Abstract
Virtual-assisted lung mapping (VAL-MAP) is a preoperative bronchoscopic multi-spot dye-marking technique using virtual images developed to assist in navigational lung resection. The technique of VAL-MAP has been shown to be safe and effective surgical assistive tool for performing pulmonary sublobar resections. The technique is applicable for treating multiple small pulmonary lesions that are hardly palpable including ground glass nodules (GGNs). It also may help shorten surgical duration in wedge resection cases. Electromagnetic navigation bronchoscopy (ENB) may eliminate the need for post-mapping computed tomography (CT) scans in logistically challenged situations. In the most recent, multicenter prospective single-arm study, conventional VAL-MAP had reasonable efficacy for obtaining good surgical margin in pulmonary sublobar resections, although the successful resection rate did not reach the primary goal most significantly due to deep resection margins. The technique of VAL-MAP in combination with microcoil may be the next step to acquire better surgical margins.
Keywords: Virtual-assisted lung mapping (VAL-MAP), lung cancer, localization, ground glass nodule (GGN)
Virtual-assisted lung mapping (VAL-MAP) is a preoperative, bronchoscopic, multi-spot dye-marking technique using virtual images developed to assist in navigational lung resection (1,2). It is a unique “mapping” technique showing a certain area to be resected rather than “marking” an approximate pleural surface to be resected. Here, we briefly describe the latest update of the technique.
Steps in conventional VAL-MAP
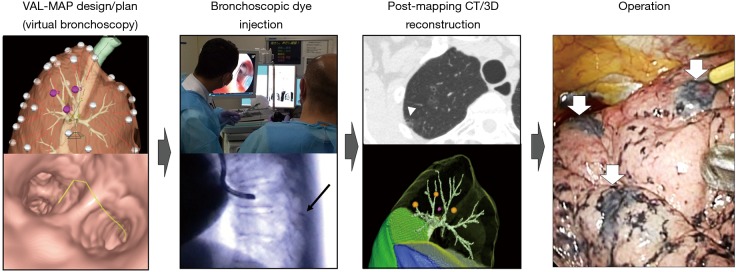
The steps in conventional VAL-MAP is shown in Figure 1. VAL-MAP consists of planning, bronchoscopic dye-marking, post-mapping CT scans, and surgical steps (2). Three-dimensional (3D) reconstruction of computed tomography (CT) data is performed to generate virtual bronchoscopy images as well as to design multiple locations to make the dye marking on the lung surface. On the day of surgery or 1 day prior to surgery, bronchoscopy is conducted under sedation and local anesthesia. A bronchoscope is progressed toward the target bronchi with the guidance of the virtual bronchoscopy images. Under fluoroscopic observation, a blunt metal-tip injection catheter preloaded with indigo carmine is advanced through the working channel of the bronchoscope to the visceral pleura and dye is injected. Dye marking can be repeated until all the planned spots are marked. Post-mapping CT is taken to confirm the actual locations of the dye marks and to map them on 3D images. Upon thoracoscopy, surgeons can easily recognize the dye marks as blue spots on the visceral pleural surface.
Figure 1.
Steps of VAL-MAP. The lung “map” was designed using radiology workstations and virtual bronchoscopy. Bronchoscopic dye injection was conducted within 3 days before surgery under fluoroscopic guidance to confirm the location of the metal-tip injection catheter (black arrow). After mapping, CT scan was taken within a few hours–days after VAL-MAP to visualize actual locations of markings (arrowhead). Using a radiology workstation, 3D images were further reconstructed, reflecting actual locations of markings. The operation was conducted using the 3D image for guidance. The white arrows indicate dye marks. The figure is reproduced with permission from reference (1). VAL-MAP, virtual-assisted lung mapping; CT, computed tomography.
Advantages of VAL-MAP
The geometric information on the lung surface helps surgeons to identify small pulmonary nodules or ground glass nodules (GGNs) that are not yet palpable. In a single institute retrospective study comparing pulmonary wedge resection cases that used preoperative VAL-MAP (n=29) and that did not (n=45), surgery duration was significantly shorter in the cases that used VAL-MAP (average 76.4±28.5 vs. 108.6±40.4 minutes), even though the former cases bore smaller pulmonary nodules (median 8.0 vs. 15.0 mm) (3). Post-mapping CT allowed surgeons to know the accurate location of the target pulmonary lesions sometimes without the need of palpation, and to determine the resection lines. The authors concluded that the technique not only had shortened the surgical duration but also had expanded the indications of resectable lesions.
The technique of VAL-MAP not only helps surgeons to identify small pulmonary nodules but also provides references for oncologically satisfactory resection lines, particularly in patients undergoing thoracoscopic sublobar anatomical lung resection, namely, pulmonary segmentectomy or subsegmentectomy. This aspect is especially important when a patient carries multiple pulmonary tumors, since resection of excessive lung volume in total may cause respiratory failure. A case has been reported lately in which VAL-MAP had successfully assisted determining the resection lines with adequate surgical margins in the thoracoscopic surgery of pulmonary left lower lobectomy combined with a wedge resection of the left upper lobe, a segmentectomy in the right upper lobe, and a complex segmentectomy in the right lower lobe for synchronous bilateral multiple lung adenocarcinomas (4). Another case in which a patient had multiple metastatic tumors in right and left lower lobes of the lung, bilateral thoracoscopic pulmonary segmentectomies were successfully performed with the aid of VAL-MAP (5). The authors claimed that the decrease in pulmonary function was minimal compared with predicted postoperative function calculated by the numbers of subsegments. In the reported case the authors also utilized the unique advantage of VAL-MAP over the use of inflation-deflation line to determine segmental planes when performing an extended segmentectomy beyond anatomical segments (6,7).
The role of post-mapping CT in VAL-MAP
Post-mapping CT seems to be essential in the conventional setting of VAL-MAP, since dye marking is not always successful in terms of the planned location or visibility of the marking after each attempt of dye injection. Post-mapping CT allows for 3D image guidance to the surgeons based on the actual location of the dye marks rather than the planned marking spots on virtual bronchoscopy. In a single-institute retrospective study investigating 43 markings in 11 patients, actual markings confirmed by post-mapping CT were deviated by an average of 3 cm from the markings predicted by virtual bronchoscopy (8). Despite this discrepancy, all targeted lesions were successfully resected owing to the 3D images generated from the post-mapping CT data. There is also a reported case that demonstrated the usefulness of post-mapping CT in which post-mapping CT unexpectedly identified additional tiny pulmonary nodules (9). Here again, the unique feature of VAL-MAP employing multiple fiducial spots on the lung surface had enabled generating auxiliary lines to estimate the location the lesions without having actual dye spots on the very next to the target lesions.
VAL-MAP using electromagnetic navigation bronchoscopy (ENB)
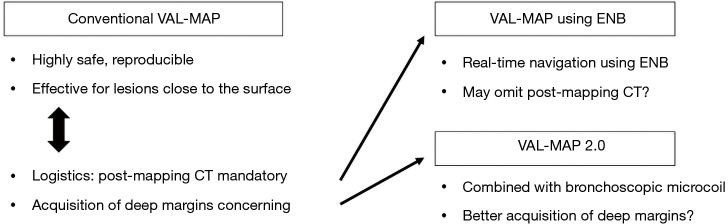
The conventional procedure of VAL-MAP is now covered by public health insurance in Japan. However, logistic feasibility may be one of the issues in conventional VAL-MAP, which involves bronchoscopy and post-mapping CT prior to surgery (Figure 2). Although dye marks are mostly identifiable if the surgery is performed within 2 days after VAL-MAP, it can still be difficult to schedule bronchoscopy and CT scans timewise in some hospitals. ENB, which also was recently covered by public health insurance in Japan, may be a solution to eliminate the need of post-mapping CT, and to perform a mapping and a surgery all at once in the operating room (10,11). ENB is an image-guided approach that uses 3D-reconstructed CT scans and sensor location technology to guide a steerable endoscopic probe to peripheral lung areas that may be beyond the reach of conventional bronchoscopes (12). In the operating room, ENB is performed under tracheal intubation and general anesthesia, and the target sites for pleural dye marking are entered into the ENB software program. Guided by the virtual bronchoscopy and ENB navigation, the probe tip is advanced to the targeted spot. Under fluoroscopic confirmation of the tip location, dye is injected on each target spot. Expecting smaller dislocation of the actual dye marks from the predicted location compared to the conventional VAL-MAP, post-mapping CT may be omitted. Following ENB, the patient is reintubated with a double-lumen endobronchial tube to undergo a thoracoscopic surgery. VAL-MAP technique has good affinity with ENB, thus is expected to become a regular practice in hospitals in which ENB is commonly performed.
Figure 2.
New generations of VAL-MAP. To overcome the limitations of conventional VAL-MAP including logistics to necessitate post-mapping CT scan and acquisition of deep resection margins, use of ENB and combination with bronchoscopic placement of microcoils (VAL-MAP 2.0) are under investigation. VAL-MAP, virtual-assisted lung mapping; CT, computed tomography; ENB, electromagnetic navigation bronchoscopy.
Roles of VAL-MAP in resecting GGNs
Multicenter studies in Japan have demonstrated that VAL-MAP is applicable for targeting multiple small pulmonary tumors, especially multi-centric early stage lung cancers that are often found as GGNs on chest CT scans in patients in east Asian countries. In a recent retrospective study, 370 GGNs in 299 patients including 257 pure and 113 mixed GGNs were analyzed (13). Among 146 wedge resections (43.6%), 99 simple segmentectomies (29.6%), and 60 complex segmentectomies (18.0%), the overall successful resection rate was 98.6%. Multiple GGNs were concurrently targeted by VAL-MAP in 53 patients (17.7%) with 123 GGNs. Multiple marks of VAL-MAP facilitated concurrent resections of multi-centric GGNs. The study revealed that 113 of 139 GGNs >5 mm in diameter (81.3%) were primary lung cancer. The authors concluded that the VAL-MAP technique may impact decision-making in terms of the timing and type of surgical resection for small GGNs given the minimal invasiveness of the technique.
Resection margins in VAL-MAP
The acquisition of sufficient resection margins is essential in sublobar resection, as they predict locoregional recurrence and ultimately affect patient survival (14-16). In the most recent prospective single-arm study focusing on whether VAL-MAP technique is efficient for obtaining sufficient surgical margins in sublobar lung resection, 213 lesions from 162 cases were primarily registered (17). Successful resection was defined as resection of the lesion with margins greater than the lesion diameter or 2 cm. The resection of 203 lesions was intended in 153 patients. Among 131 wedge resections (71.2%), 51 segmentectomies (27.7%) and 2 other procedures (1.1%), successful resection was achieved in 178 lesions (87.8%), and VAL-MAP markings successfully aided in the identification of 190 lesions (93.6%). The study showed that VAL-MAP had reasonable efficacy, although the successful resection rate did not reach the primary goal of 95% of lesions. The required resection depth was the most significant factor determining the completeness of resection following VAL-MAP.
VAL-MAP 2.0 combining microcoil placement
To better determine suitable resection lines for sublobar lung resection, especially to obtain sufficient resection depth, a novel technique of VAL-MAP (VAL-MAP 2.0) was developed by combining conventional VAL-MAP technique with a deeply placed microcoil (Figure 2), which was originally described as a localizing technique for small pulmonary nodules (18).
The locations of the dye marks and microcoil(s) are planned on 3D images. A microcoil is planned to place central to the tumor to indicate the deep resection margin. The distance from a bronchial branching to the planned microcoil location is measured on virtual bronchoscope. In the bronchoscopy suite, bronchoscopic multi-spot dye marking is first conducted. After completing three to five dye marks, one or two platinum microcoils are placed under fluoroscopic observation. The microcoil rolls up immediately at the location and is tightly wedged in the bronchus. Post-mapping CT is taken to confirm the locations of the dye marks and microcoil, and to map them on 3D images. Patients undergo surgery on the same day or 1 day after bronchoscopic mapping. During surgery, dye marks are observed first. Based on this “lung map” on the lung surface, a grasping ring forceps or stapler is applied to set a tentative resection line. Fluoroscopy is then used to examine the location of the microcoil inside the lung. If necessary, the tentative resection line is adjusted to obtain a sufficient resection margin before the stapler is fired. Finally, the resected specimen is examined under fluoroscopy to confirm complete removal of the microcoils.
A multicenter prospective clinical trial to investigate the efficacy of VAL-MAP 2.0 is currently conducted in Japan. Preliminary results demonstrated that the microcoils were successfully identified with intraoperative fluoroscopy, effectively assisting the surgeons’ decision regarding the deep resection lines. The technique of VAL-MAP 2.0 is expected to effectively assist surgeons to obtain sufficient resection margins in sublobar lung resection, even when removing deeply located lesions.
Conclusions
In conclusion, the technique of VAL-MAP has been shown to be safe and effective surgical assistive tool for performing pulmonary sublobar resections. The technique is applicable for treating multiple small pulmonary lesions including GGNs, and may help shorten surgical duration in wedge resection cases. ENB may eliminate the need for post-mapping CT scans. Conventional VAL-MAP had reasonable efficacy for obtaining good surgical margin, although the successful resection rate did not reach the primary goal. VAL-MAP in combination with microcoil may be the next step to acquire better surgical margins.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Sato M, Kuwata T, Yamanashi K, et al. Safety and reproducibility of virtual-assisted lung mapping: a multicentre study in Japan. Eur J Cardiothorac Surg 2017;51:861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. 10.1016/j.jtcvs.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 3.Kuwata T, Shinohara S, Matsumiya H, et al. Virtual-assisted lung mapping (VAL-MAP) shortened surgical time of wedge resection. J Thorac Dis 2018;10:1842-9. 10.21037/jtd.2018.03.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imanishi N, Shinohara S, Kuwata T, et al. Resection of synchronous bilateral multiple lung adenocarcinomas using virtual-assisted lung mapping. Surg Case Rep 2018;4:30. 10.1186/s40792-018-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakao K, Sato M, Nitadori JI, et al. Bilateral segmentectomies using virtual-assisted lung mapping (VAL-MAP) for metastatic lung tumors. Surg Case Rep 2017;3:104. 10.1186/s40792-017-0379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716-30. 10.21037/jtd.2016.09.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato M, Murayama T, Nakajima J. Thoracoscopic stapler-based "bidirectional" segmentectomy for posterior basal segment (S10) and its variants. J Thorac Dis 2018;10:S1179-86. 10.21037/jtd.2018.01.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato M, Nagayama K, Kuwano H, et al. Role of post-mapping computed tomography in virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2017;25:123-30. 10.1177/0218492316689351 [DOI] [PubMed] [Google Scholar]
- 9.Yanagiya M, Sato M, Kuwano H, et al. Management of lung nodules newly found by virtual-assisted lung mapping: a case report. Surg Case Rep 2017;3:49. 10.1186/s40792-017-0327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolton WD, Howe H, 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. 10.1016/j.athoracsur.2014.04.085 [DOI] [PubMed] [Google Scholar]
- 11.Awais O, Reidy MR, Mehta K, et al. Electromagnetic Navigation Bronchoscopy-Guided Dye Marking for Thoracoscopic Resection of Pulmonary Nodules. Ann Thorac Surg 2016;102:223-9. 10.1016/j.athoracsur.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 12.Weiser TS, Hyman K, Yun J, et al. Electromagnetic navigational bronchoscopy: a surgeon's perspective. Ann Thorac Surg 2008;85:S797-801. 10.1016/j.athoracsur.2007.11.052 [DOI] [PubMed] [Google Scholar]
- 13.Sato M, Kuwata T, Kitamura A, et al. The role of virtual-assisted lung mapping in the resection of ground glass nodules. J Thorac Dis 2018;10:2638-47. 10.21037/jtd.2018.05.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. 10.1016/S0003-4975(03)01511-X [DOI] [PubMed] [Google Scholar]
- 15.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. 10.1245/s10434-007-9421-9 [DOI] [PubMed] [Google Scholar]
- 16.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. 10.1016/j.athoracsur.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Kobayashi M, Kojima F, et al. Effect of virtual-assisted lung mapping in acquisition of surgical margins in sublobar lung resection. J Thorac Cardiovasc Surg 2018;156:1691-701.e5. 10.1016/j.jtcvs.2018.05.122 [DOI] [PubMed] [Google Scholar]
- 18.Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. 10.1093/ejcts/ezt220 [DOI] [PubMed] [Google Scholar]