Summary
Objective
ANGPTL4 inhibits lipoprotein lipase in adipose tissue, regulating plasma triglycerides levels. In persons with obesity plasma ANGPTL4 levels have been positively correlated with body fat mass, TG levels and low HDL. A loss‐of‐function E40K mutation in ANGPTL4 prevents LPL inhibition, resulting in lower TGs and higher HDLc in the general population. Since obesity determines metabolic alterations and consequently is a major risk factor for cardiovascular disease, the aim was to explore if obesity‐related metabolic abnormalities are modified by the ANGPTL4‐E40K mutation.
Methods
ANGPTL4‐E40K was screened in 1206 Italian participants, of which 863 (71.5%) with obesity. All subjects without diabetes underwent OGTT with calculation of indices of insulin‐sensitivity.
Results
Participants with obesity carrying the E40K variant had significantly lower TG (p = 0.001) and higher HDLc levels (p = 0.024). Also in the whole population low TGs and high HDLc were confirmed in E40K carriers. In the obese subpopulation it was observed that almost all E40K carriers were within the lowest quartile of TGs (p = 1.1 × 10−9). E40K had no substantial effect of on glucose metabolism. Finally, none of the obese E40K carriers had T2D, and together with the favourable lipid profile, they resemble a metabolically healthy obese (MHO) phenotype, compared to 38% of E40E wild‐type obese that had diabetes and/or dyslipidaemia (p = 0.0106).
Conclusions
In participants with obesity the ANGPTL4‐E40K variant protects against dyslipidemia. The phenotype of obese E40K carriers is that of a patient with obesity without metabolic alterations, similar to the phenotype described as metabolic healthy obesity.
Keywords: Genetics, lipoprotein lipase, metabolic healthy obesity (MHO), triglycerides
Introduction
ANGPTL4 is a glycosylated secreted protein of the Angiopoietin‐like family that has emerged as a likely risk factor and a possible target for the treatment of metabolic alterations, including hypertriglyceridemia, glucose intolerance and dyslipidemia‐related atherosclerotic cardiovascular disease 1, 2, 3. ANGPTL 3, 4 and 8 are regulators of plasma triglycerides levels. ANGPTL4, in particular, plays a key role in regulating lipid metabolism related to the fasting/feeding states, in concert with ANGPTL 3 and 8 4.
With regards to obesity, plasma ANGPTL4 levels have been positively correlated with body fat mass. Previous studies have shown that ANGPTL4 circulating levels are higher in patients with obesity, and correlate positively with triglyceride (TG) levels and negatively with High‐Density Lipoprotein cholesterol (HDLc) 1.
The ANGPTL4 protein is secreted mainly by liver and adipose tissue in response to fasting or hypoxia. One of its functions is inhibiting the lipolytic activity of Lipoprotein Lipase (LPL) in adipose tissue (AT); LPL “extracts” free fatty acids (FFA) from Triglycerides‐rich circulating lipoprotein (TRLs), both chylomicrons (CM) and Very Low‐Density Lipoproteins (VLDLs), thus facilitating the route towards target tissues such as adipose or muscle, modulating the storage and oxidation of lipids 5. The net effect of ANGPTL4 is therefore to maintain adequate TG and HDLc levels in plasma during the fasting/feeding states. The synthetized form of ANGPTL4 is 50‐kD protein that needs oligomerization for a proper secretion. Circulating proteins are inactive as long as they are cleaved into 37‐kD C‐terminal and 15‐kD N‐terminal fragments. C‐terminal fragments dissociate into monomers while N‐terminal rests in oligomers. These latter oligomers are necessary and sufficient for LPL inhibition 6. Inhibition is accomplished through the conversion of LPL from active dimers into inactive monomers 7. It is thought that ANGPTL4 can act both as a paracrine factor regulating LPL activity in other tissue as well as an autocrine factor inhibiting LPL activity in the tissue of its origin. Other recognized functions are related to endothelial cell function, vascularization and atherosclerosis. In mice studies, reducing levels of ANGPTL4 improves glucose tolerance coupled with increase in fat mass 8.
A missense mutation in ANGPTL4, the E40K (rs116843064), resulted strongly associated with low TGs and higher HDLc levels in plasma in different populations 9, 10. Because it is an inhibiting protein, loss‐of‐function (LoF) variants in ANGPTL4 such as E40K, act by enhancing adipose‐specific LPL activity, resulting in lowering circulating TGs, a condition per se protective against cardiovascular disease (CVD) 11. The association of ANGPTL4 E40K mutation and CVD is still controversial, but of note 4 out 5 of the larger studies revealed a protective effect (odd ratio from 0.56 (for homozygotes) ‐ 0.86) 12, 13, 14, 15, 16. While LoF variants inactivating ANGPTL4 are likely protective on CVD risk 3, no other variants in the same gene have been consistently associated with CVD risk so far.
Since obesity is a major risk factor for CVD, and in view of the impairment in metabolism and insulin regulation that are a consequence of obesity, the aim of this study was to explore if there is any interaction between the E40K variant and obesity. In particular if obesity‐related metabolic alterations (i.e dyslipidemia, disglycaemia) are modified by the presence of the variant. To our knowledge, this interaction has not been explored before. Thus, the presence of the E40K variant was examined in a very well characterized adult obese population from Italy, evaluating lipid profile, insulin regulation and metabolic indices. The hypothesis is therefore that carriers of ANGPTL4 E40K variant may be protected from obesity‐related dyslipidemia and/or other metabolic alteration, resembling the metabolic healthy obesity phenotype.
Methods
For this study, 1206 participants were selected, of which 863 (71.5%) with obesity, among patients attending the Internal Medicine outpatient clinics of Sapienza University of Rome and the Metabolic and Diabetes Unit of the Department of Experimental Medicine, Sapienza University of Rome. All participants without a previous diagnosis of diabetes underwent a standard 75 g oral glucose tolerance test (OGTT) with measurements of glucose and insulin at baseline and after 30, 60, 90, and 120 minutes. Patients were classified according to the ADA 2018 diagnostic criteria in normal glucose tolerant (NGT), with impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and affected by type 2 diabetes T2D 17. All patients have undergone a structured interview in order to collect information on family history of diabetes and cardiovascular disease and on current treatments. All participants had a complete work‐up including clinical examination, anthropometric measurements, and laboratory tests. The diagnosis of hypertension was based on the presence of elevated systolic (≥140 mmHg) and/or diastolic (≥90 mmHg) blood pressure, and/or the current use of antihypertensive medications. Patients were classified as obese if measured BMI was ≥30 kg/m2.
Laboratory measurements
The study cohort underwent fasting blood sampling to assess FBG, glycosylated haemoglobin (HbAc1), total cholesterol (TC), HDLc, TG, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) by standard laboratory methods. Insulin (Ins) was measured by radioimmunoassay (ADVIA Insulin Ready Pack 100, Bayer Diagnostics, Milan, Italy), with intra‐ and inter‐assay coefficients of variation <5%. Low‐density lipoprotein (LDL) cholesterol value was obtained using the Friedewald formula.
Surrogate indices of insulin resistance and secretion, i.e. HOMA‐IR for insulin resistance, HOMA‐B for insulin secretion and insulin sensitivity index (ISI) were calculated as previously described by Matthews et al. 18 and Matsuda et al. 19.
Genotyping assay
The E40K variant in ANGPTL4 gene was assayed using the TaqMan assay ID C_156222000_10 (Applied Biosystems, Foster City, CA USA), in a total volume of 10 μl on an EcoTM RealTime PCR System by Illumina (San Diego, CA, USA). The plate was run at 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute for 50 cycles. Positive and negative controls are included in each experiment to asses genotyping quality, which include beside two blank wells, two known heterozygotes and wild‐types samples. The genotype concordance was higher than 99%. Allele frequency was in Hardy Weinberg Equilibrium.
Statistical analysis
All statistical analyses were performed with SPSS 24.0 statistical package. Categorical variable distribution was compared by χ2 and T‐Student tests. To test for the effects of genotypes on the median of the quantitative traits, a linear regression analysis was performed (under the additive model) and the results were adjusted for the relevant covariates, including gender, age, and BMI. Normality was checked using Kolmogorov‐Smirnof. Skewed variables were logarithmically transformed before the analyses or assessed using non‐parametric test such as Mann–Whitney.
Power analysis was performed by the Genetic Association Study (GAS) Power Calculator (Johnson JL, University of Michigan 2017) 20 commonly used to compute statistical power for one‐stage genetic association studies. Lipid levels variance in this population was estimated as 0.30. Assuming an allele frequency of 0.02 and an additive model for disease risk, with a sample size of 1200 the expected power under a significance level of 0.05 was >80% to identify an OR equal or superior than 1.65.
This study was reviewed and approved by the Ethical Committee of Policlinico Umberto I, Sapienza University of Rome, and conducted in conformance with the Helsinki Declaration. Written consent was obtained from all participants before the study.
Results
Anthropometric and clinical characteristics of study population are summarized in Table 1, where the entire population is shown on the left side and, on the right, it is stratified according to E40K variant. As expected the entire population shows a high median BMI and impaired indices of insulin resistance and secretion, due to the high prevalence of patients with obesity, together with normal levels of all the other parameters. As previously reported, carriers of E40K show highly significant lower TG levels (p = 0.001), while all the other lipid parameters show non‐significant differences. Interestingly, we observed a minor allele frequency (MAF) of 1.9%, in this Italian population, rarer than in other European populations (5%) 21.
Table 1.
Clinical characteristics of Italian population (n = 1206) stratified for E40K ANGPTL4 variant
| n = 1206 | Whole population | E40E | E40K | p‐value | |||
|---|---|---|---|---|---|---|---|
| Median | (25–75)% | Median | (25–75)% | Median | (25–75)% | ||
| Sex M/F (%) | 552/654 (46/54) | ‐ | 542/641 (46/54) | ‐ | 10/13 (43/57) | ‐ | ‐ |
| Age (yrs) | 51 | (38–62) | 52 | (38–62) | 45 | (30–62) | 0.199 |
| BMI (kg/m2) | 35.4 | (29–43) | 35.2 | (29–43) | 39 | (32.5–45) | 0.079 |
| G0' (mg/dl) | 95 | (84–113) | 95 | (84–114) | 9 | (81–108) | 0.375 |
| G120′ (mg/dl) | 122 | (100–150) | 122 | (100–150) | 111 | (98–144) | 0.406 |
| INS0' (μU/mL) | 22.1 | (14.7–33.7) | 21.8 | (14.5–33.6) | 27.7 | (18–44.3) | 0.142 |
| INS120′ (μU/mL) | 106.8 | (55.7–189.3) | 106.4 | (55–187.4) | 112.7 | (66.8–200) | 0.534 |
| Col Tot (mg/dl) | 194.8 | (168.1–224.8) | 194.5 | (168.1–224) | 199.4 | (167.3–235) | 0.456 |
| HDLc (mg/dl) | 46 | (38.7–54.9) | 46 | (38.6–54.7) | 47.8 | (42.9–65.5) | 0.087 |
| TG (mg/dl) | 118.3 | (84.2–171.7) | 120 | (85–173) | 83 | (57.1–118) | 0.001 |
| LDLc (mg/dl) | 120.7 | (96–145.8) | 120.2 | (95.8–145.6) | 133.9 | (107–156) | 0.174 |
| AST (U/L) | 20 | (15–25) | 20 | (15–25) | 19.5 | (17–22) | 0.762 |
| ALT (U/L) | 28.1 | (20–42.8) | 28.1 | (20–43) | 26.6 | (21.5–38.5) | 0.840 |
| PAS (mmHg) | 130 | (120–140) | 130 | (120–140) | 130 | (120–140) | 0.357 |
| PAD (mmHg) | 80 | (80–90) | 80 | (80–90) | 85 | (70–90) | 0.634 |
| HOMA‐IR | 5.6 | (3.5–9.4) | 5.5 | (3.5–9.4) | 7.1 | (4.5–11.7) | 0.434 |
| HOMA‐B | 295.6 | (183.7–466.1) | 293.5 | (180.8–464.7) | 391.6 | (232.8–497.8) | 0.227 |
| ISI | 2.1 | (1.3–3.3) | 2.1 | (1.3–3.3) | 2.1 | (1.1–2.7) | 0.316 |
Data are expressed as median (25th–75th) percentile range, unless for gender expressed as count (Male/Female) and percentage. P‐value are calculated by Mann–Whitney.
BMI: Body Mass Index; G0': Fasting glucose from OGTT; G120': blood glucose at 120′ after OGTT; INS0': Fasting Insulin from OGTT; INS120': Circulating Insulin at 120′ after OGTT; Col Tot: Total Cholesterol; HDLc: High Density Lipoprotein cholesterol; TG: Triglycerides; LDLc: Low Density lipoprotein cholesterol; AST: aspartate transaminase; ALT: alanine transaminase; PAS: Pulmonary Artery Pressure Systolic; PAD: Pulmonary Artery Pressure Diastolic; HOMA‐IR: homeostatic model assessment of insulin resistance; HOMA‐B: homeostatic model assessment of beta‐cell function; ISI: Matsuda Insulin Sensitive Index.
Linear logistic regression in the whole population showed significant associations between E40K variant and TG and HDLc levels, as show in Table 2a. These associations stand to the adjustment for established confounding factor (such as age, sex and BMI), and result in a similar effect size but on the opposite direction, as expected for the TG ‐ HDLc relation (Table 2b).
Table 2.
Linear regression analyses of the association of ANGPTL4 E40K variant and circulating lipid levels
| a. | |||||||
|---|---|---|---|---|---|---|---|
| Beta | 95%CI | p | R 2 | ||||
| HDL: | 0.065 | 7.16E‐05 | 1.16E‐03 | 0.027 | 0.003 | ||
| TG: | −0.064 | −1.46E‐04 | −9.67E‐06 | 0.025 | 0.003 | ||
| b. | |||||||
|---|---|---|---|---|---|---|---|
| Beta | 95%CI | p | R 2 | ΔR 2 | |||
| HDL: | sex: | 0.345 | 8.345 | 11.475 | 2.4 × 10−33 | 0.119 | |
| E40K | 0.060 | 0.621 | 11.988 | 0.030 | 0.122 | 0.004 | |
| TG: | age | 0.099 | 0.304 | 1.119 | 0.001 | 0.010 | |
| E40K | −0.062 | −96.722 | −3.968 | 0.033 | 0.014 | 0.004 | |
| sex | −0.063 | −27.258 | −1.109 | 0.034 | 0.017 | 0.004 | |
a. Unadjusted. 95%CI: 95% Confidence Interval. R2: percentage of dependent‐variable variation explained by the model.
b. adjusted model for age, sex and BMI. Only significant variables are shown.
Then, the obese sub‐population (n = 863) was examined. The clinical characteristics of this sub‐population are summarized in Table 3, where a typical metabolic profile of such kind of patients is observed (high BMI, high mean fasting glucose, high HOMA‐IR and low ISI).
Table 3.
Clinical characteristics of obese Italian sub‐population (n = 863) stratified for E40K ANGPTL4 variant
| Obese population | Obese E40E | Obese E40K | p‐value | ||||
|---|---|---|---|---|---|---|---|
| n = 863 | Median | (25–75)% | Median | (25–75)% | Median | (25–75)% | |
| Sex M/F (%) | 311/552 (36/64) | 302/541 (36/64) | 9/11 (45/55) | ‐ | |||
| Age (yrs) | 47 | (35–58) | 47 | (35–58) | 46.5 | (29–60) | 0.762 |
| BMI (kg/m2) | 39.5 | (34.3–46) | 39.5 | (34–46) | 40.3 | (36–47) | 0.717 |
| G0’ (mg/dl) | 94 | (84–111) | 94 | (84–111) | 94.5 | (84–107) | 0.846 |
| G120’ (mg/dl) | 122 | (100–150) | 122 | (100–151) | 105 | (97–139.5) | 0.182 |
| INS0’ (μU/mL) | 24.1 | (15.8–36.5) | 24 | (15.7–36.2) | 29.8 | (22–49.9) | 0.143 |
| INS120’ (μU/mL) | 11.2 | (58.4–200) | 11.3 | (58.4–200) | 99.5 | (60.5–197) | 0.869 |
| Col Tot (mg/dl) | 196.4 | (172–224) | 196.4 | (173–224) | 196.5 | (160–232) | 0.854 |
| TG (mg/dl) | 119.2 | (84.9–170.2) | 121 | (86–171) | 79 | (58–111) | 0.001 |
| HDLc (mg/dl) | 47 | (40–55) | 47 | (40–55) | 48 | (42–66) | 0.235 |
| LDLc (mg/dl) | 122.1 | (99.4–145.6) | 122 | (99–146) | 128 | (97–147) | 0.741 |
| HOMA‐IR | 5.6 | (3.5–9.4) | 5.5 | (3.5–9.4) | 7.1 | (4.5–11.7) | 0.316 |
| HOMA‐B | 295.6 | (184–466) | 293.5 | (181–465) | 391.6 | (233–498) | 0.183 |
| ISI | 2.1 | (1.3–3.3) | 2.1 | (1.3–3.3) | 2.1 | (1.1–2.7) | 0.464 |
| PAS (mmHg) | 130 | (120–140) | 130 | (120–140) | 130 | (120–140) | 0.486 |
| PAD (mmHg) | 80 | (80–90) | 80 | (80–90) | 85 | (70–90) | 0.947 |
Data are expressed as median (25th–75th) percentile range, unless for gender expressed as count and percentage. P‐value are calculated by Mann–Whitney.
BMI: Body Mass Index; G0’: Fasting glucose from OGTT; G120’: blood glucose at 120’ after OGTT; INS0’: Fasting Insulin from OGTT; INS120’: Circulating Insulin at 120’ after OGTT; Col Tot: Total Cholesterol; HDLc: High Density Lipoprotein cholesterol; TG: Triglycerides; LDLc: Low Density lipoprotein cholesterol; AST: aspartate transaminase; ALT: alanine transaminase; PAS: Pulmonary Artery Pressure Systolic; PAD: Pulmonary Artery Pressure Diastolic; HOMA‐IR: homeostatic model assessment of insulin resistance; HOMA‐B: homeostatic model assessment of beta‐cell function; ISI: Matsuda Insulin Sensitive Index.
The association of E40K with lower TG‐level was also confirmed in this obese sub‐population (p = 0.001). Also the association with higher HDLc plasma levels in carriers (p = 0.024) was confirmed (Figure 1). Despites some indications from previous studies 2, 3, 9, 16 on a possible modulation of circulating insulin levels, no difference in glucose nor insulin curves derived from the OGTT in carriers versus non‐carriers was found (data not shown).
Figure 1.
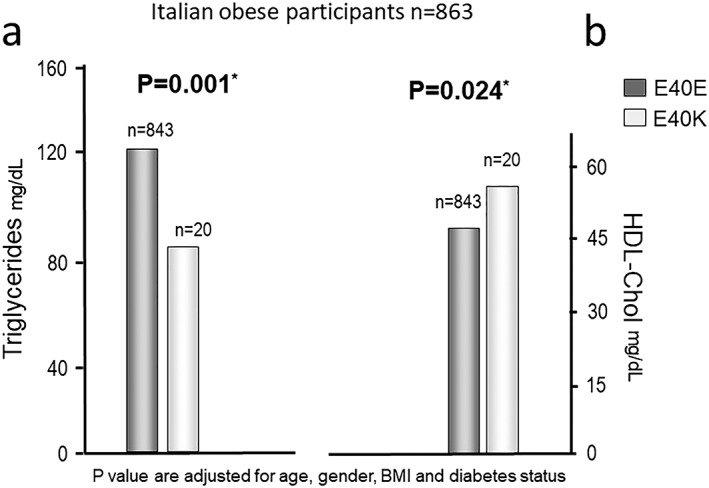
Comparison of mean lipid levels in Italian obese sub‐population. a. Triglycerides b. HDLc. P‐values are assessed by linear regression. *P values are adjusted for age, gender, BMI and diabetes status
When participants with a normal lipid profile (i.e. TGs ≤ 150 mg/dL and HDLc ≥40 or ≥ 50 mg/dL in men or women, respectively) were selected within the obese sub‐population, the frequency of these patients was around 75% in E40K carriers compared to only 25% in E40E participants (p = 0.02) (Figure 2), confirming that E40K carriers are indeed more metabolically healthy.
Figure 2.
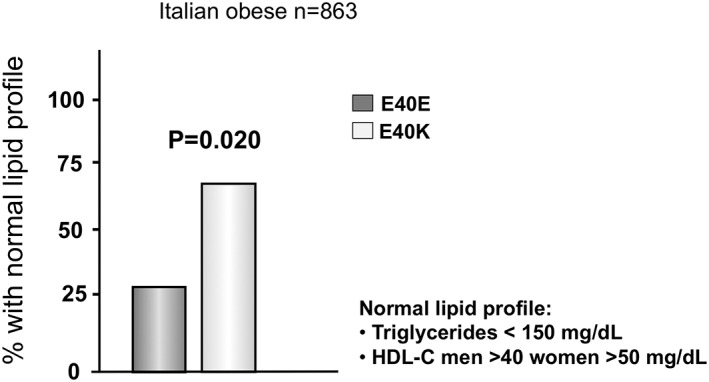
Percentage of patients with obesity with normal lipid profile stratified by E40 genotypes. Normal lipid profile was defined as Triglycerides <150 mg/dL and HDLc men >40 women >50 mg/dL
As a further demonstration of the metabolically healthier effects of E40K, in the whole cohort the frequency of E40K carriers resulted significantly higher in the lower quartile of TGs compared to the highest quartile of TGs (p = 0.017) (Figure 3). Contrariwise, the number of E40K carriers was higher in the upper quartile of distribution of the BMI (Figure 3).
Figure 3.
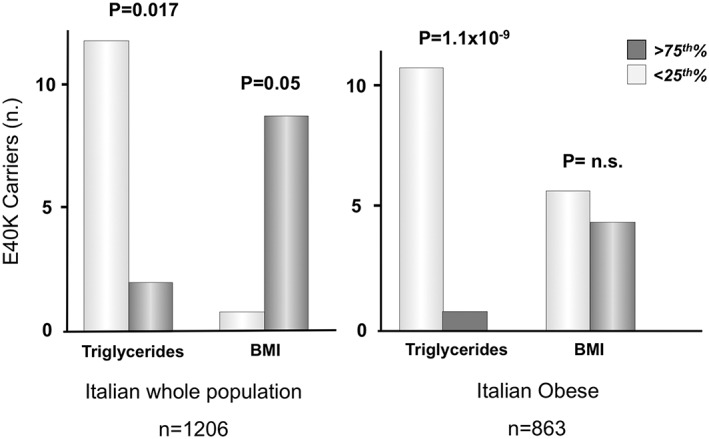
Enrichment of E40K carriers in the extremes of the quartiles (25th vs 75th) of Triglycerides and BMI in the whole population and in the obese sub‐population. 25th and 75th limits for TGs and BMI are shown in table 1 (whole population) and 3 (obese sub‐population)
In the obese sub‐population, E40K carriers were almost all within the lower quartile of TGs (p = 1.1 × 10−9), but as expected, no difference was observed in BMI quartiles, due to sub‐population selection (all with obesity) (Figure 3).
Finally, looking at participants in the lower quartile of TGs, there was a more than 6‐fold probability to find the E40K variant than in patients in the 75°, with an Odds Ratio (OR) of 6.208 [1.377–27.978; p = 0.017]. This association resisted to adjustment for recognized risk factors such as age, sex and BMI (OR = 6.225 [1.317–29.413] p = 0.021). In the obese subgroup the probability of E40K carriers of being in the 25° percentile of TGs was 5.6‐fold (p = 0.026), and after adjustment for sex and age was almost 6 (OR = 5.9; p = 0.027).
Regarding BMI, binary logistic regression analysis showed that patients in the upper quartile of BMI had a 8‐fold probability to be E40K carriers than those in the lower quartile (OR = 8.027 [0.998–64.577] p = 0.050). Adjustment for established risk factors increased the significance and the probability associated to the upper quartile of BMI to carry E40K variant to more than 10‐time higher compared lower‐quartile individuals (OR = 11.886 [1.392–101.504] p = 0.024).
Also, none of E40K carriers with obesity had type 2 diabetes. The absence of diabetes, together with the favourable lipid profile, identifies obese E40K carriers as similar to the metabolically healthy obese (MHO) patients. In contrast 38.3% (316/828) of E40E carriers with obesity had diabetes and/or dyslipidaemia. This difference between ANGPTL4 E40K carriers and wild‐types was highly significant (p = 0.0106), confirming the plausible protective effects of ANGPTL4 E40K variant on cardio‐metabolic risk factors.
Summarizing
E40K carriers show significant lower levels of TG and higher levels of HDLc than non‐carriers, both in the general population and in the obese sub‐cohort. Carriers of E40K have a high probability (6‐fold) of having low TGs and (10‐fold) of having a higher BMI. Also, no substantial effect of E40K on blood circulating levels of glucose nor insulin was confirmed. Overall, a net enrichment of E40K carriers in the “non‐pathological” extremes of several traits, such as TG and HDLc levels or MHO phenotype, was observed.
Discussion
Here it is shown that ANGPTL4 gene E40K carriers have lower levels of TGs and higher levels of HDLc than non‐carriers, both in the general population and in the obese sub‐cohort. Carriers of E40K have a high probability (6‐fold) of having low TGs and (10‐fold) of having a higher BMI. Also no substantial effect of E40K on circulating blood levels of glucose or insulin was detected. Furthermore, for the first time, it was observed that in E40K carriers with obesity, despite the high body weight (per‐se a major risk factor for CVD), the lipid profile is far better than wild‐type ANGPTL4 carriers. This findings confirm and expand previous observations in the general population 1, 3, 9, 15, 16, demonstrating that also in participants with obesity, by definition at more risk for metabolic alterations, ANGPTL4 E40K variant is capable to ameliorate lipid levels.
Based on recent molecular and cellular research, these findings fit with the proposed role for ANGPTL4 in lipid metabolism, controlling circulating TRLs levels and lipid deposition in storage (AT) and oxidative (heart and muscle) tissues. In particular, the hypothesized effect on adipose tissue, both white and brown 4, 16 is exerted principally by tissue‐specific LPL inhibition, thus maintaining the equilibrium of circulating TRLs during fast and feeding states, in concert with ANGPTL3 and 8, which show similar function mainly in muscle tissue, allowing proper nutrient partitioning. Hence, LoF in ANGTPL4 may play a role in improving the lipid profile without the risk of aberrant deposition of lipids in heart, muscle and liver tissues. Indeed, Aryal et al. observed in their AT selective ANGPTL4‐KO mouse model that skeletal muscle and hepatic lipid ectopic accumulation was significantly reduced while adipose tissue uptake of FFAs was enhanced, by augmenting TG lipolysis and oxidation. This leads, in this animal model, to the reduction of both atherosclerotic plaques and pro‐inflammatory cytokines, together with the reduction of CVD risk. Furthermore, Janssen et al. 8 observed that mice lacking ANGPTL4 displayed an increase in body weight and visceral adipose tissue mass through enhanced visceral adipose tissue lipoprotein lipase activity, without metabolic impairment.
The phenotype of E40K carriers with obesity is therefore that of a patient with obesity without significant metabolic alterations, similar to the phenotype described as metabolic healthy obesity (MHO) 22, 23. Metabolic healthy obesity defines a subgroup of individuals with obesity in which the metabolic abnormalities that commonly accompany excess adiposity are absent. However, the prognostic value of MHO is controversial. The lack of standard definitions for metabolic health and obesity as well as the dynamic properties of MHO may have contributed to these inconsistent results. For example, as reviewed by Phillips 23, the lack of a universal definition of metabolic health and differences in obesity classification (BMI vs. % body fat) account for a large proportion of the disparity in MHO prevalence. It follows that the question whether the subgroup of patients with MHO is really without an increased risk of major chronic diseases such as CVD is still open. Recently, in a large follow‐up study of 30 years of women stratified as metabolically healthy by the absence of hypertension, diabetes and hypercholesterolemia, the authors observed that women with obesity who maintained a metabolically healthy phenotype during the follow‐up still had an increased risk of CVD compared with women with a stable normal weight who were metabolically healthy 24. Similar results were obtained in the Whitehall II cohort, where MHO patients had increased mortality risk compared to the metabolically healthy normal weight participants 25. In this scenario obese ANGPTL4 E40K carriers may represent a “reference” of a metabolically healthy patients with obesity, and E40K variant in ANGPTL4 can be considered a genetic marker for this relevant but largely undefined healthy obese phenotype.
As proposed by recent studies on the effects of un‐common variants 26, an enrichment of carriers E40K variant in one extreme of the associated traits (BMI and TG) was found. In particular, carriers of E40K were more frequent in the lower quartile of TG, in the upper quartile of BMI and in the patient sub‐group that showed a normal lipid profile (i.e. TG ≤150 mg/dL and HDLc ≥40 or ≥ 50 mg/dL, in men and women respectively).
Strength of this work are the number of study participants, together with the deep characterization of the obese population. To the best of our knowledge, this is the first time that this association was assessed in a large obese cohort. Limitation can be recognized in the cross‐sectional design of this study, and only long‐term follow‐up will conclusively confirm these findings.
In conclusion, in this Italian population ANGPTL4 E40K LoF variant is confirmed to lower circulating TG and enhance HDLc levels. Carriers of E40K have a high probability (6‐fold) of having low TGs and (10‐fold) of having a higher BMI, possibly through lipid deposition in adipose tissue. No evidence of the effects on circulating levels of insulin or glucose was observed, suggesting that the molecular mechanism involved in enhancing the lipid profile, even in the obese sub‐population, is selective on LPL activity in AT. Overall, the phenotype of E40K carriers with obesity is that of an individual without significant metabolic alterations, resembling the phenotype described as metabolic healthy obesity.
From a clinical point of view, persons with obesity that are carriers of the E40K variant are certainly protected from lipid alterations. Hence, ANGPTL4 E40K LoF is a genetic marker of a healthier lipid profile in obesity, which can be used to define individual characteristics towards a more personalized medicine.
Competing interests
We declare no competing financial interests for all the authors of the study.
Funding
Financial support was provided by the following institutions: “Progetto d'Ateneo 2017” Grant (prot. RM11715C3FCB9431) from Sapienza Università di Roma (MGB); “Assegno di Ricerca” from the Italian Society for Diabetes (SID) 2013 (MGB); “Avvio alla Ricerca 2015‐16” Grant, from Sapienza Università di Roma (LB). “Avvio alla Ricerca 2016” Grant, from Sapienza Università di Roma (DB).
Disclosure
The authors declare no conflict of interest.
Bailetti, D. , Bertoccini, L. , Mancina, R. M. , Barchetta, I. , Capoccia, D. , Cossu, E. , Pujia, A. , Lenzi, A. , Leonetti, F. , Cavallo, M. G. , Romeo, S. , and Baroni, M. G. (2019) ANGPTL4 gene E40K variation protects against obesity‐associated dyslipidemia in participants with obesity. Obesity Science & Practice, 5: 83–90. 10.1002/osp4.311.
References
- 1. Olshan DS, Rader DJ. Angiopoietin‐like protein 4: A therapeutic target for triglycerides and coronary disease? J Clin Lipidol 2018; Jun; 12: 583–587. [DOI] [PubMed] [Google Scholar]
- 2. Davies BSJ. Can targeting ANGPTL proteins improve glucose tolerance? Diabetologia 2018; Jun; 61: 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gusarova V. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun 2018; Jun 13; 9: 2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang R. The ANGPTL3–4‐8 model, a molecular mechanism for triglyceride trafficking. Open Biol 2016; Apr; 6: 150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, He PP, Zhang DW, et al. Lipoprotein lipase: from gene to atherosclerosis. Atherosclerosis 2014; Dec; 237: 597–608. [DOI] [PubMed] [Google Scholar]
- 6. Yin W, Romeo S, Chang S, et al. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem 2009; May 8; 284: 13213–13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin‐like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci U S A 2006; Nov 14; 103: 17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen AWF, Katiraei S, Bartosinska B, Eberhard D, Willems van Dijk K, Kersten S. Loss of angiopoietin‐like 4 (ANGPTL4) in mice with diet‐induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 2018; Jun; 61: 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romeo S, Pennacchio LA, Fu Y, et al. Population‐based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 2007; Apr; 39: 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romeo S, Yin W, Kozlitina J, et al. Rare loss‐of‐function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest 2009; Jan; 119: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toth PP. Triglyceride‐rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag 2016; May; 6: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Talmud PJ, Smart M, Presswood E, et al. ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler Thromb Vasc Biol 2008; Dec; 28: 2319–2325. [DOI] [PubMed] [Google Scholar]
- 13. Folsom AR, Peacock JM, Demerath E, Boerwinkle E. Variation in ANGPTL4 and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Metabolism 2008; Nov; 57: 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stitziel NO, the Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators . Variants in ANGPTL4 and the Risk of Coronary Artery Disease. N Engl J Med 2016; Dec 8; 375: 2306. [DOI] [PubMed] [Google Scholar]
- 15. Dewey FE, Gromada J, Shuldiner AR. Variants in ANGPTL4 and the Risk of Coronary Artery Disease. N Engl J Med 2016; Dec 8; 375: 2305–2306. [DOI] [PubMed] [Google Scholar]
- 16. Aryal B, Singh AK, Zhang X, et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 2018; Mar 22; 3: e97918.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Standards of medical care in diabetes‐2018. Diabetes Care 2018; Jan; 41: S13–S2717. [DOI] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 19. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; Sep; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 20. Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication‐based analysis for two‐stage genome‐wide association studies. Nat Genet 2006; 38: 209–213. [DOI] [PubMed] [Google Scholar]
- 21. Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein‐coding genetic variation in 60,706 humans. Nature 2016; Aug 18; 536: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguilar‐Salinas CA, García EG, Robles L, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab 2008; Oct; 93: 4075–4079. [DOI] [PubMed] [Google Scholar]
- 23. Phillips CM. Metabolically healthy obesity across the life course: epidemiology, determinants, and implications. Ann N Y Acad Sci 2017; Mar; 1391: 85–100. [DOI] [PubMed] [Google Scholar]
- 24. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses' Health Study): 30 year follow‐up from a prospective cohort study. Lancet Diabetes Endocrinol 2018; May 30; pii: S2213–8587(18)30137–2 (Epub ahead of print); 6: 714–724. [DOI] [PubMed] [Google Scholar]
- 25. Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh‐Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care 2013; 36: 2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boyle EA, Li YI, Pritchard JK. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell 2017; Jun 15; 169: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]


