eid2302.161321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guimarães LC, et al. Inside the Pan-genome - Methods and software overview. Curr Genomics. 2015;16:245–252. doi: 10.2174/1389202916666150423002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapierre P, Gogarten JP. Estimating the size of the bacterial pan-genome. Trends Genet. 2009;25:107–110. doi: 10.1016/j.tig.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Daubin V, Ochman H. Bacterial genomes as new gene homes: the genealogy of ORFans in E. coli. Genome Res. 2004;14:1036–1042. doi: 10.1101/gr.2231904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han MS, et al. Relative Prevalence and antimicrobial susceptibility of clinical isolates of Elizabethkingia species based on 16S rRNA gene sequencing. J Clin Microbiol. 2017;55:274–280. doi: 10.1128/JCM.01637-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The species, strains, and GenBank accession numbers of microorganisms for 16S rRNA gene analysis are listed in Supplementary Table S1. The names of organisms, strains, biosample numbers, bioproject numbers, assembly numbers, isolated origins, and release dates of bacteria used in this study are shown in Supplementary Table S2. All data are available in the NCBI genome sequence repository.