Abstract
Extracellular molecules coordinate the multiple signaling pathways spatiotemporally to exchange information between cells during development. Understanding the regulation of these signal molecule-dependent pathways elucidates the mechanism of intercellular crosstalks. CCN2/CTGF is one of the CCN family members that binds BMP2, fibronectin, aggrecan, FGFR2 - regulating cartilage and bone formation, angiogenesis, wound repair etc. Tsukushi (TSK), which belongs to the Small Leucine-Rich Proteoglycan (SLRP) family, binds nodal/Vg1/TGF-β1, BMP4/chordin, Delta, FGF8, Frizzled4, and is involved in the early body formation, bone growth, wound healing, retinal stem cell regulation etc. These two secreted molecules are expressed in similar tissues and involved in several biological events by functioning as extracellular signaling modulators. Here, we examine the molecular interaction between CCN2 and TSK biochemically. Co-precipitation assay and Surface Plasmon Resonance measurement showed their direct binding with the Kd value 15.3 nM. Further, the Solid-phase Binding Assay indicated that TSK binds to IGFBP and CT domains of CCN2. Our data suggest that CCN2 and TSK exert their function together in the body formation.
Keywords: CCN2/CTGF, Tsukushi, Soluble molecule, SLRP, Vertebrate development
Introduction
Extracellular molecules provide developmental and environmental cues to individual cells. Cells detect the cues that come in the form of proteins, lipids or other small molecules, with specific receptors and the message is transmitted inside via specific signaling pathways. Specific binding between molecules and their receptors are crucial for the regulation of downstream signaling pathways (Lim et al. 2014). Interestingly, some molecules, like Cerebrus, Coco and folistatin those are members of TGF-β family secreted in embryonic developmental stages, possess the ability to bind multiple receptors and thus activate multiple signaling cascades (Bell et al. 2003; Harrington et al. 2006; Piccolo et al. 1999). With thousands of signaling molecules and their receptors, extracellular space appears to be complex and dynamic. Presence of a particular molecule does not always guarantee the activation of same downstream cascade. Several other molecules and receptors can compete and crosstalk in the binding and in turn influence the ultimate signal interpretation (Grotendorst et al. 2004).
Connective tissue growth factor (CCN2/CTGF) and Tsukushi (TSK) are two signaling proteins that are involved in multiple biological processes with distinctive adhesion structures. CCN2 is a member of CCN protein family which is composed of four distinct domains connected in tandem, i.e., IGF-binding protein-like (IGFBP), von Willebrand type C (VWC), thrombospondin type 1 repeat (TSP-1), and C-terminal (CT) domains. TSK is a member of SLRP family. They both function as signaling molecules and have the ability to interact with multiple signaling pathways (Abreu et al. 2002; Ahmad et al. 2018; Mercurio et al. 2004; Ohta 2014; Takigawa 2013, 2017, 2018). Both CCN2 and TSK can bind a number of receptors/signal intermediates, while BMP4 and TGF-β1 are the common binding partners. Major biological functions of CCN2 include skeletogenesis, fibrosis, wound healing and angiogenesis (Kubota and Takigawa 2007; Takigawa 2013, 2017, 2018). TSK is involved in various developmental processes, like early body patterning, peripheral eye development, skeletal development and wound healing (Kuriyama et al. 2006; Ohta et al. 2004, 2006, 2011; Yano et al. 2017). Skeletogenesis and wound healing require both CCN2 and TSK.
The importance of CCN2 in skeletal development has been confirmed by various studies using CTGF knockout (KO) mice. CCN2 has been reported to play vital role in mesenchymal cell condensation, terminal chondrocyte differentiation and early osteogenic differentiation (Arnott et al. 2011). The timing of CCN2 expression in different bone tissues indicates its importance at different developmental stages (Song et al. 2007). While the expression of CCN2 in different tissues is mostly regulated by TGF-β1 and BMP-2, CCN2 in turn modulates other skeletal growth factors and influences bone growth (Luo et al. 2004). The involvement of TSK in bone development has been reported recently (Yano et al. 2017). TSK KO mice shows reduced growth of long bones with overall reduction of both height and body weight. Almost all the bone regions of juvenile mice express TSK, but the absence of TSK mostly affects the columnar array of chondrocytes and the growth plate - both proliferating and hypertrophic zones. Altered expression pattern of chondrogenesis markers in TSK KO growth plate cells suggests that TSK is crucial for the regulation of chondrogenesis (Yano et al. 2017). In case of wound healing process, CCN2 and TSK appear to perform opposing functions. CCN2 regulates the fibrotic response of myofibroblasts by inducing collagen synthesis and formation of scar tissue (Shi-Wen et al. 2008). CCN2 expression in the wound sites is always preceded by the expression of TGF-β and their simultaneous expression is essential for a proper wound healing response (Igarashi et al. 1993). In contrast, TSK appears to inhibit TGF-β expression and thus reduce the TGF-β induced inflammation in the wound sites (Niimori et al. 2014). The apparently opposing expression timing of CCN2 and TSK might provide a logical explanation, but the evidences at hand are not enough to make any concluding comments regarding the contrasting effect on TGF-β signal output. We have summarized the shared features of CCN2 and TSK functions in Table 1.
Table 1.
Common biological features between CCN2 and TSK
| Biological process | Bone formation | Wound healing | ||
|---|---|---|---|---|
| Signaling protein | CCN2 | TSK | CCN2 | TSK |
| Expression | Mesenchymal cells, growth plate chondrocytes, periosteum, osteoblasts | Almost all regions, Growth plate chondrocytes, trabecular bone, cortical bone | Human foreskin fibroblasts, Myofibroblasts, γδ-T cells | Macrophages, neoepidermis, granulation tissue, Myofibroblasts, |
| Function | Mesenchymal cell condensation, terminal chondrocyte differentiation, early osteogenic differentiation | Chondrogenesis | Myofibroblast activation, increased collagen synthesis, scar tissue formation | Myofibroblast quiescence, inhibition of TGF-β mediated inflammation |
| References | (Arnott et al. 2011); Takigawa et al. 2013; (Song et al. 2007) | (Yano et al. 2017) | (Shi-Wen et al. 2008) (Igarashi et al. 1993) |
(Niimori et al. 2014) |
In this study, we have examined the molecular interaction between CCN2 and TSK. Although they belong to different protein families, the shared biological features and overlapping expression patterns encouraged us in this study. To detect the interaction, we have employed co-precipitation assay and Surface Plasmon Resonance measurement.
Materials and methods
Co-precipitation assay
A co-precipitation assay was carried out as previously described (Ohta et al. 2004). Briefly, COS-7 cells were transfected with the following constructs: V5-His-tagged mouse-TSK (M-TSK) (Ohta et al. 2011) or Flag-HA-tagged CCN2 (Aoyama et al. 2009). Transfected COS-7 cells were lysed in precipitation buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 1.5 mM CaCl2, 1.5 mM MgCl2, 0.1% Triton X-100, 0.1% CHAPS, 5% glycerol, and 0.1% BSA. Cell lysates were mixed and incubated for 12 h at 4 °C, followed by incubation with ProBond™ resins (Invitrogen). Resin beads were washed with precipitation buffer without BSA or the same buffer with high salt (450 mM NaCl), 0.5% Triton-X-100 and 0.5% CHAPS. Proteins bound to the beads were subjected to SDS–PAGE and blotted onto a nylon membrane. His-tagged and HA-tagged proteins were immuno-detected using their respective antibodies [anti-His antibody (Abcam) and anti-HA antibody (Sigma-Aldrich)].
Protein production
To obtain the pure M-TSK protein, we used silkworm-baculovirus expression system (ProCube@, http://procube.sysmex.co.jp/eng/ Corporation). The coding region without signal peptide of M-TSK was inserted into the transfer vector pM24 (Sysmex Corporation) to fuse with Flag and His tags at N and C terminus, respectively. The resultant plasmid was used for expression of recombinant Flag-M-TSK-His (rTSK) in a silkworm pupa, and the expressed rTSK was affinity-purified with 100 μg/ml FLAG peptide solution. Human recombinant CCN2 (rCCN2) was purchased from BioVendor Laboratories Inc. (Brno, Czech Republic). Recombinant IGFBP domain, VWC domain and TSP1 domain of CCN2 were produced by Brevibacillus in vivo cloning (BIC) system (Higeta Shoyu Co. Ltd.). CT domain of CCN2 was purchased from Peprotech (Rocky, NJ, USA).
Surface Plasmon Resonance (SPR) measurement
Human rCCN2 was diluted to 10 μg/ml with 10 mM sodium acetate buffer (pH 4.0) and immobilized onto CM5 sensor chips (GE Healthcare) according to standard amine coupling procedures (Aoyama and Takigawa 2017). rTSK diluted with 100 μg/ml FLAG peptide (Sigma-Aldrich) in PBS to concentrations of 46.875, 93.75, 187.5, 375 and 750 nM was injected into the flow cells. For affinity measurements, binding and dissociation were monitored with Biacore X (GE Healthcare). The data were fitted using the BIAevaluation software version 4.1 (GE Healthcare) with the single cycle kinetics support package (GE Healthcare). Binding data were globally fit to the single cycle kinetics 1:1 L binding model.
Solid-phase binding assay
Solid-phase binding assay of TSK and four domains of CCN2 was performed, as described previously (Aoyama et al. 2015). Briefly, ELISA plate coated with each domain of CCN2 was generated by incubation with 1 μg/ml of recombinant each domain of CCN2 at 4 °C overnight. After washing with wash buffer, the wells of the plate were blocked with binding buffer for 2 h at room temperature. Diluted rTSK solutions were then added to the wells, and incubated for 2 h at room temperature. Thereafter, the wells were washed and incubated with Monoclonal ANTI-FLAG® M2 antibody (Sigma-Aldrich) for 1 h at room temperature. After washing, the wells were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse Fc antibody (Sigma Aldrich, St Louis, MO) for 1 h at room temperature. The wells were added 3,3,5,5 -tetramethylbenzidine (TMB) peroxidase substrate (Sigma-Aldrich) and after 15 min HRP reaction was stopped by 1 M H2SO4. The absorbance at 450 nm of each wells were measured with SH-1000Lab microplate reader (Corona Electric Co., Ltd., Ibaraki, Japan).
Statistical analysis
Statistical analysis was performed by performing Dunnett’s t-test and the One-way ANOVA test. Data were expressed as the means of three wells ± standard deviation (SD).
Results
Direct binding between CCN2 and TSK assessed by co-precipitation assay
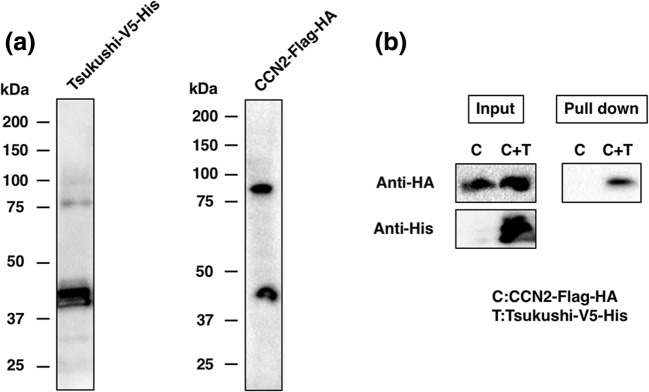
To investigate whether TSK can bind CCN2 directly, we performed co-precipitation assays. First, we examined the expression of V5-His-tagged TSK and Flag-HA-tagged CCN2 by blotting. Consisting with the previous reports (Ohta et al. 2004), Fig. 1a shows the molecular weight of TSK and CCN2 proteins with tags are 43 kDa and 46 kDa, respectively. The band with the molecular weight of about 90KDa would be a dimer as described previously (Takigawa 2013, 2017). When V5-His-tagged TSK was reacted with Flag-HA-tagged CCN2, co-precipitation with nickel chelating resins specifically pulled down CCN2 (Fig. 1b). Thus, our data show direct binding between TSK and CCN2.
Fig. 1.
Direct interaction between CCN2 and TSK. a Western blot analysis of M-TSK-V5-His and CCN2-Flag-HA. b Co-precipitation of M-TSK-V5-His and CCN2-Flag-HA. After precipitation M-TSK-V5-His with nickel chelating resins, bound CCN2 was detected by immunoblotting with anti-HA antibody
The affinity of direct binding between CCN2 and TSK examined by SPR assay
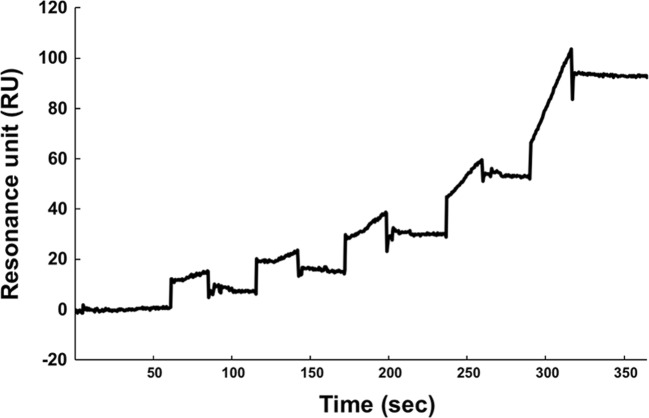
The interaction between rTSK and rCCN2 was kinetically analyzed with Surface Plasmon Resonance methodology. As shown in Fig. 2, rTSK bound on rCCN2 immobilized onto the chip. The analysis revealed that the dissociation constant value was 15.3 nM (average value of 2 independent experiments) for the binding of rCCN2 to rTSK. This affinity is comparable to the value between rCCN2 and TGF-β1 or osteoprotegerin (Aoyama et al. 2015; Khattab et al. 2015) and a little bit lower than the affinity between rCCN2 and Decorin (Kd value = 4.4 nM in Vial et al. 2011).
Fig. 2.
The binding of rCCN2 and rTSK was analyzed with SPR. The rCCN2 was coated on CM5 chip and serial dilutions of rTSK (46.875, 93.75, 187.5, 375 and 750 nM) were injected in ascending order of concentrations at 123, 246, 367, 505 and 628 s respectively. The Kd value was calculated from sensorgrams as described in Materials and methods. The data are representative of 2 independent experiments
The binding of TSK and four domains of CCN2
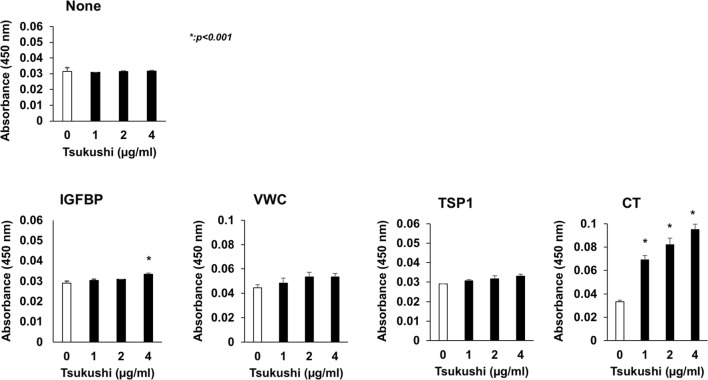
To investigate which domains are involved in the binding between rTSK and CCN2, we next carried out a solid-phase binding assay using rTSk and four independent domains of CCN2. As shown Fig. 3, rTSK bound to IGFBP and CT domains of CCN2 significantly.
Fig. 3.
The bindings of FLAG-tagged rTSK to four domains of CCN2 (IGFBP, VWC, TSP1, or CT) or none were measured with solid-phase binding assay by using anti-FLAG mouse Ab and anti-mouse IgG-HRP as described in Materials and methods. The absorbance values at 450 nm indicates the amount of HRP bound to wells coated each domain of CCN2 or none. Statistical analysis was performed by performing Dunnett’s t-test and the One-way ANOVA test, *p < 0.001, as compared with the values for wells with 0 μg/ml rTSK. Data were expressed as the means of three independent wells ± standard deviation (SD)
Discussion
The CCN family of proteins consists of six members. CCN proteins are composed of four constitutive domains (Insulin-like growth factor binding proteins-like, the Von Willebrand factor type C, thrombospondin 1 repeat, and C-terminal cystine knot) which bind different types of factors that allow specific connections to large families of cell biology regulators (Perbal 2018; Takigawa 2018). This tetramodular organization provides the basis for a very sophisticated array of biological properties with acute functions in cell growth, differentiation and communication. The SLRP family consists of five discrete subclasses and is the largest family of proteoglycans encompassing 18 distinct gene products and numerous splice variants and processed forms. The typical SLRP features include the presence of N-terminal Cys clusters. The SLRPs are ubiquitously expressed in most extracellular matrices and highly expressed during development. These SLRPs function both as structural constituent and as signalling molecules, regulating fundamental processes including migration, proliferation, innate immunity, apoptosis, autophagy and angiogenesis (Iozzo and Schaefer 2015). Previously, the molecular interaction between the members of CCN and SLRP families was reported between CCN2 and Decorin (Vial et al. 2011). CCN2 and Decorin bind each other directly, and CCN2 can induce the expression of Decorin. Further, the leucine rich repeats peptide 12 region of Decorin is involved in direct interaction and inhibits CCN2-mediated activity on C2C12 in vitro. These results suggest the existence of the molecular interaction between these families.
Here, we show that CCN2 can interact with TSK by co-precipitation experiment and Surface Plasmon Resonance measurement. In addition, the solid phase binding assay by using rTSK and four independent domains of CCN2 indicates the possibility that TSK binds to CCN2 via IGFBP and CT domains. Given their broad expression, CCN2 and TSK might also function in crosstalk in different tissues. We can find the glimpse of their mutual interaction as they share common interacting signaling molecules BMP4 and TGF-β. During the developmental processes both CCN2 and TSK play critical role that we find specially in skeletogenesis and wound healing events (Takigawa 2013, 2017, 2018; Niimori et al. 2014; Yano et al. 2017). By regulating chondrogenesis in growth plate, TSK plays a vital role in both elongation and control of the mass of bone. CCN2 is involved in condensation of mesenchymal cell, chondrocyte and osteogenic differentiation (Takigawa 2013, 2017, 2018). Both CCN2 and TSK KO mice show skeletal malformation (Ivkovic et al. 2003). On the contrary, in wound healing process these molecules show possible different strategy to each other. Following TGF-β expression, CCN2 appears at the wound site around day 6 post injury and promotes collagen synthesis and scar tissue formation in rat (Igarashi et al. 1993). In contrast, TSK expression at the wound site appears as early as 2 days post injury in mice. TSK KO mice showed increased inflammation due to upregulation of TGF-β by macrophages after wounding (Niimori et al. 2014). This indicates that TSK aids the wound healing processes by maintaining the quiescent inflammatory cells. Although expression timing is different for both molecules, still they both regulate TGF-β – for which the mechanisms are unknown. The combined effect of both these molecules in skeletogenesis and wound healing process can provide important clues for the illustration of CCN-SLRP signalling crosstalk.
Acknowledgements
The authors thank Mitsue Kumamaru and Megumi Takiguchi. We also thank all members of our labs for their technical assistance and for valuable helps. This study was supported by the Kumamoto University International Research Core for Stem Cell-based Developmental Medicine and HIGOprogram and in part by grants from the programs Grants-in-Aid for Scientific Research (B) to MT (#JP15H05014) and for Challenging Exploratory Research to MT (#JP17K19757) from Japan Society for the Promotion of Sciences, Japan.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Kunimasa Ohta and Eriko Aoyama contributed this work equally.
Contributor Information
Kunimasa Ohta, Email: ohta9203@gpo.kumamoto-u.ac.jp.
Masaharu Takigawa, Email: takigawa@md.okayama-u.ac.jp.
References
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S a I, Anam MB, Ito N, Ohta K. Involvement of Tsukushi in diverse developmental processes. J Cell Commun Signal. 2018;12:205–210. doi: 10.1007/s12079-018-0452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama E, Takigawa M. Evaluation of molecular interaction between CCN2 protein and its binding partners by surface Plasmon resonance (SPR) Methods Mol Biol. 2017;1489:169–176. doi: 10.1007/978-1-4939-6430-7_17. [DOI] [PubMed] [Google Scholar]
- Aoyama E, Hattori T, Hoshijima M, Araki D, Nishida T, Kubota S, Takigawa M. N-terminal domains of CCN family 2/connective tissue growth factor bind to aggrecan. Biochem J. 2009;420:413–420. doi: 10.1042/BJ20081991. [DOI] [PubMed] [Google Scholar]
- Aoyama E, Kubota S, Khattab HM, Nishida T, Takigawa M. CCN2 enhances RANKL-induced osteoclast differentiation via direct binding to RANK and OPG. Bone. 2015;73:242–248. doi: 10.1016/j.bone.2014.12.058. [DOI] [PubMed] [Google Scholar]
- Arnott JA, Lambi AG, Mundy C, Hendesi H, Pixley RA, Owen TA, Safadi FF, Popoff SN. The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev Eukaryot Gene Expr. 2011;21:43–69. doi: 10.1615/CritRevEukarGeneExpr.v21.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Muñoz-Sanjuán I, Altmann CR, Vonica A, Brivanlou AH. Cell fate specification and competence by coco, a maternal BMP, TGFbeta and Wnt inhibitor. Development. 2003;130:1381–1389. doi: 10.1242/dev.00344. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- Harrington AE, Morris-Triggs SA, Ruotolo BT, Robinson CV, Ohnuma S-I, Hyvönen M. Structural basis for the inhibition of activin signalling by follistatin. EMBO J. 2006;25:1035–1045. doi: 10.1038/sj.emboj.7601000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab HM, Aoyama E, Kubota S, Takigawa M. Physical interaction of CCN2 with diverse growth factors involved in chondrocyte differentiation during endochondral ossification. J Cell Commun Signal. 2015;9:247–254. doi: 10.1007/s12079-015-0290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Lupo G, Ohta K, Ohnuma S-I, Harris WA, Tanaka H. Tsukushi controls ectodermal patterning and neural crest specification in Xenopus by direct regulation of BMP4 and X-delta-1 activity. Development. 2006;133:75–88. doi: 10.1242/dev.02178. [DOI] [PubMed] [Google Scholar]
- Lim, W., Mayer, B., and Pawson, T. (2014). Cell signaling : principles and mechanisms (Garland science)
- Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, et al. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Niimori D, Kawano R, Niimori-Kita K, Ihn H, Ohta K. Tsukushi is involved in the wound healing by regulating the expression of cytokines and growth factors. J Cell Commun Signal. 2014;8:173–177. doi: 10.1007/s12079-014-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta, K. (2014). The role of Tsukushi as an extracellular signaling coordinator. In New principles in developmental processes, H. and K. Kondoh, ed. (Tokyo: Springer), pp. 227–238
- Ohta K, Lupo G, Kuriyama S, Keynes R, Holt CE, Harris WA, Tanaka H, Ohnuma S-I. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell. 2004;7:347–358. doi: 10.1016/j.devcel.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Kuriyama S, Okafuji T, Gejima R, Ohnuma S, Tanaka H. Tsukushi cooperates with VG1 to induce primitive streak and Hensen’s node formation in the chick embryo. Development. 2006;133:3777–3786. doi: 10.1242/dev.02579. [DOI] [PubMed] [Google Scholar]
- Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S, Nakagawa S, Tanaka H. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc Natl Acad Sci U S A. 2011;108:14962–14967. doi: 10.1073/pnas.1100513108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. The concept of the CCN protein family revisited: a centralized coordination network. J Cell Commun Signal. 2018;12:3–12. doi: 10.1007/s12079-018-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J Cell Physiol. 2007;210:398–410. doi: 10.1002/jcp.20850. [DOI] [PubMed] [Google Scholar]
- Takashi N, Emura K, Kubota S, Lyons KM, Takigawa M (2011) CCN family 2/connective tissue growth factor (CCN2/CTGF) promotes osteoclastogenesis via induction of and interaction with dendritic cell-specific transmembrane protein (DC-STAMP). J. Bone Miner. Res. 26 (2):351–363 [DOI] [PMC free article] [PubMed]
- Takigawa M. CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal. 2013;7:191–201. doi: 10.1007/s12079-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M (2017) The CCN proteins: An overview. CCN Proteins: Methods and Protocols, in: M. Takigawa (Ed), Methods in Molecular Biology, Springer Nature, New York, vol.1489 pp. 1–8 [DOI] [PubMed]
- Takigawa M. An early history of CCN2/CTGF research: the road to CCN2 via hcs24, ctgf, ecogenin, and regenerin. J Cell Commun Signal. 2018;12:253–264. doi: 10.1007/s12079-017-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Gutiérrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem. 2011;286:24242–24252. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Washio K, Tsumanuma Y, Yamato M, Ohta K, Okano T, Izumi Y. The role of Tsukushi (TSK), a small leucine-rich repeat proteoglycan, in bone growth. Regenerative Therapy. 2017;7:98–107. doi: 10.1016/j.reth.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]