Abstract
Seven pairs of new oxygenated aplysinopsin-type enantiomers, (+)- and (−)-oxoaplysinopsins A‒G (1‒7), two new bromotyrosine-derived alkaloids, subereamollines C and D (18 and 19), together with ten known compounds (8‒17) were isolated from the Xisha Islands sponge Fascaplysinopsis reticulata. The planar structures were determined by extensive NMR and MS spectroscopic data. Each of the optically pure enantiomers was achieved by chiral HPLC separation. The absolute configurations were assigned by the quantum chemical calculation methods. Compound 19 showed cytotoxicity against Jurkat cell lines with IC50 value of 0.88 μM. Compounds 2, 16 and 17 showed tyrosine phosphatase 1B (PTP1B) inhibition activity with IC50 value ranging from 7.67 to 26.5 μM, stronger than the positive control of acarbose and 1-deoxynojirimycin. A structural activity relationship for the aplysinopsin-type enantiomers were observed in PTP1B inhibition activity of 2 and cytotoxicity of 3 that the dextrorotary (+)-2 and (+)-3 showed stronger activity than the levorotary (−)-2 and (−)-3.
Introduction
Sponge of the genus Fascaplysinopsis is special in taxonomy that it is a rare monotypic sponge genus containing only one species F. reticulata which was originally identified as Aplysinopsis reticulata in 1912 and revised to F. reticulata in 19801. Fascaplysinopsis is important resource of marine natural products2–6 that there were more than 60 compounds been isolated since the typical aplysinopsin firstly found in 19777–12. Aplysinopsins are a class of indole alkaloids structurally architected by an indole and an imidazole moieties which showed rich structural diversity characterized by N3′-methylaplysinopsin13, brominated derivatives14, oxoforms15, and dimmeric forms16. Up to date, there are totally 30 aplysinopsins isolated, showing a diverse origin including sponge genera of Dercitus17, Smenospongia18, Verongula19 et al. as well as corals of Tubastrea20, Dendrophyllia21 and mollusc of Phestilla22. Aplysinopsins have shown pharmaceutical significance with neuromodulation, antineoplastic, antiplasmodial, and antimicrobial activities11. The geographic locations of these aplysinopsin-origin organisms are mostly focusing on Caribbean, Mediterranean Sea, as well as Indo-Pacific region.
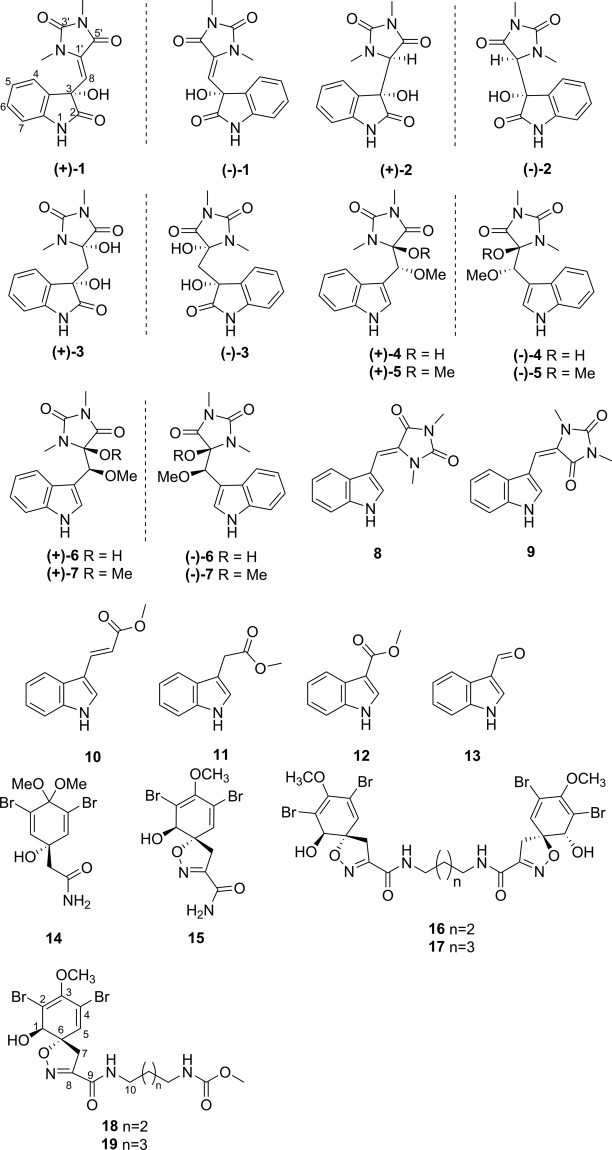
Our first investigation on XiSha Islands (Paracel Islands) F. reticulata has yielded (+)- and (−)-spiroreticulatine, a pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids in previous study23. A further study on this species yielded eighteen compounds, including seven pairs of new oxygenated aplysinopsin-type enantiomers, (+)- and (−)-oxoaplysinopsins A‒G (1‒7), two new bromotyrosine-derived alkaloids, subereamollines C and D (18 and 19), together with ten known related compounds (8‒17) (Fig. 1). The enantiomers were purified by chiral HPLC method. And all the absolute configurations were determined by comparing experimental and calculated ECD using quantum chemical calculation method. The cytotoxicity against selected tumor cell lines and tyrosine phosphatase 1B (PTP1B) inhibition activity of the isolates were assayed. Herein we report the isolation, structural elucidation, and biological activities of these compounds.
Figure 1.
Structures of 1–19 from sponge Fascaplysinopsis reticulata.
Results and Discussion
Oxoaplysinopsin A (1) was obtained as yellow, amorphous powder, possessing a molecular formula of C14H13N3O4 with 10 degrees of unsaturation as informed by its HRESIMS data. The IR spectrum suggested the presence of carbonyl (1702 cm−1) group and aromatic moiety (1603, 1497, 1449 cm−1). The 1H NMR spectrum of 1 (Table 1) displayed indole signals characterized by an imino proton (δH 10.30, br s) and four aromatic protons in an ABCD coupling system (δH 6.80, d, J = 7.6 Hz; 6.88, dd, J = 7.5, 7.5 Hz; 7.16, d, J = 6.7 Hz; and 7.18, dd, J = 7.7, 6.2 Hz)18. Besieds, an olefinic proton (δH 5.80, s), and two nitrogen-bearing methyl protons (δH 2.83, 3.04), as well as a hydroxyl proton (δH 6.85, s) were observed. The 13C NMR and DEPT spectra of 1 (Table 1) exhibited totally 14 carbon resonances which were divided into two methyls (δC 26.1, 24.4), five olefinic methines (δC 109.4, 117.5, 121.4, 123.9, and 129.2), and seven quaternary carbons including one oxygen-bearing (δC 74.9), three olefinic (δC 130.4, 132.1, 143.0), and three amide carbonyls (δC 153.3, 161.3, and 176.2). The 1D NMR data suggested compound 1 as aplysinopsin analogue of 3′-deimino-3′-oxoaplysinopsin13.
Table 1.
1H (500 MHz) and 13C NMR (125 MHz) Data for 1–3 and 6 in DMSO-d6.
| No | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |
| 1 | 10.30, br s | 10.43, br s | 10.30, br s | |||
| 2 | 176.2, C | 175.3, C | 177.9, C | |||
| 3 | 74.9, C | 76.2, C | 72.3, C | |||
| 3a | 132.1, C | 126.9, C | 128.6, C | |||
| 4 | 123.9, CH | 7.16, d, 1 H (6.7) | 124.1, CH | 7.08, d, 1 H (7.5) | 126.3, CH | 7.04, d, 1 H (7.4) |
| 5 | 121.4, CH | 6.88, dd, 1 H (7.5, 7.5) | 121.5, CH | 6.90, dd, 1 H (7.5, 7.5) | 120.9, CH | 6.88, dd, 1 H (7.5, 7.5) |
| 6 | 129.2, CH | 7.18, dd, 1 H (7.7, 6.2) | 130.1, CH | 7.22, dd, 1 H (7.7, 7.7) | 129.4, CH | 7.13, dd, 1 H (7.5, 7.6) |
| 7 | 109.4, CH | 6.80, d, 1 H (7.6) | 109.9, CH | 6.78, d, 1 H (7.7) | 109.2, CH | 6.70, d, 1 H (7.5) |
| 7a | 143.0, C | 142.6, C | 142.3, C | |||
| 8 | 117.5, CH | 5.80, s, 1 H | 41.7, CH2 | 2.57, s, 2 H | ||
| 1′ | 130.4, C | 66.1, CH | 4.40, s, 1 H | 83.4, C | ||
| 3′ | 153.3, C | 157.2, C | 155.6, C | |||
| 5′ | 161.3, C | 168.6, C | 172.3, C | |||
| 2′-NCH3 | 26.1, CH3 | 3.04, s, 3 H | 31.3, CH3 | 3.14, s, 3 H | 24.4, CH3 | 2.55, s, 3 H |
| 4′-NCH3 | 24.4, CH3 | 2.83, s, 3 H | 24.2, CH3 | 2.50, s, 3 H | 23.7, CH3 | 2.19, s, 3 H |
| 3-OH | 6.85, s | 6.62, s | 6.70, s | |||
| 1′-OH | 6.00, s | |||||
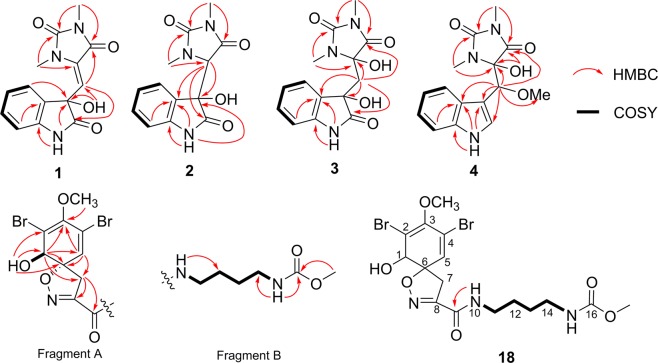
A consecutive 1H-1H COSY correlation from H-4 to H-7, together with the HMBC correlations from H-4 to C-3 (δC 74.9), and from 1-NH to C-3, C-3a (δC 132.1) and C-7a (δC 143.0) confirmed the indole moiety (Fig. 2). The HMBC correlations from NMe (δH 3.04) to C-1′ (δC 130.4) and C-3′ (δC 153.3), from the other NMe (δH 2.83) to C-3′ and C-5′ (δC 161.3) constructed the 1, 3-dimethyl-imidazolidin-2, 4-dione moiety. The residual hydroxyl group was located at C-3 evident from HMBC correlations from the hydroxyl proton to C-2 (δC 176.2), C-3, C-3a and C-8. The additional HMBC correlations from H-8 (δH 5.80) to C-2, C-3, C-1′ and C-5′ indicated that the imidazolidin and indole moieties were connected through sp2 methine C-8. Thus, the planar structure of 1 was elucidated as shown.
Figure 2.
Key COSY and HMBC correlations in compounds 1–4, and 18.
Oxoaplysinopsin B (2) was isolated as a yellow, amorphous powder. HRESIMS implied its molecular formula of C13H13N3O4, 12 atomic mass less than that of compound 1. The spectroscopic data of 2 were similar to those of compound 1 except for the disappeared methine signal of CH-8 and extra N-bearing CH-1′ (δH 4.40 and δC 66.1). HMBC correlations from H-1′ (δH 4.40) to C-2 (δC 175.3), C-3 (δC 76.2), C-3a (δC 126.9), C-3′ (δC 157.2), and C-5′ (δC 121.5), and from NMe (δH 3.14) to C-1′ (Fig. 2), suggested a direct connection of 2-oxoindole and 1, 3-dimethylimidazolidin-2, 4-dione moieties in compound 2.
Oxoaplysinopsin C (3) had molecular formula of C14H15N3O5 by HRESIMS data. The main difference of 1D NMR between compounds 3 and 1 was that a methylene signals (δH 2.57 and δC 41.7) was observed in 3 intend of olefinic methine in 1. HMBC correlations from the methylene H2-8 (δH 2.57) to C-2 (δC 177.9), C-3 (δC 72.3), C-3a (δC 128.6), C-1′ (δC 83.4), and C-5′ (δC 172.3) (Fig. 2), suggested that compound 3 was a hydroxylated product of 1.
Oxoaplysinopsins D (4) had molecular formula of C15H17N3O4 from HRESIMS data, 14 atomic mass more than that of 3. Analysis of its 1H and 13C NMR spectra (Table 2) disclosed that 4 was very similar to 3 except for an extra methoxyl group (δH/δC 3.20/57.3) and olefinic CH (δH/δC 7.15/124.6) instead of the carbonyl group of C-2 in 3. HMBC correlations (Fig. 2) from OMe group to C-8 (δC 78.7), and from H-8 (δH 4.88) to C-2 (δC 124.6), C-3 (δC 108.5), C-3a (δC 127.0), C-1′ (δC 87.4), C-5′ (δC 171.8), and OMe indicated that the structure of compound 4 was shown as depicted. Oxoaplysinopsins E (6) had the same molecular formula with that of 4. And their 1D NMR data were very similar except for slight differences around the chiral center of C-8 and C-1′. COSY and HMBC data of 6 indicated that 6 had the same planar structure with 4, indicating that they were epimers.
Table 2.
1H (500 MHz) and 13C NMR (125 MHz) Data for 4–5 in DMSO-d6.
| No | 4 | 5 | 6 | 7 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |
| 1 | 11.13, br s | 11.18, br s | 11.17, br s | 11.20, br s | ||||
| 2 | 124.6, CH | 7.15, d, 1 H (2.3) | 124.7, CH | 7.17, d, 1 H (2.2) | 125.3, CH | 7.28, s, 1 H | 125.8, C | 7.29, s |
| 3 | 108.5, C | 107.9, C | 108.3, C | 108.4, C | ||||
| 3a | 127.0, C | 127.0, C | 126.5, C | 126.8, C | ||||
| 4 | 119.0, CH | 7.54, d, 1 H (8.0) | 118.9, CH | 7.53, d, 1 H (8.0) | 120.2, CH | 7.62, d, 1 H (8.0) | 120.4, CH | 7.58, d, 1 H (8.0) |
| 5 | 118.7, CH | 6.97, dd, 1 H (8.0, 8.0) | 118.8, CH | 6.97, dd, 1 H (7.8, 7.2) | 118.9, CH | 6.98, dd, 1 H (7.5, 7.5) | 119.5, CH | 7.00, dd, 1 H (7.3, 7.7) |
| 6 | 120.9, CH | 7.05, dd, 1 H (7.2, 7.8) | 120.9, CH | 7.06, dd, 1 H (7.2, 7.9) | 121.0, CH | 7.07, dd, 1 H (7.3, 7.7) | 121.5, CH | 7.08, dd, 1 H (7.2, 7.9) |
| 7 | 111.4, CH | 7.34, d, 1 H (8.1) | 111.4, CH | 7.35, d, 1 H (8.1) | 111.5, CH | 7.36, d, 1 H (8.1) | 112.1, CH | 7.37, d, 1 H (8.1) |
| 7a | 135.7, C | 135.7, C | 136.3, C | 136.8, C | ||||
| 8 | 78.7, CH | 4.88, s, 1 H | 77.9, CH | 4.94, s, 1 H | 79.2, CH | 4.78, s, 1 H | 79.4, CH | 4.85, s, 1 H |
| 1′ | 87.4, C | 92.4, C | 86.8, C | 92.3, C | ||||
| 3′ | 155.6, C | 155.6, C | 156.6, C | 156.6, C | ||||
| 5′ | 171.8, C | 169.2, C | 173.7, C | 171.4, C | ||||
| 2′-NCH3 | 25.5, CH3 | 3.00, s, 3 H | 25.6, CH3 | 3.02, s, 3 H | 25.6, CH3 | 2.22, s, 3 H | 26.1, CH3 | 2.33, s, 3 H |
| 4′-NCH3 | 23.9, CH3 | 2.54, s, 3 H | 23.9, CH3 | 2.60, s, 3 H | 24.2, CH3 | 2.86, s, 3 H | 24.7, CH3 | 2.90, s, 3 H |
| 8-OCH3 | 57.3, CH3 | 3.20, s, 3 H | 57.1, CH3 | 3.19, s, 3 H | 57.0, CH3 | 3.18, s, 3 H | 57.6, CH3 | 3.19, s, 3 H |
| 1′-OH | 6.96, s | 6.94, s | ||||||
| 1′-OCH3 | 51.3, CH3 | 3.03, s, 3 H | 51.8, CH3 | 3.01, s, 3 H | ||||
Oxoaplysinopsin F and G (5 and 7) had the same molecular of C16H19N3O4, 14 atomic mass more than those of compounds 4 and 6. An extra methoxyl group at δH/δC 3.03/51.3 in 5 and δH/δC 3.01/51.8 in 7 suggested compounds 5 and 7 as 1′-methylated product of 4 and 6, which was confirmed by HMBC correlations from OMe to C-1′. And the empimeric relationship between 6 and 7 were also shown by the slight differences around chiral centers of C-8 and C-1′.
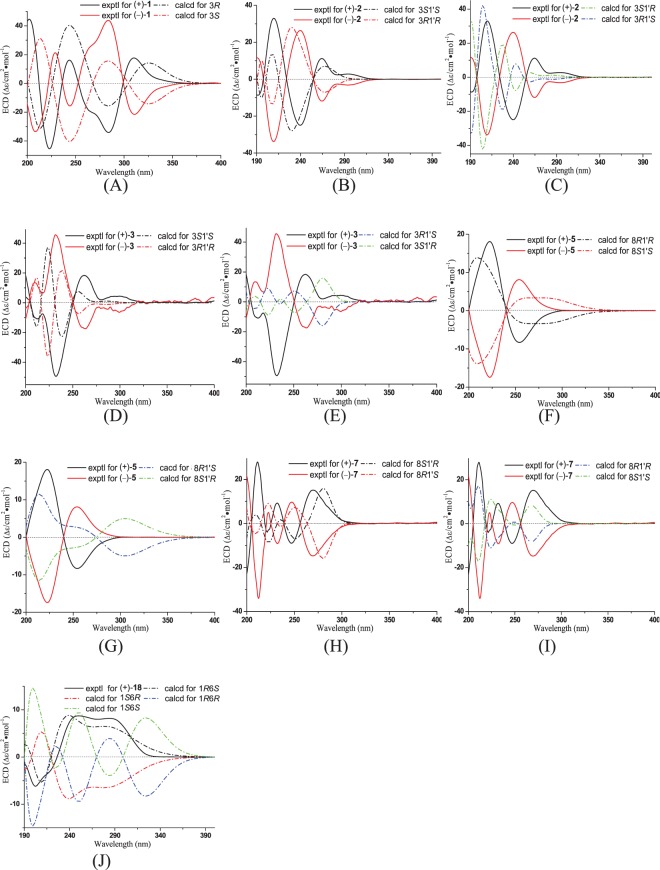
Compounds 1–7 were initially obtained as optical inactivity compounds, indicating that they were enantiomers. Chiral HPLC purification afforded seven pairs of enantiomers, (+)- and (−)-1‒(+)- and (−)-7, in a ratio of almost 1:1 (Supporting Information). The opposite optical rotation values and mirror ECD spectra for the dextrorotary and levorotary enantiomers were observed (Fig. 3). To determine their absolute configurations, ECD calculation for respective (+)- and (−)-isomers of compounds 1–7 were performed by the TDDFT/ECD method at RB3LYP/DGDZVP level (Supporting Information)23,24. The experimental ECD of (+)-1 exhibited two strong positive Cotton effect (CEs) at 242.5 and 309.0 nm and two strong negative CEs at 222.5 and 284.5 nm, in agreement with the calculated ECD spectrum for 3R configuration (Fig. 3), and showed mirror-like relationship with calculated and experimental ECD spectra for 3S configuration. Therefore, 3R and 3S were finally assigned for (+)-1 and (−)-1, respectively. Similarly, the absolute configurations of 3S, 1′S for (+)-2, 3R, 1′R for (−)-2, 3R, 1′R for (+)-3, 3S, 1′S for (−)-3, 8R, 1′R for (+)-5, 8S, 1′S for (−)-5, 8S, 1′R for (+)-7, and 8R, 1′S for (−)-7 were assigned (Fig. 3). And compounds (+)-4 and(−)-4, and (+)-6 and (−)-6 showed similar Cotton effects as respective (+)-5 and(−)-5, and (+)-7 and (−)-7 (Supporting Information), indicating that they possessed the same absolute configuration.
Figure 3.
(A) Experimental ECD spectra of (+)- and (−)-1 in MeOH and calculated ECD spectra of (3R)-1 and (3S)-1 (half width 0.3; UV-shift 5 nm). (B) Experimental ECD spectra of (+)- and (−)-2 in MeOH and calculated ECD spectra of (3R, 1′R)-2 and (3S, 1′S)-2 (half width 0.24; UV-shift -18 nm).(C) Experimental ECD spectra of (+)- and (−)-2 in MeOH and calculated ECD spectra of (3R, 1′S)-2 and (3S, 1′R)-2 (half width 0.24; UV-shift 0 nm). (D) Experimental ECD spectra of (+)- and (−)-3 in MeOH and calculated ECD spectra of (3R, 1′R)-3 and (3S, 1′S)-3 (half width 0.2; UV-shift 10 nm). (E) Experimental ECD spectra of (+)- and (−)-3 in MeOH and calculated ECD spectra of (3R, 1′S)-3 and (3S, 1′R)-3 (half width 0.2; UV-shift 10 nm). (F) Experimental ECD spectra of (+)- and (−)-5 in MeOH and calculated ECD spectra of (8R, 1′R)-5 and (8S, 1′S)-5 (half width 0.44; UV-shift 0 nm). (G) Experimental ECD spectra of (+)- and (−)-5 in MeOH and calculated ECD spectra of (8R, 1′S)-5 and (8S, 1′R)-5 (half width 0.44; UV-shift 0 nm).(H) Experimental ECD spectra of (+)- and (−)-7 in MeOH and calculated ECD spectra of (8S, 1′R)-7 and (8R, 1′S)-7 (half width 0.16; UV-shift -20 nm). (I) Experimental ECD spectra of (+)- and (−)-7 in MeOH and calculated ECD spectra of (8R, 1′R)-7 and (8S, 1′S)-7 (half width 0.16; UV-shift -20 nm). (J) Experimental ECD spectra of 18 in MeOH and calculated ECD spectra of (1R, 6′S)-18a,(1S, 6′R)-18a,(1R, 6′R)-18a, and (1S, 6′S)-18a (half width 0.5; UV-shift: -13 nm).
Subereamolline C (18) was isolated as a white, amorphous powder. The HRESIMS spectrum showed three quasi-molecular ion peaks (m/z 509.9869, 511.9846, 513.9825) in a ratio of 1:2:1, indicating that compound 18 was a dibrominated product possessing molecular formula of C16H21Br2N3O6 with 7 degrees of unsaturation. 13C NMR and DEPT spectra of 18 (Table 3) exhibited a total of 16 carbon resonances which were divided into two methoxys (δC 59.6, 51.1), five methylenes (δC 39.9, 39.4, 38.5, 26.9 and 26.2), two methines (δC 131.3, 73.6) and seven quaternary carbons (δC 158.8, 156.7, 154.5, 147.1, 120.8, 113.1, 90.1), which was similar with those of brominated phenolic compound subereaphenol A isolated from a methanol extract of Red Sea Sponge Suberea mollis25, except for the absence of oxygenated methylene in 18. HMBC correlation from 16-OCH3 (δH 3.50) to C-16 (δC 156.7) suggested the terminal methyl ester in 18 rather than the ethyl ester in subereaphenol A. Detailed analysis of COSY and HMBC data (Fig. 2) allowed the planar structure of 18 determined. The absolute configuration of 18 was suggested to be the same as subereaphenol A by comparing their optical rotation values, which was confirmed by theoretical ECD calculation of all the four candidates of 1R, 6S and 1R, 6R and their enantiomers of 18a (Supporting Information)26. The calculated CEs of (1R, 6S)-18a matched well with the experimental CEs of 18 and were image symmetrical to (1S, 6R)-18a, while they were totally different from those of (1R, 6R)-18a and (1S, 6S)-18a (Fig. 3). That allowed the absolute configuration of 18 determined as 1R, 6S.
Table 3.
1H (500 MHz) and 13C NMR (125 MHz) Data for 18 and 19 in DMSO-d6.
| No | 18 | 19 | ||
|---|---|---|---|---|
| δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |
| 1 | 73.6, CH | 3.91, d, 1 H (5.6) | 73.6, CH | 3.91, d, 1 H (5.6) |
| 2 | 113.1, C | 113.1, C | ||
| 3 | 147.1, C | 147.1, C | ||
| 4 | 120.8, C | 120.8, C | ||
| 5 | 131.3, CH | 6.58, s, 1 H | 131.3, CH | 6.59, s, 1 H |
| 6 | 90.1, C | 90.1, C | ||
| 7 | 39.4, CH2 | 3.61, d, 1 H (18.2); 3.20 d, 1 H (18.2) | 39.4, CH2 | 3.61, d, 1 H (18.3); 3.21, d, 1 H (18.3) |
| 8 | 154.5, C | 154.1, C | ||
| 9 | 158.8, C | 158.8, C | ||
| 10 | 8.49, t (5.7, 5.8) | 8.52, t (5.5, 5.6) | ||
| 11 | 38.5, CH2 | 3.13, m, 2 H | 38.7, CH2 | 3.11, m, 2 H |
| 12 | 26.2, CH2 | 1.43, m, 2 H | 29.1, CH2 | 1.44, m, 2 H |
| 13 | 26.9, CH2 | 1.38, m, 2 H | 23.6, CH2 | 1.23, m, 2 H |
| 14 | 39.9, CH2 | 2.96, m, 2 H | 28.5, CH2 | 1.38, m, 2 H |
| 15 | 7.08, t (4.9, 4.9) | 40.1, CH2 | 2.94, m, 2 H | |
| 16 | 156.7, C | 7.11, t (5.5, 5.5) | ||
| 17 | 156.7, C | |||
| 1-OH | 6.36, d (7.1) | 6.39, d (7.1) | ||
| 3-OCH3 | 59.6, CH3 | 3.65, s, 3 H | 59.6, CH3 | 3.64, s, 3 H |
| 16-OCH3 | 51.1, CH3 | 3.50, s, 3 H | ||
| 17-OCH3 | 51.5, CH3 | 3.50, s, 3 H | ||
Subereamolline D (19) possessed the molecular formula of C17H23Br2N3O6 by HRESIMS, 14 atomic mass more than that of compound 18. The 1D NMR data of compound 19 were very similar with those of 18 except for an extra CH2 group (δH 1.23, δC 23.6) in 19. 1H-1H COSY correlations of NH-10/H2-11/H2-12/H2-13/H2-14/NH-15, together with HMBC correlations from OMe, NH-15 and H2-14 to C-16 suggested methyl (5-aminopentyl)carbamate moiety in 19 rather than the methyl (4-aminobutyl)carbamate moiety in 18. And the absolute configuration of 19 was determined the same as 18 by comparing their optical rotation value and NMR data.
On comparison of the physical and spectroscopic data with published values, the known compounds were identified as (Z)-3′-deimino-3′-oxoaplysinopsin (8)15, (E)-3′-deimino-3′-oxoaplysinopsin (9)15, (E)-3-indolylpropenoate (10)27, indolyl-3-acetic acid methyl ester (11)28, 3-methoxycarbonylindole (12)29, 3-formylindole (13)30, 3, 5-dibromoverongiaquinol dimethyl ketal (14)31, purealidin R (15)31, aerothionin (16)32, and homoaerothionin (17)25.
The cytotoxicity against human lung carcinoma (A549), human cervical cancer (HeLa), human leukemia (K562), and human T-cell leukemia (Jurkat) cell lines, as well as tyrosine phosphatase 1B (PTP1B) inhibition activity of the isolates were assayed. Compound 19 showed cytotoxicity against Jurkat cell lines with IC50 value of 0.88 μM, comparable to the positive control of Doxorubicin (IC50 = 0.442 μM). Compounds 16 and 17 showed tyrosine phosphatase 1B (PTP1B) inhibition activity with IC50 value of 7.67 and 11.25 μM, respectively, stronger than the positive control of acarbose (457 μg/mL) and 1-deoxynojirimycin (31.29 μg/mL). The racemate (±)-2 (20.8 μM) and optically pure (+)-2 (18.3 μM) and (−)-2 (26.5 μM) showed a little high IC50 value in inhibition of PTP1B but were still stronger than the positive controls. The previous study showed a preliminary structure-activity relationship of the significant role of the substations in the benzene and imidazole moieties11. The present study suggested oxygen pattern rather than methylation as another contribution for bioactivity of aplysinopsins. In addition, the dextrorotary (+)-2 showed stronger activity than the levorotary enantiomers (−)-2. This firstly encountered structural activity relationship for the aplysinopsin-type enantiomers were also observed in cytotoxicity assay of compound 3 against HeLa cell lines that the dextrorotary (+)-3 had IC50 value of 27.0 μM, two fold of the levorotary enantiomers (−)-3 with IC50 value of 61.6 μM.
In summary, nine aplysinopsin-type alkaloids, nine indole analogues, and six bromotyrosine-derived alkaloids were isolated from the Xisha Islands sponge Fascaplysinopsis reticulata. Seven new aplysinopsin-type alkaloids (1‒7) and two new bromotyrosine-derived alkaloids (18 and 19) were identified by comprehensive using of NMR, MS, and quantum chemical calculation methods. Although the first and the only one aplysinopsin type indole alkaloid was isolated from F.reticulata, in previous study the dimmeric indole alkaloids fascaplysin were suggested as the main metabolites in Fascaplysinopsis sponge7–10. Furthermore, the konwn oxygenated aplysinopsin mainly focused on 3′-oxoaplysinopsin including 3′-deimino-3′-oxoaplysinopsin, and 3′-deimino-2′, 4′-bis(demethyl)-3′-oxoaplysinopsin, as well as their brominated analogues7–10. In the present study, series of 3, 8-oxoaplysinopsins (1‒7) were firstly encountered in F. reticulata. Besides, bromotyrosine-derived alkaloids were reported previously as a kind of characteristic structures solely isolated from the sponge of Verongida order8,33–35, and were recently obtained through culturing sponge Arenosclera brasiliensis derived bacterium Pseudovibrio denitrificans26. And there were totally no more than 30 bromotyrosine-derived alkaloids found in nature8. We obtained six bromotyrosine-derived alkaloids (14‒19) from the sponge F. reticulata. The result indicated a significant chemical diversity in F. reticulata which is possibly attributed to the special geography of XiSha Islands.
The series of aplysinopsin enantiomers inspired again the biosynthetic enantiodivergence evidence in natural36,37. Sponges were suggested to be potentially biosynthetic enantiodivergence, since more and more enantiomers such as purealidin R from Psammaplysilla38, plakortolides H and I from Plakortis39, and strongylodiols A–C from Petrosia (Strongylophora)40, as well as corynechromones from Sponge-Derived Strain of the Fungus Corynespora cassiicola41, and DD- and LL-diketopiperazines from respective Calyx sponge derived Pecten maximus and Isodictya sponge derived Pseudomonas aeruginosa42,43, were isolated. In present study, the firstly encountered versatile enantiomeric 3-oxoaplysinopsin (1–3), 1′-oxoaplysinopsin (3–7), or 8-oxoaplysinopsin (4–7) showed remarkable stereochemistry diversity in oxoaplysinopsins which are possibly originated from (Z)- or (E)-3′-deimino-3′-oxoaplysinopsin (8/9). That indicated a potential enantiodivergence in F. reticulata which could be unveiled through the biosynthesis and symbiot study in future.
Experimental Section
General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 digital polarimeter. UV spectra were recorded on a Beckman DU640 spectrophotometer. CD spectra were obtained on a JASCO J-810 spectropolarimeter. IR spectra were taken on a Nicolet NEXUS 470 spectrophotometer in KBr discs. NMR spectra were measured by Bruker AVANCE III 600 spectrometers. The 2.5000 ppm and 39.50 ppm resonances of DMSO were used as internal references for 1H and 13C NMR spectra, respectively. HRESIMS spectra were measured on a Micromass Q-Tof Ultima GLOBAL GAA076LC and Thermo Scientific LTQ orbitrap XL mass spectrometers. Semi-preparative HPLC utilized an ODS column [YYMC-Pack ODS-A, 100 × 250 mm, 5 µm, 1.5 mL/min]. Chiral HPLC utilized chiral analytical columns [CHIRALPAK IC column (4.6 × 250 mm, 5 µm)]. Silica gel (200–300 mesh, Qingdao, China) was used for column chromatography, and precoated Silica gel plates (GF254, Qingdao, China) were used for TLC, and spots visualized by heating SiO2 plates sprayed with 5% H2SO4 in EtOH.
Animal Material
The marine sponge Fascaplysinopsis reticulata was collected from Xisha Island of South China Sea in December 2009, and was frozen immediately after collection. The specimen was identified by Nicole J. de Voogd, National Museum of Natural History, Leiden, The Netherlands. The voucher specimen (No. XS-2009-29) was deposited at State Key Laboratory of Marine Drugs, Ocean University of China, P. R. China.
Extraction and isolation
A frozen specimen of Fascaplysinopsis reticulata (1.6 kg, wet weight) was homogenized and then extracted with MeOH four times (3 days each time) at RT. The combined solutions were concentrated in vacuo and was then subsequently desalted by redissolving with MeOH to yield a residue (69 g). The crude extract was subjected to silica gel vacuum liquid chromatography (VLC), eluting with a gradient of petroleum/acetone (from 10:0 to 1:1, v:v) and subsequently CH2Cl2/MeOH (from 20:1 to 0:1, v-v) to obtain eight fractions (Fr.1–Fr.10). Fr.3 (6.3 g) was then subjected to a silica gel CC (petroleum/ethyl acetate, from 50:1 to 1:1, v:v) to give twelve subfractions Fr.3-1–Fr.3-12. Fr.3-12 (449.0 mg) was also subjected to a silica gel CC (petroleum/acetone, from 10:1 to 1:1, v:v) to give eight subfractions Fr.3-12-1–Fr.3-12-8. Fr.3-12-7 (53 mg) was purified by semi-preparative HPLC (ODS, 5 µm, 250 × 10 mm; MeOH/H2O, 40:60, v/v; 1.5 mL/min) to afford 6 (6.1 mg) and 7 (6.0 mg). Fr.3-12-8 (37 mg) was also purified by semi-preparative HPLC (ODS, 5 µm, 250 × 10 mm; MeOH/H2O, 40:60, v/v; 1.5 mL/min) to afford 1 (1.9 mg) and 2 (1.8 mg). Fr.4 (7.0 g) was subjected to a silica gel CC (petroleum/ethyl acetate, from 20:1 to 1:1, v:v) to give twelve fractions Fr.4-1–Fr.4-12. Fr.4-10 (296.8 mg) was purified by semi-preparative HPLC (ODS, 5 µm, 250 × 10 mm; MeOH/H2O, 55:45, v/v; 1.5 mL/min) to afford 4 (8.8 mg), 5 (12.6 mg), 11 (5.5 mg), 12 (6.0 mg) and 13 (2.8 mg). Fr.5 (5.5 g) was subjected to a silica gel CC (petroleum/ethyl acetate, from 10:1 to 1:1, v:v) to give twelve fractions Fr.5-1–Fr.5-10. Fr.5-8 (693.7 mg) was also subjected to a silica gel CC (petroleum/acetone, from 20:1 to 1:1, v:v) to give seven fractions Fr.5-8-1–Fr.5-8-7. Fr.5-8-5 (77 mg) was purified by semi-preparative HPLC (ODS, 5 µm, 250 × 10 mm; MeOH/H2O, 30:70, v/v; 1.5 mL/min) to afford 3 (4.0 mg). Fr.6 (20 g) was subjected to a silica gel CC (petroleum/ethyl acetate, from 10:1 to 1:1, v:v) to give six fractions Fr.6-1–Fr.6-6. Fr.6-2 (5.2 g) was also subjected to a silica gel CC (petroleum/acetone, from 20:1 to 1:1, v:v) to give five fractions Fr.6-2-1–Fr.6-2-5. Fr.6-2-4 (456 mg) was purified by semi-preparative HPLC (ODS, 5 µm, 250 × 10 mm; MeOH/H2O, 40:70, v/v; 1.5 mL/min) to afford 8 (4.0 mg), 9 (3.0 mg) and 10 (6.3 mg). Fr.6-2-5 (875 mg) was purified by semi-preparative HPLC (ODS, 5 µm, 250 × 10 mm; MeOH/H2O, 30:70, v/v; 1.5 mL/min) to afford 14 (19.0 mg), 15 (38.0 mg), 16 (20.0 mg), 17 (36.3 mg), 18 (11.2 mg) and 19 (10.0 mg).
Cytotoxicity assay
The cytotoxicity assay of the isolated compounds were evaluated against human lung carcinoma (A549), human cervical cancer (HeLa), human leukemia (K562), and human T-cell leukemia (Jurkat) cell lines using the MTT method with Doxorubicin as the positive control44.
Tyrosine phosphatase 1B (PTP1B) inhibition activity assay
The antidiabetic activities were evaluated on the inhibition of tyrosine phosphatase 1B (PTP1B) protein which is recently of substantial interest for the treatment of type-2 diabetes mellitus45,46. 1-Deoxynojirimycin and Acarbose were used as positive controls.
Oxoaplysinopsin A (1)
yellow, amorphous powder; 1H and 13C NMR data, see Table 1; UV (MeOH) λmax (log ε): 213 (3.74), 237 (3.87), 295 (3.90) nm; IR (KBr) νmax: 3360, 1702, 1603, 1497, 1449, 1163 cm−1; HRESIMS m/z 310.0795 [M + Na]+ (calcd for C14H13N3O4Na: 310.0798). (+)-1: [α]20D = +28.3 (c 0.2, MeOH); (−)-1: [α]20D = −27.9 (c 0.2, MeOH).
Oxoaplysinopsin B (2)
Yellow, amorphous powder; 1H and 13C NMR data, see Table 1; UV (MeOH) λmax (log ε): 210 (4.02), 245 (4.01), 290 (3.99) nm; IR (KBr) νmax: 3619, 1754, 1680, 1497, 1449, 1007 cm−1; HRESIMS m/z 298.0790 [M + Na]+ (calcd for C13H13N3O4Na: 298.0798). (+)-2: [α]20D = +4.5 (c 0.2, MeOH); (−)-2: [α]20D = −3.3 (c 0.2, MeOH).
Oxoaplysinopsin C (3)
Yellow, amorphous powder; 1H and 13C NMR data, see Table 1; UV (MeOH) λmax (log ε): 211 (3.89), 250 (3.85), 295 (3.92) nm; IR (KBr) νmax: 3357, 2980, 1708, 1653, 1601, 1524, 1019 cm−1; HRESIMS m/z 328.0906 [M + Na]+ (calcd for C14H15N3O5Na: 328.0904). (+)-3: [α]20D = +5.6 (c 0.2, MeOH); (−)-3: [α]20D = −5.1 (c 0.2, MeOH).
Oxoaplysinopsin D (4)
White, amorphous powder; 1H and 13C NMR data, see Table 2; UV (MeOH) λmax (log ε): 212 (3.95), 278 (4.00) nm; IR (KBr) νmax: 3213, 1697, 1643, 1597, 1502, 1148 cm−1; HRESIMS m/z 326.1110 [M + Na]+ (calcd for C15H17N3O4Na: 326.1111). (+)-4: [α]20D = +7.4 (c 0.2, MeOH); (−)-4: [α]20D = −6.8 (c 0.2, MeOH).
Oxoaplysinopsin E (5)
White, amorphous powder; 1H and 13C NMR data, see Table 2; UV (MeOH) λmax (log ε): 215 (4.01), 270 (3.84) nm; IR (KBr) νmax: 1705, 1655, 1603, 1502, 1068 cm−1; HRESIMS m/z 340.1266 [M + Na]+ (calcd for C16H19N3O4Na: 340.1268). (+)-5: [α]20D = +6.4 (c 0.2, MeOH); (−)-5: [α]20D = −6.1 (c 0.2, MeOH).
Oxoaplysinopsin F (6)
White, amorphous powder; 1H and 13C NMR data, see Table 2; UV (MeOH) λmax (log ε): 216 (3.95), 270 (3.85) nm; IR (KBr) νmax: 3314, 1698, 1650, 1591, 1502, 1106 cm−1; HRESIMS m/z 326.1110 [M + Na]+ (calcd for C15H17N3O4Na: 326.1111). (+)-6: +10 (c 0.1, MeOH); (+)-6: −10.4 (c 0.1, MeOH).
Oxoaplysinopsin G (7)
White, amorphous powder; 1H and 13C NMR data, see Table 2; UV (MeOH) λmax (log ε): 215 (3.88), 270 (3.90) nm; IR (KBr) νmax: 1709, 1664, 1616, 1493, 1103 cm−1; HRESIMS m/z 340.1266 [M + Na]+ (calcd for C16H19N3O4Na: 340.1268). (+)-7: [α]20D = +19.2 (c 0.2, MeOH); (+)-7: [α]20D = −18.8 (c 0.2, MeOH).
Subereamolline C (18)
White, amorphous powder; [α]20D = +189.1 (c 0.2, MeOH); 1H and 13C NMR data, see Table 3; UV (MeOH) λmax (log ε): 280 (3.77), 230 (3.91), 205 (3.96) nm; HRESIMS m/z 511.9846 [M + H]+ (calcd for C16H22N3O6Br81Br: 511.9849).
Subereamolline D (19)
White, amorphous powder; [α]20D = +145.0 (c 0.2, MeOH); 1H and 13C NMR data, see Table 3; UV (MeOH) λmax (log ε): 281 (3.55), 236 (3.89), 211 (3.94) nm; HRESIMS m/z 526.0000 [M + H]+ (calcd for C17H24N3O6Br81Br: 526.0006).
Supplementary information
Aplysinopsin-type and Bromotyrosine-derived Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21572210 and 41522605), NSFC-Shandong Joint Fund for Marine Science Research Centers (Grant No. U1606403), AoShan Talents Program Supported by Qingdao National Laboratory for Marine Science and Technology (No. 2015ASTP). Special thanks are given to Professor J. Li (Ocean University of China, Qingdao, China) for the cytotoxicity tests.
Author Contributions
Q.W. and X.T. contributed equally to this study. P.L. wrote the main manuscript text. G.L. G.L. designed the project. Q.W. isolated and elucidated the structures. N.V. Identified the species. X.L. did the calculations. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Wang and Xu-Li Tang contributed equally.
Contributor Information
Ping-Lin Li, Email: lipinglin@ouc.edu.cn.
Guo-Qiang Li, Email: liguoqiang@ouc.edu.cn.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38696-3.
References
- 1.WoRMS Editorial Board World Register of Marine Species (Flanders Marine Institute). Available from, http://www.marinespecies.org at VLIZ. (2017).
- 2.Roll DM, Ireland CM, Lu HSM, Clardy J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. J. Org. Chem. 1988;53:3276–3278. doi: 10.1021/jo00249a025. [DOI] [Google Scholar]
- 3.Bishara A, et al. Salarins A and B and Tulearin A: New cytotoxic sponge-derived macrolides. Org. Lett. 2008;10:153–156. doi: 10.1021/ol702221v. [DOI] [PubMed] [Google Scholar]
- 4.Bishara A, et al. Taumycins A and B, two bioactive lipodepsipeptides from the Madagascar sponge Fascaplysinopsis sp. Org. Lett. 2008;10:4307–4309. doi: 10.1021/ol801750y. [DOI] [PubMed] [Google Scholar]
- 5.Bishara A, et al. Tausalarin C: a new bioactive marine sponge-derived nitrogenous bismacrolide. Org. Lett. 2009;11:3538–3542. doi: 10.1021/ol9011019. [DOI] [PubMed] [Google Scholar]
- 6.Aknin M, Gros E, Vacelet J, Kashman Y, Gauvin-Bialecki A. Sterols from the Madagascar sponge Fascaplysinopsis sp. Mar. Drugs. 2010;8:2961–2975. doi: 10.3390/md8122961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazlauskas R, Murphy PT, Quinn RJ, Wells RJ. Aplysinopsin, a new tryptophan derivative from a sponge. Tetrahedron Lett. 1977;18:61–64. doi: 10.1016/S0040-4039(01)92550-X. [DOI] [Google Scholar]
- 8.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2013;30:237–323. doi: 10.1039/C2NP20112G. [DOI] [PubMed] [Google Scholar]
- 9.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2014;31:160–258. doi: 10.1039/c3np70117d. [DOI] [PubMed] [Google Scholar]
- 10.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 11.Bialonska D, Zjawiony JK. Aplysinopsins-marine indole alkaloids: chemistry, bioactivity and ecological significance. Mar. Drugs. 2009;7:166–183. doi: 10.3390/md7020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharate SB, Manda S, Mupparapu N, Battini N, Vishwakarma RA. Chemistry and biology of Fascaplysin, a potent marine-derived CDK-4 inhibitor. Mini-Rev. Med. Chem. 2012;12:650–664. doi: 10.2174/138955712800626719. [DOI] [PubMed] [Google Scholar]
- 13.Kondo K, Nishi J, Ishibahi M, Kobayashi J. Two new tryptophan-derived alkaloids from the Okinawan marine sponge Aplysina sp. J. Nat. Prod. 1994;57:1008–1011. doi: 10.1021/np50109a023. [DOI] [PubMed] [Google Scholar]
- 14.Fattorusso E, Lanzotti V, Magno S, Novellino E. Tryptophan derivatives from a Mediterranean anthozoan. Astroides calycularis. J. Nat. Prod. 1985;48:924–927. doi: 10.1021/np50042a006. [DOI] [Google Scholar]
- 15.Guella G, Mancini I, Zibrowius H, Pietra F. Novel Aplysinopsin-type alkaloids from scleractinian corals of the family Dendrophylliidae of the Mediterranean and the Philippines. Configurational-assignment criteria, stereospecific synthesis, and photoisomerization. Helv. Chim. Acta. 1988;71:773–782. doi: 10.1002/hlca.19880710412. [DOI] [Google Scholar]
- 16.Iwagawa T, et al. Three novel bis(indole) alkaloids from a stony coral, Tubastraea sp. Tetrahedron Lett. 2003;44:2533–2535. doi: 10.1016/S0040-4039(03)00331-9. [DOI] [Google Scholar]
- 17.Djura P, Faulkner DJ. Synthesis of triacontanol via metathesis-hydroboration-isomerization-oxidation. J. Org. Chem. 1980;45:737–738. doi: 10.1021/jo01292a044. [DOI] [Google Scholar]
- 18.Hu JF, et al. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 2002;65:476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]
- 19.Kochanowska AJ, et al. Secondary metabolites from three Florida sponge with antidepressant activity. J. Nat. Prod. 2008;71:186–189. doi: 10.1021/np070371u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fusetani N, Asano M, Matsunaga S, Hashimoto K. Bioactive marine matabolites-xv. Isolation of Aplysinopsin from the Scleractinian coral Tubastrea Aurea as an inhibitor of development of fertilized sea urchin eggs. Comp. Biochem. Physiol. 1986;85B:845–846. [Google Scholar]
- 21.Guella G, Mancini I, Zibrowius H, Pietra F. Aplysinopsin-type alkaloids from Dendrophyllia sp., a scleractinian coral of the family Dendrophylliidae of the philippined, facile photochemical (Z/E) photoisomerization and thermal reversal. Helv. Chim. Acta. 1989;72:1444–1450. doi: 10.1002/hlca.19890720703. [DOI] [Google Scholar]
- 22.Okuda RK, Klein D, Kinnel RB, Li M, Scheuer PJ. Marine natural products: the past twenty years and beyond. Pure Appl. Chem. 1982;54:1907–1914. doi: 10.1351/pac198254101907. [DOI] [Google Scholar]
- 23.Wang Q, et al. (+)- and (−)-Spiroreticulatine, a pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China Sea sponge Fascaplysinopsis reticulata. Org. Lett. 2015;17:3458–3461. doi: 10.1021/acs.orglett.5b01503. [DOI] [PubMed] [Google Scholar]
- 24.Jiang C, et al. Carolignans from the aerial parts of Euphorbia sikkimensis and their anti-HIV activity. J. Nat. Prod. 2016;79:578–583. doi: 10.1021/acs.jnatprod.5b01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou-Shoer MI, Shaala LA, Youseef DTA, Bardr JM, Habib A-AM. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2008;71:1464–1467. doi: 10.1021/np800142n. [DOI] [PubMed] [Google Scholar]
- 26.Mándi A, Mudianta IW, Kurtán T, Garson M. Absolute configuration and conformational study of Psammaplysins A and B from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2015;78:2051–2056. doi: 10.1021/acs.jnatprod.5b00369. [DOI] [PubMed] [Google Scholar]
- 27.Guerriero A, D’Ambrosio M, Pietra F, Debitus C, Ribes O. Pteridines, Sterols, and indole derivatives from the Lithistid sponge Corallistes undulates of the coral sea. J. Nat. Prod. 1993;56:1962–1970. doi: 10.1021/np50101a015. [DOI] [Google Scholar]
- 28.Lan WJ, et al. Five new cytotoxic metabolites from the marine Fungus Neosartorya pseudofischeri. Mar. Drugs. 2016;14:18. doi: 10.3390/md14010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon J, et al. Chemical constituents isolated from the root bark of Cudrania tricuspidata and their potential neuroprotective effects. J. Nat. Prod. 2016;79:1938–1951. doi: 10.1021/acs.jnatprod.6b00204. [DOI] [PubMed] [Google Scholar]
- 30.Dashti Y, Grkovic T, Abdelmohsen UR, Hentschel U, Quinn RJ. Actinomycete metabolome induction/suppression with N-Acetylglucosamine. J. Nat. Prod. 2017;80:828–836. doi: 10.1021/acs.jnatprod.6b00673. [DOI] [PubMed] [Google Scholar]
- 31.Ciminiello P, Dell’Aversano C, Fattorusso E, Magno S, Pansini M. Chemistry of Verongida sponges. 10. Secondary metabolite composition of the Caribbean sponge Verongula gigantea. J. Nat. Prod. 2000;63:263–266. doi: 10.1021/np990343e. [DOI] [PubMed] [Google Scholar]
- 32.Nicacio KJ, et al. Cultures of the marine bacterium Pseudovibrio denitrificans Ab134 produce bromotyrosine-derived alkaloids previously only isolated from marine sponges. J. Nat. Prod. 2017;80:235–240. doi: 10.1021/acs.jnatprod.6b00838. [DOI] [PubMed] [Google Scholar]
- 33.Ciminiello P, et al. Chemistry of Verongida sponges, II. Constituents of the Caribbean sponge Aplysina fistularis forma fulva. J. Nat. Prod. 1994;57:705–712. doi: 10.1021/np50108a004. [DOI] [Google Scholar]
- 34.Ciminiello P, Aversano CD, Fattorusso E, Magno S, Pansini M. Chemisty of Verongida sponges. 9. Secondary metabolite composition of the Caribbean sponge Aplysina cauliformis. J. Nat. Prod. 1999;62:590–593. doi: 10.1021/np9805138. [DOI] [PubMed] [Google Scholar]
- 35.Compagnone RS, et al. 11-deoxyfistularin-3, a new cytotoxic metabolite from the Caribbean sponge Aplysina fistularis insularis. J. Nat. Prod. 1999;62:1443–1444. doi: 10.1021/np9901938. [DOI] [PubMed] [Google Scholar]
- 36.Ma Z, et al. Asymmetric syntheses of Sceptrin and Massadine and evidence for biosynthetic enantiodivergence. Science. 2014;346:219–224. doi: 10.1126/science.1255677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finefield JM, Sherman DH, Kreitman M, Williams RM. Enantiomeric natural products: occurrence and biogenesis. Angew. Chem. Int. Edit. 2012;51:4802–4836. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragini K, Fromont J, Piggott AM, Karuso P. Enantiodivergence in the biosynthesis of bromotyrosine alkaloids from sponges. J. Nat. Prod. 2017;80:215–219. doi: 10.1021/acs.jnatprod.6b01038. [DOI] [PubMed] [Google Scholar]
- 39.Rudi A, et al. Three new cyclic peroxides from the marine sponge Plakortis aff simplex. J. Nat. Prod. 2003;66:682–685. doi: 10.1021/np020589a. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe K, et al. Acetylenic strongylodiols from a Petrosia (Strongylophora) Okinawan marine sponge. J. Nat. Prod. 2005;68:1001–1005. doi: 10.1021/np040233u. [DOI] [PubMed] [Google Scholar]
- 41.Zhao DL, Shao CL, Gan LS, Wang M, Wang CY. Chromone derivatives from a sponge-derived strain of the Fungus Corynespora cassiicola. J. Nat. Prod. 2015;78:286–293. doi: 10.1021/np5009152. [DOI] [PubMed] [Google Scholar]
- 42.Fdhila F, Vázquez V, Sánchez JL, Riguera R. DD-diketopiperazines: antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003;66:1299–1301. doi: 10.1021/np030233e. [DOI] [PubMed] [Google Scholar]
- 43.Jayatilake GS, Thornton MP, Leonard AC, Grimwade JE, Baker BJ. Metabolites from an Antarctic sponge-associated bacterium. Pseudomonas aeruginosa. J. Nat. Prod. 1996;59:293–296. doi: 10.1021/np960095b. [DOI] [PubMed] [Google Scholar]
- 44.Alley MC, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 45.Patel OPS, et al. Naturally occurring carbazole alkaloids from Murraya koenigii as potential antidiabetic agents. J. Nat. Prod. 2016;79:1276–1284. doi: 10.1021/acs.jnatprod.5b00883. [DOI] [PubMed] [Google Scholar]
- 46.Qin S, et al. Malonylginsenosides with potential antidiabetic activities from the flower buds of Panax ginseng. J. Nat. Prod. 2017;80:899–908. doi: 10.1021/acs.jnatprod.6b00789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aplysinopsin-type and Bromotyrosine-derived Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata