Abstract
Regulation of cell type-specific gene expression is critical for generating neuronal diversity. Transcriptome analyses have unraveled extensive heterogeneity of transcribed sequences in retinal photoreceptors because of alternate splicing and/or promoter usage. Here we show that Frmpd1 (FERM and PDZ domain containing 1) is transcribed from an alternative promoter specifically in the retina. Electroporation of Frmpd1 promoter region, −505 to +382 bp, activated reporter gene expression in mouse retina in vivo. A proximal promoter sequence (−8 to +33 bp) of Frmpd1 binds to neural retina leucine zipper (NRL) and cone-rod homeobox protein (CRX), two rod-specific differentiation factors, and is necessary for activating reporter gene expression in vitro and in vivo. Clustered regularly interspaced short palindromic repeats/Cas9-mediated deletion of the genomic region, including NRL and CRX binding sites, in vivo completely eliminated Frmpd1 expression in rods and dramatically reduced expression in rod bipolar cells, thereby overcoming embryonic lethality caused by germline Frmpd1 deletion. Our studies demonstrate that a cell type-specific regulatory control region is a credible target for creating loss-of-function alleles of widely expressed genes.
Introduction
Differentiation of diverse cell types during mammalian organogenesis requires precisely coordinated spatial and temporal patterns of gene expression. Like most organisms, the human genome possesses a limited number of genes (~20 000) (1), yet 250 000 to 1 million distinct RNA and protein variants are derived from the same genetic blueprint to construct extensive complexity of architecture and function (2). How such a highly orchestrated genetic program unravels the vast potential of the genome remains one of the most elusive questions in biology. A plethora of RNA isoforms can be produced by alternative splicing, and over 95% of human genes can generate distinctive splice variants in discrete cell types, and many times even in the same cell, at defined stages of development (3–5). In the nervous system, activity-dependent alternative splicing reportedly contributes to synaptic remodeling (6). Furthermore, use of alternate promoters can confer cell type-specific regulation and function to widely expressed genes, highlighting its key role in shaping distinct cellular identities (7–9). Indeed, alternative promoter usage is prevalent as early as embryogenesis and may contribute to germ layer specification (10). Expression of cell type-specific transcripts is especially prevalent in the central nervous system and contributes to functional differences among neurons (11–13). Untangling regulatory control mechanisms that produce remarkable specificity and function is a challenging task, especially because similar or overlapping isoforms are expressed in multiple cell types.
The exquisite laminated architecture of the retina with only five major types of neurons makes it an attractive model to dissect the contribution of alternative promoter usage in generating unique cellular functions from common pools of progenitor cells (14,15). The rod and cone photoreceptors in the retina are designed for the efficient capture of light and rapid subsequent transmission of electrical signals. These highly specialized neurons originate from common precursors during evolution (16) yet have distinct physiological characteristics pertaining to detection of light, color perception and signal transmission (17). The differentiation of rod and cone subtypes is largely guided by a small set of transcription factors; these include Orthodenticle homeobox 2 (OTX2), neural retina leucine zipper (NRL), cone-rod homeobox (CRX) and thyroid hormone receptor beta (TRβ2) (18). OTX2 is critical for determining photoreceptor cell fate (19). NRL and TRβ2 together define the fate of three photoreceptor subtypes in mouse retina (20). Post-mitotic cells fated to become rods develop instead as short wavelength sensitive cone (S-cone)-like photoreceptors in the absence of Nrl (Nrl−/− mouse) (21), whereas ectopic Nrl expression drives cones to develop as rods (22). CRX is critical for the expression of both rod and cone genes (23,24) and works synergistically with NRL to establish rod morphology and function (25).
Transcriptomic and epigenomic analyses of developing mouse rods and S-cone-like photoreceptors have uncovered a large network of NRL-regulated genes enriched in the rod photoreceptors (26–28). Several hallmarks of photoreceptor morphogenesis, including outer segment and synapse formation, are initiated after postnatal day (P)6 during retinal development, concurrent with a major shift in gene expression patterns (26,29,30). We therefore focused on evaluating NRL- and CRX-regulated genes that demonstrate dramatically increased expression after P6, with a goal to elucidate molecular pathways contributing to rod morphology and/or function.
The Frmpd1 (FERM and PDZ domain containing 1) gene was selected from rod transcriptome filtering analysis because of its highly specific expression in rods but not in cones (26). Here, we demonstrate that an alternatively spliced isoform of Frmpd1 is specifically transcribed in the rod photoreceptors and rod bipolar cells of the retina. In vivo and in vitro experiments reveal that the expression of the Frmpd1 alternate isoform in rod photoreceptors is specifically controlled by NRL and CRX. We also achieved complete loss of Frmpd1 expression in rods and greatly reduced expression in bipolar cells, by deleting the genomic region including the NRL and CRX binding sites via clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9. In addition to further delineating the differentiation effector molecules in NRL and CRX regulatory network, our studies provide a useful strategy for producing cell type-specific CRISPR gene knockouts by targeting regulatory genomic regions in vivo.
Results
Frmpd1 expression increases during rod photoreceptor maturation
Frmpd1 was initially selected by filtering of transcriptome data from developing retina and flow-sorted photoreceptors because of its dramatic rod-enriched upregulation of expression after P6 (Fig. 1A). Polymerase chain reaction (PCR) amplification of Frmpd1 transcripts from a panel of adult (P21) mouse tissues revealed its high expression especially in the retina, with much lower levels in brain and lung (Fig. 1B). Fluorescent in situ hybridization studies were then performed on a developmental time series of retina sections from both Nrl+/+ and Nrl−/− mice (Fig. 1C). Consistent with ribonucleic acid sequencing (RNA-seq) data, enhanced expression of Frmpd1 transcripts was readily detected in the outer nuclear layer (ONL) as rod differentiation proceeded from P4 to P21 (Fig. 1C). In addition, Frmpd1 transcripts were evident in the inner nuclear layer (INL) close to the photoreceptor layer; co-labeling with anti-protein kinase C alpha (PKCα) antibodies revealed these cells to be rod bipolar cells (data not shown). In the cone-only Nrl−/− retina where all rods have been converted into S-cone-like photoreceptors, only bipolar cells showed Frmpd1 transcripts confirming its rod-specific expression in the photoreceptor layer (Fig. 1C).
Figure 1.

Frmpd1 is upregulated in developing rod photoreceptors. (A) Frmpd1 expression in whole retina and flow-sorted photoreceptors. Relative expression (counts per million reads mapped, CPM) of Frmpd1 gene was assessed by RNA-seq transcriptome profiling of whole wild-type retina, flow-sorted rod (Nrl-GFP) and flow-sorted S-cone-like (Nrl-GFP:Nrl−/−) photoreceptors. (B) Frmpd1 expression in various tissues. Presence of Frmpd1 mRNA was assessed in multiple tissues via PCR using P21 mouse cDNA as template. Rhodopsin (Rho) primers were used to detect for retina contamination in other samples, and Hprt was used as a loading control. (C) RNA localization of Frmpd1 during development. Localization of Frmpd1 RNA was assessed by fluorescent in situ hybridization during development of rod-dominated (Nrl+/+) and cone-dominated (Nrl−/−) mouse retina. Scale bar, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Frmpd1 transcript in the retina but not brain includes an alternative 5′-untranslated exon
RNA-seq analysis indicated that the FRMPD1 transcript in the human retina corresponds exclusively to Ensembl ID Frmpd1-203, whereas Frmpd1-202 is detected in the brain (Fig. 2A). Similar transcript isoforms were observed in the mouse retina and brain (data not shown). To identify the Frmpd1 transcriptional start site (TSS), 5′-RACE (Rapid Amplification of cDNA Ends) was performed on both mouse and human retina and brain tissues (Fig. 2B). Sanger sequencing of the 5′-RACE products confirmed that the brain Frmpd1 transcript was initiated from the annotated exon 1, whereas the retinal transcript possessed an alternate exon 1a, indicating the use of an alternate promoter (Fig. 2B). We validated these findings by identifying additional sequences beyond the TSS of both annotated and alternate 5′-untranslated exon in the brain and retina, respectively (Fig. 2C). Exons 2–16-derived sequences were identical in transcripts from both tissues, thereby maintaining the identical protein product generated from the initiation codon in exon 2. The primers specific to exon 1a amplified a product only from the retinal RNA but not from heart, brain and lung samples (Fig. 2D). Fluorescent in situ hybridization of retinal sections using an exon 1a-specific probe detected Frmpd1 expression in both rod photoreceptors and INL cells (Fig. 2E).
Figure 2.
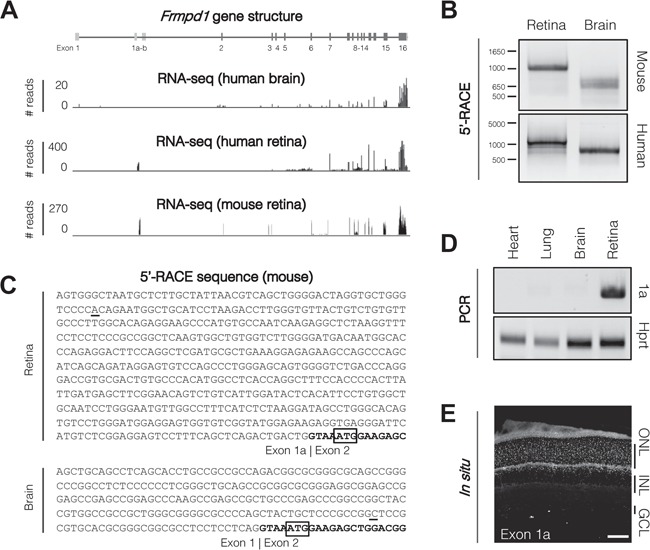
Frmpd1 is transcribed from an alternative promoter in the retina. (A) RNA-seq reveals alternate exon usage in human brain and retina. Frmpd1 exon structure for all three annotated Ensembl splice variants are shown; dark grey boxes indicate protein-coding exons and light grey boxes indicate untranslated exons. Aligned RNA-sequencing reads from adult human brain and retina are represented as a histogram. Human brain reads correspond to Ensembl human transcript ID Frmpd1–202, whereas human retina reads correspond to Frmpd1–203. (B) Tissue-specific RACE products in mouse and human. To determine TSSs used in brain and retina, a primary RACE reaction was performed using a gene-specific primer in Exon 8, followed by a nested RACE reaction using a gene-specific primer in Exon 6 to target both Frmpd1–202 and Frmpd1–203 transcript isoforms. 5′-RACE PCR products were visualized on an agarose gel, purified and cloned into a sequencing vector. (C) Frmpd1 5′-UTR sequences in murine retina and brain. Sanger sequencing of 5′-RACE products revealed the transcription of alternate 5′-UTR sequences in brain and retina. The retina transcript begins with Exon 1a, while brain begins with Exon 1. Both are spliced to Exon 2 (bold) where the translation start site is located (ATG; boxed). (D) Exon 1a expression is limited to the retina. PCR using primers specific for Exon 1a using cDNA from various P21 mouse tissues reveal that it is only expressed in the retina. (E) Localization of Exon 1a RNA in retina. Localization of Exon 1a RNA was assessed by fluorescent in situ hybridization of P21 mouse retina. Scale bar, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Frmpd1 alternative promoter activates reporter activity in the retina in vivo
To identify regulatory elements contributing to retinal Frmpd1 expression during development, we first examined the conservation of genomic sequences from upstream of brain exon 1 to the retinal alternate exon 1a and then integrated these with previously reported (31) open chromatin regions as evidenced by DNase-seq (Fig. 3A). Interestingly, this analysis revealed the presence of three putative regulatory elements [referred as conserved open chromatin region (COCR)-A–C in Fig. 3A] within +/−20 kb of the Frmpd1 retinal TSS (+1), which shows differential chromatin accessibility during retina development. COCR-A (−19 491 to −18 605) encompasses part of brain exon 1 (−18 964 to −18 913), whereas COCR-B (−505 to +382) contains part of retina exon 1a (+1 to +563). We cloned the three potential transcriptional control regions upstream of the green fluorescent protein (GFP) reporter gene and co-transfected these constructs with a CAG-mCherry plasmid (control for transfection) into neonatal mouse retina by in vivo electroporation, as illustrated (Fig. 3B). The retinas were harvested at P21 (representative of peak Frmpd1 expression levels) and imaged for expression of GFP and mCherry reporters in flatmounts. The COCR-B construct carrying the −505 to +382 sequence encompassing the upstream region and a part of exon 1a produced the highest levels of GFP expression with almost 50-fold increase compared to an empty promoter construct (Fig. 3C and D). We therefore conclude that retina-specific upregulation of Frmpd1 is directed by COCR-B control region in vivo.
Figure 3.

Identification of sequence elements required for Frmpd1 expression in vivo. (A) Frmpd1 upstream genomic sequence showing major COCRs. Conservation diagram displays genomic sequence homology among mammals, and DNase-seq diagrams display DNase hypersensitive regions in retina across various points of development. E = embryonic day. P = postnatal day. TSS = transcription start site. (B) In vivo promoter assay workflow. Putative regulatory elements COCR-A, B or C highlighted in (A) were cloned upstream of a GFP reporter. GFP reporter with no upstream promoter was used as a negative control, while mCherry under control of a CAG promoter was used as a positive transfection control. Constructs were transfected into retinal cells by in vivo electroporation at P1. At P21, retinas were harvested, flat-mounted, then imaged and relative promoter activity quantified. (C) In vivo promoter activity of conserved and accessible DNA clusters. Test COCR fragments were cloned upstream of a GFP reporter and transfected into neonatal mouse retina (P1) with CAG-mCherry transfection marker. Retinas were harvested at P21 to examine expression of the reporter quantified in (D). Scale bar, 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
NRL and CRX bind to proximal region of Frmpd1 alternate promoter
In silico examination of COCR-B revealed putative binding sites for multiple transcription factors that are expressed in the P21 retina (Fig. 4A and B). Given that loss of Nrl abrogated Frmpd1 expression in rods and NRL functions synergistically with CRX, we first evaluated the published chromatin immunoprecipitation sequencing (ChIP-seq) data for these two transcription factors (32,33) to narrow down the control elements (Fig. 4C). We selected two different retina promoter oligonucleotides (RPO1 and RPO2) carrying potential NRL- and CRX-binding sequences and generated multiple mutants, by site-directed mutagenesis, to examine specific critical elements (Fig. 4D). Electrophoretic mobility shift assays (EMSAs) using the two 32P-labeled oligonucleotide probes revealed binding of retinal nuclear proteins, and this binding was reduced or eliminated by including an excess of respective cold wild-type oligonucleotides (Fig. 4E and F). The cold mutant (M)2–M4, but not M1 and M5, oligonucleotides strongly affected the mobility shift of the radiolabeled RPO1 probe, suggesting that M1 mutation abrogates the binding site for the retinal protein(s) (Fig. 4E). The electrophoretic mobility shift of radiolabeled RPO2 was reduced by the addition of cold wild-type and other mutant oligonucleotides, and only M11 mutant did not have any effect, implicating multiple residues in RPO2 for protein binding (Fig. 4F). We note that the nucleotides required for mobility shift in RPO1 and RPO2 oligonucleotides overlap with CRX and NRL binding sites.
Figure 4.

Frmpd1 promoter region contains putative binding sites for retinal transcription factors. (A) Annotated Frmpd1 proximal promoter, indicating several overlapping predicted binding sites for various transcription factors expressed in the retina. The −505 to +382 region of DNA relative to the retina-specific transcription start site was analyzed by Matinspector to predict putative transcription factor binding sites. Red arrow indicates the TSS identified by 5′-RACE. (B) Heat map of transcription factor expression values in the retina (in Log2CPM). A heat map was generated from the transcription factors containing putative binding sites within the Frmpd1 minimal promoter region B (−505 to +382) with increased expression in flow-sorted rod photoreceptors during development. P = postnatal day. CPM = counts per million of reads mapped. (C) NRL and CRX ChIP-seq peaks lie within Frmpd1 proximal promoter region. NRL and CRX ChIP-seq reads from adult mouse retina samples were mapped to the mouse genome. Mammalian conservation and Frmpd1 promoter region are aligned to scale. The boxed region indicates −107 to +33, which was used to test for NRL and CRX binding and activation in subsequent panels. (D) NRL and CRX core binding site mutations. Several small clusters of perfectly conserved residues (asterisks) in transcription factor binding sites within the −107 to +33 region were altered to produce various RPOs for use in EMSA. (E and F) NRL and CRX directly bind to Frmpd1 proximal promoter in vitro. Radioactive-labeled RPO probes containing putative NRL or CRX binding sites were incubated with nuclear extract of P21 mouse retina. The shift created by protein-DNA binding (indicated by arrow) was reduced by the addition of unlabeled probe in 250× molar excess but to a lesser degree by unlabeled probes with critical mutations in binding site residues. Single asterisk denotes a nonspecific shift; double asterisk shows unbound 32P-labeled probe.
COCR-B sequence elements are required for promoter activation with NRL and CRX
To examine the relative contribution of CRX and NRL binding sequences within RPO1 and RPO2 to Frmpd1 promoter activation, we generated COCR-B-Luc reporter construct, containing −505 to +382 sequence upstream of a luciferase reporter gene, and mutated critical nucleotides identified in EMSA experiments (Fig. 5A; see mutants M5 and M11 in Fig. 4E and F). We then co-transfected COCR-B-Luc or the two mutant constructs with NRL and/or CRX expression vectors. As predicted, NRL and CRX activated the Frmpd1 promoter containing COCR-B sequence, and only M11 mutant (i.e. RPO2 sequence) but not M5 (i.e. RPO1 sequence) dramatically decreased the luciferase reporter activity (Fig. 5B). Electroporation of a mutant Frmpd1 promoter-GFP construct carrying RPO2-M11 mutations demonstrated a dramatic decrease in reporter activity in the retina in vivo (Fig. 5C and D). Further analysis of mutations in RPO2 sequence identified nucleotides critical for transactivation by NRL and/or CRX (Fig. 5E). Together with EMSA (Fig. 4F), these results pinpointed critical nucleotides for Frmpd1 promoter activation by NRL and CRX and demonstrate that the −8 to +33 promoter sequence is critical for NRL- and CRX-mediated expression of Frmpd1 in rod photoreceptors.
Figure 5.

NRL and CRX activate alternative Frmpd1 promoter in vitro and in vivo. (A) Schematic of Frmpd1 alternative promoter elements. Retina-specific transcription of Frmpd1 begins with Exon 1a (bp +1 to +563). COCR-B from −505 to +382 can activate GFP reporter gene expression in vivo. RPO1 and RPO2 can bind to retinal transcription factors and lie within COCR-B. (B) Modulation of Frmpd1 minimal promoter element COCR-B activity by NRL and CRX. HEK293 cells were co-transfected with COCR-B wild-type or mutant promoters driving luciferase reporter gene simultaneously with NRL and CRX expression constructs. Total DNA concentration was adjusted by empty expression vector pcDNA4/Hismax-C. Fold change is relative to reporter gene activation in the absence of NRL and CRX expression vectors. (C) In vivo promoter activity of mutated COCR-B. The wild-type or mutant COCR-B fragments were cloned upstream of a GFP reporter and transfected into neonatal mouse retina (P1) with CAG-mCherry transfection marker. Retinas were harvested at P21 to examine expression of the reporter gene, quantified in (D). Scale bar, 50 μm. (E) Modulation of Frmpd1 mutant COCR-B activities by NRL and CRX. COCR-B wild-type or mutant promoter constructs driving luciferase reporter gene were co-transfected in HEK293 cells with NRL and CRX expression plasmids. Total DNA concentration was adjusted by including empty expression vector pcDNA4/Hismax-C. Fold change is relative to reporter gene activation in the absence of NRL and CRX expression vectors.
Deletion of NRL and CRX binding sites causes rod-specific silencing of Frmpd1
We wondered whether the removal of the rod photoreceptor-specific promoter region (RPO2 but not RPO1) would overcome embryonic lethality observed by complete loss of Frmpd1 (unpublished data). CRISPR/Cas9 guide RNAs (gRNAs) were designed flanking the photoreceptor minimal promoter and exon 1a to excise this region through non-homologous end joining (NHEJ) (Fig. 6A). The RPO1 sequence, the brain TSS and the translation start site (in exon 2) were left unaltered such that only the rod photoreceptor-specific regulatory sequence of Frmpd1 was disrupted. PCR genotyping and sequencing of tail DNA confirmed a deletion of 1080 bp in the target Frmpd1 genomic DNA, encompassing the NRL and CRX binding sites (Fig. 6B and C). In situ hybridization studies using probes specific to the targeted exon 1a revealed absolutely no expression in rods with negligible transcripts in the retinal bipolar cells (arrowheads), whereas probes designed from Frmpd1 exons 3–12 showed a complete loss of mRNA in rods and very low level of expression in bipolar cells (Fig. 6D). When assessed by quantitative PCR (qPCR) using primers from exon 16, the retinas of Frmpd1Δ1a mice revealed reduced expression of Frmpd1 mRNA, and expression in other tissues remained unchanged (Fig. 6E). Retina protein lysates from Frmpd1Δ1a mice demonstrated a drastic reduction (~70%) in Frmpd1 protein levels by immunoblotting (Fig. 6F and G). As predicted, the Frmpd1 protein expression was unaffected in the brain (data not shown).
Figure 6.

Deletion of NRL and CRX binding sites causes rod-specific silencing of Frmpd1. (A) Schematic of gRNAs and genotyping primers for CRISPR/Cas9-targeted genomic region. gRNA1 and gRNA2 flanking exon 1a were designed to delete a 1 kbp fragment upon NHEJ. Primers pF, pF′ and pR were designed to detect NHEJ events. (B) PCR genotyping of genomic DNA. Genomic DNA was extracted from tail clippings and used for PCR amplification by three primers (pF, pF′, pR). A 350 bp product corresponds to the wild-type allele (+), while a 150 bp product corresponds to a deletion allele (Δ1a). (C) Sequence of CRISPR/Cas9-mediated deletion. PAM sequences (orange) and CRISPR gRNA sequences (green and blue) are highlighted. Wild-type and deletion alleles were PCR-amplified from genomic tail DNA and Sanger sequenced. Top line corresponds to wild-type genomic sequence, while bottom line corresponds to genomic sequence after Exon 1a excision by NHEJ, whereby basepairs −48 to +1033 relative to retina Frmpd1 TSS were removed from the genome. (D) Exon 1a deletion abolishes full-length Frmpd1 RNA expression in the ONL. Fluorescent in situ hybridization probes specific to Exon 1a or downstream Exons 3–12 were used to visualize Frmpd1 RNA expression in wild-type or CRISPR-targeted mouse retina. INL expression in Frmpd1Δ1a/Δ1a is indicated by arrowheads. (E) Tissue specificity of Frmpd1 RNA expression knockdown. RNA was harvested from multiple tissues from Frmpd1+/+ and Frmpd1Δ1a/Δ1a animals and used for quantitative PCR with Hprt as a loading control. (F) Frmpd1 protein expression in retina. Immunoblots from 50 μg of whole retina lysate from mice of each genotype were probed with anti-Frmpd1 antibody and anti-β-actin as a loading control. Densitometric analysis from three separate immunoblots comparing Frmpd1 expression in Frmpd1+/+ and Frmpd1Δ1a/Δ1a retina is shown in (G).
Discussion
Spatiotemporally restricted and quantititatively precise control of transcription is critical for producing cell type-specific attributes. Alternate splicing and promoter usage expand modes of gene regulation and permit the generation of unique transcribed sequences from a single gene, offering efficiency as well as functional redundancy in biological pathways. Complexities associated with the processing of visual information within retinal neurons necessitate a multitude of control mechanisms. Maintenance of physiological state and initiation of phototransduction in retinal photoreceptors exert enormous metabolic demands that are constantly responding to light, microenvironment and circadian cues. NRL and CRX direct and/or augment the expression of most rod genes by providing local control to cellular dynamics specific to rod photoreceptors. In order to elucidate how unique rod morphology and function are created during development, we have focused on delineating the regulation of photoreceptor-enriched transcription from otherwise widely expressed genes. Here we demonstrate that transcription of Frmpd1 is initiated from a retina-specific alternative promoter and define sequence elements mediating its rod-specific expression through NRL and CRX. We also show that the removal of this rod-specific regulatory region by CRISPR/Cas9 completely abolishes Frmpd1 expression in rod photoreceptors of the mouse retina. The dramatically reduced Frmpd1 expression in bipolar neurons might reflect its regulation by CRX, which is also expressed in these cells (34). Our studies thus provide a novel approach to target cell type-specific transcript isoforms of widely expressed genes in the germline by excising specific gene regulatory control elements.
Selective use of alternative promoters in different cell types or tissues to generate an identical protein product adds an additional level of spatio-temporal control on gene expression. In addition, unique 5′-untranslated regions (UTRs) of distinct alternative transcripts provide regulation through mRNA stability/turnover, localization and translation efficiency (35–37). Similar alternative promoters and UTRs that do not affect the open reading frame have been reported for many genes, e.g. runt-related transcription factor 1, OTX2 and short stature homeobox (38–40). The 5′-UTR of Frmpd1 in the retina is more AT-rich compared to Frmpd1 transcripts in other tissues (45% versus 19% AT), suggesting lesser stability of the retinal isoform. We hypothesize that faster turnover of Frmpd1 transcripts permits more stringent control over its function in response to rapid dynamics of photoreceptor microenvironment. Interestingly, Frmpd1 expression varies as much as 2-fold across the circadian cycle (data not shown), suggesting that continuous fine-tuning of its expression might be required to maintain rod photoreceptor homeostasis.
A majority of rod-expressed genes are transcriptionally activated by synergistic actions of NRL and CRX, and the presence of NRL and CRX binding elements in close proximity within a promoter-enhancer region strongly suggest rod-specific transcription (24–26,32,33,41). Dramatic upregulation of Frmpd1 transcription in developing rods from P6–P10 is indicative of contributions from additional factors, such as NR2E3 and estrogen receptor-related beta protein, that are induced during this period (Fig. 4B) and shown to contribute to rod differentiation (42–44). Notably, NR2E3 forms complexes with NR1D1 (42), which is implicated in modulating circadian rhythmicity in gene expression patterns (45). In fact, the −505 to +382 promoter region (COCR-B) of Frmpd1 that exhibited enhanced activation in the retina (Fig. 3C) includes one NR1D1 and several NR2E3 binding sites (Fig. 4A), which might further influence Frmpd1 expression dynamics in rods.
The FERM and PDZ domains are commonly detected in scaffolding proteins that coordinate anchoring of cytoskeletal and/or signaling complexes at the cell membrane (46,47). Frmpd1–4 proteins include both of these domains and are implicated in a wide range of morphogenic and signaling functions. For example, Frmpd2 interacts with Lrit1 in cone photoreceptors to modulate synaptic transmission to ON-bipolar cells (48,49). Frmpd4 or Preso1 facilitates the interaction of metabotropic glutamate receptors with adaptor protein Homer to modulate their activity at excitatory synapses (50). The presence of such a highly conserved, tissue-specific regulatory mechanism that escaped selective pressure suggests the importance of tightly controlled expression of Frmpd1 for optimal rod photoreceptor function. Though a precise role for Frmpd1 in the retina is not yet delineated, this protein can interact with G-protein signaling modulators 1 and 2 (51,52), which have been implicated in stabilizing the inactive, guanosine diphosphate-bound form of transducin alpha (53–56). Given that phototransduction in rod photoreceptors relies heavily on G-protein signaling by transducin, we postulate a key function of Frmpd1 in modulating neurotransmission in rods. Generation of a rod-specific Frmpd1-knockout mouse will now allow us to test this hypothesis.
CRISPR/Cas9 typically generates mosaic founder mice, although some founder mice may be homozygous for mutations (57). Thus, CRISPR-based methods for generating loss-of-function mouse lines can be more time effective than traditional Cre/loxP systems. However, germline disruption of a gene can result in embryonic lethality. The Cre/loxP system offers the flexibility of inducing gene disruption in a spatially and temporally regulated manner through selective Cre lines to bypass embryonic lethality (58). The CRISPR/Cas9 system has been widely used for producing loss of function alleles in mice, yet its use for cell-specific gene editing or disruption has been limited (59–61). A germline deletion of Frmpd1 resulted in death of embryos at around E10 (data not shown), thereby hampering the analysis of its function in rod photoreceptors. We were able to generate a rod-specific loss of Frmpd1 through CRISPR/Cas9-mediated deletion of its alternative promoter in the germline without altering the expression of Frmpd1 protein in brain and other tissues. Our targeted approach should have wide application for producing specific alleles of other similar transcript isoforms in the photoreceptors (62) and/or other cell types.
Gene therapy approach for dominant diseases at times requires silencing of the host allele before gene replacement using adeno-associated viral vectors (AAV) (63,64). However, this strategy cannot easily distinguish between the target allele(s) and those delivered exogenously and/or expressed in multiple cell types. Delivery of CRISPR/Cas9 via AAV has been successful in knocking down the expression of rod photoreceptor gene Nrl in vivo (65). As such, the retina offers an excellent model for testing AAV-delivered and CRISPR/Cas9-mediated targeted gene disruption, editing and therapy. A selective promoter-targeted strategy, described in this report, would permit specific host transcript isoform silencing for experimental or gene therapy purposes. Almost 600 genes that are predicted to be transcriptional targets of NRL and CRX in rod photoreceptors (33,41,66) represent attractive candidates for utilizing this targeted strategy.
Materials and Methods
Mouse lines and animal husbandry
All experiments were conducted according to protocols approved by a local Institutional Animal Care and Use Committee and adhered to the Association for Research in Vision and Ophthalmology statement for animal use in ophthalmic and vision research.
CRISPR/Cas9-mediated genome editing
Deletion of the retina-specific Frmpd1 proximal promoter in the mouse genome was achieved by CRISPR-mediated editing in C57BL/6J zygotes (67). A pair of gRNAs for Streptococcus pyogenes Cas9 (SpCas9) flanking the retina-specific Frmpd1 proximal promoter were designed using an online CRISPR tool (http://www.crisprscan.org) and synthesized by in vitro transcription (IVT), as described (68). Briefly, a forward primer containing the crRNA sequence following the T7 promoter (Table 1) and a reverse primer consisting of the last 20 bp of the trans-activating crRNA (tracrRNA) sequence were used to produce the templates, which were then transcribed into crRNA–tracrRNA (single gRNA) (69) using the MEGAshortscript T7 Transcription Kit (Thermo Fisher Scientific, Rockford, IL, USA). SpCas9 protein (100 ng/μl) was then mixed with 50 ng/μl of each gRNA in 10 mm Tris (pH 7.5) and incubated at room temperature for 15 min to assemble ribonucleoprotein particles (RNP). Assembled RNP were microinjected into zygotes from 3-week-old superovulated C57BL/6J females (Jackson Laboratory, Bar Harbor, ME, USA). These were cultured in modified Krebs–Ringer bicarbonate or KSOM medium overnight, and the next day, two-cell embryos were transferred into pseudopregnant recipient females. Pups carrying the deletion alleles were identified by PCR with primers flanking the gRNA cut sites as described (`PCR and genotyping’, below). Six of the eight F0 pups carried deletions of the target region, which varied somewhat in size. The genetic mosaic F0 founder carrying the largest deleted region was crossed with wild-type C57BL/6J mice for germline transmission of the deletion alleles. F1 founders carrying the alleles were crossed to C57BL/6J mice for at least two additional generations before heterozygous and homozygous progeny were used in the studies.
Table 1.
Forward primers used for making IVT templates
| gRNA | Forward primer (5′ → 3′) |
|---|---|
| gRNA1 | taatacgactcactataGGGGGTGACCTGCCCCTCTgttttagagctagaa |
| gRNA2 | taatacgactcactataGGATGGGGCGTGGATGCCATgttttagagctagaa |
PCR and genotyping
For genotyping, genomic DNA was extracted from tail clippings (DirectPCR Lysis Reagent, Viagen, Los Angeles, CA, USA). Wild-type and deletion alleles were detected via amplification using a 3-primer PCR reaction yielding products of 342 bp and/or 150 bp, respectively. Endpoint PCR and quantitative PCR were performed using gene-specific primers on cDNA synthesized from various mouse tissues at P21 as previously described (70). qPCR experiments were performed in biological and technical triplicate, and statistical significance determined by one-way analysis of variance (ANOVA) multiple comparisons. Primer sequences are provided in Table 2.
Table 2.
Primers used for genotyping and quantitative PCR
| Product name | Use | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|---|
| Frmpd1 promoter wild type | Genotyping | GCAGGTTCGTTCTCAAGCAT | GTTGCCCTTTGACCTTCACA |
| Frmpd1 promoter deletion | Genotyping | CCTGTGAATGAGGGCTCTGA | |
| Frmpd1 universal | PCR | GCCGCTGTATTCTGTTTGAC | GGCAGGTGAACAAGAAGAGG |
| Frmpd1 exon 1a | PCR | CGCTGAAAGGAGAGAAGCCA | GCCAACATTCCCAGGATTGC |
| Hprt | PCR | CAAACTTTGCTTTCCCTGGT | CAAGGGCATATCCAACAACA |
| Rho | PCR | TGTTCCTGCTCATCGTGCTGG | GGAAGTTGCTCATCGGCTTGC |
Fluorescent in situ hybridization
Fluorescent in situ hybridization was performed on 1 h 4% paraformaldehyde (PFA)-fixed cryosections from adult mouse retina, using reagents from Advanced Cell Diagnostics as previously described (26). Custom probes were designed to target either all Frmpd1 transcripts (exons 3–12) or exon 1a specifically (Table 3). Images are representative of at least three biological replicates.
Table 3.
In situ probe summary
| Probe name | Catalog # | Target region (bp) | ZZ pairs |
|---|---|---|---|
| Mm-Frmpd1 | 440459 | 188–1197 of NM_001081172.2 | 20 |
| Mm-Frmpd1-exon1A | 440469 | 2–478 of ENSMUSE00000674488 | 10 |
5′-RACE derived from full-length RNA
Total mouse RNA was extracted from tissues according to the manufacturer’s protocol (TRIzol LS, Thermo Fisher Scientific), while human RNA was obtained commercially (Clontech, Mountain View, CA, USA). 5′-RACE was performed according to the SMARTer RACE 5′/3′ kit manufacturer’s instructions (Clontech) using gene-specific primers (Table 4). At least 10 independent clones from each reaction were Sanger sequenced and submitted to Cap3 (http://doua.prabi.fr/software/cap3) to generate a consensus sequence.
Table 4.
Gene-specific primers used for 5′-RACE amplification
| Primer | Location | Sequence (5′ → 3′) |
|---|---|---|
| Mouse primary PCR | Exon 8 | GATTACGCCAAGCTTTGCAGAAGGTGTAGGCGGGAGATGCTGT |
| Mouse-nested PCR | Exon 6 | GATTACGCCAAGCTTCCGGGCCCGCTTCTCCTCAGTCAAGAAG |
| Human primary PCR | Exon 8 | GATTACGCCAAGCTTGATGAGTTCCTCTTCGTGCAGCAGGTGC |
| Human-nested PCR | Exon 6 | GATTACGCCAAGCTTCCGGGCCCGCTTCTCCTCAGTCAAGAAG |
In silico promoter analysis
FASTQ files from CRX transcription factor ChIP-seq were downloaded from Gene Expression Omnibus with accession IDs GSE20012 and GSE54084 (32). NRL ChIP-seq data (33) was downloaded from https://datashare.nei.nih.gov/nnrlMain.jsp. DNase-seq data was downloaded from the mouse ENCODE project (31). Low-quality bases of FASTQ reads were trimmed using Trimmomatic (71) with parameters SLIDINGWINDOW:4:15 and MINLEN:24. Cleaned FASTQ reads were then aligned to the mouse reference genome (mm10) using BWA backtrack algorithm (72). Alignments that were unmapped, having a mapping score < 20, and secondary alignments were filtered using Samtools (73) with the following parameters: ‘-q 20 -F 1796’. Mammalian conservation (Placental Mammal Basewise Conservation by PhyloP) was visualized using the December 2011 (GRCm38/mm10) UCSC Genome Browser assembly. MatInspector (Genomatix Ann Arbor, MI, USA) was used to identify predicted transcription factor binding sites within the COCRs directly upstream of Exon 1a in the mouse genome.
RNA-seq analysis
Mouse total RNA-seq (74) and flow-sorted photoreceptor (26) datasets were analyzed as reported previously (75). For human RNA-seq, adaptors and low-quality bases of FASTQ reads were trimmed with Trimmomatic (71) and aligned via the STAR (2-pass) tool to the human transcriptome (Ensembl GRCh38.85) (76). Parameters sjdbOverhang = 124 for retinal reads and sjdbOverhang = 75 for brain reads were used to generate the STAR index, with ENCODE standard options for the rest of the alignment. The resulting BAMs were sorted and indexed via SAMTOOLs and then subset using SAMTOOLs for the FRMPD1 region and re-indexed (73).
Plasmid DNA constructs and mutagenesis
Putative promoter sequences were PCR amplified from C57BL/6J mouse genomic DNA using a high-fidelity Taq polymerase (Seqamp polymerase, Clontech) and inserted into the multiple cloning site of pGL3-basic vector for in vitro luciferase assays (Promega, Madison, WI, USA). To generate in vivo GFP promoter constructs, PCR amplification of pEGFP-N1 vector backbone (Clontech) was used to remove its CMV promoter, which was then replaced by test promoter fragments. Mutations were introduced into Frmpd1 promoter constructs according to Q5 Site-Directed Mutagenesis Kit manufacturer’s instructions (New England Biolabs, Ipswich, MA, USA). Primer sequences are provided in Table 5.
Table 5.
Cloning and mutagenesis primers for Frmpd1 promoter plasmid constructs
| Product name | Use | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) |
|---|---|---|---|
| COCR-A | Promoter cloning | GACTGGGAGACAAGGG | CCGCACCTGAACCCA |
| COCR-B | Promoter cloning | CAAGGGGTATGAAGGACAG | TATCCAATGACAGACTGTTCC |
| COCR-C | Promoter cloning | CGGCACATTCTGACTCTCCA | GGTTACCTAGTCATGGCCCC |
| EGFP-N1 | CMV removal | TTCGAATTCTGCAGTCGAC | AACTAATGCATGGCGGTAATACGG |
| Mutant 1 | Mutagenesis | TTGTCATTTCCCCATCTGTCAAGGGAGGATAATATGCTTGGGGGTG | CACAGGATGCGGCGGTAACCTGCCCAGCCTCGTGCAGGC |
| Mutant 2 | Mutagenesis | TTGGACGCTCCCCATCTGTCAAGGGAGGATAATATGCTTGGGGGTG | CACAGGATGATTAAATAACCTGCCCAGCCTCGTGCAGGC |
| Mutant 3 | Mutagenesis | TTGTCATTTATATATCTGTCAAGGGAGGATAATATGCTTGGGGGTG | CACAGGATGATTAAATAACCTGCCCAGCCTCGTGCAGGC |
| Mutant 4 | Mutagenesis | TTGTCATTTCCCCAGACTGCAAGGGAGGATAATATGCTTGGGGGTG | CACAGGATGATTAAATAACCTGCCCAGCCTCGTGCAGGC |
| Mutant 5 | Mutagenesis | TTGGACGCTATATAGACTGCAAGGGAGGATAATATGCTTGGGGGTG | CACAGGATGCGGCGGTAACCTGCCCAGCCTCGTGCAGGC |
| Mutant 6 | Mutagenesis | TCTTGCTATTAACGTCAGCTGGGGACTAGGTGCTGGGTCC | GCATTAGCCCACGCGGTAGCACGGGAGCCCAGAGGGGCA |
| Mutant 7 | Mutagenesis | TCTTGCTATTAACGTCAGCTGGGGACTAGGTGCTGGGTCC | TAGTTAGCCCACTAAGCCTCACGGGAGCCCAGAGGGGCA |
| Mutant 8 | Mutagenesis | TCTCATCATTAACGTCAGCTGGGGACTAGGTGCTGGGTCC | GCATTAGCCCACTAAGCCTCACGGGAGCCCAGAGGGGCA |
| Mutant 9 | Mutagenesis | TCTTGCTATTAACAGAAGCTGGGGACTAGGTGCTGGGTCC | GCATTAGCCCACTAAGCCTCACGGGAGCCCAGAGGGGCA |
| Mutant 10 | Mutagenesis | TCTTGCTATTAACGTCAGCTGGGGACTAGGTGCTGGGTCC | GCATTATATACTGAAGCCTCACGGGAGCCCAGAGGGGCA |
| Mutant 11 | Mutagenesis | TCTCATCATTAACAGAAGCTGGGGACTAGGTGCTGGGTCC | TAGTTATATACTGCGGTAGCACGGGAGCCCAGAGGGGCA |
In vivo electroporation
Equimolar amounts (140 fmol) of each promoter driving GFP were co-transfected with 50 fmol of CAG-mCherry construct in neonatal (P1) mouse pups via in vivo electroporation as previously described (77). At P21, electroporated eyeballs were fixed in 4% PFA for 30 min before the retina was dissected out and fixed for an additional 30 min. Each retina was then flat-mounted with Fluoromount-G (Southern Biotech, Birmingham AL, USA), imaged using a Zeiss LSM 700 confocal microscope and quantified for relative activity as described (78). A minimum of three biological replicates for each construct were analyzed, and statistical significance determined by one-way ANOVA multiple comparisons.
Electrophoretic mobility shift assay
Total nuclear extract was isolated from pooled P21 mouse retina following standard protocols (Thermo Fisher Scientific, Waltham, MA, USA) and quantified by bicinchoninic acid assay (BCA) (Pierce, Waltham, MA, USA). Briefly, 10 μg nuclear extract was incubated at room temperature for 45 min with 1× Binding Buffer (LightShift, Thermo Fisher Scientific), 50 ng/μl Poly dI dC, 5 mm MgCl2 and 50 pmol cold competitor oligonucleotide in H2O up to 19 μl. Following this incubation, 0.2 pmol of 32P-labeled oligonucleotide was added and allowed to incubate at room temperature 1 h longer. Reactions were terminated by addition of loading buffer, separated on a 6% DNA retardation gel (Invitrogen, Waltham, MA, USA) and subjected to autoradiography. Images are representative of at least three independent experiments.
Luciferase assay
HEK293 cells were seeded in 450 μl complete media at a density of 50 000 cells/well into 24-well plates. Two days later, cells were transfected using Lipofectamine 2000 (Invitrogen) by adding 50 μl concentrated DNA–lipid complex directly to each well-containing cells and media for desired end concentrations of each plasmid construct per well. Forty-eight hours post-transfection, cells were harvested, and luminescence of firefly and renilla luciferases were measured according to the Dual-Luciferase Reporter Assay System (Promega) manufacturer’s protocol as previously described (79). Experiments were performed in biological triplicates, and statistical significance was determined by multiple t-tests.
Immunoblotting
Retinae were isolated from P21 mice and lysed by sonication in ice-cold radioimmunoprecipitation buffer supplemented with protease inhibitors (Roche Applied Science, Penzberg, Germany) and 1% n-dodecyl β-D-maltoside (Sigma, St. Louis, MO, USA). Supernatants were quantified by BCA protein assay according to manufacturer’s protocol (Pierce) and solubilized in 4× Laemmli buffer + β-mercaptoethanol. After denaturation, 50 μg of the total protein extract was separated by SDS-PAGE and transferred to PVDF membrane (Trans-Blot Turbo System, Bio-Rad, Hercules, CA, USA). Standard immunoblot procedure was followed as previously described (79) using primary antibodies raised against β-actin (Sigma A5316, 1:5000) and Frmpd1 (Atlas, Bromma, Sweden, HPA042934, 1:1000) and HRP-conjugated secondary antibodies raised in donkey (Millipore, St. Louis, MO, USA, 1:6000). Images are representative of at least three independent experiments. Densitometric analysis was performed in ImageJ by normalizing Frmpd1 to β-actin, and statistical significance was determined by unpaired t-test.
Acknowledgements
We are grateful to Jacob Nellissery, Laura Campello, Vijender Chaitankar, Koray Kaya, Matthew Brooks, Madeline Kwicklis, Margaret Starostik, Yide Mi and Megan Kopera for assistance with gel-shifts, bioinformatics and mouse colony management. This research utilized the high-performance computational capabilities of the Biowulf Linux cluster at NIH (http://biowulf.nih.gov).
Conflict of Interest statement. None declared.
Funding
Intramural Research program of the National Eye Institute (EY000450, EY000474); John Fell Oxford University Press (OUP) Research Fund (132/107).
References
- 1. Ezkurdia I., Juan D., Rodriguez J.M., Frankish A., Diekhans M., Harrow J., Vazquez J., Valencia A. and Tress M.L. (2014) Multiple evidence strands suggest that there may be as few as 19 000 human protein-coding genes. Hum. Mol. Genet., 23, 5866–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klerk E. and t Hoen P.A. (2015) Alternative mRNA transcription, processing, and translation: insights from RNA sequencing. Trends Genet., 31, 128–139. [DOI] [PubMed] [Google Scholar]
- 3. Pan Q., Shai O., Lee L.J., Frey B.J. and Blencowe B.J. (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet., 40, 1413–1415. [DOI] [PubMed] [Google Scholar]
- 4. Wang E.T., Sandberg R., Luo S., Khrebtukova I., Zhang L., Mayr C., Kingsmore S.F., Schroth G.P. and Burge C.B. (2008) Alternative isoform regulation in human tissue transcriptomes. Nature, 456, 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faustino N.A. and Cooper T.A. (2003) Pre-mRNA splicing and human disease. Genes Dev., 17, 419–437. [DOI] [PubMed] [Google Scholar]
- 6. Hermey G., Bluthgen N. and Kuhl D. (2017) Neuronal activity-regulated alternative mRNA splicing. Int. J. Biochem. Cell Biol., 91, 184–193. [DOI] [PubMed] [Google Scholar]
- 7. Feng G., Tong M., Xia B., Luo G.Z., Wang M., Xie D., Wan H., Zhang Y., Zhou Q. and Wang X.J. (2016) Ubiquitously expressed genes participate in cell-specific functions via alternative promoter usage. EMBO Rep., 17, 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayoubi T.A. and Van De Ven W.J. (1996) Regulation of gene expression by alternative promoters. FASEB J., 10, 453–460. [PubMed] [Google Scholar]
- 9. Davuluri R.V., Suzuki Y., Sugano S., Plass C. and Huang T.H. (2008) The functional consequences of alternative promoter use in mammalian genomes. Trends Genet., 24, 167–177. [DOI] [PubMed] [Google Scholar]
- 10. Lu X., Zhao Z.A., Wang X., Zhang X., Zhai Y., Deng W., Yi Z. and Li L. (2018) Whole-transcriptome splicing profiling of E7.5 mouse primary germ layers reveals frequent alternative promoter usage during mouse early embryogenesis. Biol. Open, 7, doi:10.7554/bio.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raj B. and Blencowe B.J. (2015) Alternative splicing in the mammalian nervous system: recent insights into mechanisms and functional roles. Neuron, 87, 14–27. [DOI] [PubMed] [Google Scholar]
- 12. Pal S., Gupta R., Kim H., Wickramasinghe P., Baubet V., Showe L.C., Dahmane N. and Davuluri R.V. (2011) Alternative transcription exceeds alternative splicing in generating the transcriptome diversity of cerebellar development. Genome Res., 21, 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reyes A. and Huber W. (2018) Alternative start and termination sites of transcription drive most transcript isoform differences across human tissues. Nucleic Acids Res., 46, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cayouette M., Poggi L. and Harris W.A. (2006) Lineage in the vertebrate retina. Trends Neurosci., 29, 563–570. [DOI] [PubMed] [Google Scholar]
- 15. Popova E.Y., Salzberg A.C., Yang C., Zhang S.S. and Barnstable C.J. (2017) Identification and prediction of alternative transcription start sites that generate rod photoreceptor-specific transcripts from ubiquitously expressed genes. PLoS One, 12, e0179230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J.W., Yang H.J., Oel A.P., Brooks M.J., Jia L., Plachetzki D.C., Li W., Allison W.T. and Swaroop A. (2016) Recruitment of rod photoreceptors from short-wavelength-sensitive cones during the evolution of nocturnal vision in mammals. Dev. Cell, 37, 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingram N.T., Sampath A.P. and Fain G.L. (2016) Why are rods more sensitive than cones? J. Physiol., 594, 5415–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swaroop A., Kim D. and Forrest D. (2010) Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci., 11, 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I. and Furukawa T. (2003) Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci., 6, 1255–1263. [DOI] [PubMed] [Google Scholar]
- 20. Ng L., Lu A., Swaroop A., Sharlin D.S., Swaroop A. and Forrest D. (2011) Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J. Neurosci., 31, 11118–11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mears A.J., Kondo M., Swain P.K., Takada Y., Bush R.A., Saunders T.L., Sieving P.A. and Swaroop A. (2001) Nrl is required for rod photoreceptor development. Nat. Genet., 29, 447–452. [DOI] [PubMed] [Google Scholar]
- 22. Oh E.C., Khan N., Novelli E., Khanna H., Strettoi E. and Swaroop A. (2007) Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc. Natl. Acad. Sci. U. S. A., 104, 1679–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furukawa T., Morrow E.M., Li T., Davis F.C. and Cepko C.L. (1999) Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat. Genet., 23, 466–470. [DOI] [PubMed] [Google Scholar]
- 24. Hennig A.K., Peng G.H. and Chen S.M. (2008) Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res., 1192, 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitton K.P., Swain P.K., Chen S., Xu S., Zack D.J. and Swaroop A. (2000) The leucine zipper of NRL interacts with the CRX homeodomain. A possible mechanism of transcriptional synergy in rhodopsin regulation. J. Biol. Chem., 275, 29794–29799. [DOI] [PubMed] [Google Scholar]
- 26. Kim J.W., Yang H.J., Brooks M.J., Zelinger L., Karakulah G., Gotoh N., Boleda A., Gieser L., Giuste F., Whitaker D.T. et al. (2016) NRL-regulated transcriptome dynamics of developing rod photoreceptors. Cell Rep., 17, 2460–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mo A., Mukamel E.A., Davis F.P., Luo C., Henry G.L., Picard S., Urich M.A., Nery J.R., Sejnowski T.J., Lister R. et al. (2015) Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron, 86, 1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aldiri I., Xu B., Wang L., Chen X., Hiler D., Griffiths L., Valentine M., Shirinifard A., Thiagarajan S., Sablauer A. et al. (2017) The dynamic epigenetic landscape of the retina during development, reprogramming, and tumorigenesis. Neuron, 94, 550–568e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daum J.M., Keles O., Holwerda S.J., Kohler H., Rijli F.M., Stadler M. and Roska B. (2017) The formation of the light-sensing compartment of cone photoreceptors coincides with a transcriptional switch. Elife, 6, doi: 10.7554/eLife.31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Assawachananont J., Kim S.Y., Kaya K.D., Fariss R., Roger J.E. and Swaroop A. (2018) Cone-rod homeobox CRX controls presynaptic active zone formation in photoreceptors of mammalian retina. Hum. Mol. Genet., 27, 3555–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilken M.S., Brzezinski J.A., La Torre A., Siebenthall K., Thurman R., Sabo P., Sandstrom R.S., Vierstra J., Canfield T.K., Hansen R.S. et al. (2015) DNase I hypersensitivity analysis of the mouse brain and retina identifies region-specific regulatory elements. Epigenetics Chromatin, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corbo J.C., Lawrence K.A., Karlstetter M., Myers C.A., Abdelaziz M., Dirkes W., Weigelt K., Seifert M., Benes V., Fritsche L.G. et al. (2010) CRX ChIP-seq reveals the cis-regulatory architecture of mouse photoreceptors. Genome Res., 20, 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hao H., Kim D.S., Klocke B., Johnson K.R., Cui K., Gotoh N., Zang C., Gregorski J., Gieser L., Peng W. et al. (2012) Transcriptional regulation of rod photoreceptor homeostasis revealed by in vivo NRL targetome analysis. PLoS Genet., 8, e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim D.S., Matsuda T. and Cepko C.L. (2008) A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J. Neurosci., 28, 7748–7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mignone F., Gissi C., Liuni S. and Pesole G. (2002) Untranslated regions of mRNAs. Genome Biol., 3, REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davuluri R.V., Suzuki Y., Sugano S. and Zhang M.Q. (2000) CART classification of human 5' UTR sequences. Genome Res., 10, 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X., Hou J., Quedenau C. and Chen W. (2016) Pervasive isoform-specific translational regulation via alternative transcription start sites in mammals. Mol. Syst. Biol., 12, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pozner A., Goldenberg D., Negreanu V., Le S.Y., Elroy-Stein O., Levanon D. and Groner Y. (2000) Transcription-coupled translation control of AML1/RUNX1 is mediated by cap- and internal ribosome entry site-dependent mechanisms. Mol. Cell Biol., 20, 2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Courtois V., Chatelain G., Han Z.Y., Le Novere N., Brun G. and Lamonerie T. (2003) New Otx2 mRNA isoforms expressed in the mouse brain. J. Neurochem., 84, 840–853. [DOI] [PubMed] [Google Scholar]
- 40. Blaschke R.J., Topfer C., Marchini A., Steinbeisser H., Janssen J.W. and Rappold G.A. (2003) Transcriptional and translational regulation of the Leri–Weill and Turner syndrome homeobox gene SHOX. J. Biol. Chem., 278, 47820–47826. [DOI] [PubMed] [Google Scholar]
- 41. White M.A., Kwasnieski J.C., Myers C.A., Shen S.Q., Corbo J.C. and Cohen B.A. (2016) A simple grammar defines activating and repressing cis-regulatory elements in photoreceptors. Cell Rep., 17, 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng H., Khanna H., Oh E.C., Hicks D., Mitton K.P. and Swaroop A. (2004) Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet., 13, 1563–1575. [DOI] [PubMed] [Google Scholar]
- 43. Peng G.H., Ahmad O., Ahmad F., Liu J. and Chen S. (2005) The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum. Mol. Genet., 14, 747–764. [DOI] [PubMed] [Google Scholar]
- 44. Onishi A., Peng G.H., Poth E.M., Lee D.A., Chen J., Alexis U., Melo J., Chen S. and Blackshaw S. (2010) The orphan nuclear hormone receptor ERRbeta controls rod photoreceptor survival. Proc. Natl. Acad. Sci. U. S. A., 107, 11579–11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mollema N.J., Yuan Y., Jelcick A.S., Sachs A.J., Alpen D., Schorderet D., Escher P. and Haider N.B. (2011) Nuclear receptor Rev-erb alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS One, 6, e17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harris B.Z. and Lim W.A. (2001) Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci., 114, 3219–3231. [DOI] [PubMed] [Google Scholar]
- 47. Moleirinho S., Tilston-Lunel A., Angus L., Gunn-Moore F. and Reynolds P.A. (2013) The expanding family of FERM proteins. Biochem. J., 452, 183–193. [DOI] [PubMed] [Google Scholar]
- 48. Sarria I., Cao Y., Wang Y., Ingram N.T., Orlandi C., Kamasawa N., Kolesnikov A.V., Pahlberg J., Kefalov V.J., Sampath A.P. et al. (2018) LRIT1 modulates adaptive changes in synaptic communication of cone photoreceptors. Cell Rep, 22, 3562–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ueno A., Omori Y., Sugita Y., Watanabe S., Chaya T., Kozuka T., Kon T., Yoshida S., Matsushita K., Kuwahara R. et al. (2018) Lrit1, a retinal transmembrane protein, regulates selective synapse formation in cone photoreceptor cells and visual acuity. Cell Rep., 22, 3548–3561. [DOI] [PubMed] [Google Scholar]
- 50. Hu J.H., Yang L., Kammermeier P.J., Moore C.G., Brakeman P.R., Tu J., Yu S., Petralia R.S., Li Z., Zhang P.W. et al. (2012) Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat. Neurosci., 15, 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. An N., Blumer J.B., Bernard M.L. and Lanier S.M. (2008) The PDZ and band 4.1 containing protein Frmpd1 regulates the subcellular location of activator of G-protein signaling 3 and its interaction with G-proteins. J. Biol. Chem., 283, 24718–24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pan Z., Shang Y., Jia M., Zhang L., Xia C., Zhang M., Wang W. and Wen W. (2013) Structural and biochemical characterization of the interaction between LGN and Frmpd1. J. Mol. Biol., 425, 1039–1049. [DOI] [PubMed] [Google Scholar]
- 53. McCudden C.R., Willard F.S., Kimple R.J., Johnston C.A., Hains M.D., Jones M.B. and Siderovski D.P. (2005) G alpha selectivity and inhibitor function of the multiple GoLoco motif protein GPSM2/LGN. Biochem. Biophys. Acta, 1745, 254–264. [DOI] [PubMed] [Google Scholar]
- 54. Nair K.S., Mendez A., Blumer J.B., Rosenzweig D.H. and Slepak V.Z. (2005) The presence of a Leu-Gly-Asn repeat-enriched protein (LGN), a putative binding partner of transducin, in ROD photoreceptors. Invest. Ophthalmol. Vis. Sci., 46, 383–389. [DOI] [PubMed] [Google Scholar]
- 55. Bernard M.L., Peterson Y.K., Chung P., Jourdan J. and Lanier S.M. (2001) Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J. Biol. Chem., 276, 1585–1593. [DOI] [PubMed] [Google Scholar]
- 56. De Vries L., Fischer T., Tronchere H., Brothers G.M., Strockbine B., Siderovski D.P. and Farquhar M.G. (2000) Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc. Natl. Acad. Sci. USA, 97, 14364–14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu E.T., Bolcun-Filas E., Grass D.S., Lutz C., Murray S., Shultz L. and Rosenthal N. (2017) Of mice and CRISPR: the post-CRISPR future of the mouse as a model system for the human condition. EMBO Rep., 18, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ablain J. and Zon L.I. (2016) Tissue-specific gene targeting using CRISPR/Cas9. Methods Cell Biol., 135, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carroll K.J., Makarewich C.A., McAnally J., Anderson D.M., Zentilin L., Liu N., Giacca M., Bassel-Duby R. and Olson E.N. (2016) A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc. Natl. Acad. Sci. USA, 113, 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Staahl B.T., Benekareddy M., Coulon-Bainier C., Banfal A.A., Floor S.N., Sabo J.K., Urnes C., Munares G.A., Ghosh A. and Doudna J.A. (2017) Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol., 35, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang S., Sengel C., Emerson M.M. and Cepko C.L. (2014) A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell, 30, 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hao H., Tummala P., Guzman E., Mali R.S., Gregorski J., Swaroop A. and Mitton K.P. (2011) The transcription factor neural retina leucine zipper (NRL) controls photoreceptor-specific expression of myocyte enhancer factor Mef2c from an alternative promoter. J. Biol. Chem., 286, 34893–34902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Millington-Ward S., Chadderton N., O'Reilly M., Palfi A., Goldmann T., Kilty C., Humphries M., Wolfrum U., Bennett J., Humphries P. et al. (2011) Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol. Ther., 19, 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rossmiller B., Mao H. and Lewin A.S. (2012) Gene therapy in animal models of autosomal dominant retinitis pigmentosa. Mol. Vis., 18, 2479–2496. [PMC free article] [PubMed] [Google Scholar]
- 65. Yu W., Mookherjee S., Chaitankar V., Hiriyanna S., Kim J.W., Brooks M., Ataeijannati Y., Sun X., Dong L., Li T. et al. (2017) Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat. Commun., 8, 14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shen S.Q., Myers C.A., Hughes A.E., Byrne L.C., Flannery J.G. and Corbo J.C. (2016) Massively parallel cis-regulatory analysis in the mammalian central nervous system. Genome Res., 26, 238–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F. and Jaenisch R. (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell, 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Varshney G.K., Pei W., LaFave M.C., Idol J., Xu L., Gallardo V., Carrington B., Bishop K., Jones M., Li M. et al. (2015) High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res., 25, 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Briner A.E., Donohoue P.D., Gomaa A.A., Selle K., Slorach E.M., Nye C.H., Haurwitz R.E., Beisel C.L., May A.P. and Barrangou R. (2014) Guide RNA functional modules direct Cas9 activity and orthogonality. Mol. Cell, 56, 333–339. [DOI] [PubMed] [Google Scholar]
- 70. Campla C.K., Breit H., Dong L., Gumerson J.D., Roger J.E. and Swaroop A. (2017) Pias3 is necessary for dorso-ventral patterning and visual response of retinal cones but is not required for rod photoreceptor differentiation. Biol. Open, 6, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bolger A.M., Lohse M. and Usadel B. (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li H. and Durbin R. (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics, 25, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. and 1000 Genome Project Data Processing Subgroup (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics, 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoshino A., Ratnapriya R., Brooks M.J., Chaitankar V., Wilken M.S., Zhang C., Starostik M.R., Gieser L., La Torre A., Nishio M. et al. (2017) Molecular anatomy of the developing human retina. Dev. Cell, 43, 763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen H.Y., Kaya K.D., Dong L. and Swaroop A. (2016) Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation. Mol. Vis., 22, 1077–1094. [PMC free article] [PubMed] [Google Scholar]
- 76. Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M. and Gingeras T.R. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kautzmann M.A., Kim D.S., Felder-Schmittbuhl M.P. and Swaroop A. (2011) Combinatorial regulation of photoreceptor differentiation factor, neural retina leucine zipper gene NRL, revealed by in vivo promoter analysis. J. Biol. Chem., 286, 28247–28255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Montana C.L., Myers C.A. and Corbo J.C. (2011) Quantifying the activity of cis-regulatory elements in the mouse retina by explant electroporation. J. Vis. Exp., 52, e2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roger J.E., Nellissery J., Kim D.S. and Swaroop A. (2010) Sumoylation of bZIP transcription factor NRL modulates target gene expression during photoreceptor differentiation. J. Biol. Chem., 285, 25637–25644. [DOI] [PMC free article] [PubMed] [Google Scholar]


