Abstract
Nowadays, using ionizing radiation (IR) is necessary for clinical, agricultural, nuclear energy or industrial applications. Accidental exposure to IR after a radiation terror or disaster poses a threat to human. In contrast to the old dogma of radiation toxicity, several experiments during the last two recent decades have revealed that intercellular signaling and communications play a key role in this procedure. Elevated level of cytokines and other intercellular signals increase oxidative damage and inflammatory responses via reduction/oxidation interactions (redox system). Intercellular signals induce production of free radicals and inflammatory mediators by some intermediate enzymes such as cyclooxygenase-2 (COX-2), nitric oxide synthase (NOS), NADPH oxidase, and also via triggering mitochondrial ROS. Furthermore, these signals facilitate cell to cell contact and increasing cell toxicity via cohort effect. Nitric oxide is a free radical with ability to act as an intercellular signal that induce DNA damage and changes in some signaling pathways in irradiated as well as non-irradiated adjacent cells. Targeting of these mediators by some anti-inflammatory agents or via antioxidants such as mitochondrial ROS scavengers opens a window to mitigate radiation toxicity after an accidental exposure. Experiments which have been done so far suggests that some cytokines such as IL-1β, TNF-α, TGF-β, IL-4 and IL-13 are some interesting targets that depend on irradiated organs and may help mitigate radiation toxicity. Moreover, animal experiments in recent years indicated that targeting of toll like receptors (TLRs) may be more useful for radioprotection and mitigation. In this review, we aimed to describe the role of intercellular interactions in oxidative injury, inflammation, cell death and killing effects of IR. Moreover, we described evidence on potential mitigation of radiation injury via targeting of these mediators.
Keywords: Radiation, Cohort effect, Bystander effect, Non-targeted effect, Radiation toxicity, Radiation disaster, Radiotherapy, Intracellular communication, Cytokines, Redox system, Mitigation, Carcinogenesis
Introduction
Nowadays, human exposure to ionizing radiation (IR) is an unavoidable phenomenon. The main source of human exposure in developed countries is medical uses. Also, the use of medical IR sources for diagnostic or therapeutic aims is growing. In addition to clinical applications, exposure to high doses of IR is one of the main threats for human space mission as well as accidental or terrorism related radiological events (Moulder 2004; Valentin 2005). A Knowledge about the mechanisms of IR-induced normal tissues toxicity is one of the main goals in radiation biology. Enhancements in this knowledge can lead to better management of radiation toxicity, improve clinical outcomes of radiotherapy, and also in mitigation of human radiation injury after a nuclear disaster. The major concerns for radiotherapy patients or irradiated people in an accidental disaster are carcinogenesis and organ failure that threaten the lives of irradiated individuals (Moulder 2003).
A large body of experiments have shown that intracellular signaling plays a significant role in radiation toxicity in irradiated cells (Prise et al. 2005). Moreover, intercellular communications between irradiated and non-irradiated cells, induce various damages via a phenomenon known as bystander effect (Azzam et al. 1998). Interestingly, in vitro studies showed that the mechanisms involved in bystander effect are very similar to directly irradiated cells (Najafi et al. 2014; Yahyapour et al. 2018c). Furthermore, inhibition of bystander effect can lead to alleviation of oxidative cellular damage in both directly irradiated and bystander cells (Zhou et al. 2005). This shows that after exposure to radiation, cells release some clastogenic factors that amplify radiation toxicity in irradiated cells and also cause damage to non-irradiated cells (Yang et al. 2005). The radiation toxicity by released signals involves two distinct processes which include the production of danger signals and response of other irradiated or non-irradiated cells to these signals (Yahyapour et al. 2018a). Based on these conclusions, it has been proposed that identification of intercellular signals can help better management of radiation toxicity in radiotherapy patients, deep space missions, as well as nuclear/radiological disasters. Although, several studies have been conducted to define the mechanisms of radiation-induced normal tissue toxicity, radiobiologists still agree that the complete involvement of signaling pathways remain to be known.
Radiation toxicity in radiotherapy
Response of normal tissues to radiotherapy is so dependent on the type of irradiated organs, as well as radiation dose. In conventional radiotherapy, 2Gy of Gamma or X-rays is a typical radiation dose in each fraction. However, in some new radiotherapy techniques such as stereotactic, higher radiation dose in each fraction is available. On the other hand, in some hyper-fractionation techniques, doses lower than 1Gy per fraction may be used. For every radiation dose, DNA is the most critical target within cells, whose interaction with IR may lead to cell death. Following exposure to a radiation dose lower than 1Gy, apoptosis which is caused by DNA damage is the most common type of cell death in irradiated organs. However, in higher doses such as in conventional or stereotactic radiotherapy, necrosis is more common compared to apoptosis (Najafi et al. 2018a). Necrosis is as a result of damage to other organelles in cells such as the membrane. The response of immune cells to each of these cell death types are different. Each of these responses have toxic effects on survived cells. Although, most of the late effects of radiotherapy such as bleeding, fibrosis, pneumonitis, heart disorders, myelopathy etc., are as a result of inflammatory responses following higher doses of IR, anti-inflammatory responses which are common after exposure to lower doses of IR are involved in some other side effects such as fibrosis (Najafi et al. 2017b). Each of these side effects may limit the required radiation dose for tumor, leading to higher possibility of tumor recurrence and reduction of therapeutic ratio.
Radiation toxicity in a nuclear/radiological disaster
Nuclear or radiological disasters are threats to human. Results of studies on an atomic bomb explosion in Hiroshima and Nagasaki showed that exposure to a high dose of IR following nuclear weapon explosion led to deaths some hours to weeks after exposure (Barnaby 1995). Moreover, exposure to a sub-lethal dose of IR can cause appearance of various disorders after some decades (Douple et al. 2011; Kamiya et al. 2015). The most sensitive organs to external exposure to gamma rays or neutron particles includes the bone marrow and gastrointestinal system (Grammaticos et al. 2013). While, in Chernobyl disaster, it has been shown that exposure to beta-emitter particles including radioactive iodine isotopes can cause severe skin burning (Peter et al. 1994). Detrimental effects of IR on these organs may lead to death after some weeks. Some sensitive and late responding organs to IR includes lung, heart, liver and kidney (Peter et al. 1994; Dorr and Meineke 2011). Some evidences proposed that exposure of these organs to a high dose of IR cause death some months to years after exposure (Yahyapour et al. 2017a). Exposure of lung tissue to a high dose of radionuclides received through inhalation, leads to pneumonitis and fibrosis that cause death after some years (Medhora et al. 2012; Mahmood et al. 2013). Although, heart fibrosis may appear 30 years after exposure. By contrast to the lung, heart disorders can be seen following exposure to a low dose of IR (Eldabaje et al. 2015; Boerma et al. 2016).
Autocrine, paracrine and endocrine signals in radiation injury
Damage to a single cell by IR can trigger some signals that amplify further free radical production in irradiated cell. Depending on the irradiated cell type, this can continue for some hours, days or months after exposure (Pazhanisamy et al. 2011; Chang et al. 2015). Hence, exposure of irradiated cells to these signals play a key role in detrimental effects of IR on normal tissues. In addition, secreted signals can migrate to other irradiated cells. Interactions between irradiated cells that further increase oxidative stress and cell death is known as cohort effect (Blyth and Sykes 2011). It plays a key role in amplification of radiation injury by the extent of time that cells are exposed to free radicals, chemokines, cytokines and others (Lorimore et al. 2001). These changes are associated with long-term change in reduction/oxidation (redox) system and cell metabolism (Yahyapour et al. 2018b). Released signals from irradiated cells can also migrate to adjacent non-irradiated cells. This phenomenon is known as bystander effect, which causes activation of redox metabolism in non-irradiated cells (Mothersill et al. 2000). Some secreted signals from irradiated cells may be released to the circulatory system and then stimulate ROS/NO production in distant organs. This phenomenon is known as non-targeted/out-of-field effect (Najafi et al. 2014).
The origin of intercellular signaling after exposure to radiation is DNA damage and cell death. Massive DNA damage and free radical production by IR that are seen following exposure to an accidental nuclear/radiological disaster or radiotherapy lead to cellular death through mitotic catastrophe, apoptosis, necrosis, senescence and autophagy (Maier et al. 2016). Among these cell death mechanisms, mitotic catastrophe cannot trigger secretion of danger signals. By contrast, other types of cell death stimulate immune system responses including both inflammatory and anti-inflammatory responses (Golden et al. 2012). Released danger signals from damaged cells are known as damage-associated molecular patterns (DAMPs). DAMPs are detected by some receptors on cell surface that are known as pattern recognition receptors (PRRs) (Krysko et al. 2012; Multhoff and Radons 2012). Toll like receptors (TLRs) are the most common PRRs in cell surface that detect DAMPs. Some experiments have shown that after an exposure, some TLRs such as TLR2, TLR4, TLR5 and TLR9 are upregulated (Piccinini and Midwood 2010). These TLRs stimulate the expression of some transcription factors such as mitogen activated protein kinases (MAPKs), nuclear factor of kappa B (NF-KB), signal transducer and activator of transcriptions (STATs), and some others (Piccinini and Midwood 2010; Karki and Igwe 2013). Therefore, depending on the TLRs-transcription factor pathway, various types of immune system mediators and cytokines are released into cells and intracellular space. As some of immune mediators are able to produce ROS/NO, these signaling pathways are associated with oxidative damage, chromosomal aberrations and cell death. Thus, secretion of danger alarms can stimulate redox system in targeted, bystander and distant non-targeted cells. Upregulation of transcription factors, immune mediators and free radicals have been reported in all these cells, while the expression of genes profile may be different (Ghandhi et al. 2008; Chaudhry and Omaruddin 2012).
Intercellular mediators in radiation toxicity
As mentioned above, after exposure to IR, DNA damage and cell death lead to the release of some danger alarm into intracellular space. Oxidized DNA in both nucleus and mitochondria is a danger alarm that is secreted to outer space of cells after damage and deletion of a part of DNA. High mobility group box 1 (HMGB1) is a key molecule that is secreted by necrosis cells. This molecule triggers inflammatory cytokines via binding to TLR4. Sulfonyl HMGB1 is another type of this molecule that is secreted following apoptosis and has no stimulatory effect on inflammatory responses (Yang et al. 2015). HMGB1 through interaction with TLR4 upregulates NF-κB and MAPKs, leading to filtration of macrophages and production of inflammatory cytokines such as IL-1, IL-6, IL-8, TNF (Park et al. 2006; Yang et al. 2010). As inflammatory cytokines stimulate ROS and NO generation via redox system, this is associated with oxidative damage in cells that are exposed to HMGB1 (Li et al. 2013). Moreover, HMGB1 is able to provoke ROS/NO production by NADPH oxidase enzymes (Klune et al. 2008). By contrast to inflammatory pathways of cell death by necrosis, removal of apoptotic bodies by macrophages cause release of anti-inflammatory cytokines such as TGF-β and IL-10 in autocrine and paracrine cells (Szondy et al. 2017; Fadok et al. 1998). On the other hand, TGF-β stimulates apoptosis through some signaling pathways such as SMAD and Rho/ROCK pathways (Jang et al. 2002; Schuster and Krieglstein 2002). TGF-β also triggers macrophages and lymphocytes to generate free radicals through some signaling pathway such as iNOS, NADPH oxidase and cyclooxygenase-2 (COX-2). Production of free radicals stimulate further release of HMGB1 by macrophages and monocytes (Tang et al. 2007).
Various studies have shown that after exposure of cells to radiation, some of these mediators are released to intracellular spaces as well as blood circulation. For example, Wang et al. showed that exposure to 4 or 12 Gy X-rays causes dramatic intracellular release of HMGB1 in human skin fibroblast and bronchial epithelial cells, as well as in irradiated rats (Wang et al. 2016). Elevated level of both inflammatory and anti-inflammatory cytokines such as IL-1, IL-2, IL-6, IL-8, IL-33, TGF-β and TNF-α, as well as some siRNAs, miRNAs and vesicles after exposure to IR have been confirmed by several studies (Yahyapour et al. 2017b; Prise and O'Sullivan 2009). Cell-free chromatin, which may be produced after interaction of IR with DNA is another intracellular mediator that can trigger inflammation and oxidative damage in other cells (Mittra et al. 2015). Studies confirmed that oxidized DNA which originates from the nucleus or mitochondrial DNA is able to stimulate inflammation and oxidative stress in adjacent cells (Havaki et al. 2015; Kostyuk et al. 2012). Similar to HMGB1, oxidized DNA can bind to TLR9 and then upregulate inflammatory mediators and cytokines, leading to free radical production in a positive feedback loop (Yahyapour et al. 2017b). An experiment by Mittra et al. revealed that co-culture of dying cells along with live cells cause induction of bystander effect via transition of cell-free chromatin. They showed a rapid entrance of cell free chromatins into live cells and induction of DNA damage. Moreover, they showed that injection of dead Jurkat cells to mice cause stimulation of inflammation and upregulation of inflammatory mediators such as NF-κB, IL-6, TNFα and IFN-γ in different tissues. However, injection of live cells had less effect (Mittra et al. 2017).
These studies show that there is a potent link between radiation-induced DNA damage, cell death, inflammation and oxidative damage. Cytokines, siRNAs, miRNAs, vesicles, HMGB1 and cell-free DNA fragments are intracellular mediators. These mediators amplify cellular damages by chronic ROS/NO production in irradiated cells. Furthermore, these mediators can affect other non-irradiated cells, leading to bystander effect. Although the severity of oxidative damage in bystander cells is lower than directly irradiated cells, this effect increases the risk of carcinogenesis. Inhibition of these mediators can be proposed to ameliorate oxidative stress in directly irradiated, bystander cells and non-targeted tissues (Ghobadi et al. 2017; Fardid et al. 2017; Najafi et al. 2017a).
Intercellular connections
For several years, it has been known that normal activity of cells in tissues depends on communication between cells. After exposure to IR and production of toxic agents or clastogenic factors, intercellular communications which link adjacent cells to each other facilitate the propagation of toxic products between irradiated cells (Autsavapromporn et al. 2011). Also, intercellular communications can transfer clastogenic factors to non-irradiated/bystander cells. Connexin channels including connexin26 (Cx26) and Cx43, are the most important junctions that amplify radiation toxicity or induce bystander effect in non-irradiated cells (de Toledo et al. 2017; Azzam et al. 2001).
Zhao et al. in an in-vitro study showed that exposure of adenocarcinoma HeLa cells to IR upregulates Cx26 in both irradiated and bystander cells. This was associated with increased micronuclei formation. They showed that there is a crosstalk between COX-2 and Cx26 in irradiated and bystander cells. COX-2 upregulation in irradiated cells induces Cx26 in bystander cells, leading to COX-2 expression and mutation in these cells (Zhao et al. 2014). Although, the role of connexins and gap junctions in mediating radiation injury has been confirmed, it has also shown cell specific and radiation quality effects (Autsavapromporn et al. 2013a, 2013b). However, this may not be seen in all cell types (Mothersill and Seymour 1998).
Cytokines
Cytokines are products of immune system cells including macrophages and lymphocytes T. These molecules are the most important mediators for interrelation between immune cells, and also response to DAMPs. In response to IR, cytokines play a central role in promoting various side effects such as inflammation, fibrosis and carcinogenesis. Increased level of cytokines is a marker for radiation toxicity and its modulation can be proposed for alleviating side effects of radiotherapy. Depending on the type of cell death, exposure to IR stimulates the production of inflammatory cytokines such as IL-1, IL-2, IL-6, IL-8, IL-18, IL-33, TNF-α and IFN-γ, or anti-inflammatory cytokines which include TGF-β and IL-10. These cytokines stimulate several signaling pathways that induce inflammation and oxidative damage in irradiated as well as bystander cells. Upregulation of TGF-β in non-targeted tissues can trigger COX-2 and ROS production (Cheki et al. 2018).
Epigenetic modulators
In the last two decades, several studies have shown that miRNAs and siRNAs play a key role in response of normal tissues to IR. Although there has not been any clear understanding of how IR upregulates or downregulates the expression of some miRNAs and siRNAs, it has been confirmed that after exposure to IR, these mediators are released from irradiated cells to intracellular space (Xu et al. 2015). Also, miRNAs and siRNAs can transfer to other cells through vesicles. The role of epigenetic modulators in radiation-induced free radical production in irradiated and bystander cells have been studied. Similar studies on the role of these mediators in radiation induced DNA damage and genomic instability in distant non-targeted tissues have been conducted (Mothersill and Seymour 2012).
Let-7 subfamily of miRNAs that upregulates after exposure of cells to IR are involved in free radical production (He and Jiang 2016). A well-known effect of miRNAs in ROS production and oxidative stress is increasing super oxidase level via suppression of antioxidant enzymes. A good example is upregulation of mir-21 in both targeted and bystander cells. mir-21, which is itself triggered by TGF-β inhibits SOD2 gene expression, leading to decreasing SOD2 activity and damage to irradiated and bystander cells by superoxide (Jiang et al. 2014; Tian et al. 2015; Xu et al. 2014). Suppression of SOD activity and GSH level have been revealed in non-targeted lung tissues (Ghobadi et al. 2017; Najafi et al. 2016).
Cohort/Bystander/Non-targeted signals induce Redox system and amplify radiation injury in irradiated and non-irradiated cells
As previously mentioned, interaction between cells amplify radiation toxicity. The most critical products of intracellular signals involved in radiation toxicity are free radicals. In irradiated organs, intracellular signals are involved in early and late side effects of IR. There are some immune mediators such as COX-2, NADPH oxidase and iNOS that produce ROS and NO after stimulation by cytokines or others. Moreover, some organelles including mitochondria, Endoplasmic reticulum and lysosomes amplify DNA damage after interaction with free radicals.
COX-2
COX-2 is an isoenzyme that plays a central role in inflammation. It also has a key role in radiation injury and continuous ROS production after exposure to IR. Thus, targeting COX-2 has been proposed for protection and mitigation of radiation injury (Cheki et al. 2018). It has been confirmed that COX-2 is involved in radiation toxicity in irradiated and bystander cells, as well as in non-targeted tissues. In-vitro studies have shown that COX-2 can be stimulated by IGF-1–COX-2 and TNF-α–PKC–COX-2 pathways (Zhou et al. 2005; Fang et al. 2016). While in-vivo studies have shown that COX-2 in the lung and bronchia tissues of mice is upregulated up to 30 fold via TGF-βR-COX-2 pathway (Chai et al. 2012, 2013). However, these studies showed that TNF-α is not involved in non-targeted effect. Based on these studies, so far, it has been shown that IGF-1 and TNF-α are the most important mediators in radiation toxicity by COX-2 pathway in targeted cells.
Nitric oxide synthase (NOS)
NO is an important mediator within and between cells in the immune system, cardiovascular, and nervous systems (Calabrese et al. 2007). It is generated from arginine by metabolism of some enzymes known as nitric oxide synthase (NOS). NO is one of the most important mediators that play a key role in oxidative damage in targeted and bystander cells (Tomita et al. 2015; Han et al. 2007). NO has a low weight and higher half-life compared to other free radicals. Hence, enabling it to migrate to distant cells. These properties make it to reach to bystander cells without the need of gap junctions (Cali et al. 2015). NO plays an important role in various signaling pathways of the immune system. In addition, it can be produced by some enzymes such as inducible form of NO synthetize (iNOS). Although, other forms of NO synthesis such as constitutive NOS (cNOS) may be involved in early effects of IR, it seems that iNOS is the main source of NO production for long time following exposure to IR (Hong et al. 2013).
Upregulation of iNOS and subsequent NO production in irradiated and bystander cells are due to inflammatory signaling pathways. In hematopoietic system, which has the most critical organs in radiation disasters, the activation of macrophages can lead to NO production by iNOS for days or weeks after exposure (Hildebrandt et al. 2003). A study showed that irradiation of macrophages upregulates iNOS expression and increases NO production, leading to chromosome damage in bystander cells. Suppression of this enzyme by its selective inhibitor causes a significant reduction in DNA damage (Ghosh et al. 2008). Although, exact signaling pathways of NO production by iNOS in targeted and bystander cells remain to be elucidated, some studies proposed that IL-1β, TNF-α and TGF-β are the main activators (Wu et al. 2017). The role of NO in non-targeted effect is not yet studied.
NADPH oxidase
NADPH oxidase includes five NOX subfamilies (NOX1-5) and two dual oxidases (DUOX1-2). These enzymes are producers of H2O2, which help to kill foreign bodies in cells. Moreover, in responses to a wide range of cytokines and growth factors. Studies have revealed that both NOX and DUOX subfamilies have involvement in radiation toxicity in irradiated cells. Also, some limited studies proposed that NOX system may be involved in radiation toxicity in bystander cells (Azzam et al. 2002). After activation, these enzymes have high stability, leading to continuous free radical generation after exposure to IR (Battino et al. 1999). Studies proposed that inflammatory cytokines such as IL-1β, TNF-α and TGF-β are stimulators of NOX1-5, while DUOX1-2 is activated by IFN-γ, IL-4 and IL-13 (Battino et al. 1999; Cho et al. 2017).
A study showed that NOX2 and NOX4 are the main sources of free radical production in bone marrow cells. This study revealed that exposure to IR can upregulate NOX4 for 2 months, leading to the death of bone marrow stem cells and genomic instability (Pazhanisamy et al. 2011). As hematopoietic system failure is a main reason of death after an accidental radiation disaster, modulation of this enzyme may help mitigate death and possible radiation diseases in these organs. In addition to hematopoietic syndrome, suppression of NOX1-5 and DUOX1-2 system pathways have been proposed for mitigation of radiation-induced lung injury, mucositis, enteritis, fibrosis, and etc. (Ameziane-El-Hassani et al. 2015; Piwkowska et al. 2013; Wu and Doroshow 2014a; Najafi et al. 2018b).
Mitochondria
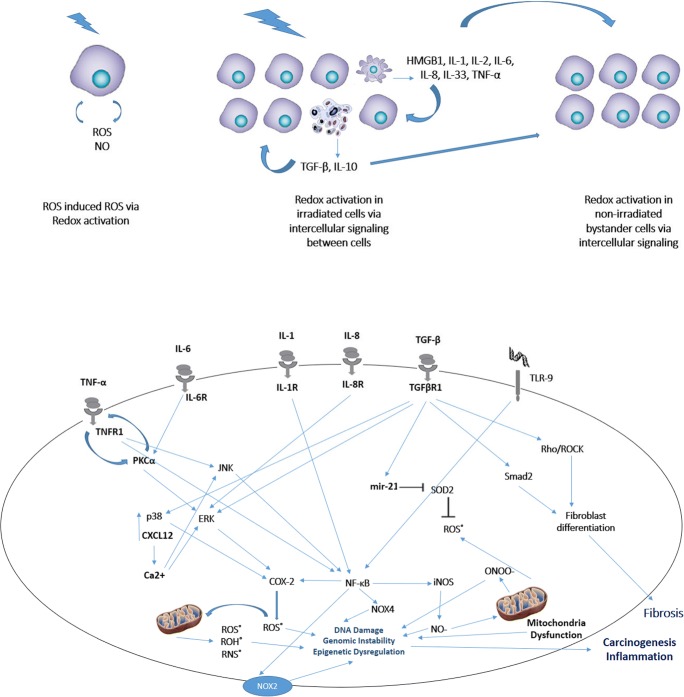
Mitochondria is an ATP source for the energy of cells. This procedure is a result of oxidative phosphorylation that occurs in the mitochondrial inner membrane. The mitochondrial DNA (mtDNA) is located close to the mitochondrial inner membrane and produces superoxide by oxidative phosphorylation after exposure. Experiments have shown that mutation in mtDNA can increase superoxide production by oxidative phosphorylation (Brand et al. 2004; Yoshida et al. 2012). After irradiation, an Increase in Ca2+ ions also increases ROS production by the mitochondria (Leach et al. 2001). Under normal conditions, ROS produced by the mitochondria are neutralized by mitochondria antioxidant enzymes such as catalase, SOD2, glutathione (GSH) and glutathione peroxidase (GPx). Oxidative stress occurs when the production of ROS exceeds the levels of these enzymes. Excessive free radical production after damage to mtDNA can react with DNA and intracellular organelles, leading to lipid peroxidation, protein hydroxylation and chromosomal aberrations. Studies have confirmed the key role of the mitochondria in radiation-induced inflammation and chronic oxidative stress in targeted and bystander cells (Tartier et al. 2007; Zhou et al. 2008a, 2008b, 2011; Zorov et al. 2006). In addition, targeting of mitochondrial ROS has been proposed for mitigation of radiation injury (Rwigema et al. 2011) (Fig. 1).
Fig. 1.
Mechanisms of radiation toxicity via activation of redox system by intra and intercellular mediators. a; a single irradiated cell induces ROS production which amplify radiation toxicity, leading to more production of ROS. Necrosis and apoptosis cause stimulation macrophages and lymphocytes T to produce several inflammatory or anti-inflammatory cytokines. This is associated with increased redox activity in irradiated cells, as well as in other adjacent non-irradiated cells.
Suppression of Intercellular-Redox signaling for mitigation of radiation injury
Suppression of cytokines
Suppression of cytokines is an interesting strategy for the amelioration of radiation toxicity. Elevated levels of cytokines including inflammatory and pro-fibrotic cytokines are a result of immunologic cell death (apoptosis and necrosis). Hence, inhibition of these cytokines with their antagonists or via reduction of cell death can ameliorate inflammation and fibrosis induced by IR (Yahyapour et al. 2017a). Using selective inhibitors or other agents have been studied for evaluating possible mitigatory effects of cytokines targeting. Among various cytokines that are increased by IR, IL-4, IL-13 and TGF-β have been proposed for mitigation of radiation fibrosis (Gauter-Fleckenstein et al. 2010b). These cytokines can upregulate some months after exposure to IR, leading to stimulation of fibrosis mediators and collagen deposition in intercellular space (Groves et al. 2016; Wu and Doroshow 2014b). This suggests that chronic stimulation of ROS production by Redox interactions play a key role in the development of fibrosis. IL-4 induces expression of DUOX2, while IL-13 stimulates DUOX1 gene expression. These changes are associated with long term ROS production by these enzymes (Ameziane-El-Hassani et al. 2015). In addition, upregulation of these enzymes can stimulate upregulation of TGF-β. TGF-β is one of the most important players of radiation toxicity in directly irradiated as well as non-irradiated bystander cells (Anscher 2010). TGF-β regulates several signaling pathways that are involved in fibrosis. Among these pathways, TGF-β-Rho/ROCK, TGF-β-Smad2, and TGF-β-NOX4 promote fibrosis after exposure to IR (Dancea et al. 2009; Martin et al. 2000). In addition to these pathways, various studies have proposed that inflammatory cytokines amplify fibrosis induced by IR (Medhora et al. 2012; Oikonomou et al. 2006; Robbins and Zhao 2004).
An in-vivo study by Chung et al. have shown that IL-13 is a potential target for mitigation of radiation-induced lung fibrosis. They showed that exposure to 5 daily fractions of 6 Gy lead to an increase in IL-13, but not for IL-4 in the lung 16 weeks after irradiation. Moreover, their results showed that IL-13 upregulation stimulates TGF-β, leading to phosphorylation and activation of smad2/smad3 signaling and other pro-fibrosis factors such as fibrillin-1, matrix metalloproteinase-2 (MMP-2), and MMP-3. Furthermore, they revealed that targeting of IL-13 can mitigate radiation death induced by lung fibrosis via downregulation of these pathways (Chung et al. 2016). Although, this study proposed no role for IL-4 in lung injury, some other studies have proposed a role for this cytokine (Huaux et al. 2003). Groves et al. showed that IL-4 stimulates macrophage accumulation and activity in irradiated lung mice. They revealed that while inhibition of IL-4 does not prevent fibrosis, it can promote the maintenance of macrophages in lung tissue following irradiation (Groves et al. 2016).
Targeting of TGF-β is the most studied approach for amelioration of radiation injury in normal tissues, as well as increasing therapeutic ratio through inhibition of angiogenesis and tumor growth. In addition to fibrosis progression, TGF-β stimulates oxidative damage via upregulation of ROS producing enzymes such as NOX2, NOX4 and COX-2, and also via stimulation of NO producing enzymes like iNOS. These changes may continue for some days to months after exposure to IR. In the bone marrow, which is the most critical organ in a radiation disaster, ROS generation by activation of NOX2 and NOX4 can continue for some months and involves hematopoietic syndrome and genomic instability. Inhibition of TGF-β has shown reduced oxidative damage and cell death in irradiated bone marrow of mice. Zhang et al. have shown that treatment of mice with SB431542 (a selective TGF-β inhibitor) reduces expression of NOX2 and NOX4, and also free radical production in bone marrow mononuclear cells following exposure to 2-4 Gy of IR. Results also showed an increase in NOX1, but it was independent of TGF-β. The most effective concentration was 1 μM SB431542. This increases the ability of CFU-GM by 60% (Zhang et al. 2013).
In addition to stimulation of ROS/NO production and DNA damage, TGF-β is a potent player in late effects of IR, including fibrosis (Boerma et al. 2013). As earlier mentioned, TGF-β can induce fibrosis via some different signaling pathways. Expression of TGF-β and lung fibrosis is increased after exposure (Rube et al. 2000). Rabender et al. investigated selective targeting of TGF-β for mitigation of radiation-induced fibrosis in the lung and heart after exposure to 11.5 Gy. IPW-5371 as a selective inhibitor of TGF-β was administered 24 h after irradiation at a dose of 10 mg/kg or 30 mg/kg per day. This process continued for 6 or 20 weeks in irradiated groups. Results showed a significant increase in survival and reducing fibrosis. Meanwhile, results indicated that higher dose of TGF-β inhibitor for longer periods of time was more effective. The median survival of mice was increased from 135 days in irradiated mice without TGF-β inhibitor to 180 days in irradiated plus treated mice. Several analyses showed a significant improvement in respiratory function, reduced heart injury, decreased collagen deposition and attenuation of TGF-β/Smad3 signaling (Rabender et al. 2016). In another study by Flechsig et al., they showed that targeting of TGF-β receptor I by LY2109761 produced similar results. Results of these studies was associated with decreased pneumonitis and lung fibrosis, leading to prolonged survival (Flechsig et al. 2012). Other studies confirmed the inhibition of TGF-β via different agents such as activin receptor-like kinase-5 inhibitor, adenoviral vectors, TGF-β antibody, halofuginone (Koh et al. 2015; Nishioka et al. 2004, 2015; Anscher et al. 2006; Xavier et al. 2004).
Some other studies proposed that IL-17 has a role in the development of radiation fibrosis. Wang et al. showed that after irradiation of mice lung with 15 Gy the level of this cytokine elevates after 1 week and reaches its peak after 4 weeks. They showed that attenuation of this cytokine by dexamethasone leads to reducing macrophage infiltration and lung injury (Wang et al. 2014b). In another study, they evaluated targeting of IL-17 on radiation-induced fibrosis and pneumonitis. They used an IL-17 antibody (4 μg, 4 days/month) before exposure for 4 months after chest irradiation with 15 Gy (Wang et al. 2014a). Moreover, inhibition of IL-17 can reduce expression of TGF-β and IL-13 (pro-fibrotic cytokines) and augment that of IFN-γ (Mi et al. 2012).
In addition to selective inhibitors, some studies proposed that attenuation of cytokines by other agents ameliorate late effects of IR such as pneumonitis and fibrosis. Genistein or quercetin as an isoflavonoid, flaxseed as a lignan and EUK-207 as an SOD-catalase mimetic, have shown ability to mitigate thorax radiation injury and reduce TGF-β signaling (Mahmood et al. 2013; Horton et al. 2013; Pietrofesa et al. 2013; Lee et al. 2009). This was confirmed for other SOD mimetic, MnTE-2-PyP(5+) (Yakovlev et al. 2010; Gauter-Fleckenstein et al. 2010a; Archambeau et al. 2013). Due to the synergic effect on tumor therapy after targeting some of these cytokines, agents such as LY2109761 and ZnSO4 can be proposed as an adjuvant in radiotherapy (Zhang et al. 2011; Melisi et al. 2008; Hardee et al. 2012; Reeves et al. 2007).
Targeting of TLRs
TLRs are mediators between DNA/cell damage and inflammation. Among the known TLRs; TLR2, TLR4, TLR5 and TLR9 are involved in inflammation signaling pathways and redox activation by IR (Yahyapour et al. 2017a). Targeting of these receptors have been proposed for radiation mitigation and protection of normal tissues in some studies. Shakhov et al. examined an agonist of TLR2 for mitigation of radiation injury in mice after whole body irradiation with 9 or 10 Gy gamma rays. Results showed that pretreatment with TLR2 agonist before exposure to 10 Gy gamma rays increases survival by more than 80%, while using placebo does not lead to survival. Moreover, they showed that agonist administration 24 to 48 h after irradiation increases survival more potently. The best time for administering TLR2 agonist was 1 h after irradiation. Their results showed an increase in survival (73% against 7%). More analyses showed an amelioration of inflammatory responses and recovery of hematopoietic system following irradiation (Shakhov et al. 2012). Similar results have been obtained for TLR2/6 agonist (Kurkjian et al. 2017). Targeting of TLR4 has shown ability to mitigate radiation toxicity in spleen, testis, intestine and bone marrow. This was associated with increased survival, reducing apoptosis and protection of CD34+ hematopoietic stem cells (HSC), and suppression of inflammatory and pro-fibrotic cytokines such as IL-1, IL-4, IL-6, IL-13 and TNF-α (Guo et al. 2017).
TLR5 is another target that has shown both radioprotection and mitigation effects. A single administration of CBLB502 (a TLR5 agonist) has shown ability to protect against both hematopoietic and gastrointestinal syndrome following irradiation of mice with 10 or 13 Gy. Results showed that while exposure to 13 Gy caused no survival in irradiated mice, treatment with CBLB502 gave more than 80% survival. Interestingly, survival for mice treated with 150 mg/kg amifostine was lower than CBLB502 (87% against 54%) (Burdelya et al. 2008). Treatment with TLR5 agonist has shown increased survival by 2-3 fold via amelioration of hematopoietic and gastrointestinal syndrome (Krivokrysenko et al. 2015). In addition to early radiosensitive organs in hematopoietic and gastrointestinal systems, targeting of TLR5 has been proposed for mitigation of radiodermatitis and mucositis (Toshkov et al. 2017).
Targeting of ROS/NO producing enzymes
ROS/NO producing enzymes are some immune enzymes that are involved in defense against pathogens as well as inflammatory responses. Moreover, NO and ROS play key roles in various signaling pathways. Overproduction of these enzymes after exposure to IR, and subsequent products induce the production of ROS and NO over a long period of time. Hence, attenuation of these enzymes may be proposed for mitigation of radiation injury. Among these enzymes, COX-2 inhibition has been studied. However, its protective effect may be due to inhibition of inflammatory mediators and cytokines (Cheki et al. 2018). Inhibition of COX-2 has shown ability to mitigate pneumonitis, arthritis, and inflammatory cytokines such as TNF and IL-1 (Khayyal et al. 2009). In addition, COX-2 suppression by celecoxib may reduce oxidative damage in bone marrow cells (Hosseinimehr et al. 2017). Claude et al. evaluated the mitigatory effect of targeting nitric oxide synthase enzymes in C57BL/6 NHsd mice after exposure to 9.5 Gy X-rays. They showed that targeting of NOS enzymes by MCF201-89 with a dose of 10 mg/kg can mitigate lethal dose of radiation. They showed that treatment of mice with MCF201-89 increases survival from 20% up to 80% (Rwigema et al. 2011). It has been shown that suppression of COX-2 and iNOS by melatonin is associated with mitigation of oxidative damage in non-targeted lung tissues of rats (Ghobadi et al. 2017; Fardid et al. 2017).
NADPH oxidase are ROS producing enzymes whose role in IR-induced injury have been widely studied in recent years. However, studies for mitigation of normal tissues injury by suppression of these enzymes are limited. Inhibition of NOX-2 in mice brain has shown ability to ameliorate oxidative injury and inflammatory markers such as TNF-α and MCP-1 (Cho et al. 2017). Another study by Collins-Underwood showed that upregulation of NADPH oxidase in brain cells results from angiotensin II stimulation. In addition, they proposed that targeting of these enzymes can ameliorate neurovascular syndrome and brain injury following exposure to IR (Collins-Underwood et al. 2007). Saka et al. showed that among different subfamilies of NADPH oxidase, NOX4 is upregulated following irradiation of mouse embryonic fibroblasts. They showed that inhibition of NOX4 can reduce ROS production and recruitment of inflammatory cells (Sakai et al. 2018). Targeting of NOX4 has also been proposed for the mitigation of radiation injury in fibroblast cells (Weyemi et al. 2015). These studies indicated that overproduction of these enzymes are associated with increased risk of carcinogenesis, even though it seems that targeting of ROS/NO producing enzymes can reduce the risk of carcinogenesis following an accidental disaster, or second primary cancers after radiotherapy.
Targeting of mitochondria
As mentioned earlier, mtDNA mutation leads to an excessive ROS production by mitochondria. ROS produced by the mitochondria can interact with NO, resulting in nitroxide production. Mutations in complex II in mitochondria resulting from superoxide production can lead to genomic instability by IR (Dayal et al. 2009). Hence, targeting of mitochondrial ROS and nitroxide free radicals can mitigate radiation injury. An in-vitro study by Jiang et al. showed that targeting mitochondrial nitroxide free radicals by [2-(1-oxyl-2,2,6,6-tetramethyl-piperidin-4-ylimino)-ethyl] -triphenyl-phosphonium (TPEY-Tempo) reduces mitochondrial apoptosis and increases cell survival. Mouse embryonic cells were irradiated with 10 Gy gamma rays and then incubated by 10 μM TPEY-Tempo. Results showed that TPEY-Tempo neutralize nitroxide and prevent the release of cytochrome C, leading to decreasing apoptosis. Furthermore, they showed that the major mitigatory effect of TPEY-Tempo is not a result of direct scavenging of free radicals and is mediated by mitochondria-targeted nitroxides (Jiang et al. 2009). Similar results have been shown for S-conjugated 4-amino-2,2,6,6-tetramethyl-piperidine-N-oxyl (hemi-GS-TEMPO) on same cells (Jiang et al. 2008). The mitochondria-targeted nitroxide (JP4-039) is another agent that has been shown to mitigate murine hematopoietic progenitor cells (Rajagopalan et al. 2009).
The mitigatory effect of mitochondria targeting has also been shown in in-vivo studies. Results of a study by Claude et al. revealed the mitigatory effect of JP4-039 in a C57BL/6 NHsd mice model. Total body of mice was irradiated with 9.5 Gy and 24 h after exposure received 5 mg/kg JP4-039. This method increased survival from 20% to 70% (Rwigema et al. 2011). Another study showed that treatment with 10 mg/kg JP4-039 after exposure to 9-9.25 Gy total body irradiation mitigates hematopoietic syndrome by increasing bone marrow progenitor cells (Goff et al. 2011). JP4-039 has shown ability to ameliorate radiation-induced distant bone marrow suppression in mice, which gives an indication of its potential to suppress non-targeted signals (Willis et al. 2018; Berhane et al. 2014). JP4-039 can be proposed for clinical radioprotection of normal tissues in patients with cancer since it has shown no protective effect on some tumor cell types in mice (Shinde et al. 2016).
Summary and conclusion
The aim of this review was to clarify the mechanisms by which IR secrete intracellular mediators that cause cellular damage and tissue failure following exposure to radiation. The most important final product of these mediators that amplify radiation toxicity are free radicals such as ROS and NO. Knowledge of these mediators which are involved in radiation toxicity can help mitigate death and other organ damages in radiation disaster. This can also help to alleviate normal tissue damage in radiotherapy, thus improve cancer therapy outcome. Among various type of intracellular mediators, inflammatory cytokines such as IL-1β, TNF-α and TGF-β, pro-fibrotic cytokines such as IL-4 and IL-13, microRNAs including mir21, HMGB1 and oxidized DNA are known. These mediators induce free radical production and some other signaling pathways that extend the acute reaction of normal tissues.
The long term generation of intracellular mediators that can be seen during chronic inflammation has a central role in late effects of radiotherapy such as fibrosis, pneumonitis, enteritis, mucositis, bleeding as well as carcinogenesis. Furthermore, modulation of these mediators is a potential strategy for mitigation of possible radiological/nuclear disasters. In this situation, based on the injured organ, each of these mediators can be proposed for alleviation of radiation damage that may threaten the life of exposed people. Therefore, targeting these mediators or signaling pathways reduces risk of carcinogenesis.
The simplest strategy for the mitigation of radiation injury is administration of a high dose of antioxidants such as selenium, ascorbic acid, melatonin, resveratrol etc. Similarly, treatment with some mitochondrial antioxidants have shown promising results. Among various agents, mitochondrial ROS antioxidants have shown promising results. The application of these antioxidants in radiotherapy is associated with doubt, hence this strategy is not recommended in clinical use of IR. However, the targeting of other mediators is a more useful strategy. For example, targeting of TGF-β, IL-4 and IL-13 has been proposed for alleviation of fibrosis and bone marrow toxicity, while the inhibition of IL-1β and TNF-α can reduce inflammatory reactions such as pneumonitis, arthritis, mucositis, dermatitis and others.
Targeting of some TLRs including TLR2, TLR4, TLR5 and TLR9 is other strategy, which has shown interesting results. Inhibition of these mediators, in addition to amelioration of inflammation in irradiated organs, reduce activity of ROS producing enzymes, including COX-2, NADPH oxidase subfamilies, iNOS, and others. Moreover, superoxide production by mitochondria can be reduced. In a study, it has shown more protective effect and survival compared to amifostine. Hence, their administration can be proposed as a potent antagonist.
Studies conducted to define the role of gap junctions in radiation toxicity in cohort and bystander effect proposed that inflammation facilitates migration of clastogenic factors to adjacent cells. Therefore, inhibition of these mediators can reduce tissue toxicity through amelioration of inflammation, reduction of redox system and suppression of cell to cell contact.
So far, it has been confirmed that mir21 is a key player in oxidative damage by suppression of SOD2 activity. Stimulation of antioxidant enzymes by some agents such as melatonin and selenium can reverse inhibition of SOD activity. For some complicated situations such as in whole body exposure in a radiological or nuclear accident, using a combination of agents to neutralize free radicals and ameliorate inflammatory responses is a more useful strategy.
Compliance with ethical standards
Conflict of interest
No
References
- Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart J-M, De Deken X, Miot F, Diallo I, De Vathaire F, Schlumberger M, Dupuy C. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A. 2015;112:5051–5056. doi: 10.1073/pnas.1420707112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher MS. Targeting the TGF-β1 Pathway to Prevent Normal Tissue Injury After Cancer Therapy. The Oncologist. 2010;15:350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006;65:876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Archambeau JO, Tovmasyan A, Pearlstein RD, Crapo JD, Batinic-Haberle I. Superoxide dismutase mimic, MnTE-2-PyP(5+) ameliorates acute and chronic proctitis following focal proton irradiation of the rat rectum. Redox Biol. 2013;1:599–607. doi: 10.1016/j.redox.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, de Toledo SM, Little JB, Jay-Gerin JP, Harris AL, Azzam EI. The role of gap junction communication and oxidative stress in the propagation of toxic effects among high-dose alpha-particle-irradiated human cells. Radiat Res. 2011;175:347–357. doi: 10.1667/RR2372.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, de Toledo SM, Jay-Gerin JP, Harris AL, Azzam EI. Human cell responses to ionizing radiation are differentially affected by the expressed connexins. J Radiat Res. 2013;54:251–259. doi: 10.1093/jrr/rrs099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, Suzuki M, Plante I, Liu C, Uchihori Y, Hei TK, Azzam EI, Murakami T. Participation of gap junction communication in potentially lethal damage repair and DNA damage in human fibroblasts exposed to low- or high-LET radiation. Mutat Res. 2013;756:78–85. doi: 10.1016/j.mrgentox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from α-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- Barnaby F. The effects of the atomic bombings of Hiroshima and Nagasaki. Med War. 1995;11:1–9. doi: 10.1080/07488009508409217. [DOI] [PubMed] [Google Scholar]
- Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: the challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–476. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]
- Berhane H, Shinde A, Kalash R, Xu K, Epperly MW, Goff J, Franicola D, Zhang X, Dixon T, Shields D, Wang H, Wipf P, Li S, Gao X, Greenberger JS. Amelioration of radiation-induced oral cavity mucositis and distant bone marrow suppression in fanconi anemia Fancd2-/- (FVB/N) mice by intraoral GS-nitroxide JP4-039. Radiat Res. 2014;182:35–49. doi: 10.1667/RR13633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res. 2011;176:139–157. doi: 10.1667/rr2548.1. [DOI] [PubMed] [Google Scholar]
- Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One. 2013;8:e70479. doi: 10.1371/journal.pone.0070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma M, Sridharan V, Mao XW, Nelson GA, Cheema AK, Koturbash I, Singh SP, Tackett AJ, Hauer-Jensen M. Effects of ionizing radiation on the heart. Mutat Res. 2016;770:319–327. doi: 10.1016/j.mrrev.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, Gudkov AV. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Cali B, Ceolin S, Ceriani F, Bortolozzi M, Agnellini AH, Zorzi V, Predonzani A, Bronte V, Molon B, Mammano F. Critical role of gap junction communication, calcium and nitric oxide signaling in bystander responses to focal photodynamic injury. Oncotarget. 2015;6:10161–10174. doi: 10.18632/oncotarget.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Calaf G, Zhou H, Ghandhi S, Elliston C, Wen G, Nohmi T, Amundson S, Hei T. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br J Cancer. 2012;108:91–98. doi: 10.1038/bjc.2012.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Lam R, Calaf G, Zhou H, Amundson S, Hei T. Radiation-induced non-targeted response in vivo: role of the TGFβ-TGFBR1-COX-2 signalling pathway. Br J Cancer. 2013;108:1106–1112. doi: 10.1038/bjc.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Feng W, Wang Y, Luo Y, Allen AR, Koturbash I, Turner J, Stewart B, Raber J, Hauer-Jensen M, Zhou D, Shao L. Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice. Radiat Res. 2015;183:240–248. doi: 10.1667/RR13887.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry MA, Omaruddin RA. Differential regulation of microRNA expression in irradiated and bystander cells. Mol Biol (Mosk) 2012;46:634–643. [PubMed] [Google Scholar]
- Cheki M, Yahyapour R, Farhood B, Rezaeyan A, Shabeeb D, Amini P, Rezapoor S, Najafi M (2018) COX-2 in Radiotherapy; a potential target for radioprotection and radiosensitization. Curr Mol Pharmacol. 10.2174/1874467211666180219102520 [DOI] [PubMed]
- Cho HJ, Lee WH, Hwang OMH, Sonntag WE, Lee YW. Role of NADPH oxidase in radiation-induced pro-oxidative and pro-inflammatory pathways in mouse brain. Int J Radiat Biol. 2017;93:1257–1266. doi: 10.1080/09553002.2017.1377360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SI, Horton JA, Ramalingam TR, White AO, Chung EJ, Hudak KE, Scroggins BT, Arron JR, Wynn TA, Citrin DE (2016) IL-13 is a therapeutic target in radiation lung injury. Sci Rep 6 [DOI] [PMC free article] [PubMed]
- Collins-Underwood JR, Zhao W, Kooshki M, Robbins M. Modulation of NADPH oxidase by ionizing radiation and its role in radiation-induced oxidative stress and inflammation in brain endothelium. Cancer Res. 2007;67:1381–1381. [Google Scholar]
- Dancea HC, Shareef MM, Ahmed MM (2009) Role of Radiation-induced TGF-beta Signaling in Cancer Therapy. Mol Cell Pharmacol 1 [DOI] [PMC free article] [PubMed]
- Dayal D, Martin SM, Owens KM, Aykin-Burns N, Zhu Y, Boominathan A, Pain D, Limoli CL, Goswami PC, Domann FE, Spitz DR. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–745. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Toledo SM, Buonanno M, Harris AL, Azzam EI. Genomic instability induced in distant progeny of bystander cells depends on the connexins expressed in the irradiated cells. Int J Radiat Biol. 2017;93:1182–1194. doi: 10.1080/09553002.2017.1334980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med. 2011;9:126. doi: 10.1186/1741-7015-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douple EB, Mabuchi K, Cullings HM, Preston DL, Kodama K, Shimizu Y, Fujiwara S, Shore RE. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep. 2011;5(Suppl 1):S122–S133. doi: 10.1001/dmp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldabaje R, Le DL, Huang W, Yang LX. Radiation-associated Cardiac Injury. Anticancer Res. 2015;35:2487–2492. [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Xu A, Wu L, Hei TK, Hong M. The role of protein kinase C alpha translocation in radiation-induced bystander effect. Sci Rep. 2016;6:25817. doi: 10.1038/srep25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardid R, A S, Mosleh-Shirazi MA, Sharifzadeh S, Okhovat MA, Najafi M, Rezaeyan A, Abaszadeh A. Melatonin ameliorates the production of COX-2, iNOS, and the formation of 8-OHdG in non-targeted lung tissue after pelvic irradiation. Cell J. 2017;19:324–331. doi: 10.22074/cellj.2016.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, Peschke P, Hahn EW, Grone HJ, Yingling J, Lahn M, Wirkner U, Huber PE. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–3627. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Rebouças JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP 5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandhi SA, Yaghoubian B, Amundson SA. Global gene expression analyses of bystander and alpha particle irradiated normal human lung fibroblasts: Synchronous and differential responses. BMC Med Genet. 2008;1:63–63. doi: 10.1186/1755-8794-1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobadi A, Shirazi A, Najafi M, Kahkesh MH, Rezapoor S (2017) Melatonin ameliorates radiation-induced oxidative stress at targeted and nontargeted lung tissue. J Med Phys 42(4):241–244 [DOI] [PMC free article] [PubMed]
- Ghosh S, Maurya DK, Krishna M. Role of iNOS in bystander signaling between macrophages and lymphoma cells. Int J Radiat Oncol Biol Phys. 2008;72:1567–1574. doi: 10.1016/j.ijrobp.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Goff JP, Epperly MW, Dixon T, Wang H, Franicola D, Shields D, Wipf P, Li S, Gao X, Greenberger JS. Radiobiologic effects of GS-nitroxide (JP4-039) on the hematopoietic syndrome. In Vivo. 2011;25:315–323. [PMC free article] [PubMed] [Google Scholar]
- Golden E, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti S (2012) The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2 [DOI] [PMC free article] [PubMed]
- Grammaticos P, Giannoula E, Fountos GP. Acute radiation syndrome and chronic radiation syndrome. Hell J Nucl Med. 2013;16:56–59. [PubMed] [Google Scholar]
- Groves AM, Johnston CJ, Misra RS, Williams JP, Finkelstein JN. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int J Radiat Biol. 2016;92:754–765. doi: 10.1080/09553002.2016.1222094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Chen Y, Lei X, Xu Y, Liu Z, Cai J, Gao F, Yang Y. Monophosphoryl lipid a attenuates radiation injury through TLR4 activation. Oncotarget. 2017;8:86031–86042. doi: 10.18632/oncotarget.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Wu L, Chen S, Bao L, Zhang L, Jiang E, Zhao Y, Xu A, Hei TK, Yu Z. Constitutive nitric oxide acting as a possible intercellular signaling molecule in the initiation of radiation-induced DNA double strand breaks in non-irradiated bystander cells. Oncogene. 2007;26:2330–2339. doi: 10.1038/sj.onc.1210024. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res. 2012;72:4119–4129. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaki S, Kotsinas A, Chronopoulos E, Kletsas D, Georgakilas A, Gorgoulis VG. The role of oxidative DNA damage in radiation induced bystander effect. Cancer Lett. 2015;356:43–51. doi: 10.1016/j.canlet.2014.01.023. [DOI] [PubMed] [Google Scholar]
- He J, Jiang B-H. Interplay between Reactive oxygen Species and MicroRNAs in Cancer. Curr Pharmacol Rep. 2016;2:82–90. doi: 10.1007/s40495-016-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt G, Loppnow G, Jahns J, Hindemith M, Anderegg U, Saalbach A, Kamprad F. Inhibition of the iNOS pathway in inflammatory macrophages by low-dose X-irradiation in vitro. Is there a time dependence? Strahlenther Onkol. 2003;179:158–166. doi: 10.1007/s00066-003-1044-x. [DOI] [PubMed] [Google Scholar]
- Hong C-W, Kim Y-M, Pyo H, Lee J-H, Kim S, Lee S, Noh JM. Involvement of inducible nitric oxide synthase in radiation-induced vascular endothelial damage. J Radiat Res. 2013;54:1036–1042. doi: 10.1093/jrr/rrt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JA, Li F, Chung EJ, Hudak K, White A, Krausz K, Gonzalez F, Citrin D. Quercetin inhibits radiation-induced skin fibrosis. Radiat Res. 2013;180:205–215. doi: 10.1667/RR3237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinimehr SJ, Fathi M, Ghasemi A, Shiadeh SN, Pourfallah TA. Celecoxib mitigates genotoxicity induced by ionizing radiation in human blood lymphocytes. Res Pharm Sci. 2017;12:82–87. doi: 10.4103/1735-5362.199051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol. 2003;170:2083–2092. doi: 10.4049/jimmunol.170.4.2083. [DOI] [PubMed] [Google Scholar]
- Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol. 2002;4:51–58. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- Jiang J, Belikova NA, Hoye AT, Zhao Q, Epperly MW, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int J Radiat Oncol Biol Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, Kagan VE. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. The role of TGF-β1–miR-21–ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br J Cancer. 2014;111:772–780. doi: 10.1038/bjc.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama K, Takamura N, Zaharieva EK, Kimura Y, Wakeford R. Long-term effects of radiation exposure on health. Lancet. 2015;386:469–478. doi: 10.1016/S0140-6736(15)61167-9. [DOI] [PubMed] [Google Scholar]
- Karki R, Igwe OJ (2013) Toll-like receptor 4-mediated nuclear factor kappa B activation is essential for sensing exogenous oxidants to propagate and maintain oxidative/nitrosative cellular stress. PloS one 8 [DOI] [PMC free article] [PubMed]
- Khayyal MT, El-Ghazaly MA, El-Hazek RM, Nada AS. The effects of celecoxib, a COX-2 selective inhibitor, on acute inflammation induced in irradiated rats. Inflammopharmacology. 2009;17:255–266. doi: 10.1007/s10787-009-0014-z. [DOI] [PubMed] [Google Scholar]
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh RY, Lim CL, Uhal BD, Abdullah M, Vidyadaran S, Ho CC, Seow HF. Inhibition of transforming growth factor-beta via the activin receptor-like kinase-5 inhibitor attenuates pulmonary fibrosis. Mol Med Rep. 2015;11:3808–3813. doi: 10.3892/mmr.2015.3193. [DOI] [PubMed] [Google Scholar]
- Kostyuk SV, Ermakov AV, Alekseeva AY, Smirnova TD, Glebova KV, Efremova LV, Baranova A, Veiko NN. Role of extracellular DNA oxidative modification in radiation induced bystander effects in human endotheliocytes. Mutat Res. 2012;729:52–60. doi: 10.1016/j.mrfmmm.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Krivokrysenko VI, Toshkov IA, Gleiberman AS, Krasnov P, Shyshynova I, Bespalov I, Maitra RK, Narizhneva NV, Singh VK, Whitnall MH, Purmal AA, Shakhov AN, Gudkov AV, Feinstein E. The Toll-Like Receptor 5 Agonist Entolimod Mitigates Lethal Acute Radiation Syndrome in Non-Human Primates. PLoS One. 2015;10:e0135388. doi: 10.1371/journal.pone.0135388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Kurkjian CJ, Guo H, Montgomery ND, Cheng N, Yuan H, Merrill JR, Sempowski GD, Brickey WJ, Ting JP. The Toll-Like Receptor 2/6 Agonist, FSL-1 Lipopeptide, Therapeutically Mitigates Acute Radiation Syndrome. Sci Rep. 2017;7:17355. doi: 10.1038/s41598-017-17729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JK, Van Tuyle G, Lin P-S, Schmidt-Ullrich R, Mikkelsen RB. Ionizing Radiation-induced, Mitochondria-dependent Generation of Reactive Oxygen/Nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009;8:47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Tang D, Lotze MT. Menage a Trois in stress: DAMPs, redox and autophagy. Semin Cancer Biol. 2013;23:380–390. doi: 10.1016/j.semcancer.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20:7085–7095. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat Res. 2013;179:125–134. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier P, Hartmann L, Wenz F, Herskind C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int J Mol Sci. 2016;17:102. doi: 10.3390/ijms17010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Lefaix J-L, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- Medhora M, Gao F, Jacobs ER, Moulder JE. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 2012;17:66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Li Z, Liu H, Hu ZW, Hua F. Blocking IL-17A protects against lung injury-induced pulmonary fibrosis through promoting the activation of p50NF-kappaB. Yao Xue Xue Bao. 2012;47:739–744. [PubMed] [Google Scholar]
- Mittra I, Khare NK, Raghuram GV, Chaubal R, Khambatti F, Gupta D, Gaikwad A, Prasannan P, Singh A, Iyer A, Singh A, Upadhyay P, Nair NK, Mishra PK, Dutt A. Circulating nucleic acids damage DNA of healthy cells by integrating into their genomes. J Biosci. 2015;40:91–111. doi: 10.1007/s12038-015-9508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra I, Samant U, Sharma S, Raghuram GV, Saha T, Tidke P, Pancholi N, Gupta D, Prasannan P, Gaikwad A, Gardi N, Chaubal R, Upadhyay P, Pal K, Rane B, Shaikh A, Salunkhe S, Dutt S, Mishra PK, Khare NK, Nair NK, Dutt A. Cell-free chromatin from dying cancer cells integrate into genomes of bystander healthy cells to induce DNA damage and inflammation. Cell Death Discov. 2017;3:17015. doi: 10.1038/cddiscovery.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Are Epigenetic Mechanisms Involved in Radiation-Induced Bystander Effects? Front Genet. 2012;3:74. doi: 10.3389/fgene.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Stamato TD, Perez ML, Cummins R, Mooney R, Seymour CB. Involvement of energy metabolism in the production of 'bystander effects' by radiation. Br J Cancer. 2000;82:1740–1746. doi: 10.1054/bjoc.2000.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE. Pharmacological intervention to prevent or ameliorate chronic radiation injuries. Semin Radiat Oncol. 2003;13:73–84. doi: 10.1053/srao.2003.50007. [DOI] [PubMed] [Google Scholar]
- Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Radons J (2012) Radiation, inflammation, and immune responses in cancer. Front Oncol 2 [DOI] [PMC free article] [PubMed]
- Najafi M, Fardid R, Hadadi G, Fardid M. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 2014;4:163–172. [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Fardid R, Takhshid MA. Radiation-Induced Oxidative Stress at Out-of-Field. Cell J. 2016;18:340–345. doi: 10.22074/cellj.2016.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Salajegheh A, Rezaeyan A. Bystander Effect and Second Primary Cancers following Radiotherapy: What are its Significances? J Med Phys. 2017;42:55–56. doi: 10.4103/jmp.JMP_124_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology. 2017;25:403–413. doi: 10.1007/s10787-017-0332-5. [DOI] [PubMed] [Google Scholar]
- Najafi M, Motevaseli E, Shirazi A, Geraily G, Rezaeyan A, Norouzi F, Rezapoor S, Abdollahi H (2018a) Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int J Radiat Biol 94:335–356 [DOI] [PubMed]
- Najafi M, Cheki M, Rezapoor S, Geraily G, Motevaseli E, Carnovale C, Clementi E, Shirazi A (2018b) Metformin: prevention of genomic instability and cancer: a review. Mutat Res Genet Toxicol Environ Mutagen 827:1–8. 10.1016/j.mrgentox.2018.01.007 [DOI] [PubMed]
- Nishioka A, Ogawa Y, Mima T, Jin YJ, Sonobe H, Kariya S, Kubota K, Yoshida S, Ueno H. Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector. Int J Radiat Oncol Biol Phys. 2004;58:1235–1241. doi: 10.1016/j.ijrobp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Nishioka A, Ogawa Y, Kariya S, Hamada N, Nogami M, Inomata T, Ueno H. Reduction of fibroproliferative changes in irradiated rat lung with soluble transforming growth factor-beta receptor. Mol Med Rep. 2015;11:2659–2663. doi: 10.3892/mmr.2014.3064. [DOI] [PubMed] [Google Scholar]
- Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE (2006) Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS One 1 [DOI] [PMC free article] [PubMed]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Pazhanisamy SK, Li H, Wang Y, Batinic-Haberle I, Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis. 2011;26:431–435. doi: 10.1093/mutage/ger001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter RU, Braun-Falco O, Birioukov A, Hacker N, Kerscher M, Peterseim U, Ruzicka T, Konz B, Plewig G. Chronic cutaneous damage after accidental exposure to ionizing radiation: the Chernobyl experience. J Am Acad Dermatol. 1994;30:719–723. doi: 10.1016/s0190-9622(08)81501-0. [DOI] [PubMed] [Google Scholar]
- Piccinini A, Midwood K (2010) DAMPening inflammation by modulating TLR signalling. Mediat Inflamm 2010 [DOI] [PMC free article] [PubMed]
- Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, Gallagher-Colombo SM, Solomides CC, Cengel KA, Christofidou-Solomidou M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwkowska A, Rogacka D, Jankowski M, Angielski S. Metformin reduces NAD(P)H oxidase activity in mouse cultured podocytes through purinergic dependent mechanism by increasing extracellular ATP concentration. Acta Biochim Pol. 2013;60:607–612. [PubMed] [Google Scholar]
- Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–528. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- Rabender C, Mezzaroma E, Mauro AG, Mullangi R, Abbate A, Anscher M, Hart B, Mikkelsen R. IPW-5371 Proves Effective as a Radiation Countermeasure by Mitigating Radiation-Induced Late Effects. Radiat Res. 2016;186:478–488. doi: 10.1667/RR14403.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Pierce JG, Kagan VE, Wipf P, Kanai AJ, Greenberger JS. In Vivo. 2009. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair; pp. 717–726. [PMC free article] [PubMed] [Google Scholar]
- Reeves A, Zagurovskaya M, Gupta S, Shareef MM, Mohiuddin M, Ahmed MM. Inhibition of transforming growth factor-beta signaling in normal lung epithelial cells confers resistance to ionizing radiation. Int J Radiat Oncol Biol Phys. 2007;68:187–195. doi: 10.1016/j.ijrobp.2006.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- Rwigema JC, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Goff JP, Franicola D, Dixon T, Frantz MC, Wipf P, Tyurina Y, Kagan VE, Wang H, Greenberger JS. Two strategies for the development of mitochondrion-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80:860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Yamamori T, Yoshikawa Y, Bo T, Suzuki M, Yamamoto K, Ago T, Inanami O. NADPH oxidase 4 mediates ROS production in radiation-induced senescent cells and promotes migration of inflammatory cells. Free Radic Res. 2018;52:92–102. doi: 10.1080/10715762.2017.1416112. [DOI] [PubMed] [Google Scholar]
- Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, Panoskaltsis-Mortari A, Orschell CM, Baker PS, Gudkov A, Feinstein E. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2) PLoS One. 2012;7:e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde A, Berhane H, Rhieu BH, Kalash R, Xu K, Goff J, Epperly MW, Franicola D, Zhang X, Dixon T, Shields D, Wang H, Wipf P, Parmar K, Guinan E, Kagan V, Tyurin V, Ferris RL, Zhang X, Li S, Greenberger JS. Intraoral Mitochondrial-Targeted GS-Nitroxide, JP4-039, Radioprotects Normal Tissue in Tumor-Bearing Radiosensitive Fancd2(-/-) (C57BL/6) Mice. Radiat Res. 2016;185:134–150. doi: 10.1667/RR14035.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szondy Z, Sarang Z, Kiss B, Garabuczi E, Koroskenyi K. Anti-inflammatory Mechanisms Triggered by Apoptotic Cells during Their Clearance. Front Immunol. 2017;8:909. doi: 10.3389/fimmu.2017.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67:5872–5879. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Yin X, Wang L, Wang J, Zhu W, Cao J, Yang H. The key role of miR-21-regulated SOD2 in the medium-mediated bystander responses in human fibroblasts induced by alpha-irradiated keratinocytes. Mutat Res. 2015;780:77–85. doi: 10.1016/j.mrfmmm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Tomita M, Matsumoto H, Funayama T, Yokota Y, Otsuka K, Maeda M, Kobayashi Y. Nitric oxide-mediated bystander signal transduction induced by heavy-ion microbeam irradiation. Life Sci Space Res (Amst) 2015;6:36–43. doi: 10.1016/j.lssr.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Toshkov IA, Gleiberman AS, Mett VL, Hutson AD, Singh AK, Gudkov AV, Burdelya LG. Mitigation of Radiation-Induced Epithelial Damage by the TLR5 Agonist Entolimod in a Mouse Model of Fractionated Head and Neck Irradiation. Radiat Res. 2017;187:570–580. doi: 10.1667/RR14514.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin J. Protecting people against radiation exposure in the event of a radiological attack. A report of The International Commission on Radiological Protection. Ann ICRP. 2005;35(1-110):iii–iiv. doi: 10.1016/j.icrp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wang B-Z, Wang L-P, Han H, Cao F-L, Li G-Y, Xu J-L, Wang X-W, Wang L-X. Interleukin-17A antagonist attenuates radiation-induced lung injuries in mice. Exp Lung Res. 2014;40:77–85. doi: 10.3109/01902148.2013.872210. [DOI] [PubMed] [Google Scholar]
- Wang, L. P., Wang, Y. W., Wang, B. Z., Sun, G. M., Wang, X. Y. & Xu, J. L. 2014b. Expression of interleukin-17A in lung tissues of irradiated mice and the influence of dexamethasone. ScientificWorldJournal, 2014, 251067. [DOI] [PMC free article] [PubMed]
- Wang L, He L, Bao G, He X, Fan S, Wang H. Ionizing Radiation Induces HMGB1 Cytoplasmic Translocation and Extracellular Release. Guo Ji Fang She Yi Xue He Yi Xue Za Zhi. 2016;40:91–99. [PMC free article] [PubMed] [Google Scholar]
- Weyemi U, Redon CE, Aziz T, Choudhuri R, Maeda D, Parekh PR, Bonner MY, Arbiser JL, Bonner WM. Inactivation of NADPH oxidases NOX4 and NOX5 protects human primary fibroblasts from ionizing radiation-induced DNA damage. Radiat Res. 2015;183:262–270. doi: 10.1667/RR13799.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, J., Epperly, M. W., Fisher, R., Zhang, X., Shields, D., Hou, W., Wang, H., Li, S., Wipf, P., Parmar, K., Guinan, E., Steinman, J. & Greenberger, J. S. 2018. Amelioration of Head and Neck Radiation-Induced Mucositis and Distant Marrow Suppression in Fanca(-/-) and Fancg(-/-) Mice by Intraoral Administration of GS-Nitroxide (JP4-039). Radiat Res. [DOI] [PMC free article] [PubMed]
- Wu Y, Doroshow JH. Abstract 5358: IL-4/IL-13 induce Duox2/DuoxA2 expression and reactive oxygen production in human pancreatic and colon cancer cells. Cancer Res. 2014;74:5358. [Google Scholar]
- Wu, Y. & Doroshow, J. H. 2014b. IL-4/IL-13 induce Duox2/DuoxA2 expression and reactive oxygen production in human pancreatic and colon cancer cells. AACR.
- Wu Q, Allouch A, Martins I, Modjtahedi N, Deutsch E, Perfettini J-L. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed J. 2017;40:200–211. doi: 10.1016/j.bj.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, Samuni AM, Felici A, Reiss M, Yarkoni S, Sowers A, Mitchell JB, Roberts AB, Russo A. Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem. 2004;279:15167–15176. doi: 10.1074/jbc.M309798200. [DOI] [PubMed] [Google Scholar]
- Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014;11:1161–1170. doi: 10.4161/rna.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang T, Xu S, Xu S, Wu L, Wu Y, Bian P. Radiation-induced epigenetic bystander effects demonstrated in Arabidopsis thaliana. Radiat Res. 2015;183:511–524. doi: 10.1667/RR13909.1. [DOI] [PubMed] [Google Scholar]
- Yahyapour R, Amini P, Rezapoor S, Rezaeyan A, Farhood B, Cheki M, Fallah H, Najafi M (2017a) Targeting of inflammation for radiation protection and mitigation. Curr Mol Pharmacol. 10.2174/1874467210666171108165641 [DOI] [PubMed]
- Yahyapour R, Motevaseli E, Rezaeyan A, Abdollahi H, Farhood B, Cheki M, Najafi M, Villa V (2017b) Mechanisms of radiation bystander and non-targeted effects: implications to radiation carcinogenesis and radiotherapy. Curr Radiopharm 11(1):34–45 [DOI] [PubMed]
- Yahyapour R, Amini P, Rezapour S, Cheki M, Rezaeyan A, Farhood B, Shabeeb D, Musa AE, Fallah H, Najafi M. Radiation-induced inflammation and autoimmune diseases. Mil Med Res. 2018;5:9. doi: 10.1186/s40779-018-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyapour R, Motevaseli E, Rezaeyan A, Abdollahi H, Farhood B, Cheki M, Rezapoor S, Shabeeb D, Musa AE, Najafi M, Villa V (2018b) Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin Transl Oncol. 10.1007/s12094-017-1828-6 [DOI] [PubMed]
- Yahyapour R, Salajegheh A, Safari A, Abbasi S, Amini P, Rezaeyan A, Amraee A, Najafi M (2018c) Radiation-induced non-targeted effect and carcinogenesis; Implications in Clinical Radiotherapy. J Biomed Phys Eng. 10.22086/jbpe.v0i0.713 [PMC free article] [PubMed]
- Yakovlev VA, Rabender CS, Sankala H, Gauter-Fleckenstein B, Fleckenstein K, Batinic-Haberle I, Jackson I, Vujaskovic Z, Anscher MS, Mikkelsen RB, Graves PR. Proteomic analysis of radiation-induced changes in rat lung: Modulation by the superoxide dismutase mimetic MnTE-2-PyP(5+) Int J Radiat Oncol Biol Phys. 2010;78:547–554. doi: 10.1016/j.ijrobp.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., Wang, H., Chavan, S. S. & Andersson, U. 2015. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Molecular Medicine, 21, S6-S12. [DOI] [PMC free article] [PubMed]
- Yoshida T, Goto S, Kawakatsu M, Urata Y, Li T-s. Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res. 2012;46:147–153. doi: 10.3109/10715762.2011.645207. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, Lahn M, Huber PE. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–7167. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang Y-a, Meng A, Yan H, Wang X, Niu J, Li J, Wang H. Inhibiting TGFβ1 has a protective effect on mouse bone marrow suppression following ionizing radiation exposure in vitro. J Radiat Res. 2013;54:630–636. doi: 10.1093/jrr/rrs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, de Toledo SM, Hu G, Hei TK, Azzam EI. Connexins and cyclooxygenase-2 crosstalk in the expression of radiation-induced bystander effects. Br J Cancer. 2014;111:125–131. doi: 10.1038/bjc.2014.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Lien Y-C, Davidson M, Hei TK. Mitochondrial Function and NF-κB Mediated Signaling in Radiation-Induced Bystander Effects. Cancer Res. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Lien Y-C, Davidson M, Hei TK. Mitochondrial function and nuclear factor-κB–mediated signaling in radiation-induced bystander effects. Cancer Res. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]