Abstract
Cancer-associated fibroblasts (CAFs) have been considered as major players in tumor growth and malignancy. In colorectal cancer (CRC), CAFs are attendance in high affluence and little is known about how they impact tumor progression. An increasing number of studies indicated that dysregulation of human urothelial carcinoma associated 1 (UCA1) is associated with progression of tumor and metastasis in various cancers including CRC. Nonetheless, the possible mechanisms of UCA1 actuation in CRC remain poorly understood. To address this, we elucidated the effects of conditioned medium from SW480 CRC cells/Normal fibroblast co-culture (CAF-CM) on UCA1 expression, and the cell proliferation, EMT, invasion and migration of the treated CRC cell were evaluated in vitro. Our study indicated that CAFs dramatically stimulated cell proliferation and migration of CRC cell. Furthermore, CAFs induced the EMT phenotype in CRC cell, with an associated change in the expression of EMT markers including vimentin, E-cadherin, N-cadherin and metastasis-related genes (MMPs). Moreover, we found an increased percentage of CRC cell in the S and G2/M phase induced by CAFs. Our results revealed that CAFs could induce upregulation of UCA1, leading to upregulation of mTOR. Up-regulation of UCA1/mTOR axis suppressed p27 and miR-143 while the expression of Cyclin-D1 and KRAS were significantly increased compared with control. Furthermore, UCA1 silencing in treated CRC cell suggested that upregulation of UCA1, which was induced by CAFs, regulates the expression of downstream key effectors. Taken together, these results highlight the vital role of cooperation between lncRNA UCA1 and mTOR in proliferation and metastasis which support the hypothesis that CAFs may be a prominent therapeutic target of stroma-based therapy in CRC treatment.
Keywords: Cancer-associated fibroblasts (CAFs), lncRNA UCA1, mTOR, KRAS, miR-143, Colorectal cancer
Introduction
Notwithstanding the fact that fatality rate of Colorectal cancer (CRC) has decreased in recent decades, at this time yet CRC is the third main reason for malignant tumor death in the United States and the third most current diagnosed cancer in both males and females worldwide (Siegel et al. 2017). Around 774,000 losses of life estimated from CRC in 2015 by The World Health Organization (WHO) (Organization 2017). Metastasis to lung and liver is the main reason of mortality in CRC patients (Conti and Thomas 2011). Although progress in screening tests, surgery and therapy options have substantially decreased the mortality and incidence rate in last decades, nevertheless the greater part of cases diagnosed with metastatic CRC are yet cureless (Edwards et al. 2010).
Evidence from various high-throughput genomic platforms has cleared that human genome is extensively transcribed and larger part of transcriptome contains non-coding RNAs (ncRNAs), including long non-coding RNAs (lncRNAs) and microRNAs (Madar et al. 2013; Wang and Chang 2011). Increasing number of studies indicated that lncRNAs play as the master regulator in numerous vital biological processes including tumor initiation, progression and metastasis or tumor suppression in various types of cancers (Augsten 2014; Wang et al. 2017a; Gandellini et al. 2015; Patel et al. 2016; Shiga et al. 2015). Urothelial carcinoma associated 1 (UCA1) was incipiently discovered as an oncogene in bladder cancer (Wang et al. 2006, 2008). Recently upregulation of UCA1 in multiple cancers including gastric cancer, hepatocellular carcinoma, glioma, breast cancer, tongue and esophageal squamous cell carcinomas and ovarian cancer has been reported and proposed as a predictive biomarker for cancers (Hong et al. 2016; He et al. 2017; Wang et al. 2017b; Fang et al. 2014; Xue et al. 2016). Accumulating evidence has revealed an upregulation of UCA1 transcripts in CRC tissues and cells, which could direct cell proliferation, cell cycle distribution, metastasis and inhibition of cell apoptosis (Bian et al. 2016; Ni et al. 2015; Han et al. 2014). On the other hand, one of the downstream effectors of lncRNA UCA1 is the mammalian target of rapamycin (mTOR), and hyperactivation of mTOR signaling pathway has been reported as one of the main causes of cell proliferation, EMT (Epithelial–mesenchymal transition), migration and metastasis in various cancers (Zhang et al. 2017; Cao et al. 2015; Francipane and Lagasse 2014), converging the UCA1 and mTOR signaling functions in pathobiology of CRC.
Almost all researches on CRC in last decades were largely centered on genetic alterations or epigenetic modifications of malignant cell itself, on the contrary recent studies have suggested that solid tumors are truly heterogeneous tissues and interactions between cancer cells and tumor stroma have capability to induce the cancer initiation, development and ultimately metastasis in CRC (Herrera et al. 2013; Armaghany et al. 2012). Included in surrounding stromal cells, cancer-associated fibroblasts (CAFs) are the majority cells in tumor microenvironment and play a crucial role in tumor growth, progression, metastasis, angiogenesis and immune responses (Tommelein et al. 2015; Augsten 2014). CAFs was originally described as a heterogeneous subpopulation of fibroblasts, which are activated by tumor cells and display particular markers that could be consider as prognostic biomarkers in cancers (Paulsson and Micke 2014). CAFs secretome contains a wide range of factors inducing chemokines, cytokines, exosomes and growth factors by which they influence cancer cells and facilitate cancer-promoting processes (Han et al. 2015; Li et al. 2017).
To gain insights into the paracrine effects of CAFs on CRC, we explored the impact of CAF-CM on proliferation, EMT, invasion, migration and cell cycle distribution of CRC (SW480) cell. Furthermore, to unveil the mechanism by which CAFs exert these effects, we investigated the expression of UCA1 as well as its downstream effectors mTOR, cyclin D1, p27, KRAS and miR-143. Finally, we approved the CAFs effects by knocking down of the UCA1 expression in CAF-CM-treated SW480 cells.
Materials and Methods
Cell culture and fibroblast isolation
Human colorectal cancer SW480 cell line was purchased from National Cell Bank of Iran (NCBI) and grown in complete DMEM-high glucose (Gibco) supplemented with 10% fetal bovine serum (FBS) and Penicillin- Streptomycin (P/S) solution (0.1 U/mL penicillin and 0.1 μg/ mL streptomycin) (Gibco) at 37 °C in humidified air with 5% CO2. Human colorectal normal fibroblast (NF) was established from human non-malignant colon tissue as described previously (Soon et al. 2013). The primary fibroblasts were maintained in DMEM-high glucose with 10% FBS at 37 °C in a humidified incubator containing 5% CO2.
Direct co-culture and Conditioned medium experiments
To detect the paracrine effects of CAFs on SW480 cells, co-culture conditioned medium was produced as described previously (Steinbichler et al. 2016). About 2 × 104 cells/ml NF were seeded in 250 ml cell culture flask (Orange, Belgium, Germany) and incubated overnight at 37 °C to allow fibroblast cells to adhere. Afterward, SW480 cells were seeded on the monolayer of NF. After 3 days, cells were washed twice by PBS and cultured with fresh medium for 48 h, then the Conditioned Medium (CM) was collected. Co-cultured-CM was centrifuged for 5 min at 1300 rpm to avoid the presence of any cells, then sterile-filtered and stored at −80 °C for further experiments. SW480 cells were seeded in 12-well plates (Orange, Belgium, Germany) at a density of 1.3 × 104 cells/well in 1 ml DMEM glucose supplemented with FBS (10%) and P/S and allowed to adhere overnight. Cells were treated with CM, which was diluted twofold with fresh DMEM with 10% FBS, for 4 days. SW480 cells grown in DMEM-high glucose containing 10% FBS and P/S served as a control.
Total RNA isolation and expression analysis by real-time PCR
Total RNA was extracted from SW480 cells using the TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer’s protocol. RNA concentration was examined using a Nanodrop ND-1000 (Nanodrop Technologies, ilmington, Delaware, USA) and the RNA integrity was verified by 1.5% agarose gel electrophoresis. For each sample, 1.5 μg of total RNA was treated with DNase I (Fermentase, Lithuania) to remove any residual contaminating genomic DNA. Single-stranded cDNA was generated using a PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan) following manufacturer’s protocol. Real-time quantitative PCR was performed by specific primers for target genes or miR-143 using RealQ Plus 2× Master Mix Green, High ROX™ (Ampliqon, Denmark) on an ABI Step One Plus Real-Time PCR System (CA, USA). All of the reactions, including the no-template controls, were run in triplicate and final volume of 20 μl. Results were normalized with respect to GAPDH and calculated using the ΔΔCt method. Changes in gene expression were expressed using the 2-ΔΔCt method. The size of Real-time PCR products was confirmed by electrophoresis in 1.5% agarose gels. U6 was used as an internal control for miRNA.
RNA interference transfection
SW480 cells were seeded in 6 well plates at 6 × 10 5 cells/well for 24 h. The transfection of SW480 cells was carried out with 4 specifically targeting lncRNA UCA1 siRNAs simultaneously (named as UCA1-siRNA) or negative control (NC siRNA), siRNA with scramble sequence, using Lipofectamine RNAiMAX (Invitrogen, USA) according to manufacturer’s protocol. UCA1 siRNAs were purchased from GenePharma (Shanghai, China). After transfection, medium was replaced with CAF-CM or carrier control. Cells were harvested for further analysis after 48 h.
Western blot analysis
Cells were harvested and then lysed by protein lysis buffer containing 20 mM Tris-Hcl pH 7.4, 5 mM EDTA acid, 1% triton X-100, 150 mM NaCl, 1% dithiothreithol and protease inhibitor cocktail (Roche). Cell extracts were separated by 12% SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk in Tris-buffered saline polysorbate 20 (TBST) for 1.5 h at room temperature. Afterwards target protein probed with corresponding primary antibodies against mTOR, KRAS, p-p27Ser10, E-cadherin, N-cadherin (Santa Cruz Biotechnology, USA), Cyclin D1, p27 (BD Bioscience, USA) and β-actin (Sigma-Aldrich, USA), followed by incubation with appropriate secondary HRP-conjugated secondary antibody for 1 h and finally subjected to chemiluminescence. β-actin protein was used as endogenous control.
Cell Proliferation assay
Cell proliferation assay was performed using MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide) assay (Sigma) and trypan blue staining. Briefly, SW480 cells were harvested from exponential phase cultures growing in DMEM, counted and seeded onto 96-well plates at the density of 9 × 103 cells/well in 200 μl DMEM and allowed to adhere overnight. Medium replaced with CAF-CM, NF-CM or CAF-CM + siRNA and incubated for 24 or 48 h. Cells treated with an equal volume of DMEM with 10% FBS or CAF-CM + NC siRNA, served as controls and a group without cells served as the blank. After indicated time intervals, cells were incubated with MTT reagent (2 mg/ml) for 4 h and then solubilized with dimethyl sulfoxide (DMSO). The optical density was measured in an ELISA reader at 490 nm. For cell counting assay SW480 cells were seeded into 24-well plates at 7 × 104 cells per well and allowed to adhere for 24 h. Before adding assay media, cells were starved with serum-free media for 24 h and washed briefly in PBS. The cells then incubated with CAF-CM, NF-CM or none for 24 h and 48 h. After trypsinization, viable cells were counted by using trypan blue dye (Sigma-Aldrich Corp., St. Louis, MO, USA) and a hemocytometer. Experiments were performed in four independent sets containing at least four biological repeats and expressed as mean ± SEM.
Transwell migration assay
Transwell plates (SPL) with a pore size of 8.0 μm were used to perform cell migration assay. Briefly, SW480 cells (1 × 105/well) were incubated with CAF-CM, NF-CM or CAF-CM + siRNA and control medium for 48 h. The cells were trypsinized, washed, added to 100 μL of serum-free medium, and then placed in the upper chamber of the inserts. H-DMEM (500 μL) containing 10% FBS was added to the lower chamber as the chemotactic factor. After culture with 5% CO2 at 37 °C for 48 h, the cells on the upper surface were removed with a cotton swab, while migrated cells on the lower surface of the insert were washed in PBS and fixed with 95% ethanol and stained with 0.1% crystal violet. The number of migrated cells was counted under an inverted microscope in five randomly selected fields of the fixed cells.
Scratch wound healing assay
The migratory behavior of treated SW480 cells was assayed by means of a wound healing assay. SW480 cells were plated at a density of 8 × 104 cells/well in 24-well plates and allowed to grown to confluency. After 16 h of serum starvation (DMEM-high glucose supplemented with 1% FBS) to synchronize, cells were wounded with 200 μl pipette tip. The wounded monolayers were washed once with the PBS to remove the debris or the detached cells. Thereupon, SW480 cells were incubated with CAF-CM or NF-CM and DMEM with 10% FBS as control for 48 h. Wound borders and cells migration were photographed at 0, 24 and 48 h. Next the images were analyzed using Wimasis WimScratch online software.
Transwell invasion assay
Transwell assay was performed on SW480 cells to estimate the effects of CAF-CM or CAF-CM + UCA1-siRNA on the invasion of cancer cells. Matrigel was diluted in serum free-cold DMEM medium and 100 μl of the diluted matrigel was added into transwells containing 8 μm pores and dried out at 37 °C for 4 h. Previously treated and control cells were seeded into the upper chamber containing serum-free DMEM and 0.5 ml medium with 10% FBS was added to the lower chamber as chemoattractant. Cells were given 48 h to invade through matrigel then transwells were removed from the 24-well plate and stained with 0.1% crystal violet. The non-invaded cells on the top of the transwell were scraped off using a cotton swab. Cells were counted in five different fields with an inverted microscope.
Cell cycle assay
SW480 cells were seeded on 6-well plates at a density of 4 × 105 cells/well. Cells were starved with serum-free media for 24 h and then exposed to CAF-CM or DMEM supplemented with 10% FBS (as control). After 48 h cells were harvested, washed twice with PBS, then fixed in 75% ethanol overnight at 4 °C. Ethanol was removed from fixed cells by centrifugation. Cell pellets were stained with 0.5 ml PI/RNase staining buffer (Sigma-Aldrich Inc.) according to the manufacturer’s protocol. Cells in G0/G1, G2/M and S phase were analyzed by flow cytometry (BD FACS Caliber), using FlowJo Software version 7.6.1. The experiment was repeated four times to ascertain reproducibility.
Data analysis
The GraphPad Prism 5.0 software was used for statistical analysis. The results were presented as the means ± SD for three or more independent experiments. Comparisons between two groups were performed using student’s t-test and comparisons between multiple groups were analyzed by one-way ANOVA. Unless otherwise mentioned, all experiments were performed at least three times independently. The differences were considered to be statistically significant at P-value <0.05.
Results
CAFs promote cell proliferation of human CRC cell
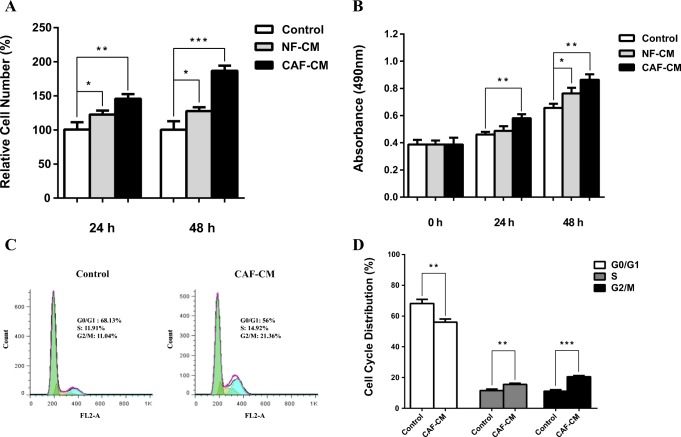
To determine the role of CAFs in regulating CRC cell proliferation, SW480 cells were treated with CAF-CM and cell proliferation was monitored using trypan blue staining and MTT assays at 24 h and 48 h. As seen in Fig. 1, CAFs remarkably promoted cell proliferation after 48 h in treated CRC cell. Whereas treatment of CRC cell with NF-CM had slightly effect on cell proliferation. We further verified this observation by cell cycle analysis (Fig. 1c). Results indicated that the cells in G1 phase were significantly decreased due to CAF-CM treatment compared with the control group cells. In addition, S and G2/M population were significantly increased in CRC cell which incubated with CAF-CM (Fig. 1c, d), suggesting an important role of CAFs in regulating the G1/S transition.
Fig. 1.
Effect of CAFs on cell proliferation and cell cycle progression of CRC cell. a Cell proliferation assessed by trypan blue exclusion. The cells were treated with CAF-CM or NF-CM for 24 h and 48 h and the cell proliferation compared with control group. Asterisk denotes significant increase in proliferation compared with control cells. b MTT assay was applied for ascertaining proliferation rate in SW480 cells treated with CAF-CM or NF-CM. The optical density of each well was measured with a microplate spectrophotometer at 490 nm and compared with control group. The optical density of the CAF-CM treated group significantly increased after 24 h and 48 h. c Effect of CAFs on cell cycle distribution. CAFs significantly decreased G0/G1 phase population in CAF-CM treated CRC cell compared with control group whereas the proportion of S and G2/M phase population significantly increased and d Histogram showing the percentages of control and treated cells in each phase of the cell cycle after 48 h. *: P < 0.05; **: P < 0.01; ***: P < 0.001
CAFs provoke the migration and invasion of human CRC cell
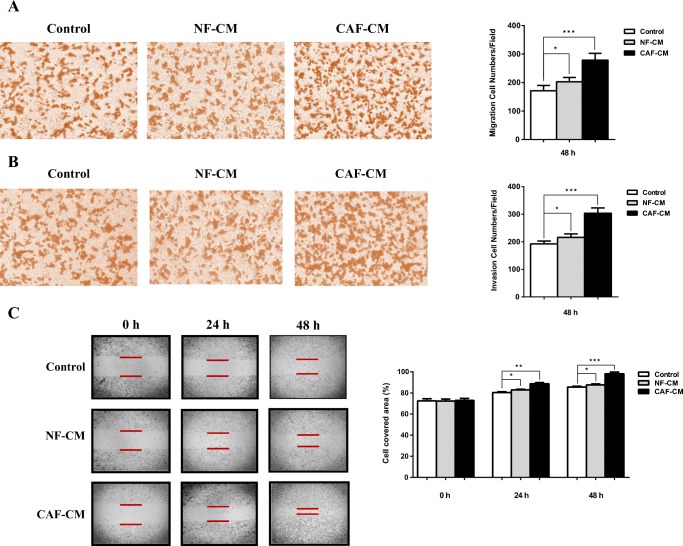
Transwell assay was used to examine whether conditioned medium form CAFs could affect the cell migration and invasion characteristics of SW480 cells. As shown in Fig. 2a, CAFs induced significant increase in cell migration of CRC cell after 48 h compared with control groups. Furthermore, to confirm the results of transwell migration assay, we performed the wound healing assay. CAFs strikingly increased cell migration of SW480 cells, which resulted in notably faster healing of the wound in comparison with that of control cells, which was consistent with the results of transwell migration assay. The results of transwell invasion assay revealed that CAFs also raised the number of invaded cells attaching on bottom chamber efficaciously (Fig. 2b). Collectively, these results suggest that CAFs secrete some factors which can promote migration and invasion of CRC cell in a paracrine manner.
Fig. 2.
CAFs promoted migration and invasion of CRC cell. a Transwell migration assay used to determine migration ability of CAF-CM or NF-CM treated CRC cell. After 48 h CAFs significantly induced migration of treated SW480 cells compared with NF-CM and control groups. Cell migration was measured by counting cells per area. b To assess the cell invasion ability transwell invasion assay was performed. CRC cell cultured with CAF-CM markedly induced more invasiveness compared to NF-CM and control groups. b To further analyze cell migration ability, scratch wound healing assay was performed on CAF-CM treated SW480 cells and compared with NF-CM and control groups. Represented images were showed on the left and data analysis represented on the right. *: P < 0.05; **: P < 0.01; ***: P < 0.001
CAFs direct EMT programming genes in human CRC cell
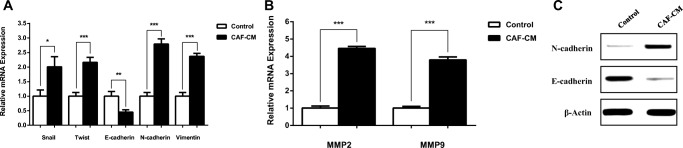
Increasing migration and invasion features of tumor cells have been reported to be related to EMT (Epithelial–mesenchymal transition), which has been shown to facilitate the carcinogenesis. In order to assess whether CAFs can affect the EMT programming in SW480 CRC cells, the expression of EMT-inducing transcription factors (EMT-TFs) SNAI2 and TWIST was assessed. Moreover, the expression of epithelial cell marker E-cadherin and the mesenchymal markers Vimentin and N-cadherin were evaluated using Real-time PCR or immunoblotting. Results verified that CAFs induce the EMT-TFs and consequently E-cadherin was remarkably down-regulated, meanwhile vimentin and N-cadherin significantly up-regulated at mRNA or protein level in SW480 cells by CAF-CM, compared to the control group (Fig. 3a). To further confirm the role of CAFs in metastasis of CAF-CM-treated CRC cell, mRNA level of MMPs was evaluated. CAFs significantly induced the upregulation of metastasis-related genes, MMP2 and MMP9, in treated group compared with control (Fig. 3b). Our results revealed that CAFs secretome increased the metastasis potential by inducing EMT phenotype and EMT programming in treated CRC cell.
Fig. 3.
Effects of CAFs on EMT programing in treated CRC cell. SW480 cells were cultured in CAF-CM for 48 h. a The mRNA levels of EMT-TFs (Snail and Twist) and EMT markers were detected by qRT-PCR. b Quantitative PCR analysis of metastasis-related genes, MMPs (MMP2 and MMP9), in CAF-CM-treated CRC cell and control group. c The protein expression level of EMT hallmarks, E-cadherin and N-cadherin, were detected by western blot assay. *: P < 0.05;**: P < 0.01; ***: P < 0.001
CAFs induce UCA1 upregulation in human CRC cell
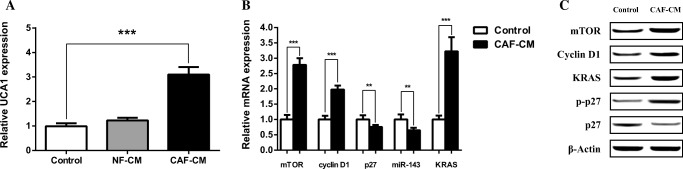
Increasing number of studies have revealed that UCA1 acts as oncogene and is upregulated in many tumors, which has ability to influence cell proliferation, cell cycle progression, EMT and migration of various cancers inclusive of CRC (Xue et al. 2016). Accordingly, to delineate whether CAFs affect CRC through the upregulation of UCA1, we performed qRT-PCR assay to investigate the expression levels of UCA1 in CAF-CM treated, NF-CM-treated and untreated SW480 cells. Comparison of expression levels demonstrated significant enhancement of UCA1 expression in the CAF-CM treated group compared with the NF-CM and control groups. These results suggested that CAFs may impact the human CRC via upregulating the UCA1 expression (Fig. 4a).
Fig. 4.
Influence of CAFs on UCA1 expression and its downstream key effectors. a CAFs induced significant upregulation of UCA1 in CAF-CM-treated SW480 cells compared with control and NF-CM groups. b Transcript levels of UCA1 downstream effectors in SW480 cells treated by CAF-CM in comparison with control group. c Protein levels of the UCA1 downstream effectors analyzed with western blot in both CAF-CM-treated and control groups. **: P < 0.01; ***: P < 0.001
Cell proliferation effectors in downstream of lncRNA UCA1 are affected by CAFs secretome
To understand the way by which CAFs affect SW480 cell proliferation and cell cycle progression we investigated the effects of CAF-CM on mTOR, cyclin D1 and p27 expression which are downstream key effectors of UCA1. SW480 cells were treated for 48 h by CAF-CM and analyzed using qRT-PCR and western blotting. Compared to the control group, expression of mTOR and Cycline D1 was significantly upregulated whereas p27 was downregulated both in mRNA and protein levels (Fig. 4b, c). Furthermore CAFs significantly decreased phosphorylated p27 level, which was consistent with mTOR upregulation (Fig. 4c). Collectively these results suggest that UCA1 upregulation by CAFs modulates cell proliferation and cell cycle progression of SW480 cell through the activation of UCA1/mTOR axis.
EMT, invasion and migration effectors in downstream of lncRNA UCA1 are affected by CAFs secretome
It was reported that UCA1 and mTOR both target miR-143, which is a tumor suppressor gene and proved to be downregulated in many cancers including CRC. miR-143 targets KRAS, an oncoprotein involving in different cancer cell characteristics including EMT, invasion and migration (Shao et al. 2014; Boutin et al. 2017). Accordingly, we evaluated the effects of CAF-CM on mTOR/miR-143/KRAS axis in SW480 cells. To this end, we determine the correlation between mTOR mRNA levels and miR-143 expression levels in the treated CRC cell and control groups. The qRT-PCR revealed downregulation of miR-143 but upregulation of mTOR and KRAS in CAF-CM treated cells compared with the control (Fig. 4b). The upregulation of mTOR and KRAS was approved by western blotting (Fig. 4c). As Fig. 4b shows, the expression of mTOR and KRAS was negatively correlated with the expression of miR-143. Collectively, these results suggested that UCA1 is an important upstream effector of mTOR which in collaboration with the mTOR, suppresses the miR-143, leading to rise of KRAS protein.
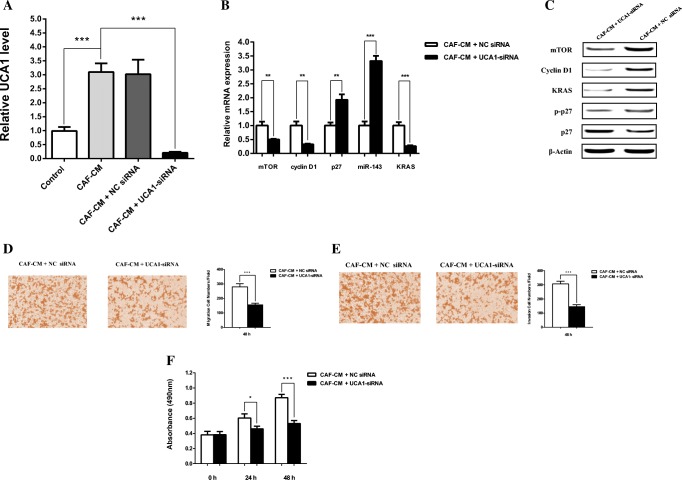
UCA1 knockdown confirmed its involvement in CAFs-mediated SW480 cell proliferation, EMT, invasion and migration
To further assess whether UCA1 regulates the expression of mTOR, cyclin D1, p27 and miR-143, we knocked down the expression of UCA1 by siRNAs in CAF-CM treated SW480 cells (Fig. 5). Not surprisingly, our results revealed that silencing of UCA1 significantly decreased mTOR and cyclin D1 transcripts level. Moreover, UCA1 silencing was inversely correlated with miR-143 and p27 mRNA levels. In line with qRT-PCR results, western blot assays showed that mTOR, KRAS and cyclin D1 were partially attenuated by knocking down of UCA1 while the level of p27 protein was increased in CRC cell.
Fig. 5.
UCA1 siRNA suppressed the expression of the downstream key effectors in CAF-CM treated SW480 cells. a SW480 cells were cultured in normal DMEM (as control), CAF-CM, CAF-CM + NC siRNA and CAF-CM + UCA1 siRNA for 48 h then UCA1 mRNA level evaluated by qRT-PCR. b Effects of UCA1-siRNA and NC siRNA transfection on mRNA level of downstream key molecules. CAF-CM-treated SW480 cells were transfected with UCA1-siRNA or NC siRNA and transcript levels of mTOR, cyclin D1, p27, miR-143 and KRAS was evaluated by qRT-PCR. c CAF-CM-treated SW480 cells were transfected with UCA1-siRNA or NC siRNA and the protein levels of mTOR, cyclin D1, p27, P-p27 and KRAS were detected by western blot analysis. d, e Transfected SW480 cells cultured in CAF-CM and transwell assay was performed to determine the suppression effects of UCA1-siRNA on migration and invasion in CRC cell after 48 h. UCA1 knockdown significantly decreased the migration and invasion in CRC cell. f UCA1 knockdown significantly inhibited the cell proliferation of SW480 cells compared with NC siRNA group after 24 h and 48 h, as measured by MTT assay. *: P < 0.05; **: P < 0.01; ***: P < 0.001
To verify the effects of UCA1 silencing, we evaluated the cell migration and invasion characteristics of SW480 cells treated with CAF-CM + UCA1-siRNA or CAF-CM + NC siRNA by transwell assay. The results confirmed that UCA1 silencing significantly decreased the migration and invasion of SW480 cells treated with CAF-CM + UCA1-siRNA. Moreover, UCA1 silencing remarkably decreased the cell proliferation in CAF-CM-treated group as revealed by MTT cell proliferation assay. Together, these data support the potential role of UCA1, as a principal regulator for the key effectors of cell proliferation and metastasis in CAF-CM-treated CRC cell.
Discussion
Cancer cells go hand in hand with cancer-associated host cells, which mutually have dynamic interactions within cancer microenvironment (de Wever et al. 2014). Activated fibroblasts around tumors, CAFs, are of the most abundant residents of solid tumor stroma that during tumorigenesis and metastasis not only participate in constructing a scaffold for tumor, but also play pivotal role in tumor growth and malignant behavior (Paulsson and Micke 2014; Gonda et al. 2010). Here we investigated the impact of CAFs on the cell proliferation and metastasis of the SW480 cells and explained the molecular mechanism by which CAFs communicate with tumor cells using SW480 cell line as a model for CRC. Conditioned medium from direct co-culture of fibroblast-SW480 cell was used to assess the effects of CAFs on CRC cells.
Firstly, the effect of CAFs on cell proliferation and cell cycle progression was analyzed. Our results indicated that CAFs remarkably enhanced cell proliferation in CAF-CM treated group compared with fibroblast-CM. Moreover, cell cycle analysis of CAF-CM-treated SW480 cell indicated a significant transition from G1 to S as well as an increase in G2/M phases. Taken together these results suggest the proliferative effect of CAFs on CRC cell.
To explore the impact of CAFs on CRC cell metastasis, transwell migration and invasion assay, as well as scratch wound healing assay were performed. We found that CAFs significantly enhanced migration and invasion of CRC cell in vitro. To farther investigate the metastatic effect of CAFs, we examined the expression of EMT-related genes. The EMT, mediated by various momentous pathways, plays vital role in tumor migration, invasion and metastasis. Consistent with the results of migration and invasion assays, our findings revealed that the EMT transcription factors Snail and Twist induced by CAFs. Accordingly, E-cadherin expression was significantly decreased, whereas, N-cadherin and vimentin expressions were significantly increased, indicating the progression of EMT by CAFs in CRC cell.
Matrix metalloproteinases (MMPs) play crucial roles in CRC invasion and metastasis. We showed increased transcript levels of MMP2 and MMP9 (gelatinase sub-family of MMPs) in CAF-CM-treated SW480 cells. Collectively these results suggest that co-culturing of fibroblasts with SW480 cells directs them as CAFs which are capable of inducing EMT, invasion and migration in CRC cell.
To further decipher the underlying mechanism leading to malignancy behavior of CAF-CM-treated CRC cell, we studied the key molecules and pathways, which have been proved to enhance cell proliferation and metastasis in cancer cells. Accumulating evidence indicates the crucial roles of lncRNAs in tumor progression and metastasis. Several findings have revealed that lncRNA UCA1 involves in tumor development and metastasis. It implicates in cell proliferation, EMT, migration and cell cycle progression in CRC and promotes cell growth via suppression of the tumor suppressor p27 in breast tumor as well as the G1/S transition through cyclin D1 in gastric cancer cells (Han et al. 2014; Huang et al. 2014; Wang et al. 2017c). Upregulation of the oncogene lncRNA UCA1 was originally reported in bladder cancer cells (Wang et al. 2006) and have been proved to involve in cell growth, proliferation, cell invasion, drug resistance, EMT, metastasis and regulating the cell cycle progression in a wide range of cancers including CRC (Xue et al. 2016). Accordingly, to test whether UCA1 involves in malignant behaviors of CRC, we evaluated the expression level of UCA1 in SW480 cells treated with CAF-CM. As expected, our results indicated that CAFs significantly induced upregulation of UCA1 in CRC cell, which suggests a crucial role for UCA1 in regulation of cell proliferation and metastasis in CRC. Moreover, we analyzed the expression of UCA1 downstream effectors including mTOR, cyclin D1, miR-143, Kras and p27, which have been evidenced as key players in tumor progression.
mTOR is a proto-oncogene that acts as a central regulator of pivotal biological activities and tumor progression, which could be activated by UCA1. Accumulating evidence showed that activation of mTOR plays such a key role in CRC cell proliferation, autophagy, cell cycle, EMT, migration and metastasis via various mechanisms (Balk and Knudsen 2008; Faivre et al. 2006; Schwaederlé et al. 2015; Cao et al. 2015; Fang et al. 2017; Hong et al. 2008; Francipane and Lagasse 2014; Li et al. 2015; Gulhati et al. 2011). mTOR seems to have a multifaceted effects on cell cycle progression and cell proliferation. On the one hand, mTOR enhances the cyclin D1, on the other hand, regulates p27 phosphorylation and suppresses miR-143. Downregulation of miR-143 as a tumor suppressor gene have been reported in CRC (Slaby et al. 2007; Akao et al. 2010; Kulda et al. 2010). Here we showed that CAFs-induced upregulation of UCA1 is positively related with upregulation of mTOR in both mRNA and protein levels, which suggests that UCA1 as an upstream effector regulates the mTOR expression in CRC cell. Besides, mTOR phosphorylates and suppresses p27 protein via both Akt and SGK in cancer cells (Hong et al. 2008; Hollander et al. 2011; Faivre et al. 2006; Toker 2008). On the other hand recent studies demonstrated that UCA1 plays key roles in cancer cell growth and proliferation by suppressing p27 and promoting the expression of cyclin D1 (Huang et al. 2015; Hu et al. 2016; Wang et al. 2017c). In line with these findings, our results indicated a positive correlation between expressions of UCA1/mTOR and cyclin D1, and a negative correlation between UCA1 and p27. mTOR upregulation was also negatively correlated with phosphorylated p27 (p-p27). These results suggest that activation of UCA1/mTOR axis by CAFs regulates the p27 and cyclin D1 in favor of the cell proliferation and cell cycle progression.
KRAS plays major roles in adenoma to carcinoma transition (Fang and Richardson 2005; Michor et al. 2005; Chen et al. 2009) and has been evidenced as a driver of invasion and retainer of metastasis in CRC (Boutin et al. 2017). Recently, tumor suppressor miR-143 was reported as a significant inhibitor of KRAS in CRC tumorigenesis and prostate cancer cells (Chen et al. 2009; Xu et al. 2011). In addition, recent studies indicated that mTOR can repress and directly interact with miR-143 (Li et al. 2014; Tuo et al. 2015), and eventually restores the expression of downstream effectors including KRAS in CRC cells (Chen et al. 2009). In agreement, our results indicated an inverse relation between the mTOR and miR-143 as well as miR-143 and KRAS expression levels, suggesting that upregulation of mTOR leading to suppression of miR-143, which subsequently reinstates KRAS mRNA and results in upregulation of KRAS. While our data suggest that mTOR directly suppresses the miR-143, a recent study suggested that UCA1 also directly suppresses miR-143, highlighting the important role of UCA1/miR-143 axis in breast cancer as well (Tuo et al. 2015). In line with this study our data demonstrated a negative correlation between UCA1 and miR-143 as well, which provides another evidence that besides mTOR, miR-143 can also be directly targeted by UCA1 in CRC (Fig. 6). EMT have been considered as a vital phase for metastasis in various tumor cells (Kudo-Saito et al. 2009). Previous results, as also demonstrated here, showed an association between UCA1 and EMT markers (Cheng et al. 2015). Consistent with these results we showed that CAFs-caused upregulation of UCA1, regulated the expression of EMT-related markers and might induce EMT.
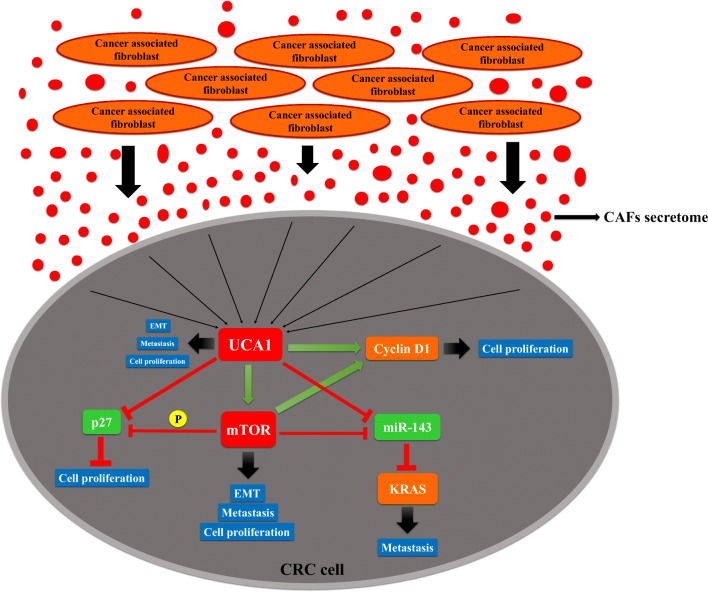
Fig. 6.
Schematic representation of the suggested pathway by which CAFs induce cell proliferation, EMT and metastasis in CRC cell. CAF-CM induces the upregulation of UCA1 in sw480 cells and leading to upregulation of mTOR. UCA1 in cooperation with mTOR regulates downstream key effectors and induces Cyclin-D1 and suppresses p27 and miR-143, subsequently, directs the cell proliferation, EMT and metastasis of CRC cell
UCA1 silencing was performed to determine whether CAFs impact CRC cell through the upregulation of UCA1. We found that knockdown of UCA1 remarkably increased p27, miR-143 and decreased mTOR, Cyclin-D1 and KRAS mRNA and protein levels in treated CRC cell compared to control. UCA1 silencing results, suggested that effects of CAFs on the expression of downstream key molecules are mediated, at least in part, by the upregulation of UCA1. To clarify the effects of UCA1 silencing, cell proliferation, invasion and migration of the treated CRC cells were also evaluated. These experiments confirmed that CAFs affect migration, invasion and cell proliferation of CRC cell through the upregulation of UCA1.
In conclusion, our study indicated that CRC cell activates fibroblasts as CAFs and in return, CAFs secretome promotes cell proliferation and metastasis of CRC cell. We showed that CAFs induced the oncogenic effects of UCA1 in CRC. Moreover, UCA1 and mTOR induced cyclin D1 and suppressed miR-143 and p27. Our findings provide a significant prospect that CAFs served as an inducer of UCA1, which in cooperation with mTOR regulates the downstream key effectors of cell proliferation and metastasis in CRC cell.
Acknowledgements
This work was supported by the research deputy of University of Tabriz.
Abbreviations
- CAF
Cancer-associated fibroblasts
- CAF-CM
Cancer-associated fibroblasts conditioned medium
- CRC
Colorectal cancer cell
- DMSO
Dimethyl sulfoxide
- EMT
Epithelial-to-mesenchymal transition
- LncRNA
Long noncoding RNA
- MMP
Matrix metalloproteinases
- NF-CM
Normal fibroblasts conditioned medium
- qRT-PCR
Quantitative reverse transcription–polymerase chain reaction
- UCA1
Urothelial carcinoma associated 1
- siRNA
Small interfering RNA
- TFs
Transcription factors
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh T, Kojima K, Nakashima R, Kitade Y, Naoe T. Role of anti-oncomirs miR-143 and-145 in human colorectal tumors. Cancer Gene Ther. 2010;17:398. doi: 10.1038/cgt.2009.88. [DOI] [PubMed] [Google Scholar]
- Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointestinal cancer research: GCR. 2012;5:19. [PMC free article] [PubMed] [Google Scholar]
- Augsten M (2014) Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol:4 [DOI] [PMC free article] [PubMed]
- Balk SP, Knudsen KE (2008) AR, the cell cycle, and prostate cancer. Nucl Recept Signal 6 [DOI] [PMC free article] [PubMed]
- Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu Y, Feng Y, Liu H, Fei B, Mao Y (2016) LncRNA—UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep 6 [DOI] [PMC free article] [PubMed]
- Boutin AT, Liao W-T, Wang M, Hwang SS, Karpinets TV, Cheung H, Chu GC, Jiang S, Hu J, chang k. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017;31:370–382. doi: 10.1101/gad.293449.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M, Wang X-A, Zhang F, Jiang L, Zhang Y. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett. 2015;360:141–150. doi: 10.1016/j.canlet.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, Cai X, Wang K, Wang G, Ba Y. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- Cheng N, Cai W, Ren S, Li X, Wang Q, Pan H, Zhao M, Li J, Zhang Y, Zhao C. Long non-coding RNA UCA1 induces non-T790M acquired resistance to EGFR-TKIs by activating the AKT/mTOR pathway in EGFR-mutant non-small cell lung cancer. Oncotarget. 2015;6:23582. doi: 10.18632/oncotarget.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti J, Thomas G. The role of tumour stroma in colorectal cancer invasion and metastasis. Cancers. 2011;3:2160–2168. doi: 10.3390/cancers3022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wever O, Van Bockstal M, Mareel M, Hendrix A, Bracke M (2014) Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin Cancer Biol:33–46 [DOI] [PubMed]
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, seeff l C. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- Fang Y, Bao W, Rao Q, Wang X, Xia Q, Shen Q, Zhou X, Yao B. TFE3 regulates renal adenocarcinoma cell proliferation via activation of the mTOR pathway. Mol Med Rep. 2017;16:2721–2725. doi: 10.3892/mmr.2017.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Wu L, Wang L, Yang Y, Meng Y, Yang H. Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: a possible correlation with cancer metastasis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:89–95. doi: 10.1016/j.oooo.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Francipane MG, Lagasse E. mTOR pathway in colorectal cancer: an update. Oncotarget. 2014;5:49. doi: 10.18632/oncotarget.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandellini P, Andriani F, Merlino G, D’aiuto F, Roz L, Callari M (2015) Complexity in the tumour microenvironment: Cancer associated fibroblast gene expression patterns identify both common and unique features of tumour-stroma crosstalk across cancer types. Semin Cancer Biol:96–106 [DOI] [PubMed]
- Gonda TA, Varro A, Wang TC, Tycko B (2010) Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy. Semin Cell Dev Biol:2–10 [DOI] [PMC free article] [PubMed]
- Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O'connor KL, Gao T. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yang Y-N, Yuan H-H, Zhang T-T, Sui H, Wei X-L, Liu L, Huang P, Zhang W-J, Bai Y-X. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46:396–401. doi: 10.1097/PAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang Y, Jia T, Sun Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumor Biol. 2015;36:1385–1394. doi: 10.1007/s13277-015-3230-8. [DOI] [PubMed] [Google Scholar]
- He Z, Wang Y, Huang G, Wang Q, Zhao D, Chen L. The lncRNA UCA1 interacts with miR-182 to modulate glioma proliferation and migration by targeting iASPP. Arch Biochem Biophys. 2017;623:1–8. doi: 10.1016/j.abb.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Herrera M, Islam AB, Herrera A, Martín P, García V, Silva J, Garcia JM, Salas C, Casal I, de Herreros AG. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res. 2013;19:5914–5926. doi: 10.1158/1078-0432.CCR-13-0694. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace r, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Hong H-H, Hou L-K, Pan X, Wu C-Y, Huang H, Li B, Nie W. Long non-coding RNA UCA1 is a predictive biomarker of cancer. Oncotarget. 2016;7:44442. doi: 10.18632/oncotarget.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J-J, Song W, Zhang S-D, Shen X-H, Qiu X-M, Wu H-Z, Gong P-H, Lu S, Zhao Z-J, He M-L. HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci Rep. 2016;6:23521. doi: 10.1038/srep23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhou N, Watabe K, Lu Z, Wu F, Xu M, Mo Y. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1) Cell Death Dis. 2014;5:e1008. doi: 10.1038/cddis.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhou N, Watabe K, Lu Z, Wu F, Xu M, Mo Y. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1) Cell Death Dis. 2015;5:e1008. doi: 10.1038/cddis.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Kulda V, Pesta M, Topolcan O, liska V, Treska V, Sutnar A, Rupert K, Ludvikova M, Babuska V, Holubec L. Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet. 2010;200:154–160. doi: 10.1016/j.cancergencyto.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Li W, Chang J, Wang S, Liu X, Peng J, Huang D, Sun M, Chen Z, Zhang W, Guo W. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget. 2015;6:24448. doi: 10.18632/oncotarget.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang X, Wang J, Li M, Cao C, Tan J, Ma D, Gao Q. TGFβ1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget. 2017;8:96035. doi: 10.18632/oncotarget.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li X, Wu S, Xue M, Chen W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR–STAT3/microRNA143 pathway. Cancer Sci. 2014;105:951–955. doi: 10.1111/cas.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’–more than meets the eye. Trends Mol Med. 2013;19:447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Michor F, Iwasa Y, Lengauer C, Nowak MA (2005) Dynamics of colorectal cancer. Semin Cancer Biol:484–493 [DOI] [PubMed]
- Ni B, Yu X, Guo X, Fan X, Yang Z, Wu P, Yuan Z, Deng Y, Wang J, Chen D. Increased urothelial cancer associated 1 is associated with tumor proliferation and metastasis and predicts poor prognosis in colorectal cancer. Int J Oncol. 2015;47:1329–1338. doi: 10.3892/ijo.2015.3109. [DOI] [PubMed] [Google Scholar]
- Organization WH. World health statistics 2016: monitoring health for the SDGs, sustainable development goals. 2016. Geneva: WHO Library Cataloguing-in-Publication Data; 2017. [Google Scholar]
- Patel JS, Hu M, Sinha G, Walker ND, Sherman LS, Gallagher A, Rameshwar P. Non-coding RNA as mediators in microenvironment–breast cancer cell communication. Cancer Lett. 2016;380:289–295. doi: 10.1016/j.canlet.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Paulsson J, Micke P (2014) Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol:61–68 [DOI] [PubMed]
- Schwaederlé M, Daniels GA, Piccioni DE, Fanta PT, Schwab RB, Shimabukuro KA, Parker BA, Kurzrock R. Cyclin alterations in diverse cancers: Outcome and co-amplification network. Oncotarget. 2015;6:3033. doi: 10.18632/oncotarget.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers. 2015;7:2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- Soon PS, Kim E, Pon CK, Gill AJ, Moore K, Spillane AJ, Benn DE, Baxter RC. Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr Relat Cancer. 2013;20:1–12. doi: 10.1530/ERC-12-0227. [DOI] [PubMed] [Google Scholar]
- Steinbichler TB, Metzler V, Pritz C, Riechelmann H, Dudas J. Tumor-associated fibroblast-conditioned medium induces CDDP resistance in HNSCC cells. Oncotarget. 2016;7:2508. doi: 10.18632/oncotarget.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. mTOR and Akt signaling in cancer: SGK cycles in. Mol Cell. 2008;31:6–8. doi: 10.1016/j.molcel.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, de Wever O (2015) Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer Frontiers in oncology:5 [DOI] [PMC free article] [PubMed]
- Tuo Y, Li X, Luo J. Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci. 2015;19:3403–3411. [PubMed] [Google Scholar]
- Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, Wu X, Zhou Q, Xu K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8:76116. doi: 10.18632/oncotarget.18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-S, Zhang Z, Wang H-C, Cai J-L, Xu Q-W, Li M-Q, Chen Y-C, Qian X-P, Lu T-J, Yu L-Z. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–4858. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Wang F, Zhang L, Lu J-C (2017b) Long non-coding RNA UCA1 can predict tumor lymph node metastasis. Tumor Biol 39(1010428317706208) [DOI] [PubMed]
- Wang Z-Q, Cai Q, Hu L, He C-Y, Li J-F, Quan Z-W, Liu B-Y, Li C, Zhu Z-G. Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis. 2017;8:e2839. doi: 10.1038/cddis.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350:207–213. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- Xue M, Chen W, Li X. Urothelial cancer associated 1: a long noncoding RNA with a crucial role in cancer. J Cancer Res Clin Oncol. 2016;142:1407–1419. doi: 10.1007/s00432-015-2042-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang B, Wang H-Y, Chang A, Zheng XS (2017) Emerging Role of MicroRNAs in mTOR Signaling. Cell Mol Life Sci:1–13 [DOI] [PMC free article] [PubMed]