Abstract
The evolutionarily conserved Wnt signaling pathway regulates physiological hematopoiesis, a process of formation of blood cells and has been shown to play crucial role in the development of both myeloid and lymphoid malignancies. The Wnt signaling pathway can be broadly divided into canonical and non-canonical pathways. In the present study, we investigated the pathobiology of leukemia by studying the expression profile of Wnt proteins, receptors, key signaling intermediates and endogenous Wnt antagonist involved in canonical and non-canonical pathways in the bone marrow (BM) hematopoietic stem/progenitor cell (HSPC) compartment of experimental leukemic mice. Cell adhesion molecule N-Cadherin and leukemic BM microenvironment with reference to Wnt were also studied. We used ENU, a potent carcinogen, to induce leukemia in wild type Swiss albino mice and malignant transformation was cofirmed by peripheral blood and BM studies. Flow cytometric expression analysis revealed profound up-regulation of canonical Wnt3a/β-catenin/CyclinD1 signaling axis along with N-Cadherin whereas down-regulation of non-canonical Wnt5a/Ca2+/CaMKII signaling axis in the leukemic HSPC compartment. Subsequent use of anti-Wnt3a antibody in the in vitro clonogenicity assay uncovered that anti-Wnt3a antibody preferentially inhibited the growth and number of the primitive leukemic hematopoietic CFU-GEMM and BFU-E colonies. Stromal cells derived from the leukemic BM also exhibited aberrant Wnt3a and Wnt5a protein expression. Taken together, alteration of canonical and non-canonical Wnt signaling pathways in the HSPC compartment along with classical Wnt protein expression pattern in the leukemic stromal microenvironment resulted in progression of leukemia.
Keywords: ENU, Leukemia, N-Cadherin, Wnt signaling, Wnt3a, Wnt5a
Introduction
The Wnt pathway, an evolutionarily conserved developmental signaling pathway, plays an important role during embryogenesis and also regulates crucial aspects of adult hematopoiesis. It is a complex signaling pathway and signaling output depends on the ligand-receptor interaction and cellular context. To date, approximately 19 Wnt proteins and 10 Frizzled (Fzd) receptors have been identified. Signaling activation and subsequent signal transduction in the cytoplasm relies on Wnt ligand binding with the extracellular domain of Fzd receptor (Lento et al. 2013; Kim et al. 2014; Zhan et al. 2017; Bigas et al. 2013; Luis et al. 2012; Richter et al. 2017). Wnt signaling can be broadly divided into β-catenin dependent canonical pathway and β-catenin independent non-canonical pathways. Wnt3a is a classical canonical Wnt ligand, which upon binding to Fzd receptor and LRP5/6 co-receptor causes nuclear translocation of β-catenin that promotes the associated target gene expression to regulate cellular function. Unlike canonical, non-canonical Wnt signaling pathway is more diverse and comparatively less characterized. Wnt5a, a classical non-canonical Wnt ligand, upon binding with Fzd receptor releases intracellular Calcium (Ca2+) ion and activates Ca2+ responsive regulators such as Calcineurin, CaMKII and PKC in order to exert cellular function (Lento et al. 2013; Schreck et al. 2014; Many and Brown 2014; De 2011). Wnt5a also interacts with ROR2, a member of the ROR family orphan receptor tyrosine kinases, to transduce β-catenin independent signaling via JNK, RhoA etc. and has been shown to inhibit canonical β-catenin pathway (Minami et al. 2010; Yan et al. 2016).
During neoplastic transformation normal cellular mechanisms are either hyper-activated or suppressed lead to alteration of cell fate. Leukemia - a hematopoietic malignancy -characterized by unconstrained production of hematopoietic precursor cells which don’t differentiate into mature blood cells and accumulate as leukemic blasts in the bone marrow microenvironment and eventually release into the peripheral circulation. Increased expression of β-catenin and constitutive activation of the canonical Wnt pathway has been detected in neoplastic cells of both hematopoietic and non-hematopoietic origin (Wang et al. 2010; Ysebaert et al. 2006; Polakis 2012). On the contrary, the exact role of non-canonical Wnt signaling pathway in normal as well as in malignant condition is elusive. It has been shown that non-canonical Wnt signaling pathway can inhibit canonical Wnt/β-catenin pathway [Topol et al. 2003; Mikels and Nusse 2006; Ishitani et al. 2003] and Wnt5a acts as a tumor suppressor in hematological neoplasms (Martín et al. 2010; Ying et al. 2007; Ng et al. 2015; Zhou et al. 2017; Liang et al. 2003). However, tumor promoting activity of Wnt5a has also been detected in few human malignancies (Bellon et al. 2013; Bakker et al. 2013). Although both canonical and non-canonical Wnt pathways have been separately studied, the crosstalk between the two has recently gained much attention as a fundamental mechanism controlling many biological processes such as hematopoietic stem cell ageing and function, drug induced differentiation of leukemia cell line, protein expression in the blood-brain barrier cells, regulation of osteogenic differentiation potential of periodontal ligament stem cells, drug resistance in colon cancer cells etc. (Florian et al. 2013; Zang et al. 2014; Pinzón-Daza et al. 2014; Liu et al. 2016; Bordonaro et al. 2011). In all of the above mentioned conditions different components of both the canonical and non-canonical Wnt pathways are simultaneously fine tuned either by endogeneous or exogeneous forces to control different cell fate.
In the present study, we investigated the pathobiology of leukemia by studying the altered cross-talk between the canonical Wnt/β-catenin, non-canonical Wnt/Ca2+ and Wnt/ROR2 signaling pathways in the bone marrow hematopoietic stem/progenitor cell (HSPC) compartment of ENU induced experimental leukemic mice. Outcome of the present study indicates multiple deregulated points of Wnt signaling pathway in leukemia which can be utilized for future therapeutic purposes.
Materials and Methods
Mice and leukemia induction
Inbred Swiss albino mice (Mus Musculus) were maintained in the animal facility at the Calcutta School of Tropical Medicine under pathogen free standard experimental conditions (22 ± 2 °C temperature, controlled humidity and 12 h dark-light cycle) and were given standard diet and water ad libitum. For leukemia induction, 5–10 days old litter pups (N = 30) were given a single intraperitoneal ENU (N-ethyl-N-nitrosourea, Sigma, Cat No-N3385) injection at a dose rate of 80 mg/kg body weight (Chatterjee et al. 2016a; Law et al. 2001; Basak et al. 2010; Chatterjee et al. 2016b). The control group (N = 30) received 0.9% intraperitoneal saline injection. The animal groups were monitored carefully for the period of 4–7 months for leukemia development. All animal protocols followed were approved by the Institutional Animal Ethical Committee (IAEC).
Peripheral blood hemogram
Blood was collected from the tail vein of control and leukemic mice into heparinised vials ~180 days after the ENU administration. The total leukocyte count and differential leukocyte count were performed as per our previously described protocol (Chattopadhyay et al. 2016a; Pereira and Law 2018).
Bone marrow (BM) isolation and single cell preparation
Control and leukemic mice were sacrificed ~180 days after the ENU administration by cervical dislocation and long bones (femur and tibia) were removed. The BM was flushed out by 24 gauge needle into ice-cold RPMI-1640 (Sigma) with 10% FBS (Lonza). The BM cells were washed thrice, repeat pipetted and passed through 100 μm wire mesh to prepare single cell suspension.
Marrow smear study
Marrow smears were prepared, stained with May-Grunwald Giemsa and examined under the light microscope (Olympus CH20i) for morphological analysis.
Cytochemistry
Myeloperoxidase (MPO): BM smears were incubated in buffer formal acetone and then in 3,3'-Diaminobenzidine (DAB) solution for 15–20 min, counterstained with Hematoxylin and examined under light microscope.
Nonspecific-esterase (NSE): BM smears were incubated in a solution containing pararosaniline-sodium nitrite and α-napthyl acetate for 20 min, counter-stained with Hematoxylin and examined under light microscope.
Immunophenotyping
2X106 paraformaldehyde (PFA) fixed control and leukemic BM cells were stained with following antibodies: anti-CD117-FITC (BD Pharmingen), anti-CD22-FITC, anti-CD79a-FITC (Biolegend), anti-CD10-FITC (Santa Cruz Biotechnology), anti-CD3-PE (Biolegend). Data acquired by BD-FACS Calibur and analyzed by Cell Quest Pro software. Forward scatter (FSC) vs antigen expression strategy (Weir et al. 2007) was used for analysis and antigen expression was considered as positive by comparing with the control.
Flow cytometry
Flow cytometry was performed to analyze expression profile of Wnt signaling components in control and leukemic condition as per our previously described protocol (Chattopadhyay et al. 2016a; Daw et al. 2016; Chatterjee et al. 2016c; Chattopadhyay et al. 2016b). 2X106 PFA fixed control and leukemic BM cells were distributed in polystyrene tubes and stained with following primary antibodies: anti-Wnt5a and anti-Wnt3a (Santa Cruz Biotechnology) [Wnt proteins], anti-Fzd5, anti-Fzd7 (Santa Cruz Biotechnology) and anti-ROR2 (Cell Signaling Technology) [Receptors], anti-β-catenin (Santa Cruz Biotechnology) and anti-phospho-CaMKII (Thr 286) (Cell Signaling Technology) [Signaling intermediates], anti-CyclinD1-FITC (Abcam) [Target protein], anti-Dkk1 (Santa Cruz Biotechnology) [Antagonist], anti-N-Cadherin (Santa Cruz Biotechnology) [Cell adhesion molecule]. Cell membrane permeabilization was performed by 90% chilled methanol prior to antibody staining for detection of intracellular signaling molecules (except for surface proteins such as Fzd7, Fzd5, ROR2 and N-Cadherin). Untagged primary antibodies were tagged with secondary antibody attached to fluorochrome Alexa Fluor 488 (Invitrogen). For intracellular Ca2+ level [Second messenger] measurement, 2X106 BM cells were stained with Fluo-4 AM (Molecular Probes) as per the manufacturer’s instruction. Data acquired by BD FACS Calibur and analyzed by Cell Quest Pro software. The expression level of the signaling molecules were quantified in the low FSC (forward scatter) versus low SSC (side scatter) gated zone in the flow cytometry dot plot, which is the Sca-1 enriched murine BM HSPC compartment (Chattopadhyay et al. 2016a).
Hematopoietic clonogenicity assay with anti-Wnt3a antibody
Clonogenicity assay was performed in methylcellulose-based semisolid media which consisted of 1.2% methylcellulose (Sigma), 30% FBS, 1% BSA (Sigma), 0.02% β-ME and growth factors [100 ng/ml recombinant mouse (rm) SCF, 50 ng/ml rm IL-3 and 50 ng/ml rm GM-CSF]. RBC lysed control and leukemic BM cells were plated at a concentration of 5X105 cells/ml with and without anti-Wnt3a antibody (100 ng/ml) (Santa Cruz Biotechnology) in triplicate and cultured at 37 °C in a 5% CO2 incubator. After 16 days the numbers of hematopoietic colonies (viz, CFU-GEMM, CFU-G, CFU-GM, BFU-E and CFU-E) were scored manually under the inverted microscope.
Fluorescence imaging
N-Cadherin expression was studied by immunofluorescence imaging. BM cells were stained with anti-N-Cadherin primary antibody (Santa Cruz Biotechnology; 1:300 dilution) and Alexa Fluor 488 tagged secondary antibody (1:600 dilution) as per the protocol described previously (Chattopadhyay et al. 2016b). Cells were counterstained with DAPI shield (Sigma) and examined by confocal microscope (Leica TCS SP8 Laser Scanning Confocal Microscope).
Long-term BM culture (LT-BMC)
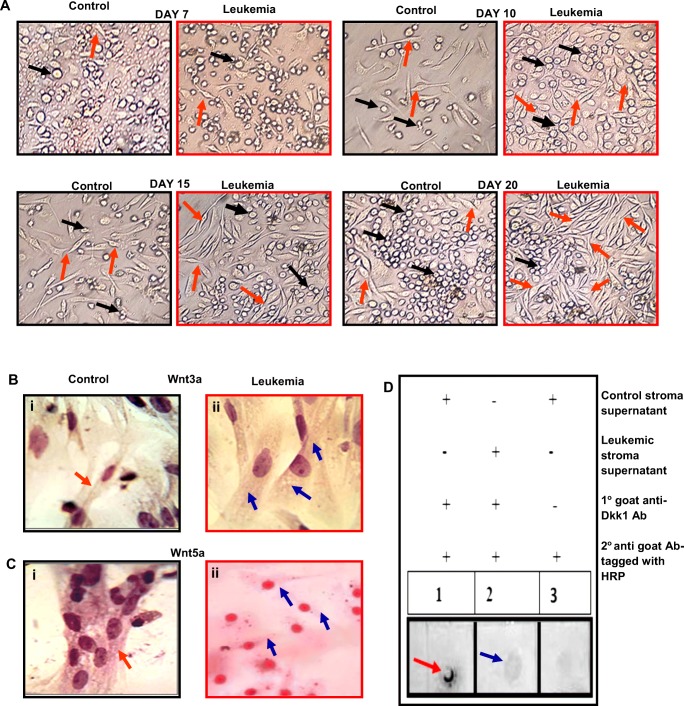
BM cells at a concentration of 2X106 cells/ml were plated in six well cell culture plates (Corning) containing 3 ml RPMI-1640, 30% FBS, 1% BSA, 0.02% β-ME and cultured at 37 °C in a 5% CO2 incubator. After 72 h, the non-adherent cells were removed by washing and the media was replenished every 3 days to grow adherent BM stromal cells. The culture plates were monitored at day 7, 10, 15 and 20 for cellular growth pattern assessment and photographed (Chaklader and Law 2015).
Immunocytochemistry (ICC)
LT-BMC was used to grow BM stromal cells. Stromal cells were stained with anti-Wnt3a and anti-Wnt5a antibody (1:300 dilution) overnight at 4oC according to the protocol of (Chaklader and Law 2015). Cells were washed and stained the following day for 2 h with HRP tagged secondary antibody (1:600 dilution) (Invitrogen). The reaction product was developed chromogenically by DAB and examined under light microscope after counterstaining with Hematoxylin.
Dot blot assay
Dot blot assay was performed as per the standard protocol to determine the level of Dkk1 in the BM stromal cell culture supernatant. Briefly, the culture supernatant was collected, blotted onto nitrocellulose membrane and further processed conventionally. Next, the membrane was stained with anti-Dkk1 primary antibody (1:300 dilution; Santa Cruz Biotechnology) followed by HRP tagged secondary antibody (1:600 dilution) and the reaction product was developed using HRP-DAB based chromogenic method.
Statistics
Statistical analysis was performed using unpaired student’s t-test and quantitative values were represented as Mean ± SD. In addition, one-way ANOVA followed by Posthoc Tukey tests were used when differences between more than two groups were evaluated. For all comparisons, P ≤ 0.05 was considered as significant difference. Each experiment was repeated thrice.
Results
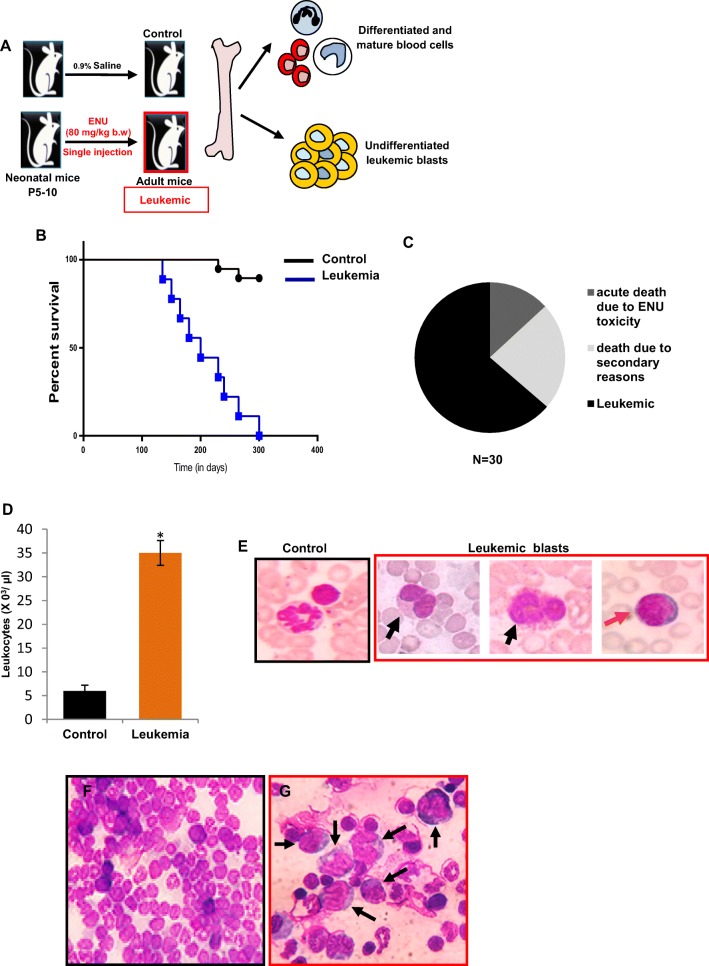
Neonatal ENU administration leads to development of mixed type leukemia
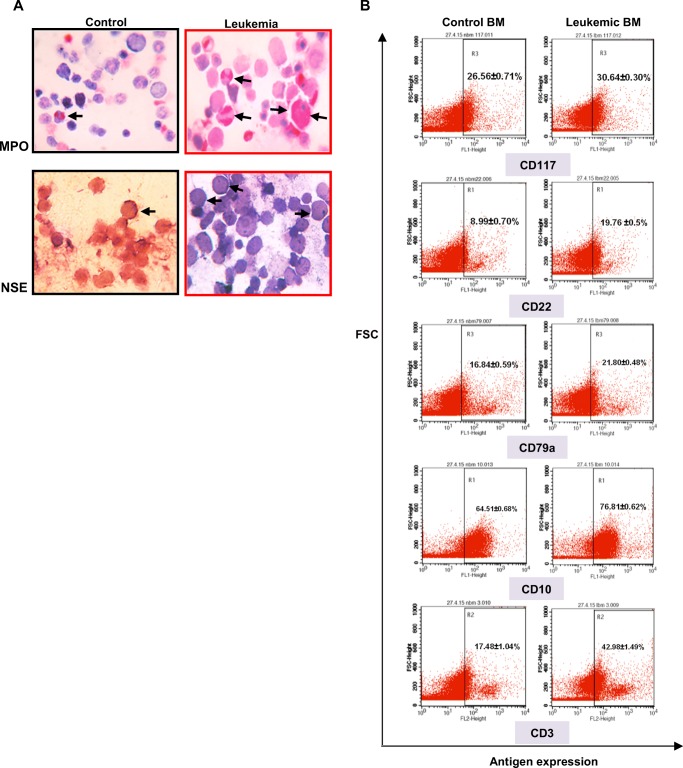
Neonatal [P5–10] ENU administered mice showed signs of leukemia development within a period of 4–7 months after injection (Fig. 1a) and Kaplan-meier analysis revealed the median survival was 200 days (Fig. 1b). Therefore, to investigate the pathological features and Wnt signaling, we choose ~180 days as the optimum time required for neoplastic transformation. 63.66% mice developed leukemia after ENU induction and rest of the mice died due to acute ENU toxicity (13.13%) and secondary reasons (23.21%) (Fig. 1c). Peripheral blood count was considered as the initial predictive tool for the confirmation of leukemic onset, which showed a significant leukocytosis (Ctrl 6 ± 1.2X103 and Leukemia 35 ± 2.6X103 cells/μl) [P < 0.05] (Fig. 1d) along with massive infiltration of morphologically distinct myeloblasts and lymphoblasts (Fig. 1e) (total blast count: 34.29 ± 4.26%). These findings initially indicated mixed type leukemia development. Unlike control (Fig. 1f) examination of the BM compartment showed clear sign of hematopoietic malignancy i.e. huge accumulation of undifferentiated precursor cells and very few numbers of residual differentiated hematopoietic cells (Fig. 1g). Next, we studied the lineage specific antigens according to the revised WHO guidelines (Vardiman et al. 2009). Myeloblasts were identified by MPO and NSE cytochemistry in addition to CD117 expression. Leukemic cells were CD117 positive (Fig. 2b right panel), strongly MPO (enzyme activity exclusively present in the azurophilic granules of myeloid cells) positive (Fig. 2a upper right panel) and a few cells showed NSE (membrane bound enzymes exclusively present in the monocytes) positivity (Fig. 2a lower right panel), altogether confirmed presence of myeloblasts. B-lymphoblasts were identified primarily by the expression of CD22, a specific antigen for B-lymphoid cells, in addition to CD79a and CD10 in the leukemic BM (Fig. 2b right panel). T-lymphoblasts, on the other hand, were identified by the expression of CD3, an antigen unique to T-lymphoid cells (Fig. 2b right panel). To sum up, we have observed both myeloblasts and lymphoblasts in the leukemic blood and antigen expression in the BM, which indicated the development of mixed type leukemia in the adult mice after neonatal ENU induction.
Fig. 1. Pathological features of ENU induced experimental leukemic mice.
a Outline of experimental strategy to induce leukemia in 5–10 days old Swiss albino litter pups by single intraperitoneal injection of ethyl-nitrosourea (ENU) at dose rate of 80 mg/kg b.w. b Kaplan-meier survival plot of ENU-induced leukemic mice (N = 18; median survival = 200 days). c Pie chart represented the percentage of mice (63.66%) developed leukemia after ENU induction. Rest of the mice died due to acute ENU toxicity (13.13%) and due to secondary reasons (23.21%). d The total leukocyte count of control and leukemic mice. Leukemic peripheral blood showed significant leukocytosis [*P < 0.05] (e) Representative control and leukemic blood films. Neutrophil and lymphocyte in control blood. Morphologically distinct leukemic blasts such as large cells with irregular nuclei indicated myeloblasts (black arrow) and small cells with round nuclei indicated lymphoblasts (orange arrow) [Magnification 1000X]. Representative bone marrow (BM) smears of (f) control and (g) leukemic mice. Leukemia BM showed accumulation of undifferentiated leukemic blasts (black arrows) [Magnification 400X]
Fig. 2. Cytochemistry and immunophenotyping.
aLeft panel: Control BM showed very few numbers of MPO and NSE positive cells. Right panel: Leukemia BM showed large numbers of intense MPO and few NSE positive cells (black arrows) [Magnification 1000X]. b Leukemia BM showed positive expression for myeloid (CD117) as well as lymphoid (B-lymphoid: CD22, CD79a and CD10 and T-lymphoid: CD3) lineage specific antigens (right panel). Control BM showed basal level of antigen expression (left panel)
Expression profile of canonical Wnt/β-catenin signaling pathway in the leukemic HSPC compartment
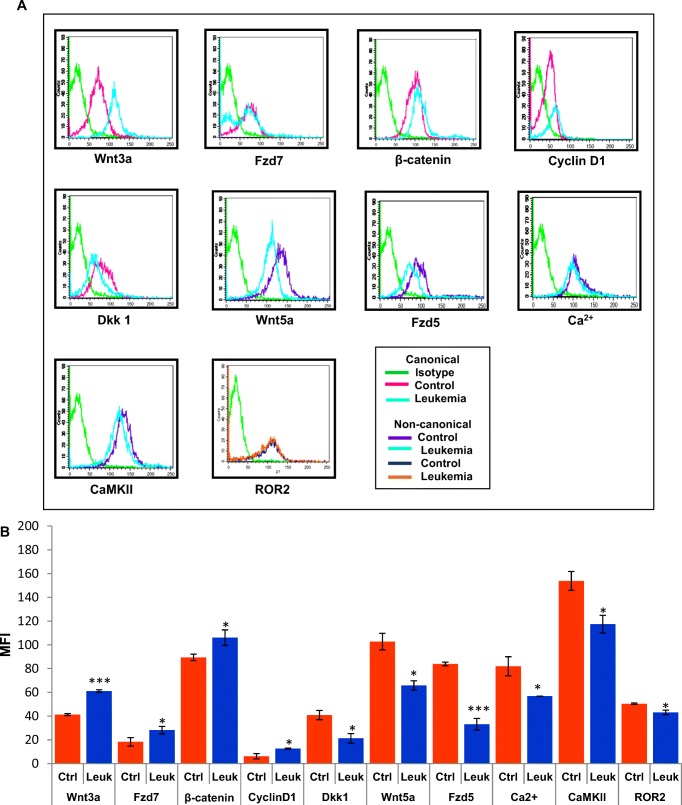
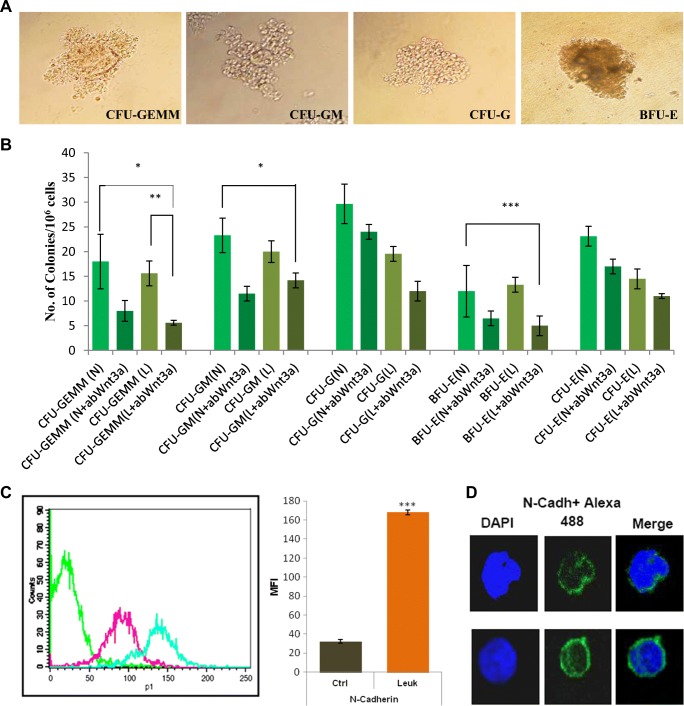
For comparative protein expression analysis between control and leukemic BM cells histogram overlay plots were used. Quantitative values obtained from the flow cytometric analysis were presented in the form of MFI (Mean Fluorescence Intensity), which denoted protein expression level in individual cells. MFI values indicated that leukemic HSPC compartment expressed significantly higher Wnt3a (MFI: Ctrl 41.28 ± 0.88 and Leukemia 61.07 ± 1.09) [P < 0.0001] and Fzd7 (MFI: Ctrl 18.25 ± 3.55 and Leukemia 28.18 ± 3.16) [P < 0.05] when compared to control. Downstream of Wnt3a/Fzd7 total intracellular β-catenin (MFI: Ctrl 89.39 ± 2.77 and Leukemia 106.11 ± 6.42) [P < 0.05] and CyclinD1, the direct target gene of β-catenin, (MFI: Ctrl 6.17 ± 2.24 and Leukemia 12.62 ± 0.43) [P < 0.05] expression level were also significantly higher in the leukemic HSPC compartment. On the contrary, Dkk1 (Dickkopf family member), a potent canonical Wnt antagonist that inhibits Wnt3a signaling by directly binding to LRP5/6 co-receptor, expression in the leukemic condition was notably lower (MFI: Ctrl 40.78 ± 3.88 and Leukemia 21.28 ± 4) [P < 0.05] (Fig. 3a-b). In vitro clonogenicity assay uncovered that supplementation of anti-Wnt3a antibody significantly inhibited the growth of primitive hematopoietic colonies such as multipotent CFU-GEMM (Colony-forming unit of granulocyte/erythrocyte/macrophage/megakaryocyte) and BFU-E (Burst-forming unit of erythroid cells). However, no significant changes were observed in case of comparatively matured colony numbers such as CFU-GM (Colony-forming unit of granulocyte/macrophage), CFU-G (Colony-forming unit of granulocyte) and CFU-E (Colony-forming unit of erythroid cells) after supplementation of anti-Wnt3a antibody (Fig. 4a-b).
Fig. 3.
Deregulation of Wnt signaling pathway in the leukemic hematopoietic stem/progenitor (HSPC) compartment. a-b Representative histogram overlay plots and bar diagrams showed expression levels of canonical (Wnt3a, Fzd7, β-catenin, CyclinD1 and Dkk1) and non-canonical (Wnt5a, Fzd5, Ca2+, CaMKII and ROR2) Wnt signaling pathway components in the control and leukemic HSPC compartment. MFI (Mean Fluorescence Intensity) values indicated significant up-regulation of Wnt3a, Fzd7, β-catenin, CyclinD1 whereas down-regulation of Dkk1, Wnt5a, Fzd5, CaMKII, ROR2 expression and Ca2+ level in the leukemic condition [*P < 0.05; *** < 0.0001]
Fig. 4.
Proliferation retardation of primitive leukemic hematopoietic progenitor colonies by anti-Wnt3a antibody and N-Cadherin expression in the leukemic marrow. a Representative CFU-GEMM, CFU-GM, CFU-G and BFU-E colonies in methylcellulose based semi-solid media [Magnification 400X]. b Bar diagrams represented the number of control and leukemic hematopoietic progenitor colonies with and without anti-Wnt3a antibody. The numbers of leukemic CFU-GEMM and BFU-E colonies were decreased significantly after anti-Wnt3a antibody supplementation. c Histogram overlay plot and bar diagram showed significant up-regulation of N-Cadherin in the leukemic HSPC compartment. d Representative immunofluorescence images showed N-Cadherin expression in the control and leukemic marrow cells. N-cadherin expression was higher in the leukemic marrow cells (lower panel) [Scale bar 10.0 μm]
Expression profile of non-canonical Wnt/Ca2+ and Wnt/ROR2 signaling pathway in the leukemic HSPC compartment
Flow cytometric analysis showed significant decline of Wnt5a (MFI: Ctrl 102.72 ± 7 and Leukemia 65.82 ± 3.95) [P < 0.05] and Fzd5 (MFI: Ctrl 83.9 ± 1.56 and Leukemia 33.11 ± 4.87) [P < 0.0001] expression in the leukemic HSPC compartment. Next, we studied the intracellular second messenger Ca2+ level by Fluo-4 AM (AM-acetoxymethyl ester), a cell permeable, non-fluorescent ester dye which gives green fluorescence after cleavage of AM ester and binding of free Fluo-4 with Ca2+ ion. Leukemic HSPC compartment showed decreased Fluo-4 fluorescence intensity (MFI: Ctrl 81.94 ± 8.03 and Leukemia 56.8 ± 0.08) [P < 0.05] which was directly proportional to low Ca2+ level. The finding also confirmed that Wnt5a transduces signal through the Ca2+ pathway in the leukemic hematopoietic cells. Consequently, downstream of Wnt5a/Ca2+ pathway decreased expression of CaMKII (phosphorylated at Thr 286 - the active form), a Ca2+ dependent kinase and a vital Wnt5a signaling intermediate (MFI: Ctrl 153.81 ± 7.89 and Leukemia 117.45 ± 7.45) [P < 0.05] was observed. On the other hand, decreased ROR2 expression (MFI: Ctrl 50.41 ± 0.65 and Leukemia 43.09 ± 1.87) [P < 0.05] in the leukemic marrow cells was also observed. (Fig. 3a-b).
Expression pattern of cell adhesion molecule N-Cadherin in the leukemic marrow
Differential expression of CAM molecules (Cell adhesion molecule) regulate behaviour and aggressiveness of variety of solid tumors (Chaklader et al. 2013). Here, we studied N-Cadherin (CD325), a Ca2+ dependent CAM and a member of cadherin superfamily, expression in the hematopoietic malignancy. Flow cytometric analysis showed huge up-regulation of N-Cadherin expression in the leukemic primitive HSPC compartment when compared to control (MFI: Ctrl 32.25 ± 1.98 and Leukemia 168.05 ± 2.51) [P < 0.0001] (Fig. 4c). Confocal microscopy also confirmed higher N-Cadherin expression in the leukemic cells (Fig. 4d lower panel). The finding indicated malignancy associated changes in adhesion molecule expression to modulate cell-microenvironmental interaction.
Assessment of leukemic BM stromal microenvironment and its correlation to Wnt
LT-BMC is a powerful technique to study BM microenvironmental scenario in vitro by utilizing the ability of a specific marrow cell population to form adherent stromal cell layer (representative of microenvironmental stromal cells in vivo) under defined culture condition (Ramakrishnan et al. 2013; Chaklader and Law 2015). Comparative LT-BMC of control and leukemic BM derived cells showed different scenario. Control marrow showed systematic generation and transformation of stromal precursors to spindle shaped stromal fibroblasts, which proliferated gradually on successive days in culture (Fig. 5a). Leukemic marrow showed comparatively early generation and rapid transformation of stromal precursor cells to stromal fibroblasts as compared to control. The leukemic stromal cells also exhibited higher proliferation potential on successive days in culture and consequently formed homogeneous stromal fibroblast mesh-work (Fig. 5a). The results indicated malignancy associated altered BM stromal microenvironmental scenario to support the growth and survival of highly proliferating leukemic clones. In addition, ICC was performed to study expression pattern of classical Wnt proteins in BM derived stromal cells in control and leukemic condition. Leukemic marrow derived stromal cells exhibited higher Wnt3a (Fig. 5b) and lower Wnt5a protein expression (Fig. 5c) when compared to control. Dot blot assay showed significantly low level of Dkk1 in the leukemic stromal cell culture supernatant in comparison to control (Fig. 5d). These results indicated the influence of malignant microenvironment behind deregulation of Wnt signaling pathway in the leukemic marrow.
Fig. 5.
Malignant transformation alters BM hematopoietic stromal microenvironment and stroma derived Wnt. a Representative images of dayswise LT-BMC of control and leukemic mice recorded at different time points, viz day 7, 10, 15 and 20. Left panel (inside black border): control BM derived stromal cell generation and their gradual proliferation pattern. Right panel (inside red border): Leukemic BM derived stromal cell generation and their proliferation pattern. Early generation and rapid transformation of stromal precursors to stromal fibroblasts and their higher proliferation potential in leukemic condition [orange arrow: stromal fibroblasts; black arrow: stromal precursors]. b-c Representative ICC images. Wnt3a expression in Bi. Control and Bii. Leukemic BM derived stromal cells. Leukemic stromal cells showed higher Wnt3a protein expression. Wnt5a expression in Ci. Control and Cii. Leukemic BM derived stromal cells. Leukemic stromal cells showed lower Wnt5a protein expression. The brown depositions were the reaction product of substrate DAB and HRP tagged with secondary antibody and its intensity was directly proportional to the protein expression level [Magnification 1000X]. d. Comparative dot blot assay showed decreased level of Dkk1 in the leukemic stromal cell culture supernatant. [red arrow: control; blue arrow: leukemia]
Discussion
Leukemia, a hematopoietic catastrophe, develops due to sequential malignant transformation of blood forming hematopoietic stem/progenitor cells (HSPC) under the influence of the hematopoiesis supporting microenvironment (Greim et al. 2014; Anthony and Link 2014; Askmyr et al. 2011). In the present study, we emphasized on the yet unexplored crosstalk between canonical and non-canonical Wnt signaling pathway in the HSPC compartment in leukemic condition.
The leukemic mouse model was developed by neonatal ENU induction in Swiss albino mice. The rationale behind the selection of neonatal period as the optimum time for ENU administration was twofold. It is the most crucial period when hematopoietic stem cells (HSCs) usually engage themselves to engraft in the BM to establish adult definitive hematopoiesis, after completing its journey from yolk sack to fetal liver via AGM (Aorta-Gonad-Mesonephros) and placenta during their pre-natal life. Unlike adult quiescent HSCs, the highly proliferating neonatal HSCs are comparatively more susceptible to damage by genotoxic agents like ENU as well as they exhibit minimal drug efflux efficacy. In addition, neonatal ENU injection mutates the majority of HSCs before homing to BM. Eventually these mutated clones migrate to the appropriate niches in BM, proliferates and initiates malignant hematopoiesis. This phenomena leads to irreversible leukemia development and propagation. Leukemia progression in mice was initially documented in the peripheral blood, which showed huge overshoot of the total leukocyte count and mobilization of leukemic blasts, the cardinal signs of leukemic onset. Further analysis revealed co-existence of morphologically distinct myeloblasts and lymphoblasts, which initially hinted towards development of mixed type leukemia. To further confirm we have performed cytochemistry and immunphenotyping based on highly selective lineage specific markers according to revised WHO guidelines (Vardiman et al. 2009) such as MPO, NSE, CD117 for myeloids, CD22, CD79a, CD10 for B-lymphoids and CD3 for T-lymphoids. Leukemic cells showed lineage specific antigen expression for both myeloids and lymphoids, which together with peripheral blood results indicated mixed type leukemia development (Law et al. 2001; Basak et al. 2010) in the adulthood after neonatal ENU challenge. We speculate that ENU, administered in the neonatal period, mutated the primitive hematopoietic population with multilineage differentiation potential, which consequently affected both the myeloid and lymphoid lineages in the later stages of life.
Next, we tried to uncover the underlying pathobiology of ENU induced leukemia by studying the Wnt signaling pathway in the primitive HSPC compartment of the above mentioned leukemic mice. Up-regulation of canonical Wnt signaling was supported by increased expression of Wnt3a protein and Fzd7 receptor in the leukemic condition, which led to stabilization and eventual increase of total cellular β-catenin level as confirmed by flow cytometry. Signaling up-regulation was also supported by the declined expression of canonical Wnt antagonist Dkk1. Wnt signaling controls multiple cellular mechanisms in a context dependent manner and the present study identified that spike in canonical Wnt facilitated the excessive proliferation of leukemic clones via up-regulation of CyclinD1, the direct target gene of β-catenin, which is required for cell cycle entry and subsequent cell proliferation (Shtutman et al. 1999; Stacey 2003). A previous report by our group also has shown that leukemic cells are highly proliferating in nature (Chatterjee et al. 2009d). After unveiling the canonical Wnt scenario in the leukemic BM HSPC compartment, we focused on the non-canonical Wnt pathways such as Wnt5a/Ca2+ and Wnt5a/ROR2. Unlike Wnt3a, Wnt5a expression in the leukemic HSPC compartment was significantly low, which was preferable for leukemic cells but detrimental for patients or the experimental subjects as Wnt5a acts as a tumor suppressor in hematological malignancies. However the exact mechanism of Wnt5a mediated anti-tumor activity is elusive. We observed down-regulation of Wnt5a/Ca2+ signaling axis and CaMKII (Ca2+/Calmodulin dependent protein kinase II) expression in the leukemic HSPC compartment, which was supported by the findings of Liang et al. who has shown that Wnt5a signals through non-canonical Wnt/Ca2+ pathway to activate CaMKII (Liang et al. 2003). Activated CaMKII phosphorylates and inactivates CREB (cAMP response element-binding protein) and suppresses CREB associated gene transcription such as CyclinD1 which contains a c-AMP response element in its promoter (Wu and McMurray 2001; Guo et al. 2011) and in turn inhibits proliferation. In our leukemic model, CyclinD1 elevation as a result of activated canonical Wnt3a/β-catenin signaling also partly supported by down-regulation of Wnt5a/Ca2+/CaMKII pathway. Moreover, Wnt5a induces ROR2 (an orphan receptor tyrosine kinase) expression and kinase activity and Wnt5a/ROR2 signaling antagonizes canonical Wnt signaling by inhibiting β-catenin/TCF (T-cell factor, a co-activator of β-catenin) transcription activation potential and eventually CyclinD1 expression (Yuan et al. 2011; Mikels et al. 2009). ENU induced leukemic cells showed decreased ROR2 expression along with Wnt5a. Thus, down-regulated Wnt5a/ROR2 signaling further facilitated proliferation of leukemic clones via CyclinD1 expression and unhindered canonical Wnt/β-catenin signaling. Next, we tried to suppress, in part, up-regulated Wnt3a/β-catenin pathway by anti-Wnt3a antibody in the in vitro hematopoietic clonogenicity assay to see whether leukemic primitive hematopoietic compartment was responsive to Wnt3a protein for abnormal proliferation. We observed that the numbers of primitive leukemic hematopoietic CFU-GEMM and BFU-E colonies were reduced due to supplementation of anti-Wnt3a antibody, probably due to lack of functional Wnt3a protein availability to bind to the Fzd receptor of the responding cells.
We also identified overexpressed N-Cadherin (CD325), a cell adhesion molecule, in the leukemic HSPC compartment, which is required for adhesion and retention of primitive leukemic cells in the BM microenvironment to get a shelter and in turn provides protection from chemotherapeutic challenge (Zhang et al. 2013). Thus, up-regulation of N-cadherin after ENU administration in the leukemic condition was probably to get more microenvironmental support through the homophilic interaction with N-Cadherin expressing stromal cells. Moreover, N-Cadherin positive cells produce increased numbers of hematopoietic colonies with cytokine stimulation due to higher proliferative capacity and few reports claimed that leukemic stem cell activity is present in the N-Cadherin positive population which engrafts well in the immunodeficient mice upon transplantation (Pawar et al. 2015; Zhi et al. 2016; Qiu et al. 2014).
In addition to alteration of Wnt signaling in the leukemic HSPC compartment, malignant stromal microenvironment also adversely influenced paracrine Wnt protein expression pattern. LT-BMC showed early generation of stromal cells from the leukemic BM (as compared to control), which proliferated rapidly on successive days and formed homogenous stromal network. The result indicated malignancy associated alteration in BM stromal microenvironmental scenario to support growth and survival of highly proliferating leukemic clones. Brenner et al. 2017 also has shown that patient derived leukemic cells co-cultered with human MSCs exhibit higher proliferation capacity and decreased cell death rate. Although neoplastic cells are well equipped with intrinsic growth and survival mechanisms, the interaction of leukemic cells with microenvironment is indispensable for leukemia development and propagation. Leukemic microenvironment provides a favourable ambience for malignant cell growth by releasing several soluble factors in addition to direct physical contact via modulation of membrane bound receptors and adhesion molecules. Studies have shown that stroma derived Wnt can modulate the activity of hematopoietic cells (Fleming et al. 2008; Schaniel et al. 2011). In our study, we observed the involvement of stroma derived Wnt behind alteration of Wnt signaling pathway in the ENU induced leukemic hematopoietic cells. Leukemic stromal cells showed differential expression of Wnts such as increased canonical Wnt ligand - Wnt3a and decreased non-canonical Wnt ligand - Wnt5a along with reduced Wnt antagonist Dkk1, which further supported up-regulation of canonical Wnt3a signaling and down-regulation of noncanonical Wnt5a signaling in the leukemic hematopoietic cells.
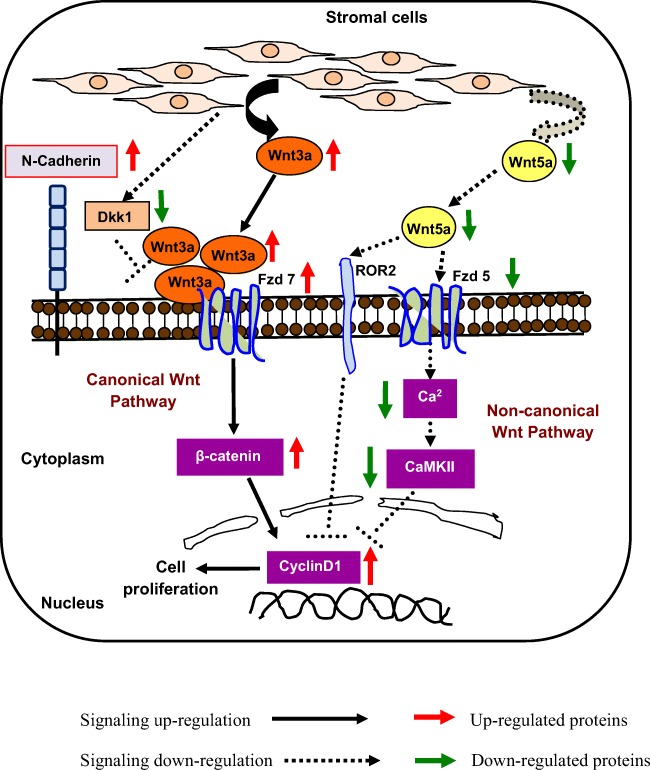
Taken together, our study demonstrated that uncoordinated interplay between canonical and non-canonical Wnt signaling pathways in the leukemic HSPC compartment was supported by deregulation of leukemic cell intrinsic mechanisms as well as alteration of BM stromal microenvironment (Fig. 6), which contributed to the leukemic disease progression in the ENU induced experimental leukemic mice. The present investigation revealed the mechanistic interplay between different branches of developmentally significant Wnt signaling pathway in hematopoietic malignant condition that can be employed in future to understand disease mechanisms involved in other malignant conditions as well as in developmental disorders.
Fig. 6.
Schematic representation of deregulated Wnt signaling pathways in the BM of ENU induced experimental leukemic mice
Acknowledgements
Authors are thankful to the Director, Calcutta School of Tropical Medicine for supporting the work. We are also thankful to Leica Microsystem and Dept. of Biological Sciences, Presidency University for providing facilities of confocal microscope.
Abbreviations
- BM
Bone marrow
- HSPC
Hematopoietic stem/progenitor cell
- ENU
N-ethyl-N-nitrosourea
- MPO
Myeloperoxidase
- NSE
Nonspecific-esterase
- Ctrl
Control
- Leuk
Leukemia
- CFU-GEMM
Colony-forming unit of granulocyte/erythrocyte/macrophage/megakaryocyte
- BFU-E
Burst-forming unit of erythroid cells
- CFU-GM
Colony-forming unit of granulocyte/macrophage
- CFU-G
Colony-forming unit of granulocyte
- CFU-E
Colony-forming unit of erythroid cells
Funding
This work was supported by the INSPIRE Fellowship, Dept. of Science and Technology, Govt. of India [Fellowship Number: DST/INSPIRE Fellowship/2013/283(IF130354)]; and the Council for Scientific and Industrial Research (CSIR), Govt. of India [Grant Number: No. 37(1429)/10/EMRII].
Compliance with ethical standards
Conflict of Interest
Authors unanimously declare no potential conflict of interest.
References
- Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35(1):32–37. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askmyr M, Quach J, Purton LE. Effects of the bone marrow microenvironment on hematopoietic malignancy. Bone. 2011;48(1):115–120. doi: 10.1016/j.bone.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Bakker ERM, Das AM, Helvensteijn W, Franken PF, Swagemakers S, van der Valk MA, ten Hagen TL, Kuipers EJ, van Veelen W, Smits R. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis. 2013;34(11):2629–2638. doi: 10.1093/carcin/bgt215. [DOI] [PubMed] [Google Scholar]
- Basak P, Chatterjee S, Das P, Das M, Pereira JA, Dutta RK, Chaklader M, Chaudhuri S, Law S. Leukemic stromal hematopoietic microenvironment negatively regulates the normal hematopoiesis in mouse model of leukemia. Chin J Cancer. 2010;29(12):969–979. doi: 10.5732/cjc.010.10431. [DOI] [PubMed] [Google Scholar]
- Bellon M, Ko NL, Lee MJ, Yao Y, Waldmann TA, Trepel JB, Nicot C. Adult T-cell leukemia cells overexpress Wnt5a and promote osteoclast differentiation. Blood. 2013;121(25):5045–5054. doi: 10.1182/blood-2012-07-439109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigas A, Guiu J, Gama-Norton L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol Dis. 2013;51(4):264–270. doi: 10.1016/j.bcmd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Bordonaro M, Tewari S, Cicco CE, Atamna W, Lazarova DL. A Switch from Canonical to Noncanonical Wnt Signaling Mediates Drug Resistance in Colon Cancer Cells. PLoS One. 2011;6(11):e27308. doi: 10.1371/journal.pone.0027308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AK, Nepstad I, Bruserud Ø. Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front Immunol. 2017;8:106. doi: 10.3389/fimmu.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaklader M, Law S. Alteration of hedgehog signaling by chronic exposure to different pesticide formulations and unveiling the regenerative potential of recombinant sonic hedgehog in mouse model of bone marrow aplasia. Mol Cell Biochem. 2015;401:115. doi: 10.1007/s11010-014-2299-5. [DOI] [PubMed] [Google Scholar]
- Chaklader M, Pan A, Law A, Chattopadhayay S, Chatterjee R, Law S. Differential remodeling of cadherins and intermediate cytoskeletal filaments influence microenvironment of solid and ascitic sarcoma. Mol Cell Biochem. 2013;382:293–306. doi: 10.1007/s11010-013-1750-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Basak P, Das M, Das P, Pereira JA, Dutta RK, Chaklader M, Chaudhuri S, Law S. Kinetic impairment of haemopoietic stem cells in experimentally induced leukemia and aplastic anemia: an inverse correlation. J Stem Cells. 2009;4(3):179–189. [PubMed] [Google Scholar]
- Chatterjee R, Chattopadhyay S, Law S. Alteration of classical and hematopoiesis specific p53 pathway in the bone marrow hematopoietic stem/progenitor compartment facilitates leukemia progression in experimental mice. Leuk Res. 2016;47:70–77. doi: 10.1016/j.leukres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Chatterjee R, Chattopadhyay S, Sanyal S, Daw S, Law S. Pathophysiological scenario of hematopoietic disorders: a comparative study of aplastic anemia, myelodysplastic syndrome and leukemia in experimental animals. Proc Zool Soc. 2016;69(1):114–124. [Google Scholar]
- Chatterjee R, Chattopadhyay S, Law S. Deregulation of vital mitotic kinase–phosphatase signaling in hematopoietic stem/progenitor compartment leads to cellular catastrophe in experimental aplastic anemia. Mol Cell Biochem. 2016;422(1–2):121–134. doi: 10.1007/s11010-016-2811-1. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Chatterjee R, Law S. Noncanonical Wnt5a-Ca(2+)-NFAT signaling axis in pesticide induced bone marrow aplasia mouse model: A study to explore the novel mechanism of pesticide toxicity. Environ Toxicol. 2016;31(10):1163–1175. doi: 10.1002/tox.22123. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Chaklader M, Chatterjee R, Law A, Law S. Differential expression of mitotic regulators and tumor microenvironment influences the regional growth pattern of solid sarcoma along the cranio-caudal axis. Exp Cell Res. 2016;340(1):91–101. doi: 10.1016/j.yexcr.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Daw S, Chatterjee R, Law A, Law S. Analysis of hematopathology and alteration of JAK1/STAT3/STAT5 signaling axis in experimental myelodysplastic syndrome. Chem Biol Interact. 2016;260:176–185. doi: 10.1016/j.cbi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin. 2011;43(10):745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Celso CL, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA, Scharffetter-Kochanek K, Kestler HA, Zheng Y, Geiger H. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503(7476):392–396. doi: 10.1038/nature12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greim H, Kaden DA, Larson RA, Palermo CM, Rice JM, Ross D, Snyder R (2014) The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Ann N Y Acad Sci 1310(1): 7–31. 10.1111/nyas.12362 [DOI] [PMC free article] [PubMed]
- Guo ZY, Hao XH, Tan FF, Pei X, Shang LM, Jiang XL, Yang F. The elements of human cyclin D1 promoter and regulation involved. Clin Epigenetics. 2011;2(2):63–76. doi: 10.1007/s13148-010-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AD, Stachura DL, Traver D. Cell signaling pathways involved in hematopoietic stem cell specification. Exp Cell Res. 2014;329(2):227–233. doi: 10.1016/j.yexcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S, Maiti D, Palit A, Majumder D, Basu K, Chaudhuri S, Chaudhuri S. Facilitation of functional compartmentalization of bone marrow cells in leukemic mice by biological response modifiers: an immunotherapeutic approach. Immunol Lett. 2001;76(3):145–152. doi: 10.1016/s0165-2478(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Lento W, Congdon K, Voermans C, Kritzik M, Reya T. Wnt Signaling in Normal and Malignant Hematopoiesis. Cold Spring Harb Perspect Biol. 2013;5(2):a008011. doi: 10.1101/cshperspect.a008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4(5):349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Liu N, Shi HG, Zhang W, Gu B. The crosstalk between canonical and noncanonical Wnt signaling pathway in osteoblast differentiation of periodontal ligament stem cells in inflammatory microenvironments. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016;51(11):673–679. doi: 10.3760/cma.j.issn.1002-0098.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Luis TC, Ichii M, Brugman MH, Kincade P, Staal FJ. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26:414–421. doi: 10.1038/leu.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many AM, Brown AMC. Both Canonical and Non-Canonical Wnt Signaling Independently Promote Stem Cell Growth in Mammospheres. PLoS One. 2014;9(7):e101800. doi: 10.1371/journal.pone.0101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín V, Valencia A, Agirre X, Cervera J, San Jose-Eneriz E, Vilas-Zornoza A, Rodriguez-Otero P, Sanz MA, Herrera C, Torres A, Prosper F, Román-Gómez J. Epigenetic regulation of the non-canonical Wnt pathway in acute myeloid leukemia. Cancer Sci. 2010;101(2):425–432. doi: 10.1111/j.1349-7006.2009.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R. Ror2 Receptor Requires Tyrosine Kinase Activity to Mediate Wnt5A Signaling. J Biol Chem. 2009;284(44):30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Oishi I, Endo M, Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn. 2010;239(1):1–15. doi: 10.1002/dvdy.21991. [DOI] [PubMed] [Google Scholar]
- Ng OH, Firtina S, Can I, Karakaş Z, Ağaoğlu L, Doğru Ö, Celkan T, Akçay A, Yıldırmak Y, Timur Ç, Özbek U, Sayitoğlu M. A Possible Role for WNT5A Hypermethylation in Pediatric Acute Lymphoblastic Leukemia. Turk J Haematol. 2015;32(2):127–135. doi: 10.4274/tjh.2013.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar RD, Cui W, Lin TL, Aljitawi OS. N-Cadherin Immunoexpression In Patients With Acute Myeloid Leukemia. Blood. 2015;126:4944. [Google Scholar]
- Pereira JA, Law S. Microenvironmental scenario of the bone marrow of inorganic arsenic-Exposed experimental mice. Biol Trace Elem Res. 2018;181(2):304–313. doi: 10.1007/s12011-017-1022-2. [DOI] [PubMed] [Google Scholar]
- Pinzón-Daza ML, Salaroglio IC, Kopecka J, Garzòn R, Couraud PO, Ghigo D, Riganti C. The cross-talk between canonical and non-canonical Wnt-dependent pathways regulates P-glycoprotein expression in human blood-brain barrier cells. J Cereb Blood Flow Metab. 2014;34(8):1258–1269. doi: 10.1038/jcbfm.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt Signaling in Cancer. Cold Spring Harb Perspect Biol. 2012;4(5):a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Jia Y, Xing H, Yu T, Yu J, Yu P, Tang K, Tian Z, Wang H, Mi Y, Rao Q, Wang M, Wang J. N-Cadherin and Tie2 positive CD34+ CD38−CD123+ leukemic stem cell populations can develop acute myeloid leukemia more effectively in NOD/SCID mice. Leuk Res. 2014;38(5):632–637. doi: 10.1016/j.leukres.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A, Torok-Storb B, Pillai MM. Stem Cell Niche. Totowa, NJ: Humana Press; 2013. Primary marrow-derived stromal cells: isolation and manipulation; pp. 75–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Traver D, Willert K. The role of Wnt signaling in hematopoietic stem cell development. Crit Rev Biochem Mol Biol. 2017;52(4):414–424. doi: 10.1080/10409238.2017.1325828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA. Wnt inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118(9):2420–2429. doi: 10.1182/blood-2010-09-305664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck C, Bock F, Grziwok S, Oostendorp RA, Istvánffy R. Regulation of hematopoiesis by activators and inhibitors of Wnt signaling from the niche. Ann N Y Acad Sci. 2014;1310:32–43. doi: 10.1111/nyas.12384. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The CyclinD1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15(2):158–163. doi: 10.1016/s0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 30. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EG, Ansari-Lari MA, Batista DA, Griffin CA, Fuller S, Smith BD, Borowitz MJ. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia. 2007;21(11):2264–2270. doi: 10.1038/sj.leu.2404848. [DOI] [PubMed] [Google Scholar]
- Wu X, McMurray CT. Calmodulin kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J Biol Chem. 2001;276(3):1735–1741. doi: 10.1074/jbc.M006727200. [DOI] [PubMed] [Google Scholar]
- Yan L, Du Q, Yao J, Liu R. ROR2 inhibits the proliferation of gastric carcinoma cells via activation of non-canonical Wnt signaling. Exp Ther Med. 2016;12(6):4128–4134. doi: 10.3892/etm.2016.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J, Li H, Chen YW, Srivastava G, Gao Z, Tao Q. WNT5A is epigenetically silenced in hematologic malignancies and inhibits leukemia cell growth as a tumor suppressor. Blood. 2007;110:4130–4131. doi: 10.1182/blood-2007-06-094870. [DOI] [PubMed] [Google Scholar]
- Ysebaert L, Chicanne G, Demur C, De Toni F, Prade-Houdellier N, Ruidavets JB, Mansat-De Mas V, Rigal-Huguet F, Laurent G, Payrastre B, Manenti S, Racaud-Sultan C. Expression of β-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20(7):1211–1216. doi: 10.1038/sj.leu.2404239. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Niu CC, Deng G, Li ZQ, Pan J, Zhao C, Yang ZL, Si WK (2011, 27(1)) The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells. Int J Mol Med:63–69. 10.3892/ijmm.2010.560 [DOI] [PubMed]
- Zang S, Liu N, Wang H, Wald DN, Shao N, Zhang J, Ma D, Ji C, Tse W. Wnt signaling is involved in 6-benzylthioinosine-induced AML cell differentiation. BMC Cancer. 2014;14(1):886. doi: 10.1186/1471-2407-14-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, Shultz L, Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt–β-catenin signaling. Blood. 2013;121(10):1824–1838. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi L, Gao Y, Yu C, Zhang Y, Zhang B, Yang J, Yao Z. N-cadherin aided in maintaining the characteristics of leukemic stem cells. Anat Rec (Hoboken) 2016;299(7):990–998. doi: 10.1002/ar.23345. [DOI] [PubMed] [Google Scholar]
- Zhou HR, FU HY, WU DS, Zhang YY, Huang SH, Chen CJ, Yan JG, Huang JL, Shen JZ. Relationship between epigenetic changes in Wnt antagonists and acute leukemia. Oncol Rep. 2017;37:2663–2671. doi: 10.3892/or.2017.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]