Abstract
Background
Dyspnoea is a common symptom in advanced cancer, with a prevalence of up to 70% among patients at end of life. The cause of dyspnoea is often multifactorial, and may cause considerable psychological distress and suffering. Dyspnoea is often undertreated and good symptom control is less frequently achieved in people with dyspnoea than in people with other symptoms of advanced cancer, such as pain and nausea. The exact mechanism of action of corticosteroids in managing dyspnoea is unclear, yet corticosteroids are commonly used in palliative care for a variety of non‐specific indications, including pain, nausea, anorexia, fatigue and low mood, despite being associated with a wide range of adverse effects. In view of their widespread use, it is important to seek evidence of the effects of corticosteroids for the management of cancer‐related dyspnoea.
Objectives
To assess the effects of systemic corticosteroids for the management of cancer‐related breathlessness (dyspnoea) in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Science Citation Index Web of Science, Latin America and Caribbean Health Sciences (LILACS) and clinical trial registries, from inception to 25 January 2018.
Selection criteria
We included randomised controlled trials that included adults aged 18 years and above. We included participants with cancer‐related dyspnoea when randomised to systemic corticosteroids (at any dose) administered for the relief of cancer‐related dyspnoea or any other indication, compared to placebo, standard or alternative treatment.
Data collection and analysis
Five review authors independently assessed trial quality and three extracted data. We used means and standard deviations for each outcome to report the mean difference (MD) with 95% confidence interval (CI). We assessed the risk of bias and quality of evidence using GRADE. We extracted primary outcomes of sensory‐perceptual experience of dyspnoea (intensity of dyspnoea), affective distress (quality of dyspnoea) and symptom impact (burden of dyspnoea or impact on function) and secondary outcomes of serious adverse events, participant satisfaction with treatment and participant withdrawal from trial.
Main results
Two studies met the inclusion criteria, enrolling 157 participants (37 participants in one study and 120 in the other study), of whom 114 were included in the analyses. The studies compared oral dexamethasone to placebo, followed by an open‐label phase in one study. One study lasted seven days, and the duration of the other study was 15 days.
We were unable to conduct many of our predetermined analyses due to different agents, dosages, comparators and outcome measures, routes of drug delivery, measurement scales and time points. Subgroup analysis according to type of cancer was not possible.
Primary outcomes
We included two studies (114 participants) with data at one week in the meta‐analysis for change in dyspnoea intensity/dyspnoea relief from baseline. Corticosteroid therapy with dexamethasone resulted in an MD of lower dyspnoea intensity compared to placebo at one week (MD –0.85 lower dyspnoea (scale 0–10; lower score = less breathlessness), 95% CI ‐1.73 to 0.03; very low‐quality evidence), although we were uncertain as to whether corticosteroids had an important effect on dyspnoea as results were imprecise. We downgraded the quality of evidence by three levels from high to very low due to very serious study limitations and imprecision.
One study measured affective distress (quality of dyspnoea) and results were similar between groups (29 participants; very low‐quality evidence). We downgraded the quality of the evidence three times for imprecision, inconsistency, and serious study limitations.
Both studies assessed symptom impact (burden of dyspnoea or impact on function) (113 participants; very low‐quality evidence). In one study, it was unclear whether dexamethasone had an effect on dyspnoea as results were imprecise. The second study showed more improvement for physical well‐being scores at days eight and 15 in the dexamethasone group compared with the control group, but there was no evidence of a difference for FACIT social/family, emotional or functional scales. We downgraded the quality of the evidence three times for imprecision, inconsistency, and serious study limitations.
Secondary outcomes
Due to the lack of homogenous outcome measures and inconsistency in reporting, we could not perform quantitative analysis for any secondary outcomes. In both studies, the frequency of adverse events was similar between groups, and corticosteroids were generally well tolerated. The withdrawal rates for the two studies were 15% and 36%. Reasons for withdrawal included lost to follow‐up, participant or carer (or both) refusal, and death due to disease progression. We downgraded the quality of evidence for these secondary outcomes by three levels from high to very low due to serious study limitations, inconsistency and imprecision.
Neither study examined participant satisfaction with treatment.
Authors' conclusions
There are few studies assessing the effects of systemic corticosteroids on cancer‐related dyspnoea in adults with cancer. We judged the evidence to be of very low quality that neither supported nor refuted corticosteroid use in this population. Further high‐quality studies are needed to determine if corticosteroids are efficacious in this setting.
Plain language summary
Corticosteroids for the management of cancer‐related breathlessness in adults with cancer
Background
Breathlessness (dyspnoea) is a common symptom in advanced cancer. Breathlessness may be due to a combination of different causes including lung cancer, metastatic disease elsewhere in the body (for example, cancer in the abdomen pushing up the diaphragm), or cancer‐related conditions affecting the nerves or muscles associated with breathing. Pain and psychological conditions (such as fear and anxiety) or pre‐existing lung disease may make symptoms worse. People with cancer report breathlessness is associated with higher psychological distress and poorer quality of life. Medicines can be used to treat breathlessness in this population, and one common medicine used is corticosteroids. In this review, we evaluated how effective systemic corticosteroids are in treating cancer‐related breathlessness in adults, compared to any control.
Study characteristics
We searched the literature in January 2018. We found two studies, enrolling 157 participants in total, that tested the effect of systemic corticosteroids on breathlessness in adults with cancer, compared to a dummy medicine (placebo). One study lasted seven days, and the other study lasted 15 days. Both studies compared a corticosteroid (oral (by mouth) dexamethasone) to a dummy medicine with no properties to reduce breathlessness, which we included in our analyses.
We were interested in the primary outcomes of participant‐reported breathlessness intensity, quality and burden. We were also interested in the secondary outcomes of serious side effects, participant satisfaction with treatment and participant withdrawal from trial.
Key results
We could not complete many of our planned analyses due to the small number of studies, the different medicines and comparisons, and outcomes that the studies reported. We did conduct one analysis of 114 participants to assess change in breathlessness intensity/relief from baseline. We found that corticosteroids had no beneficial effect compared to a dummy medicine on reducing breathlessness intensity in people with cancer.
We found that the frequency of side effects was similar between groups, and corticosteroids were generally well tolerated. None of the studies measured participant satisfaction with treatment. Participant withdrawals were 15% and 36% in the two studies.
Quality of evidence
The current evidence was based on only two studies with a small number of participants. We rated the quality of the evidence from these studies using four levels: very low, low, moderate or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident in the results. We judged the quality of the evidence in this review to be very low, downgraded due to problems with study quality and too few data. We are very uncertain of the results. More high‐quality studies are needed to determine if corticosteroids are effective for dyspnoea in people with cancer.
Summary of findings
Summary of findings 1. Systemic corticosteroids compared with placebo for the management of cancer‐related breathlessness (dyspnoea).
| Systemic corticosteroids compared with placebo forthe management of cancer‐related breathlessness (dyspnoea) | ||||||
|
Patient or population: adults with cancer‐related breathlessness (dyspnoea) Settings: inpatients and outpatients Intervention: dexamethasone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Dexamethasone | |||||
|
Breathlessness (dyspnoea) at 1 week (intensity) Scale 0–10; lower score = less breathlessness |
The mean score for intensity of breathlessness at baseline was 4.7 and 3 for the control groups. The mean difference in the intensity of breathlessness at 1 week was –1.3 and –0.58 for the control groups. |
The mean score for intensity of breathlessness at baseline was 5.0 and 4.09 for the intervention groups. The mean difference in the intensity of breathlessness at 1 week was –1.8 and –1.56 for the intervention groups. |
— | 114 (2 studies) |
⊕⊝⊝⊝ Very lowa | — |
|
Breathlessness (dyspnoea) at 1 week (quality) Cancer Dyspnea Scale |
Small reduction in effort, discomfort and anxiety. | Small reduction in effort and anxiety, with a slight increase in discomfort. | — | 29 (1 study) |
⊕⊝⊝⊝ Very lowb | |
|
Breathlessness (dyspnoea) at 1 week (burden) EORTC QLQ‐C30, FACIT, ESAS |
Different measurement tools used in each study. Significant reduction in burden at 1 week for dexamethasone group compared to control group observed only for the FACIT physical subscale in one study. | — | 113 (2 studies) |
⊕⊝⊝⊝ Very lowc | ||
| Adverse events | Severity of adverse events was not properly defined making it difficult to assess this outcome. The frequency of adverse events was similar between groups. The intervention was well tolerated in corticosteroid group. | — | 157 (2 studies) |
⊕⊝⊝⊝ Very lowc | ||
| Patient satisfaction with treatment | No data | No data | No data | No data | No data | Not possible to GRADE this outcome due to a lack of data |
| Participant withdrawal from trial | 54 withdrawals out of 173 participants (31%) for the two studies. Rate of withdrawal was similar between placebo and dexamethasone groups. | — | 173 (2 studies) |
⊕⊝⊝⊝ Very lowc | ||
|
CI: confidence interval. GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded the quality of the evidence once for imprecision due to the small number of participants in the included studies, and twice for very serious study limitations (likely selection and attrition bias).
bDowngraded the quality of the evidence three times: once for imprecision due to the small number of participants in the included study, once for inconsistency, and once for serious study limitations (likely attrition bias).
cDowngraded the quality of the evidence three times: once for imprecision due to the small number of participants in the included studies, once for inconsistency, and once for serious study limitations (likely selection and attrition bias).
Background
Description of the condition
Dyspnoea (breathlessness) is a "subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity" (American Thoracic Society 1999). It is "one of the most common and most feared symptoms amongst cancer patients" (Hui 2013), increases with disease progression (Solano 2006), and occurs in up to 70% of people with advanced cancer at end of life (Bruera 2000; Dudgeon 2001; Hui 2015; Kutner 2001; Mercadante 2017; Tishelman 2007; Viola 2008). Dyspnoea has a negative impact on a patient's quality of life. It interferes with daily life activities (ERS Monograph 2016; Mercadante 2017; Tanaka 2002a), and has been associated with fatigue, anxiety and depression, and decreased function and quality of life, since it may precipitate both physical and psychological distress (Ben‐Aharon 2012; ERS Monograph 2016; Mularski 2010; Seow 2011; Tanaka 2002b; Williams 2006). It may cause significant suffering for patients and their families (Bernhard 1991; Booth 2008), and be a source of substantial healthcare expenditure (Booth 2003; Booth 2015; ERS Monograph 2016; Johnson 2014; Seamark 2004; Skaug 2009). It is frightening for many patients who report feeling that they are suffocating, choking (Skevington 1997), short of breath, unable to get a breath or drowning (eTG 2016; Kloke 2015; Parshall 2012; Wilcock 2002).
Good symptom control is less frequently achieved in people with dyspnoea than in people with other symptoms of advanced cancer, such as pain and nausea (Yennurajalingam 2015). When disease is advanced, patients may experience episodes of acute breathlessness (Mercadante 2017), "superimposed on a background level of continuous breathlessness" (Johnson 2016). Episodes of breathlessness may be predictable (generally caused by physical exertion) or unpredictable (Johnson 2016; Simon 1990).
The pathophysiology of dyspnoea is complex and is not fully understood (Booth 2008; Burki 2010; ERS Monograph 2016; Hui 2013; Manning 1995; Parshall 2012). A constellation of sensory inputs may contribute to the multiple sensations of dyspnoea, which may include the "sensations of work or effort, tightness, and air hunger/unsatisfied inspiration" (Parshall 2012). Tightness is relatively specific to stimulation of airway receptors in conjunction with bronchoconstriction, while intensity of air hunger/unsatisfied inspiration is magnified by imbalances among inspiratory drive, efferent activation (outgoing motor command from the brain), and feedback from afferent receptors throughout the respiratory system (Parshall 2012). In the palliative care setting, the cause of dyspnoea is often multifactorial (ERS Monograph 2016), with an unpredictable response to treatment (Lin 2012). Indeed, the subjective experience of dyspnoea is influenced by "multiple physical, psychological, social and spiritual factors, and may induce secondary physiological and behavioral responses" (Lok 2016). The concept of 'total dyspnoea' – similar to that of 'total pain' – may provide a framework in the multidimensional assessment and management of breathlessness (Abernethy 2008; ERS Monograph 2016), as each of these factors may contribute to the perceived severity of a person's dyspnoea (Banzett 2008; Chin 2016; De Peuter 2004; Evans 2002; Parshall 2012).
Common pulmonary causes of dyspnoea in cancer may include progressive metastatic disease, lymphangitis carcinomatosa, pleuritis carcinomatosa, pleural effusion, interstitial lung disease, parenchymal lung involvement, pulmonary embolism, infection, atelectasis, airway obstruction and pre‐existing pulmonary disease (Booth 2014; Chan 2004; eTG 2016; Kvale 2007; Manning 1995). Systemic causes of dyspnoea may include anaemia, hypoxaemia, uraemia or acidaemia, congestive cardiac failure, pericarditis or pericardial effusion, pulmonary hypertension, sepsis, cardiovascular/physical deconditioning, muscle weakness or neuromuscular conditions (Booth 2014; Parshall 2012). Other common causes include pain, ascites, hepatomegaly, obesity, lymphadenopathy, superior vena cava obstruction, treatment‐related adverse effects (e.g. pneumonitis or fibrosis following chemotherapy or radiotherapy) and pre‐existing lung disease (e.g. asthma or chronic obstructive pulmonary disease (COPD)). Psychological drivers or psychogenic causes, such as panic disorder, anxiety and distress, may also contribute to the genesis of breathlessness or further compound symptoms, or both (Giardino 2010; Kunik 2005; Moore 1999; Nardi 2009; Parshall 2012; Perna 2004; Rassovsky 2006; Smoller 1996; Williams 2010). The symptoms of dyspnoea are usually managed following careful assessment of the potential cause and impact on the person's experience, and treatment of any reversible causes (Chin 2016; Manning 1995). Dyspnoea that appears suddenly is more likely to be reversible than progressive longstanding dyspnoea that is related to disease progression as it is likely to be related to a treatable acute event such as infection or pulmonary embolism (eTG 2016). Therefore, it is important to consider assessment for potentially reversible causes of dyspnoea (eTG 2016). As the sensation of dyspnoea is mediated by the central nervous system (Herigstad 2011), strategies that address psychosocial stressors or psychological triggers are also key, to "reduce the impact of the sensation of breathlessness, even when it cannot be removed" (Booth 2015). Therefore, non‐pharmacological techniques are of central importance in the management of breathlessness (Booth 2015; Farquhar 2014), and active management of psychosocial issues such as anxiety, depression, carer stress and distress, and the implementation of non‐pharmacological self‐management strategies such as physical and mental activity, relaxation techniques, breathing exercises, education and information should be a priority (Booth 2015). Modification of the patient's environment, activity pacing and energy conservation (Sackley 2009), and anxiety reduction training (Lai 2010), may also maximise comfort, improve respiratory efficiency, and reduce fear and anxiety (De Peuter 2004; eTG 2016; Farquhar 2014; Higginson 2014; Kamal 2012). For example, the use of a fan is one of the most important and effective non‐pharmacological interventions in the management and relief of breathlessness (Bausewein 2008; Galbraith 2010). Johnson and colleagues postulated that "as skeletal muscle (not limited to the muscles of respiration) is intimately involved in the genesis of breathlessness, reduced activity leads to reduced muscle bulk so that over time, breathlessness will be triggered by less and less exertion breathlessness" (Johnson 2014). Exercise‐based rehabilitation, a complex intervention that incorporates cognitive and behavioural management strategies (Parshall 2012), pulmonary rehabilitation, and other integrated, complex intervention services for breathlessness may thus be of use or benefit for people with dyspnoea (Booth 2006; Booth 2011; Farquhar 2010; Farquhar 2014). From an anxiety reduction training point of view, cognitive behavioural therapy, simple relaxation therapy, distraction methods, music or mindfulness may also help people feel more control (Lok 2016), and 'gain mastery' over their breathlessness (Booth 2014). For severe, chronic, refractory or intractable dyspnoea, non‐pharmacological methods may be supplemented by pharmacological treatments. These may include oral or parenteral opioids (Ben‐Aharon 2012; Johnson 2014; Parshall 2012; Viola 2008), benzodiazepines (if the person is experiencing significant anxiety), alongside other non‐pharmacological strategies (ERS Monograph 2016), oxygen (if a person is hypoxic) (Parshall 2012), and steroids (eTG 2016; Kamal 2012). Systemic corticosteroids are also commonly used for specific antitumour effect in conditions such as lymphangitis carcinomatosa or airway obstruction by tumour (Elsayem 2007).
Description of the intervention
Systemic corticosteroids are commonly used in palliative care practice for symptom control of fatigue (Yennurajalingam 2013), nausea and vomiting (Vayne‐Bossert 2017), anorexia, cachexia and pain, relief of spinal cord compression, reduction of vasogenic oedema from brain metastasis and resolution from malignant bowel obstruction (Hardy 2001; Lin 2012; Mercadante 2001; Paulsen 2014; Shih 2007). The corticosteroid used most commonly in palliative care is dexamethasone, due to its potency, long duration of action allowing once‐daily dosing and the ability to administer it subcutaneously (eTG 2016). The balance of benefit versus risk of harm must be carefully considered. Adverse effects are usually dose and duration related, and include insomnia, mental disturbances (including depression, mania, psychosis or delirium), hyperglycaemia, increased susceptibility to infection, gastric irritation, Cushingoid features and proximal myopathy (eTG 2016).
How the intervention might work
Dyspnoea, or the sensation of breathlessness, is closely related to the sensation of respiratory effort experienced via the activation of proprioceptive pathways during respiration (Dorman 2009). While the respiratory centre in the medulla controls breathing, dyspnoea is the result of cortical stimulation (Dorman 2009; Hui 2013). Both lung and central chemoreceptors detect abnormalities in blood gases (hypoxia, increased partial pressure of carbon dioxide), and together with lung and respiratory muscle mechanoreceptors (responding to stretching and pulmonary irritants), stimulate the medullary respiratory centre. The activity of the chemoreceptors, mechanoreceptors and respiratory centre can also stimulate the cerebral cortex, thus directly contributing to the sensation of dyspnoea (Dorman 2009; Hui 2013). Cancer, in a similar manner to COPD, is characterised by a significant inflammatory component that includes airway wall infiltration of macrophages and T lymphocytes, increased lung tumour necrosis factor‐alpha and interleukin (IL)‐8, elevated serum IL‐6, C‐reactive protein, increased peripheral neutrophil activation (Hui 2016), and inflammatory cytokines (Wang 2010). Corticosteroids have potent anti‐inflammatory activity that may explain their ability to alleviate dyspnoea, as people with advanced cancer often have an elevated inflammatory response that can contribute to dyspnoea both peripherally and centrally (Hui 2016). The usefulness of the anti‐inflammatory activity of corticosteroids in managing acute exacerbations of COPD is well established (Barczyk 2004; Brightling 2000; Culpitt 2003; Falk 2008; Wood‐Baker 2005). However, as dyspnoea is likely to be multifactorial in the context of cancer, corticosteroids may work more effectively in some instances (e.g. where there is a process of inflammation), but not as well in other instances.
Why it is important to do this review
Dyspnoea is a common and devastating symptom in people with cancer that often worsens in the last months of life and may be difficult to treat (Dudgeon 2001; Hui 2015; Hui 2016; Mercadante 2017; Tishelman 2007). Systemic corticosteroids are commonly used in palliative care, particularly for people with advanced malignant disease, for a variety of symptom control indications including pain, nausea, vomiting, anorexia, fatigue and dyspnoea (Lin 2012; Shih 2007), despite the fact that steroids may be associated with significant adverse effects (Matsuo 2011), especially following long‐term use. However, there is little objective evidence in the literature to support the use of systemic corticosteroids for symptom control (ERS Monograph 2016; Viola 2008), and concerns have been raised about the 'uncontrolled' use of steroids in people with cancer (Haywood 2015; Levy 2016; Vayne‐Bossert 2017).
Objectives
To assess the effects of systemic corticosteroids for the management of cancer‐related breathlessness (dyspnoea) in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised double‐blind controlled trials (RCTs) with full journal publication, as well as online clinical trial results and summaries of otherwise unpublished clinical trials or abstracts with sufficient data for analysis. We excluded studies that were non‐randomised, case reports and clinical observations, and short abstracts (usually meeting reports).
Types of participants
Participants with cancer with cancer‐related dyspnoea, aged 18 years and above.
Types of interventions
Systemic corticosteroids at any dose, administered for the relief of cancer‐related dyspnoea or other cancer‐related symptoms (where dyspnoea was also measured), compared to placebo or any active comparator including supportive care or alternate non‐pharmacological treatment. We excluded studies assessing inhaled corticosteroids.
Types of outcome measures
Primary outcomes
Our primary outcome was the effect of systemic corticosteroids on breathlessness (dyspnoea), as assessed by the American Thoracic Society 'Domains of Dyspnoea Measurement' (Parshall 2012).
Domain 1: Sensory‐perceptual experience – intensity of dyspnoea
Definition: measures of what breathing feels like to the patient or research subject. Examples include:
Domain 2: Affective distress – quality of dyspnoea
Definition: measures of how distressing breathing feels. Focus can be either immediate (e.g. unpleasantness) or evaluative (e.g. judgements of meaning or consequence). Examples include:
single‐item ratings of severity of distress or unpleasantness; and
multi‐item scales of emotional responses such as anxiety such as the Hospital Anxiety and Depression Scale (HADS), or State‐Trait Anxiety Inventory (STAI).
Domain 3: Symptom impact‐burden of dyspnoea/impact on function
Definition: measures of how dyspnoea affects functional ability, employment (disability), quality of life or health status. Examples include:
unidimensional rating of disability or activity limitation such as the Medical Research Council (MRC) Dyspnoea Scale (Mahler 1988);
unidimensional or multidimensional ratings of functional ability (Pulmonary Functional Status and Dyspnoea Questionnaire (PFSDQ) (Lareau 1998); and
multidimensional scales of quality of life/health status.
We aimed to use both standardised, mean pre–post change in breathlessness scores after the intervention (comparator), as well as post‐intervention standardised mean difference (SMD) in breathlessness scores between intervention and comparator groups. We aimed to also summarise breathlessness outcomes separately, to delineate between breathlessness measured 'now' versus 'on average over the past 24 hours' or as described by the validated outcome measure.
We aimed to obtain standardised means when seeking to summarise and compare studies that use different breathlessness measures (regardless of the scoring system).
Secondary outcomes
Serious adverse events – any untoward medical occurrence that at any dose resulted in death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability/incapacity or was a congenital anomaly/birth defect (ICH 1994).
Participant satisfaction with treatment.
Participant withdrawal.
Search methods for identification of studies
We attempted to identify as many trials as possible that met the inclusion criteria with our search strategy, without limitation by language, publication type, status or date.
Electronic searches
We searched the following databases without language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL) 2018, Issue 1 in the Cochrane Library (Appendix 1);
MEDLINE (Ovid) 1966 to 25 January 2018 (Appendix 2);
Embase.com 1970 to 25 January 2018 (Appendix 3);
CINAHL (EBSCO) 1982 to 25 January 2018 (Appendix 4);
Science Citation Index (ISI Web of Science) 1899 to 25 January 2018 (Appendix 5);
Latin America and Caribbean Health Sciences (LILACS) 1982 to 25 January 2018 (Appendix 6).
Medical subject headings (MeSH) or equivalent and text word terms were used. Where appropriate, MeSH terms and other subject headings were exploded. We applied a modified version of the Cochrane filter for the identification of RCTs, as published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Searches were tailored to individual databases.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials. In addition, we checked the bibliographic references and cited sources of any relevant identified studies to find additional trials not identified by the electronic searches. To identify any unpublished or grey literature, we searched the Internet, using the Google Scholar search engine (www.googlescholar.com), with selected terms from the above strategy. If only the abstract was published, we attempted to contact the authors for further details, or source the unpublished paper. One of the review authors (KR), who is an Information Specialist, conducted the searches. All searches are current as of 25 January 2018.
Data collection and analysis
Selection of studies
Four review authors (JH, PG, PVB, JD) independently assessed the titles and abstracts of all studies identified by the search for potential inclusion. Each of these authors independently selected all potentially relevant studies for inclusion by applying the selection criteria outlined in the 'Criteria for considering studies for this review' section. We then compared these lists, discussed any differences and either included or excluded the papers based on a majority decision. A PRISMA study flow diagram is included in Figure 1 (Moher 2009), which documents the screening process as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
1.
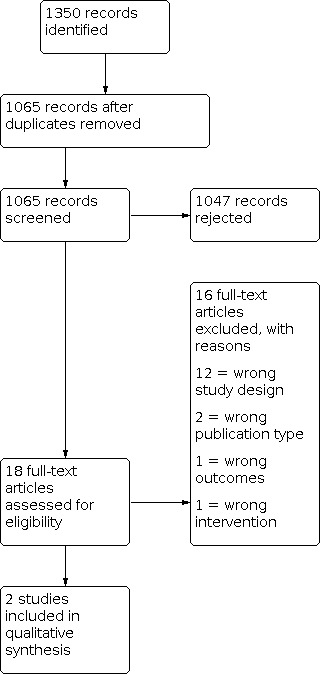
PRISMA study flow diagram.
Data extraction and management
Three review authors (AH, SK, JD) independently extracted data using a standard form, and checked for agreement before entry into Review Manager 5 (Review Manager 2014). The authors extracted data that included information about the year of the study, study design, number of participants treated, participant demographic details, type of cancer, drug and dosing regimen, study design (placebo or active control) and methods, study duration and follow‐up, outcome measures (measurement of dyspnoea and other relevant outcomes), withdrawals and adverse events. We resolved potential disagreements by discussion.
Assessment of risk of bias in included studies
Five review authors (AH, SK, JH, PG, JD) independently assessed the risk of bias of each included study using the 'Risk of bias' assessment method outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved potential disagreements by discussion. For each study, we assessed the risk of bias for the following domains.
Random sequence generation (checking for selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); or unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
Blinding of participants and personnel (checking for performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). Studies that were not double‐blinded were considered to have high risk of bias.
Blinding of outcome assessment (checking for detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved); unclear risk of bias (study stated that outcome assessors were blind to treatment allocation but lacked a clear statement on how it was achieved). Studies where outcome assessment was not blinded were considered as having a high risk of bias.
Incomplete outcome data (checking for attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or investigators used 'baseline observation carried forward' analysis, or both); unclear risk of bias (investigators used 'last observation carried forward' analysis); or high risk of bias (investigators used 'completer' analysis).
Selective reporting (checking for reporting bias). We assessed whether primary and secondary outcome measures were prespecified, and whether they were consistent with those reported. We assessed the methods as: low risk of bias (study protocol was available and all of the study's prespecified primary and secondary outcomes that were of interest were reported in the prespecified way, or if the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified); high risk of reporting bias (not all of the study's prespecified primary outcomes were reported; one or more primary outcomes were reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely so that they could not be entered into a meta‐analysis; the study report did not include results for a key outcome that would be expected to have been reported for such a study.
Size of study (checking for bias confounded by small size). We assessed studies as being at low risk of bias (200 or more participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
For dichotomous outcomes between groups, we estimated and compared the risk ratio (RR) and 95% confidence intervals (CIs). For continuous outcomes, we measured arithmetic mean and standard deviation (SD) and reported the mean difference (MD) between groups, with 95% CI. When an outcome was derived with different instruments measuring the same construct, we used the SMD with 95% CIs.
Unit of analysis issues
We only included studies that randomised individual participants. For trials containing multiple arms, we only included pair‐wise comparisons of each intervention arm to the control arm.
Dealing with missing data
We ascertained how the investigators analysed the data from withdrawals, where possible. It was not possible to assess the impact of missing data in sensitivity analyses due to the low study numbers. In all cases, we aimed to perform intention‐to‐treat (ITT) analyses.
Assessment of heterogeneity
There may be an effect of differences between participants, environment (inpatient versus outpatient) and outcome measures. We assessed heterogeneity by using the I² statistic. We considered I² values above 50% to represent substantial heterogeneity, in line with Higgins 2011, and attempted to assess potential sources of heterogeneity through subgroup analyses.
Assessment of reporting biases
We had planned to interpret the results in light of a visual inspection of a funnel plot, but were unable to do so due to the lack of studies.
Data synthesis
We entered the data extracted from the included studies into Review Manager 5, which we used for data synthesis (Review Manager 2014). We had planned to pool data for each continuous outcome and calculate the MD as an estimate of effect size, using a random‐effects model with 95% CIs, but were unable to do so due to the lack of studies.
Quality of the evidence
Two review authors (AH, SK) planned to independently rate the quality of evidence for dyspnoea relief using the three 'Domains of Dyspnoea Measurement' of dyspnoea, serious adverse events, participant satisfaction with treatment, and participant withdrawal from trial. We used the GRADE system to assess the quality of the available evidence using the GRADEpro Guideline Development Tool software (GRADEpro GDT 2015), and the guidelines provided in Chapter 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We presented the findings in a 'Summary of findings' table.
The GRADE approach employs five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system defines the quality of a body of evidence as the extent to which one can be confident of an estimate of effect, namely:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident that the true effect lies close to that of the estimate of the effect;
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
High: randomised trials or double‐upgraded observational studies.
Moderate: downgraded randomised trials or upgraded observational studies.
Low: double‐downgraded randomised trials or observational studies.
Very low: triple‐downgraded randomised trials, downgraded observational studies or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide CIs);
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
dose–response gradient.
We decreased the grade rating by one (–1) or two (–2) (up to a maximum of –3 to 'very low') for:
serious (–1) or very serious (–2) limitation to study quality;
important inconsistency (–1);
some (–1) or major (–2) uncertainty about directness;
imprecise or sparse data (–1); or
high probability of reporting bias (–1).
'Summary of findings' table
We included a ‘Summary of findings’ table to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on dyspnoea relief, as measured by the three domains of dyspnoea, i.e. intensity of dyspnoea (sensory‐perceptual experience, as measured by ratings of breathlessness intensity), quality of dyspnoea (affective distress, as measured by ratings of severity of distress), and burden of breathlessness/impact on function (as measured by ratings of functional ability, quality of life or health status), adverse events, patient satisfaction with treatment, and participant withdrawal from trial.
Subgroup analysis and investigation of heterogeneity
There were insufficient data available to perform subgroup analyses based on type of systemic corticosteroid, dose, type of cancer and length of the trial.
Sensitivity analysis
We had planned to examine the robustness of the meta‐analyses by conducting sensitivity analyses using different components of the 'Risk of bias' assessment – particularly those relating to selection bias, and trial size, to see if any of these factors influenced the results. We had also planned to investigate variation across studies (heterogeneity) by comparing a random‐effects model with a fixed‐effect model. We were unable to perform any sensitivity analyses due to the low number of studies included in the meta‐analysis, and the small number of participants in each comparison.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The PRISMA diagram outlines the number of records identified in the search and screening process for these papers (Figure 1). In the initial database search, we identified 1350 records. None were identified through other sources. Of these, 285 were duplicates and we rejected 1047 based on information given in the title and abstract. We identified 18 publications for full‐text retrieval, and excluded 16 of these records during screening. The reasons for exclusion of each study are described in the Characteristics of excluded studies table. Two placebo‐controlled studies met the inclusion criteria for this review (Hui 2016; Yennurajalingam 2013). We evaluated the results relative to dyspnoea intensity/dyspnoea relief at one week from baseline (day seven and eight), since this was the only time that could be standardised across both trials.
Included studies
We identified two studies meeting the inclusion criteria (Hui 2016; Yennurajalingam 2013). These two studies enrolled 157 participants (37 and 120 participants per study), of whom 114 were included in the analyses. The studies compared oral dexamethasone to placebo. One study lasted seven days, and the duration of the other study was 15 days. A detailed description of the included studies can be found in the Characteristics of included studies table.
Primary disease sites
We have shown the primary disease sites in Table 2. Eligibility criteria in the included trials did not specify a particular cancer.
1. Primary sites of disease.
| Study | Breast | Head, neck and lung | Gastrointestinal | Gynaecological | Genitourinary | Sarcoma | Not specified |
| Hui 2016 | — | 37 | — | — | — | — | 4 |
| Yennurajalingam 2013 | 13 | 45 | 39 | 9 | 10 | 9 | 7 |
Types of studies
We included studies in which corticosteroids were used for cancer‐related dyspnoea, or any other indication. The studies assessed dyspnoea relief intensity, quality and burden/impact on function.
Dyspnoea as a primary endpoint
Of the two included studies, one measured change in dyspnoea intensity as a primary endpoint (Hui 2016). The other study measured fatigue (a change in the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT‐F) subscale) as the primary outcome (Yennurajalingam 2013).
Types of corticosteroids studied
Both studies used oral dexamethasone. One study compared dexamethasone (8 mg twice a day for four days, then 4 mg twice a day for three days) to placebo (Hui 2016). The other study compared dexamethasone (8 mg/day) to placebo (Yennurajalingam 2013).
Dyspnoea measurement tools
The studies used different measurement tools to measure dyspnoea relief intensity, quality, burden/impact on function and quality of life.
Dyspnea Numeric Rating Scale 'now' (NRS) 0 to 10 (0 = no shortness of breath) (Hui 2016).
Edmonton Symptom Assessment Scale (ESAS) 0 to 10 (0 = no shortness of breath) – dyspnoea mean past 24 hours; fatigue, drowsiness and appetite (Hui 2016; Yennurajalingam 2013).
Modified Dyspnea Borg Scale (0 to 10) (0 = no shortness of breath at all) (Hui 2016).
Cancer Dyspnea Scale 1 to 5 (higher score indicates a greater intensity of dyspnoea) (Hui 2016).
European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire‐Core 30 – dyspnoea past week (Hui 2016).
Global Symptom Evaluation (Hui 2016).
Excluded studies
We excluded 16 studies and provided reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed each study using the Cochrane 'Risk of bias' tool. We presented overall findings in the 'Risk of bias' graph (Figure 2), which presents the authors' judgements about each risk of bias domain as percentages across all included studies. We have shown the authors' judgements about each risk of bias domain for each included study in the 'Risk of bias' summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Both included studies reported that they were randomised. Only one study described the method used to generate their random sequence (Hui 2016), thus we judged the second study to have an unclear risk of bias (Yennurajalingam 2013).
Allocation concealment
For allocation concealment, only one study used pharmacy randomisation and was at low risk in this regard (Hui 2016). The other study was at unclear risk, as there was insufficient information on the method of allocation concealment (Yennurajalingam 2013).
Blinding
Both studies were at low risk, with blinding of participants, personnel and outcome assessments reported (Hui 2016; Yennurajalingam 2013).
Incomplete outcome data
The two studies were at high risk for attrition bias, as greater than 10% of participants could not be evaluated, thereby leaving a gap in the evidence (i.e. 6/19 participants receiving the intervention and 3/18 participants receiving placebo (Hui 2016); 19/62 participants receiving the intervention, and 17/58 participants receiving placebo (Yennurajalingam 2013)).
Selective reporting
Neither study identified reporting gaps. Therefore, we judged these studies at low risk for reporting bias.
Other potential sources of bias
Small studies are thought to be at increased risk of bias as they are unlikely to be adequately powered. Neither study was large enough to be at low risk of bias (more than 200 participants per arm). We judged one study to have an unclear risk of bias due to sample size (50 to 199 participants per arm) (Yennurajalingam 2013). The other study was at high risk of bias because of their small number of participants (fewer than 50 participants per treatment arm) (Hui 2016).
Effects of interventions
See: Table 1
Primary outcome
Participant‐reported dyspnoea intensity, quality and burden
For the meta‐analysis, both studies provided change in dyspnoea intensity score (on a scale of 0 to 10; lower score = less breathlessness) and the standard deviation at one week (Hui 2016; Yennurajalingam 2013). The studies included 157 participants at baseline and 114 participants after one week of corticosteroid treatment (dexamethasone). After one week, the dexamethasone group reported less dyspnoea intensity than the control group (MD –0.85, 95% CI –1.73 to 0.03; P = 0.06; Analysis 1.1), although we were uncertain as to whether corticosteroids truly had an important effect on dyspnoea as results were imprecise.
1.1. Analysis.

Comparison 1: Breathlessness (dyspnoea) at one week, Outcome 1: Breathlessness (dyspnoea)
Both studies used a numeric rating scale to assess the intensity of dyspnoea 'now' – an 11‐point dyspnoea numeric rating scale (0 to 10).
We judged the quality of the evidence for the primary outcome of dyspnoea relief (intensity) to be very low (see Table 1). We downgraded the quality of the evidence once for imprecision due to the small number of participants in the included studies, and twice for very serious study limitations (likely selection and attrition bias).
One study measured affective distress/quality of dyspnoea (Hui 2016), using the Cancer Dyspnea Scale effort, discomfort and anxiety variables (Domain 2 of Parshall's 'Domains of Dyspnoea Measurement') and results were similar between groups. We judged the quality of the evidence for the primary outcome of dyspnoea relief (quality) to be very low. We downgraded the quality of the evidence three times for imprecision due to the small number of participants in the included study, inconsistency, and serious study limitations (likely attrition bias).
Both studies assessed impact of dyspnoea on function/quality of life. Hui 2016 assessed dyspnoea quality and burden/impact on function and quality of life using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36 (EORTC QLQ‐C30) dyspnoea 'past week,' which favoured dexamethasone compared to placebo at day four, but not at day seven. However, it was unclear whether dexamethasone had a significant effect on dyspnoea as results were imprecise. In Yennurajalingam 2013, FACIT physical well‐being scores showed significantly better improvement at days eight (P = 0.007) and 15 (P = 0.002) in the dexamethasone group compared with the control group, however there were no significant changes for FACIT social/family, emotional or functional scales. We judged the quality of the evidence for the primary outcome of dyspnoea relief (burden) to be very low. We downgraded the quality of the evidence three times for imprecision due to the small number of participants in the included studies, inconsistency, and serious study limitations (likely selection and attrition bias).
Secondary outcomes
Serious adverse events
The severity of adverse events was not properly defined, making it difficult to assess this outcome. Hence we evaluated reported ‘adverse events’. In both studies, the frequency of adverse events was similar between groups, and dexamethasone was generally well tolerated. We judged the quality of the evidence for this outcome to be very low. We downgraded the quality of the evidence three times due to serious study limitations (likely selection and attrition bias), inconsistency, and imprecision due to the small number of participants in the included studies.
Participant satisfaction with treatment
Neither study measured participant satisfaction with treatment.
Participant withdrawal
There were 6/41 (15%) participant withdrawals in Hui 2016, and 48/132 (36%) in Yennurajalingam 2013. We judged the quality of the evidence for this outcome to be very low. We downgraded the quality of the evidence three times from high to very low due to serious study limitations (likely selection and attrition bias), inconsistency, and imprecision due to the small number of participants in the included studies.
Discussion
Summary of main results
The objective of this systematic review was to assess the effects of corticosteroids on dyspnoea in adults with cancer‐related dyspnoea. Two studies with 157 participants met the inclusion criteria, of whom 114 were evaluable. The included studies assessed dexamethasone (4 mg/day or up to 16 mg/day) compared to placebo.
Both studies were evaluated for relief of dyspnoea in the meta‐analysis (Hui 2016; Yennurajalingam 2013). We reported data after one week of intervention, since this was the only time that could be standardised across both trials. The following conclusion regarding the effectiveness of corticosteroids for relief of dyspnoea should be interpreted with consideration of the small number of eligible studies with small numbers of participants in each treatment arm, and difference in dexamethasone dose. The quality of evidence was very low due to very serious study limitations and imprecision.
There was no clear difference in favour of dexamethasone over placebo (MD –0.85, 95% –1.73 to 0.03) for dyspnoea at one week of intervention (P = 0.06).
There were insufficient data to evaluate different subgroups, such as drug type, route of administration, dosage and different primary disease types.
Overall completeness and applicability of evidence
We identified two studies that met the inclusion criteria and included these studies in the meta‐analysis for dyspnoea relief/change in dyspnoea intensity from baseline. There was a lack of studies for planned comparisons, and insufficient data for subgroup analyses. The results were also influenced by differences in dosages, comparators and heterogeneity of study populations. The studies excluded people with overlying conditions that would be expected to respond to corticosteroids (e.g. COPD and superior vena cava obstruction). Comparators included in the meta‐analysis included dexamethasone compared to placebo. We also included trials where dyspnoea was not the primary outcome.
Quality of the evidence
The quality of evidence for breathlessness intensity was very low, downgraded once for imprecision due to the small number of participants in the included studies, and twice for very serious study limitations (likely selection and attrition bias). Both studies measured participant‐reported dyspnoea relief/intensity of dyspnoea at similar time points, and burden of dyspnoea/impact on function and quality of life (Hui 2016; Yennurajalingam 2013). Only one study measured affective distress/quality of dyspnoea (Hui 2016). We judged the quality of the evidence for the outcomes breathlessness quality and burden to be very low, downgraded three times for imprecision due to the small number of participants in the included studies, inconsistency, and serious study limitations.
We judged the quality of the evidence for the outcomes adverse events and withdrawals to be very low, downgraded three times due to serious study (likely selection and attrition bias), inconsistency, and imprecision due to the small number of participants in the included studies. Neither study measured participant satisfaction with treatment.
Other concerns regarding quality of the evidence included the fact that one study was an inadequately powered pilot study with greater than 10% dropouts in both arms prior to day seven (Hui 2016). The other study had a very high participant withdrawal from trial, with approximately 30% dropouts by day 15 (Yennurajalingam 2013). Therefore, the current body of evidence does not allow a robust conclusion. We have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect.
Potential biases in the review process
To minimise bias, three review authors independently extracted data and five authors assessed risk of bias. Due to the lack of studies we were unable to determine if there was evidence of small‐study effects.
Agreements and disagreements with other studies or reviews
We found no other studies or reviews that assessed the quality of evidence and effects of systemic corticosteroid use for the management of cancer‐related dyspnoea in adults.
Authors' conclusions
Implications for practice.
For people with dyspnoea
There is insufficient evidence to support or refute the suggestion that systemic corticosteroids have any efficacy in cancer‐related breathlessness (dyspnoea) in adults.
For clinicians
There is insufficient evidence to support or refute the suggestion that systemic corticosteroids have any efficacy in cancer‐related breathlessness (dyspnoea) in adults. This may be particularly relevant when considering the potential toxicity of corticosteroids, especially following prolonged use.
For policy makers
There is insufficient evidence to support or refute the suggestion that systemic corticosteroids have any efficacy in cancer‐related breathlessness (dyspnoea) in adults.
For funders
There is insufficient evidence to support or refute the suggestion that systemic corticosteroids have any efficacy in cancer‐related breathlessness (dyspnoea) in adults (that is not related to a number of specific respiratory or inflammatory (or both) conditions).
Implications for research.
General implications
This review has highlighted a marked paucity of research in the subject area. Future robust, double‐blind randomised trials with significant numbers of participants (e.g. over 200 per treatment arm) are needed to evaluate the safety and effectiveness of systemic corticosteroids in the management of cancer‐related dyspnoea in adults.
Design
There are few randomised controlled trials assessing the benefit of systemic corticosteroids in cancer‐related dyspnoea. There is a need for further research to observe the effects of systemic corticosteroids on all three domains of dyspnoea measurement (i.e. dyspnoea intensity, dyspnoea quality and dyspnoea burden/impact on function) alongside participant satisfaction with treatment, and assessment of serious adverse events. Further trials with increased number of participants are needed to evaluate the effectiveness of corticosteroids for the management of dyspnoea in adults with cancer‐related breathlessness. Comparators should include placebo versus combinations of dexamethasone with other antidyspnoea agents, since the mechanism of cancer‐related dyspnoea is not well understood and involves multiple sites of action in the body.
Measurement (endpoints)
There is currently no standard outcome measure for the measurement of cancer‐related dyspnoea. This must be determined prior to future studies. Appropriate time points for measuring the effect of corticosteroids on breathlessness also need to be determined.
What's new
| Date | Event | Description |
|---|---|---|
| 22 February 2021 | Review declared as stable | See Published notes |
History
Protocol first published: Issue 6, 2017 Review first published: Issue 2, 2019
Notes
An updated search in January 2021 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be assessed for updating in two years. If appropriate we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Acknowledgements
We acknowledge the input of the Cochrane Airways Group in clarifying the outcomes and Ke Cheng PhD, School of Acupuncture‐Moxibustion and Tuina, Shanghai University of Traditional Chinese Medicine, China for assistance with translation.
The authors would like to thank the following peer reviewers: Richella Ryan; Adrian Tookman; Janet Waddell. We also thank the consumer reviewer who did not wish to be named.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pain, Palliative and Supportive Care (PaPaS). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees
#2 MeSH descriptor: [Betamethasone] explode all trees
#3 MeSH descriptor: [Fludrocortisone] explode all trees
#4 MeSH descriptor: [Dexamethasone] explode all trees
#5 MeSH descriptor: [Methylprednisolone] explode all trees
#6 MeSH descriptor: [Prednisolone] explode all trees
#7 MeSH descriptor: [Triamcinolone] explode all trees
#8 MeSH descriptor: [Beclomethasone] explode all trees
#9 (corticoid* or corticosteroid* or glucocorticoid* or betamethasone or fludrocortisone or cortisone or deflazacort or dexamethasone or hydrocortisone or methylprednisolone or prednisolone or triamcinolone or beclomethasone)
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Dyspnea] explode all trees
#12 (dysp* or breathless* or ((short or labor* or difficult*) near/3 breath*)):ti,ab
#13 #11 or #12
#14 MeSH descriptor: [Neoplasms] explode all trees
#15 (cancer* or carcinoma* or malignan* or adenocarcinoma* or mesothelioma* or tumour* or tumor*)
#16 #14 or #15
#17 #10 and #13 and #16
Appendix 2. MEDLINE via Ovid search strategy
1. exp Adrenal Cortex Hormones/
2. (corticoid* or corticosteroid* or glucocorticoid*).tw.
3. (adrenal adj2 hormone*).tw.
4. Betamethasone/
5. betamethasone.tw.
6. Fludrocortisone/
7. fludrocortisone.tw.
8. Cortisone/
9. cortisone.tw.
10. deflazacort.tw.
11. Dexamethasone/
12. dexamethasone.tw.
13. Hydrocortisone/
14. hydrocortisone.tw.
15. Methylprednisolone/
16. methylprednisolone.tw.
17. Prednisolone/
18. prednisolone.tw.
19. Triamcinolone/
20. triamcinolone.tw.
21. exp Mometasone Furoate/
22. exp Fluticasone/
23. exp Beclomethasone/
24. exp Budesonide/
25. exp Fluocinolone Acetonide/ai [Antagonists & Inhibitors]
26. exp Androstadienes/
27. exp Pregnenediones/
28. exp Pregnadienediols/
29. budesonide.tw.
30. mometasone.tw.
31. beclomethasone.tw.
32. flunisolide.tw.
33. fluticasone.tw.
34. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33
35. "malignan*".tw.
36. "tumour*".tw.
37. "tumor*".tw.
38. "cancer*".tw.
39. "carcinoma*".tw.
40. "adenocarcinoma*".tw.
41. exp Neoplasms/
42. 35 or 36 or 37 or 38 or 39 or 40 or 41
43. exp Dyspnea/
44. "dyspn*".tw.
45. (short* adj2 breath*).tw.
46. (breath* adj2 difficult*).tw.
47. (labo*r* adj2 breath*).tw.
48. "breathless*".tw.
49. 43 or 44 or 45 or 46 or 47 or 48
50. randomized controlled trial.pt.
51. controlled clinical trial.pt.
52. randomized.ab.
53. randomised.ab.
54. placebo.ab.
55. randomly.ab.
56. trial.ab.
57. groups.ab.
58. drug therapy.fs.
59. 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58
60. exp animals/ not humans.sh.
61. 59 not 60
62. 34 and 42 and 49 and 61
Appendix 3. Embase search strategy
(('corticosteroid'/exp OR ('betamethasone'/exp OR betamethasone:ti,ab) OR ('cortisone'/exp OR cortisone:ti,ab) OR ('deflazacort'/exp OR deflazacort:ti,ab) OR ('fludrocortisone'/exp OR fludrocortisone:ti,ab) OR ('dexamethasone'/exp OR dexamethasone:ti,ab) OR ('hydrocortisone'/exp OR hydrocortisone:ti,ab) OR ('methylprednisolone'/exp OR methylprednisolone:ti,ab) OR ('prednisolone'/exp OR prednisolone:ti,ab) OR ('triamcinolone'/exp OR triamcinolone:ti,ab)) OR beclometasone:ti,ab OR beclomethasone:ti,ab)
AND
(('neoplasm'/exp OR tumor*:ti,ab OR tumour*:ti,ab OR cancer*:ti,ab OR carcinoma*:ti,ab OR adenocarcinoma*:ti,ab OR malignan*:ti,ab) OR mesothelioma)
AND
(random*:ab,ti OR placebo*:de,ab,ti OR (double NEXT/1 blind*):ab,ti)
AND
('cheyne stokes breathing'/exp OR dyspn*:ti,ab OR breathless*:ti,ab OR (short* NEAR/2 breath*):ti,ab OR 'dyspnea'/exp/mj)
Appendix 4. CINAHL search strategy
S47 S34 NOT S46
S46 S34 AND S45
S45 S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44
S44 (MH "Quantitative Studies")
S43 (MH "Placebos")
S42 TX placebo
S41 TX random* W1 allocat*
S40 (MH "Random Assignment")
S39 TX random* control* trial*
S38 TX (trebl* OR tripl* OR doubl* OR singl*) N1 (blind* OR mask*)
S37 TX clinic* N1 trial*
S36 PT Clinical Trial
S35 (MH "Clinical Trials+")
S34 S32 AND S33
S33 S20 AND S23
S32 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S31 TX breathless*
S30 TX labo#r* N2 breath*
S29 labo#r* N2 breath*
S28 TX breath N2 difficult*
S27 TX short* n2 breath*
S26 TX cheyne stokes
S25 TX dyspn*
S24 (MH "Dyspnea+")
S23 S21 OR S22
S22 TX malignan* OR tumor* OR tumour* OR cancer* OR carcinoma* OR adenocarcinoma* OR mesothelioma*
S21 (MH "Neoplasms+")
S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S19 TX beclomethasone
S18 (MH "Beclomethasone")
S17 (MH "Triamcinolone")
S16 TX triamcinolone
S15 (MH "Prednisolone+")
S14 TX methylprednisolone
S13 (MH "Methylprednisolone")
S12 TX hydrocortisone
S11 (MH "Hydrocortisone")
S10 (MH "Dexamethasone")
S9 TX dexamethasone
S8 TX deflazacort
S7 TX cortisone
S6 TX fludrocortisone
S5 TX betamethasone
S4 (MH "Betamethasone")
S3 TX adrenal N2 hormon*
S2 TX corticoid* OR corticosteroid* OR glucocorticoid*
S1 (MH "Adrenal Cortex Hormones+")
Appendix 5. Science Citation Index (ISI Web of Science) search strategy
TOPIC: (corticoid* OR corticosteroid* OR glucocorticoid* OR betamethasone OR Fludrocortisone OR cortisone OR dexamethasone OR hydrocortisone OR methylprednisolone OR prednisolone OR triamcinolone OR beclomethasone) ANDTOPIC: (cancer* OR carcinoma* OR adenocarcinoma* OR malignan* OR tumour* OR tumor*) ANDTOPIC: (dyspn* OR breath*)
Appendix 6. LILACS search strategy
1. w:((dyspnoea OR dyspnea) AND (cancer OR malignancy OR malignant OR tumours OR tumors OR carcinoma) AND (corticosteroids OR glucocorticoids OR dexamethasone OR hydrocortisone OR methylprednisolone OR prednisolone OR betamethasone)) AND (instance:"regional") AND ( type_of_study:("clinical_trials"))
tw:((mh:(adrenal cortex hormones)) OR (tw:(corticosteroid* OR corticoid* OR glucocorticoid* OR betamethasone OR fludrocortisone OR cortisone OR dexamethasone OR hydrocortisone OR methylprednisolone OR prednisolone OR triamcinolone OR beclomethasone)) AND (mh:(neoplasms)) OR (tw:(cancer* OR malignan* OR carcinoma* OR adenocarcinoma* OR tumour* OR tumor* OR mesothelioma)) AND (mh:(respiratory physiological phenomena)) OR (tw:(dyspn* OR "cheyne stokes" OR breathless*))) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("cohort" OR "case_control" OR "clinical_trials"))
Data and analyses
Comparison 1. Breathlessness (dyspnoea) at one week.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Breathlessness (dyspnoea) | 2 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.73, 0.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hui 2016.
| Study characteristics | ||
| Methods | Randomised, double‐blind, parallel, placebo‐controlled trial Single‐institution Country: USA Year: 2013–2015 Study duration: randomised trial for 7 days with an open‐label extension phase for another 7 days |
|
| Participants | 37 adults (19 intervention, 18 control) with a diagnosis of cancer with clinical or radiological evidence of lung involvement. Exclusion criteria: people with delirium, oxygen saturation < 90% despite supplemental oxygen > 6 L/minute, diabetes mellitus uncontrolled on oral hypoglycaemic agents or insulin, severe anaemia, open wound that had not healed, infection requiring antibiotics or major surgery within the past 2 weeks, chronic obstructive pulmonary disease exacerbation and heart failure exacerbation, receiving active or recent chronic systemic corticosteroids (> 14 days), or using megestrol acetate, or receiving chemotherapy or expected to start within 1 week of study enrolment. |
|
| Interventions | Participants randomised into 2 groups. Intervention: dexamethasone 8 mg (2 × 4 mg capsules) orally twice a day for 4 days, then 4 mg given orally twice a day for 3 days Control: identical‐appearing placebo capsules After 1 week, all participants received dexamethasone 4 mg orally twice a day for 7 days in an open‐label extension. |
|
| Outcomes | Primary outcome: proportion of participants who completed the blinded phase of the study (35/37 participants). Dyspnoea assessed at baseline, days 4 (SD 2) and days 7 (SD 2) using the ESAS, Modified Dyspnea Borg Scale, Cancer Dyspnea Scale and 1 item on dyspnoea from the EORTC Quality of Life Questionnaire. Secondary outcomes: fatigue, drowsiness and appetite using ESAS. |
|
| Notes | Dexamethasone was associated with rapid improvement in dyspnoea and was well tolerated; however, sample size was small and the study was not powered for between‐arm comparisons. Funding: MD Anderson Cancer Center Institutional Research Grant. Authors also supported in part from American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research and National Institutes of Health Grants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Used a secured website for allocation that was only accessible to the study pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo capsules were identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both participants and research staff conducting the study assessments were blinded to the randomisation sequence and the study intervention." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Used 'completer' analysis. 2 participants did not complete the 7‐day intervention and 4 participants did not complete from the placebo group. |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | Sample size: 37 participants; < 50 participants per treatment arm. |
Yennurajalingam 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Outpatients at 3 study centres Country: USA Year: not described Study duration: 14 days |
|
| Participants | 120 (62 intervention, 58 control) people with advanced cancer with ≥ 3 symptoms during the previous 24 hours (e.g. fatigue, pain, nausea, loss of appetite, depression, anxiety or sleep disturbance) with mean intensity of ≥ 4/10 on the ESAS. | |
| Interventions | Participants randomised into 2 groups Intervention: dexamethasone 4 mg orally twice per day for 14 days. Control: placebo orally twice per day for 14 days. |
|
| Outcomes | Primary outcome: fatigue, measured by a change in the FACIT‐F subscale from baseline to day 15. Secondary outcomes: anorexia, anxiety, depression, shortness of breath and symptom distress scores. Used the FACIT‐F, ESAS, Hospital Anxiety and Depression Scale, and Functional Assessment of Cancer Therapy‐Anorexia‐Cachexia instruments. |
|
| Notes | Dexamethasone was more effective than placebo in improving cancer‐related fatigue and quality of life. There was a non‐significant improvement in shortness of breath at days 8 and 15. Funding: American Cancer Society Mentored Research Scholar Grant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All members of the research team except the investigational pharmacist and statistician were blinded to treatment assignment throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19/62 participants receiving dexamethasone were not evaluable. 17/58 participants receiving placebo were not evaluable. |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | Unclear risk | Sample size: 120 participants; 50–199 participants per treatment arm. |
EORTC: European Organization for Research and Treatment of Cancer; ESAS: Edmonton Symptom Assessment System; FACIT‐F: Functional Assessment of Chronic Illness – Fatigue; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davidson 2010 | Not an RCT. Literature review on management of refractory dyspnoea. |
| Delgado 1986 | Did not study breathlessness. |
| Elsayem 2007 | Case studies only. |
| Jantarakupt 2005 | Not an RCT. Literature review on dyspnoea management in lung cancer. |
| Lui 2013 | Not a double‐blind study. |
| McCannon 2012 | Not an RCT. Literature review about dyspnoea management in lung cancer. |
| North 2003 | Wrong intervention. Used intrapleural administration of methylprednisolone acetate for malignant pleural effusion. |
| Ripamonti 1999 | Not an RCT. Literature review of dyspnoea in advanced cancer. |
| Simoff 2013 | Not an RCT. Literature review of symptom management in people with lung cancer. |
| Simon 2012 | Systematic review of refractory dyspnoea, but found no studies of corticosteroids. |
| Skřičková 2013 | Not an RCT. Literature review about management of respiratory symptoms in people with advanced lung cancer. |
| Thomas 2002 | Not an RCT. Literature review on management of dyspnoea. |
| Viola 2008 | Systematic review of management of dyspnoea in people with cancer. Found no studies of systemic corticosteroids. |
| White 1984 | Not an RCT. All participants received corticosteroids for bleomycin‐induced pneumonitis. |
| Yennu 2015 | Abstract only. Studied symptom clusters, and not breathlessness as an individual outcome. |
| Yennurajalingam 2016 | Studied symptom clusters, and not breathlessness as an individual outcome. |
RCT: randomised controlled trial.
Differences between protocol and review
We had planned for two authors to independently extract data and perform assessments. At review stage, three review authors (AH, SK, JD) independently extracted data from the studies. Five review authors (AH, SK, JH, PG, JD) independently assessed the risk of bias of each included study.
We stated our intention to search the metaRegister of controlled trials (mRCT) (www.controlled-trials.com/mrct), but this database is under review and not available to search.
Contributions of authors
| Draft the protocol | AH, JD |
| Develop and run the search strategy | KR PaPaS Information Specialist provided support |
| Obtain copies of studies | KR |
| Select which studies to include | JH, PG, PVB, JD |
| Extract data from studies | AH, SK, JD |
| Enter data into Review Manager 5 | AH |
| Assess risk of bias | AH, SK, JH, PG, JD |
| Carry out the analysis | AH, SK |
| Interpret the analysis | JH, PG, AH, SK, JD |
| Draft the final review | AH, KR, JD |
| Update the review | AH, JH, PG, KR |
Sources of support
Internal sources
-
In kind and operational funds, Australia
Mater Research – The University of Queensland, School of Pharmacy and Menzies Health Institute Queensland, Griffith University, The Mater Palliative Care Research Fund and St Vincent's Hospital Brisbane.
External sources
No sources of support supplied
Declarations of interest
AH: none known.
JD: none known; JD is a trainee physician in palliative medicine and manages patients with dyspnoea due to advanced cancer.
PG: none known; PG is a specialist palliative medicine physician and manages patients with dyspnoea due to advanced cancer.
SK: none known.
KR: none known.
PVB: none known; PVB is a specialist palliative medicine physician and manages patients with dyspnoea due to advanced cancer.
JH: none known; JH is a specialist palliative medicine physician and manages patients with dyspnoea due to advanced cancer. JH receives royalties from Oxford University Press.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Hui 2016 {published data only}
- Hui D, Kilgore K, Frisbee-Hume S, Park M, Tsao A, Delgado Guay M, et al. Dexamethasone for dyspnea in cancer patients: a pilot double-blind, randomized, controlled trial. Journal of Pain and Symptom Management 2016;52(1):8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yennurajalingam 2013 {published data only}
- Yennurajalingam S, Frisbee-Hume S, Palmer JL, Delgado-Guay MO, Bull J, Phan AT, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. Journal of Clinical Oncology 2013;31(25):3076-82. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Davidson 2010 {published data only}
- Davidson PM, Currow DC. Management of refractory dyspnoea: evidence-based interventions. Cancer Forum 2010;34(2):86-90. [Google Scholar]
Delgado 1986 {published data only}
- Delgado GL, Stefanuto W, Novo NF. Combination of anti-inflammatory agents in antineoplastic chemotherapy [Associaçäo de antiinflamatórios à quimioterapia antineoplásica]. Revista Paulista de Medicina 1986;104(2):93-8. [PubMed] [Google Scholar]
Elsayem 2007 {published data only}
- Elsayem A, Bruera E. High-dose corticosteroids for the management of dyspnea in patients with tumor obstruction of the upper airway. Supportive Care in Cancer 2007;15(12):1437-9. [DOI] [PubMed] [Google Scholar]
Jantarakupt 2005 {published data only}
- Jantarakupt P, Porock D. Dyspnea management in lung cancer: applying the evidence from chronic obstructive pulmonary disease. Oncology Nursing Forum 2005;32(4):785-97. [DOI] [PubMed] [Google Scholar]
Lui 2013 {published data only}
- Liu F, Wang M-L, Yuan H-H, Wang Y, Jiang B. Effects of morphine, methylprednisolone and aminophylline on management of dyspnea in patients with advanced cancer. Journal of Shanghai Jiaotong University (Medical Science) 2013;33(6):823-26. [Google Scholar]
McCannon 2012 {published data only}
- McCannon J, Temel J. Comprehensive management of respiratory symptoms in patients with advanced lung cancer. Journal of Supportive Oncology 2012;10(1):1-8. [DOI] [PubMed] [Google Scholar]
North 2003 {published data only}
- North SA, Au HJ, Halls SB, Tkachuk L, Mackey JR. A randomized, phase III, double-blind, placebo-controlled trial of intrapleural instillation of methylprednisolone acetate in the management of malignant pleural effusion. Chest 2003;123(3):822-27. [DOI] [PubMed] [Google Scholar]
Ripamonti 1999 {published data only}
- Ripamonti C. Management of dyspnea in advanced cancer patients. Supportive Care in Cancer 1999;7(4):233-43. [DOI] [PubMed] [Google Scholar]
Simoff 2013 {published data only}
- Simoff MJ, Lally B, Slade MG, Goldberg WG, Lee P, Michaud GC, et al. Symptom management in patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e455S-97S. [DOI] [PubMed] [Google Scholar]
Simon 2012 {published data only}
- Simon ST, Koskeroglu P, Bausewein C. Pharmacological therapy of refractory dyspnoea: a systematic literature review. Der Schmerz 2012;26(5):515-22. [DOI] [PubMed] [Google Scholar]
Skřičková 2013 {published data only}
- Skřičková J, Merta Z. Management of respiratory symptoms in patients with advanced lung cancer. Lung Cancer Management 2013;2(4):317-25. [Google Scholar]
Thomas 2002 {published data only}
- Thomas JR, Von Gunten CF. Clinical management of dyspnoea. Lancet Oncology 2002;3(4):223-8. [DOI] [PubMed] [Google Scholar]
Viola 2008 {published data only}
- Viola R, Kiteley C, Lloyd N S, Mackay J A, Wilson J, Wong R K S. The management of dyspnea in cancer patients: a systematic review. Supportive Care in Cancer 2008;16(4):329-37. [DOI] [PubMed] [Google Scholar]
White 1984 {published data only}
- White DA, Stover DE. Severe bleomycin-induced pneumonitis. Clinical features and response to corticosteroids. Chest 1984;86(5):723-8. [DOI] [PubMed] [Google Scholar]
Yennu 2015 {published data only}
- Yennu S, Williams JL, Chisholm GB, Bruera E. The effects of dexamethasone and placebo on symptom clusters in advanced cancer patients: a preliminary report. Journal of Clinical Oncology 2015;33(29):187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yennurajalingam 2016 {published data only}
- Yennurajalingam S, Williams JL, Chisholm G, Bruera E. Effects of dexamethasone and placebo on symptom clusters in advanced cancer patients: a preliminary report. Oncologist 2016;21(3):384-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abernethy 2008
- Abernethy AP, Wheeler JL. Total dyspnoea. Current Opinion in Supportive and Palliative Care 2008;2(2):110-3. [DOI] [PubMed] [Google Scholar]
American Thoracic Society 1999
- American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. American Journal of Respiratory and Critical Care Medicine 1999;159(1):321-40. [DOI] [PubMed] [Google Scholar]
Banzett 2008
- Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. American Journal of Respiratory and Critical Care Medicine 2008;177(12):1384-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barczyk 2004
- Barczyk A, Sozanska E, Trzaska M, Pierzchala W. Decreased levels of myeloperoxidase in induced sputum of patients with COPD after treatment with oral glucocorticoids. Chest 2004;126(2):389-93. [DOI] [PubMed] [Google Scholar]
Bausewein 2008
- Bausewein C, Booth S, Gysels M, Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD005623. [DOI: 10.1002/14651858.CD005623.pub2] [DOI] [PubMed] [Google Scholar]
Ben‐Aharon 2012
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncologica 2012;51(8):996-1008. [DOI] [PubMed] [Google Scholar]
Bernhard 1991
- Bernhard J, Ganz PA. Psychosocial issues in lung cancer patients (Part 2). Chest 1991;99(2):480-5. [DOI] [PubMed] [Google Scholar]
Booth 2003
- Booth S, Silvester S, Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: using a qualitative approach to describe the experience of patients and carers. Palliative & Supportive Care 2003;1(4):337-44. [DOI] [PubMed] [Google Scholar]
Booth 2006
- Booth S, Farquhar M, Gysels M, Bausewein C, Higginson IJ. The impact of a breathlessness intervention service (BIS) on the lives of patients with intractable dyspnea: a qualitative phase 1 study. Palliative & Supportive Care 2006;4(3):287-93. [DOI] [PubMed] [Google Scholar]
Booth 2008
- Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nature Clinical Practice. Oncology 2008;5(2):90-100. [DOI] [PubMed] [Google Scholar]
Booth 2011
- Booth S, Moffat C, Farquhar M, Higginson IJ, Burkin J. Developing a breathlessness intervention service for patients with palliative and supportive care needs, irrespective of diagnosis. Journal of Palliative Care 2011;27(1):28-36. [PubMed] [Google Scholar]
Booth 2014
- Booth S, Burkin J, Moffat C, Spathis A. Managing Breathlessness in Clinical Practice. Berlin: Springer, 2014. [Google Scholar]
Booth 2015
- Booth S, Farquhar M, Spathis A, Bausewein C, Chin C, Pattinson K. Re: the management of chronic breathlessness: more emphasis on non-pharmacological treatments and the role of the CNS. BMJ 2015;349:g7617. [Google Scholar]
Borg 1982
- Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise 1982;14(5):377-81. [PubMed] [Google Scholar]
Brightling 2000
- Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000;356(9240):1480-5. [DOI] [PubMed] [Google Scholar]
Bruera 2000
- Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J. The frequency and correlates of dyspnea in patients with advanced cancer. Journal of Pain and Symptom Management 2000;19(5):357-62. [DOI] [PubMed] [Google Scholar]
Burki 2010
- Burki NK, Lee LY. Mechanisms of dyspnea. Chest 2010;138(5):1196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2004
- Chan KS, Sham MM, Tse DM, Thorsen AB. Palliative medicine in malignant respiratory diseases. In: Doyle D, Hanks G, Cherney N, editors(s). Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press, 2004:1107-44. [Google Scholar]
Chin 2016
- Chin C, Booth S. Managing breathlessness: a palliative care approach. Postgraduate Medical Journal 2016;92(1089):393-400. [DOI] [PubMed] [Google Scholar]
Culpitt 2003
- Culpitt SV, Rogers DF, Shah P, De Matos C, Russell RE, Donnelly LE, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2003;167(1):24-31. [DOI] [PubMed] [Google Scholar]
De Peuter 2004
- De Peuter S, Van Diest I, Lemaigre V, Verleden G, Demedts M, Van den Bergh O. Dyspnea: the role of psychological processes. Clinical Psychology Review 2004;24(5):557-81. [DOI] [PubMed] [Google Scholar]
Dorman 2009
- Dorman S, Jolley C, Abernethy A, Currow D, Johnson M, Farquhar M, et al. Researching breathlessness in palliative care: consensus statement of the National Cancer Research Institute Palliative Care Breathlessness Subgroup. Palliative Medicine 2009;23(3):213-27. [DOI] [PubMed] [Google Scholar]
Dudgeon 2001
- Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. Journal of Pain and Symptom Management 2001;21(2):95-102. [DOI] [PubMed] [Google Scholar]
ERS Monograph 2016
- Bausewein C, Currow C, Johnson MJ. Palliative Care in Respiratory Disease: ERS Monograph 73. Sheffield (UK): European Respiratory Society, 2016. [Google Scholar]
eTG 2016
- Palliative Care Expert Group. Therapeutic Guidelines: Palliative Care, version 4. Melbourne: Therapeutic Guidelines Limited, 2016. [Google Scholar]
Evans 2002
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. Journal of Neurophysiology 2002;88(3):1500-11. [DOI] [PubMed] [Google Scholar]
Falk 2008
- Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2008;5(4):506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farquhar 2010
- Farquhar M, Higginson IJ, Fagan P, Booth S. Results of a pilot investigation into a complex intervention for breathlessness in advanced chronic obstructive pulmonary disease (COPD): brief report. Palliative and Supportive Care 2010;8(2):143-9. [DOI] [PubMed] [Google Scholar]
Farquhar 2014
- Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Medicine 2014;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galbraith 2010
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. Journal of Pain and Symptom Management 2010;39(5):831-8. [DOI] [PubMed] [Google Scholar]
Giardino 2010
- Giardino ND, Curtis JL, Abelson JL, King AP, Pamp B, Liberzon I, et al. The impact of panic disorder on interoception and dyspnea reports in chronic obstructive pulmonary disease. Biological Psychology 2010;84(1):142-6. [DOI] [PubMed] [Google Scholar]
Gift 1989
- Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabilitation Nursing 1989;14(6):323-5. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 21 June 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hardy 2001
- Hardy JR, Rees E, Ling J, Burman R, Feuer D, Broadley K, et al. A prospective survey of the use of dexamethasone on a palliative care unit. Palliative Medicine 2001;15(1):3-8. [DOI] [PubMed] [Google Scholar]
Haywood 2015
- Haywood A, Good P, Khan S, Leupp A, Jenkins-Marsh S, Rickett K, et al. Corticosteroids for the management of cancer-related pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD010756. [DOI: 10.1002/14651858.CD010756.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herigstad 2011
- Herigstad M, Hayen A, Wiech K, Pattinson KT. Dyspnoea and the brain. Respiratory Medicine 2011;105(6):809-17. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higginson 2014
- Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respiratory Medicine 2014;2(12):979-87. [DOI] [PubMed] [Google Scholar]
Hui 2013
- Hui D, Morgado M, Vidal M, Withers L, Nguyen Q, Chisholm G, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. Journal of Palliative Medicine 2013;16(3):274-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hui 2015
- Hui D, dos Santos R, Chisholm GB, Bruera E. Symptom expression in the last seven days of life among cancer patients admitted to acute palliative care units. Journal of Pain and Symptom Management 2015;50(4):488-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH 1994
- ICH Expert Working Group. ICH Harmonised Tripartite Guideline. Clinical safety data management: definitions and standards for expedited reporting E2A. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf (accessed 21 June 2017).
Johnson 2014
- Johnson MJ, Currow DC, Booth S. Prevalence and assessment of breathlessness in the clinical setting. Expert Review of Respiratory Medicine 2014;8(2):151-61. [DOI] [PubMed] [Google Scholar]
Johnson 2016
- Johnson MJ, Hui D, Currow DC. Opioids, exertion, and dyspnea: a review of the evidence. American Journal of Hospice & Palliative Care 2016;33(2):194-200. [DOI] [PubMed] [Google Scholar]
Kamal 2012
- Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP. Dyspnea review for the palliative care professional: treatment goals and therapeutic options. Journal of Palliative Medicine 2012;15(1):106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kloke 2015
- Kloke M, Cherny N, ESMO Guidelines Committee. Treatment of dyspnoea in advanced cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology 2015;26(Suppl 5):v169-73. [DOI] [PubMed] [Google Scholar]
Kunik 2005
- Kunik ME, Roundy K, Veazey C, Souchek J, Richardson P, Wray NP, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005;127(4):1205-11. [DOI] [PubMed] [Google Scholar]
Kutner 2001
- Kutner JS, Kassner CT, Nowels DE. Symptom burden at the end of life: hospice providers' perceptions. Journal of Pain and Symptom Management 2001;21(6):473-80. [DOI] [PubMed] [Google Scholar]
Kvale 2007
- Kvale PA, Selecky PA, Prakash UB. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(3 Suppl):368s-403s. [DOI] [PubMed] [Google Scholar]
Lai 2010
- Lai WS, Chao CS, Yang WP, Chen CH. Efficacy of guided imagery with theta music for advanced cancer patients with dyspnea: a pilot study. Biological Research for Nursing 2010;12(2):188-97. [DOI] [PubMed] [Google Scholar]
Lareau 1998
- Lareau SC, Meek PM, Roos PJ. Development and testing of the modified version of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ-M). Heart & Lung 1998;27(3):159-68. [DOI] [PubMed] [Google Scholar]
Levy 2016
- Levy M, Smith T, Alvarez-Perez A, Back A, Baker JN, Beck AC, et al. Palliative Care Version 1. Journal of the National Comprehensive Cancer Network : JNCCN 2016;14(1):82-113. [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin RJ, Adelman RD, Mehta SS. Dyspnea in palliative care: expanding the role of corticosteroids. Journal of Palliative Medicine 2012;15(7):834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lok 2016
- Lok CW. Management of breathlessness in patients with advanced cancer: a narrative review. American Journal of Hospice & Palliative Care 2016;33(3):286-90. [DOI] [PubMed] [Google Scholar]
Mahler 1988
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93(3):580-6. [DOI] [PubMed] [Google Scholar]
Manning 1995
- Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. New England Journal of Medicine 1995;333(23):1547-53. [DOI] [PubMed] [Google Scholar]
Matsuo 2011
- Matsuo N, Morita T, Iwase S. Efficacy and undesirable effects of corticosteroid therapy experienced by palliative care specialists in Japan: a nationwide survey. Journal of Palliative Medicine 2011;14(7):840-5. [DOI] [PubMed] [Google Scholar]
Mercadante 2001
- Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Supportive Care in Cancer 2001;9(5):386-9. [DOI] [PubMed] [Google Scholar]
Mercadante 2017
- Mercadante S, Fusco F, Caruselli A, Cartoni C, Masedu F, Valenti M, et al. Background and episodic breathlessness in advanced cancer patients followed at home. Current Medical Research and Opinion 2017;33(1):155-60. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1999
- Moore MC, Zebb BJ. The catastrophic misinterpretation of physiological distress. Behaviour Research and Therapy 1999;37(11):1105-18. [DOI] [PubMed] [Google Scholar]
Mularski 2010
- Mularski RA, Campbell ML, Asch SM, Reeve BB, Basch E, Maxwell TL, et al. A review of quality of care evaluation for the palliation of dyspnea. American Journal of Respiratory and Critical Care Medicine 2010;181(6):534-8. [DOI] [PubMed] [Google Scholar]
Nardi 2009
- Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respiratory Physiology & Neurobiology 2009;167(1):133-43. [DOI] [PubMed] [Google Scholar]
Parshall 2012
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al, American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. American Journal of Respiratory and Critical Care Medicine 2012;185(4):435-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulsen 2014
- Paulsen O, Klepstad P, Rosland JH, Aass N, Albert E, Fayers P, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. Journal of Clinical Oncology 2014;32(29):3221-8. [DOI] [PubMed] [Google Scholar]
Perna 2004
- Perna G, Caldirola D, Namia C, Cucchi M, Vanni G, Bellodi L. Language of dyspnea in panic disorder. Depression and Anxiety 2004;20(1):32-8. [DOI] [PubMed] [Google Scholar]
Rassovsky 2006
- Rassovsky Y, Abrams K, Kushner MG. Suffocation and respiratory responses to carbon dioxide and breath holding challenges in individuals with panic disorder. Journal of Psychosomatic Research 2006;60(3):291-8. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager. Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sackley 2009
- Sackley CM, den Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339:b3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seamark 2004
- Seamark DA, Blake SD, Seamark CJ, Halpin DM. Living with severe chronic obstructive pulmonary disease (COPD): perceptions of patients and their carers. An interpretative phenomenological analysis. Palliative Medicine 2004;18(7):619-25. [DOI] [PubMed] [Google Scholar]
Seow 2011
- Seow H, Barbera L, Sutradhar R, Howell D, Dudgeon D, Atzema C, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. Journal of Clinical Oncology 2011;29(9):1151-8. [DOI] [PubMed] [Google Scholar]
Shih 2007
- Shih A, Jackson KC 2nd. Role of corticosteroids in palliative care. Journal of Pain and Palliative Care Pharmacotherapy 2007;21(4):69-76. [PubMed] [Google Scholar]
Simon 1990
- Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. American Review of Respiratory Disease 1990;142(5):1009-14. [DOI] [PubMed] [Google Scholar]
Skaug 2009
- Skaug K, Eide GE, Gulsvik A. Hospitalisation days in patients with lung cancer in a general population. Respiratory Medicine 2009;103(12):1941-8. [DOI] [PubMed] [Google Scholar]
Skevington 1997
- Skevington SM, Pilaar M, Routh D, Macleod RD. On the language of breathlessness. Psychology and Health 1997;12(5):677-89. [Google Scholar]
Smoller 1996
- Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. American Journal of Respiratory and Critical Care Medicine 1996;154(1):6-17. [DOI] [PubMed] [Google Scholar]
Solano 2006
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. Journal of Pain and Symptom Management 2006;31(1):58-69. [DOI] [PubMed] [Google Scholar]
Tanaka 2002a
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Prevalence and screening of dyspnea interfering with daily life activities in ambulatory patients with advanced lung cancer. Journal of Pain and Symptom Management 2002;23(6):484-9. [DOI] [PubMed] [Google Scholar]
Tanaka 2002b
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Factors correlated with dyspnea in advanced lung cancer patients: organic causes and what else? Journal of Pain and Symptom Management 2002;23(6):490-500. [DOI] [PubMed] [Google Scholar]
Tishelman 2007
- Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. Journal of Clinical Oncology 2007;25(34):5381-9. [DOI] [PubMed] [Google Scholar]
Vayne‐Bossert 2017
- Vayne-Bossert P, Haywood A, Good P, Khan S, Rickett K, Hardy JR. Corticosteroids for adult patients with advanced cancer who have nausea and vomiting (not related to chemotherapy, radiotherapy, or surgery). Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012002. [DOI: 10.1002/14651858.CD012002.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, Behavior, and Immunity 2010;24(6):968-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilcock 2002
- Wilcock A, Crosby V, Hughes A, Fielding K, Corcoran R, Tattersfield AE. Descriptors of breathlessness in patients with cancer and other cardiorespiratory diseases. Journal of Pain and Symptom Management 2002;23(3):182-9. [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams CM. Dyspnea. Cancer Journal 2006;12(5):365-73. [DOI] [PubMed] [Google Scholar]
Williams 2010
- Williams M, Cafarella P, Olds T, Petkov J, Frith P. Affective descriptors of the sensation of breathlessness are more highly associated with severity of impairment than physical descriptors in people with COPD. Chest 2010;138(2):315-22. [DOI] [PubMed] [Google Scholar]
Wood‐Baker 2005
- Wood-Baker RR, Gibson PG, Hannay M, Walters EH, Walters JA. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD001288. [DOI: 10.1002/14651858.CD001288.pub4] [DOI] [PubMed] [Google Scholar]
Yennurajalingam 2015
- Yennurajalingam S, Bruera E. Cancer Management Handbook. Fatigue and Dyspnea. Cancer Network 2015. [www.cancernetwork.com/cancer-management/fatigue-and-dyspnea]


