Abstract
Isolation of progenitors with regenerative potential and their in vitro induction to specific lineage may be necessary for effective cell transplantation outcome. Earlier, we standardized specific niche for derivation of neural progenitor cells (NPCs) from circulating mononuclear cells to neural like cells (NLC) in vitro, for applications in neural regeneration. The current study analysed the prospective involvement of signaling mechanism for in vitro lineage commitment of circulating NPCs. Preferred mechanism selected was Wnt-like signaling because this is one of the pathways implicated in the central nervous system (CNS) development and homeostasis. We sought to determine the activation of Wnt3a-specific genes in the standardized NPC culture system. To start with, it was found that when standardized NPC culture niche was supplemented with Wnt 3a protein, no additional morphological changes happen. Chemical inhibitors of the pathway retarded NPC to NLC conversion both in the absence and presence of supplemented Wnt-3a. In earlier studies, involvement of the niche constituents- fibronectin (FN), laminin (La) and fibrin (Fib)- for NPC growth and differentiation was established. In an attempt to study the role of these adhesive proteins by adding antibodies against FN, La & Fib together, molecular level signaling changes seen were comparable to that occur in response to Wnt3a chemical inhibitor. Therefore, induction of Wnt 3a-like signal from the matrix-dependent niche constituents may be implicated in the differentiation of NPC to NLC. The results substantiate the potential applications of the fibrin-based composite niche in neural engineering for regeneration.
Keywords: Neural progenitor cell, Neural lineage, Wnt signaling, Biomimetic niche, Differentiation, Regeneration, Matrix proteins
Introduction
The use of stem cells in regenerative medicine is making rapid progress, which has enabled identification of the merits and demerits of different types of cells for clinical use. Adult stem cells (ASCs) are suitable candidates for use in nervous system repair because they can be used to develop autologous cell-based therapies (Porat et al. 2006); (Hsu et al. 2013). The cells isolated from adult tissue are mostly heterogeneous and multipotent. Therefore, to develop an effective therapeutic protocol, it is important to obtain pure stem/progenitor population and to lineage-commit the multipotent cells into the desired cell type prior to transplantation.
Peripheral blood mononuclear cells (PBMNCs) are an easily accessible ASC source. The protocol to obtain pure, lineage-committed proliferating neural progenitor cells (NPCs) from PBMNCs and their differentiation to neural-like cells (NLC) has been described earlier (Tara and Krishnan 2015). Importance of fibronectin (FN), fibrin (fib) and laminin (La) in the niche-dependent differentiation of NPC to NLC was established in those studies. The lineage commitment progressed slowly and stable expressions of various neural-specific markers may be promising for their application in transplantation therapy. The stability and fate of the cells after transplantation may largely depend on the biochemical pathways that direct cell proliferation and differentiation (Tanabe 2015); (Vandervelde et al. 2005). Ideally, favorable conditioning of stem cells to obtain transplantable population should involve natural signaling pathways responsible for homeostasis of the specific tissue of interest. Therefore, establishing the signaling program and its regulation becomes a part of neural engineering for regeneration of nervous tissues.
It is well known that the secreted Wnt family of proteins play an active role in cell signaling both during nervous system development and in the mature CNS homeostasis. It has been proven that Wnt 3a signaling inhibits the maintenance of neural stem cells and promotes the differentiation of embryonic stage neural stem cells into different CNS cell lineages (Muroyama et al. 2004). It has been reported that Wnt 3a signaling promotes the acquisition of pluripotency during reprogramming of somatic cells to induced-pluripotent stem cells (Marson et al. 2008). In another study, Wnt 3a from conditioned media was found to maintain embryonic stem cells (ESC) in the proliferation/self-renewal state without differentiation (Singla et al. 2006) implicating its role in both stem cell maintenance and differentiation. A number of external cues and intrinsic cellular programs seem to be involved in neural development both in vivo and in vitro. A well-balanced biomimetic approach may program the stem/progenitor cells to neuronal lineage in a regulated manner for regenerative purposes.
Many investigators have reported chemical-induced, spontaneous differentiation but cell stability and proliferation potential were major concerns which limited application of such methods for regenerative purposes. Our previous study (Tara and Krishnan 2015) demonstrated that NLCs developed from PBMNCs are proliferative till 16d and expressed neural-specific markers progressively. The results were favourable for planning transplantation therapy by harvesting cells at progenitor level during initial period of culture. Therefore, delineating signaling mechanism may be an important step to ensure that the transplanted cells participate in regeneration of sustainable and functional tissue. So this study aimed to understand the involvement of Wnt signaling which is implicated in homeostasis. We explored more specifically the role of Wnt 3a in regulating the NPC to NLC differentiation in the standardized culture system. We hypothesized that the niche may comprise protein moieties to elicit Wnt-like signals resulting in the transformation of NPCs to NLCs. The steps followed were: 1) supplement Wnt proteins to study if the differentiation process is accelerated and 2) block the signal using Wnt inhibitor and (3) inhibit using antibodies against matrix proteins. The parameters studied were, specifically the up/down regulation of the Wnt-β-catenin cascade, outcome w.r.t. morphology changes and neural-specific marker expressions.
Materials and methods
Preparation of cell-specific culture matrix
To prepare a biomimetic niche for NPC differentiation, the TCPS surface was deposited with a fibrin matrix containing fibrinogen, fibronectin and laminin (Tara and Krishnan 2015). Briefly, the fibrinogen composite was layered on the thrombin pre-adsorbed surface to obtain a thin (< 1 μm) layer of fibrin clot. Fibrin-deposited dishes were lyophilized in a sterile atmosphere in a freeze-drier (Modulyo 4 K, Edwards, UK). Since the fibrin-based matrix prepared in this manner was able to support stable differentiation, it was thought to elicit Wnt signals, and the cells cultured on it were designated as ‘Test’(‘T’) in this study.
PBMNC isolation and types of cultures
The PBMNC isolation and cell culture was done using published protocols (Tara and Krishnan 2015); (Wexler et al. 2009) with slight modifications.The buffy coat (discards from the blood bank obtained with IEC approval (SCT/IEC/FEB-2013, dated 25.02.2013)) was layered onto Histopaque-1077 and centrifuged to separate PBMNCs. In the earlier protocol, PBMNCS were seeded on to complete neural induction medium comprising MCDB 131, 20% FBS, 10 μgml−1 HE, 1 μgml−1 PGF, 10 IU ml−1 heparin sulphate, 0.44 mg ml−1 glutamine, 25 mM KCl and 1 x antibiotics. In the current study, serum was depleted and growth factors in the media were replaced with recombinant bFGF to reduce the interference of multiple unknown factors from these components on signaling. Briefly, the PBMNCs obtained were seeded in MCDB 131 medium containing 1% FBS, 10 IU ml−1 heparin sulphate, 1 mg ml−1hyaluronic acid and 0.44 mg ml−1 glutamine in an uncoated dish for 24 h. The partially attached cells were then flushed out in 1% FBS supplemented medium seeded on the ‘T’ matrix and cultured for 48 h. The cells were then cultured in serum-free MCDB medium containing 20 ng/ml basic fibroblast growth factor (bFGF, R&D Systems, Minneapolis, MN, USA) for another 48 h to promote proliferation. The growth factor was withdrawn after 48 h and supplemented with 25 mM KCl, and then allowed to grow for another 48 h. To study the possible augmentation effect of Wnt 3a, 150 ng of the protein obtained from R&D systems, USA, was added at the time of cell transfer to the ‘T’ matrix and it was designated as the ‘T-Wnt 3a+’ culture (positive control). A Wnt inhibitor (PNU 74654) [Tocris Bioscience, USA] at a concentration of 25 ng ml−1 was added after 48 h of transfer to the ‘T’ culture and was designated as ‘T-Wnt 3a−’ or (negative control). The ‘T’ matrix was pre-incubated with polyclonal antibodies (Tara and Krishnan 2015) against the matrix constituents fibronectin (FN), fibrinogen (Fib) and laminin (La), singly or in combination, to study the specific effect of these adhesive proteins on Wnt 3a signaling. The cultures on the blocked matrices were denoted as ‘T-FN−’, ‘T-Fib−’, ‘T-La−’ and ‘T-(Fib+FN + La)−’ and the fate of the cells in each matrix was analyzed separately. The cells in all different culture systems were observed periodically using a phase contrast microscope (DMIRB, Leica Microsystems, Wetzlar, Germany) and micrographs were taken using Leica Application Suite (LAS) [Leica, Germany] software. For replication and reproducibility analysis, PBMNC isolates from different donors were cultured and the effect of each condition on Wnt signalling was studied..
Estimation of cell length
More than 100 cells were selected in the phase contrast micrographs of each culture type and cell length was quantified using ImageJ software (ImageJ, National Institutes of Health, USA;http://imagej.nih.gov/ij; Java 1.8.0_25). Cultures from more than three different donors were analyzed and the average cell length was estimated.
Quantitative real-time polymerase chain reaction (qRT-PCR)
During specified periods of the PBMNC culture, upregulation and downregulation of Wnt target genes and selected neural gene markers was evaluated using reverse transcriptase-quantitative real-time polymerase chain reaction qRT-PCR. Briefly, total RNA on the 8th day of culture was extracted using Trizol (Invitrogen, USA) according to the manufacturer’s instructions and quantified using a Qubit RNA assay kit on a Qubit 2.0 fluorometer (Invitrogen, USA). One μg of RNA was converted to cDNA using the Superscript III reverse transcriptase enzyme (Invitrogen). 250 ng of cDNA was mixed with respective forward and reverse primers using the specific primers provided in Table 1 and added to the SYBR-Green I master mix-No ROX (Eurogentec, SanDiego, CA, USA). Forty cycles of reaction were performed using the Chromo 4 system (MJ Research, now part of Bio-Rad, USA). For each gene, an assessment of quality was performed by examining PCR melt curves after real-time PCR to ensure specificity. The cycle threshold (Ct) value of the target gene was analyzed after normalization to the Ct value of GAPDH. Fold change (2–ΔΔCt) was calculated by comparing the expression levels of mRNA extracted from all the culture types (1.2). The housekeeping control used was GAPDH. The molecular size of the products obtained was verified using agarose gel electrophoresis (AGE).
Table 1.
Primer sequence of marker genes used for studying the expression of Wnt transducers and neural differentiation in the PBMNC culture
| Genes | Mol. weight | Primer sequence (5′-3′) |
|---|---|---|
| Nes | 200 bp | F-GCCCTGACCACTCCAGTTTA R-GGAGTCCTGGATTTCCTTCC |
| β-III tub | 148 bp | F-GCTCAGGGGCCTTTGGACATCTCTT R-TTTTCACACTCCTTCCGCACCACATC |
| GAPDH | 210 bp | F-GCTTGTCATCAATGGAAATCCC R-TCCACACCCATGACGAACATG |
| Syn | 184 bp | F-CCAACAAGACCGAGAGTG R-ATGGAGTAGAGGAAGGCAAA |
| OSP | 283 bp | F-ACTGCTGCTGACTGTTCTTC R-GTAGAAACGGTTTTCACCAA |
| NSE | 188 bp | F-CTGATGCTGGAGTTGGATGG R-CCATTGATCACGTTGAAGGC |
| GFAP | 70 bp | F-ACATCGAGATCGCCACCTACA R-GTCTGCACGGGAATGGTGAT |
| Axin 2 | 202 bp | F-AGTCAGCAGAGGGACAGGAA R-AGCTCTGAGCCTTCAGCATC |
| LEF | 187 bp | F-GACGAGATGATCCCCTTCAA R-AGGGCTCCTGAGAGGTTTGT |
| Cyc D1 | 241 bp | F-ACCTGGATGCTGGAGGTCT R-GCTCCATTTGCAGCAGCTC |
| PCNA | 115 bp | F-AGTGGAGAACTTGGAAATGGAA R-GAGACAGTGGAGTGGCTTTTGT |
Western blotting
Western blotting was used to analyze expression of various proteins in the cell lysate prepared from all types of cultures. Briefly, 50 μg of the respective mercaptoethanol-reduced protein was loaded in each lane in SDS-PAGE (10%) and the separated proteins were transferred to polyvinylidene di fluoride (PVDF) membrane using the semi-dry method at 0.8 mA/cm2 current using the Biorad Turbo Transblot system (Bio Rad, USA).
To develop the specific bands, the blots were blocked in 1% BSA for 1 h. The blot was cut into separate strips, with each strip having 3 lanes (T, ‘T-Wnt 3a−’ and ‘T-(Fib+FN + La)−’) and incubated overnight in 1:200 dilutions of primary antibodies against Nestin (260 kDa, R&D systems, Minneapolis, MN, USA), β-III tub (55 kDa, Epitomics, an Abcam company, Burlingame, CA, USA), Synaptophysin (R&D systems, Minneapolis, MN, USA), MAP-2 (SantaCruz, Heidelberg, Germany), Syn (R&D, Minneapolis, MN, USA), Ki 67 (Abcam, Cambridge Science Park, Cambridge, UK), O4 (R&D, Minneapolis, MN, USA), GFAP (BD Bioscience, USA) and β-actin (Thermo Fisher Scientific, USA). The blots were then washed and incubated for 1 h in corresponding HRP conjugated secondary antibodies at a dilution of 1:500 and the bands were visualized by adding PBS containing 0.05% diaminobenzidine (DAB), 0.1% H2O2 and 0.04% nickel chloride (pH 7.5). Once the best contrast was achieved, reaction was stopped and imaging was done using gel documentation system Alpha Imager, (Protein Simple, USA). Band intensity measurements were also carried out using the band analysis tool from Alpha Imager.
Statistical analysis
At least 3 experiments derived from different donor NPCs were used for establishing repeatability of all qualitative observations. For all quantitative analysis, average and standard deviation (S.D.) also were also calculated using and the results from at least 3 independent experiments using different donor cells and are presented in graphs. ANOVA followed by Tukey-Kramer test and t-test (Graph Pad Software, La Jolla, California, USA) was used to compare the quantitative results. Significance is labelled in the graphs with ‘***’ (P < 0.0001); ‘**’ (P < 0.01); and ‘*’ (P < 0.05).
Results
Cell culture analysis
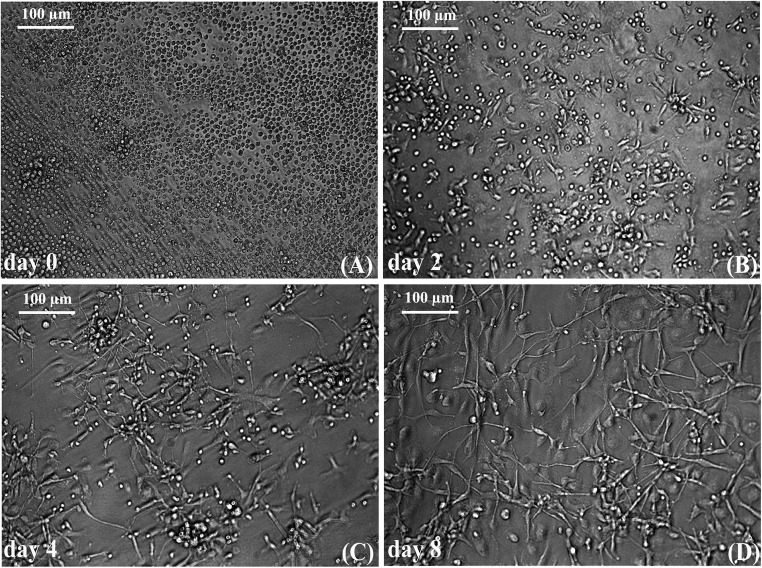
Cells with neuronal morphology was obtained within 8d of culture in the ‘T’ fibrin matrix (Fig.1) as seen previously (Tara and Krishnan 2015). Soon after seeding the isolated cell population in the ‘T’ matrix, the progenitor cell population was found to be colonized (Fig.1a). By 2d, slightly elongated cells were seen extending from majority of the cell colonies (Fig. 1b). With media changes, most of the non-specific cells were eliminated and by 4d, the cells further elongated to attain the typical neuronal-like morphology (Fig. 1c). By 8d, increased cell length with visible neurite-like branching and cell-cell contact was evident (Fig. 1d).
Fig. 1.
Micrographs of neuron like cells in culture on the test matrix: a Day0 PBMNCs seeded onto the test matrix showing cell colonies; b The colonies of cells giving rise to NLCs by 2d of culture on the test matrix; c Non-specific cells are removed and the NLCs are seen elongated; d The cells further elongate and branch out, filling the entire culture dish by day8
Effect of supplemented Wnt 3a protein/inhibitor
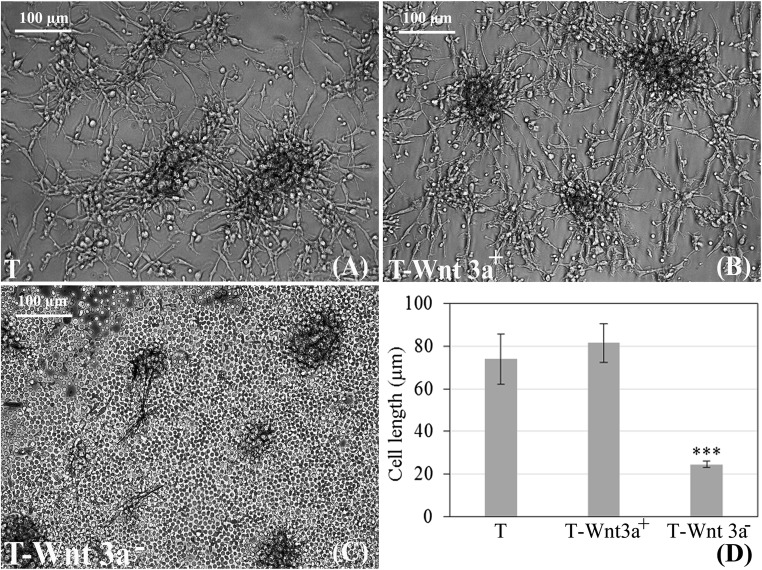
Colony formation was observed in ‘T’ and in the positive control (Fig. 2a–c) from which neural cells sprouted out, migrated and filled up the entire culture dish. The neural morphology was comparable in the ‘T’ and ‘T-Wnt 3a+‘cultures (Fig. 2a, b). Commercial Wnt inhibitor adversely affected cell morphology. There was a drastic decrease in the ability of cells in the negative control to elongate. Most of the cells remained round and they detached from the ‘T-Wnt 3a−’ surface within 9 days of culture (Fig. 2c).The length of the few remaining elongated cells in the ‘T-Wnt 3a-‘culture was comparatively much smaller (Fig. 2d). The cell length was ~75 μm upon culture in ‘T’. Even though in ‘T-Wnt3a+’, cell length increased marginally to ~80 μm, the difference was not statistically significant. However, the cell length decreased significantly to ~25 μm (P < 0.0001) in ‘T-Wnt 3a−’.
Fig. 2.
Micrographs showing effect of Wnt 3a signals on neural morphology: a & b No morphological difference in the derived NLCs between the test (T) and the Wnt 3a added (T-Wnt3a+) cultures are seen; c Drastic difference in cell morphology in presence of added Inhibitor (T-Wnt 3a−). d Quantitative data of neural cell length showing the difference between the test matrix, T-Wnt 3a+ and T-Wnt 3a− cultures. *** marks P < 0.0001 estimated by ANOVA
Effect of inhibition with antibodies
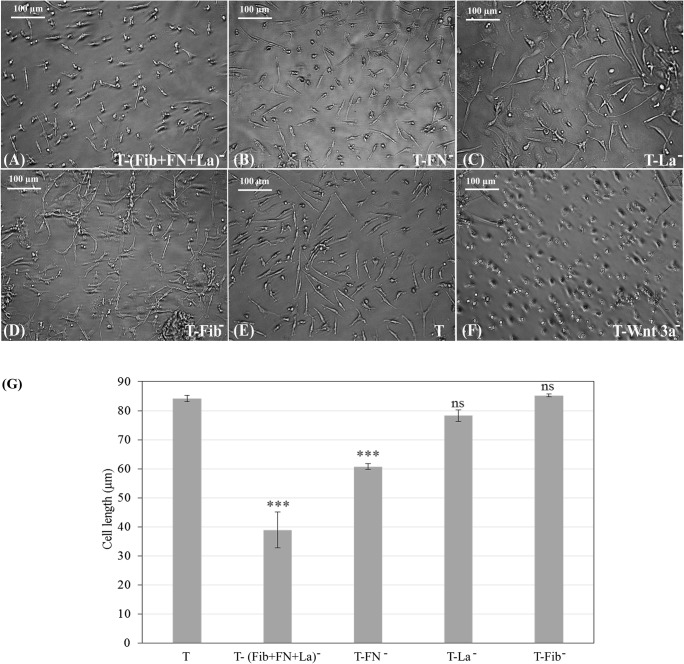
Blocking of protein moieties responsible for cell adhesion individually or collectively inhibited Wnt 3a-like signaling from the matrix, as evident from the cell morphology (Fig. 3a–f). The cell growth in ‘T’ (Fig. 3e) was compared with other antibody-treated cultures. Cell attachment and elongation was affected considerably in ‘T-FN−’ culture (Fig. 3b). Cells were more severely affected in ‘T-(Fib+FN + La)−’ and the effect was similar to that in ‘T-Wnt 3a−’, which is the negative control (Fig. 3f). In ‘T-Fib−‘and ‘T-La−’, the effect on cell morphology was nominal (Fig. 3b–d).
Fig. 3.
Micrographs showing morphology of cells grown on differently treated culture substrates: a ‘T’ substrate treated with cocktail of all 3 specific antibodies together i.e. [T-anti(Fib+ FN + La)−], b Anti-Fibronectin treated matrix [T-(FN)−], c Anti laminin treated matrix [T-(La)−], d Anti-Fibrin treated matrix [T-(Fib)−], e Test (standard) matrix (T), f Chemical inhibitor-treated matrix (T-Wnt 3a−). Culture substrates were pre-incubated with each in separate wells before seeding the cells. Scale bar = 100 μm (g) Quantitative data of neural cell length on inhibited matrices in comparison to test matrix (‘T’). *** marks P < 0.0001 estimated by ANOVA
Expression of Wnt and β-catenin proteins & Wnt target genes
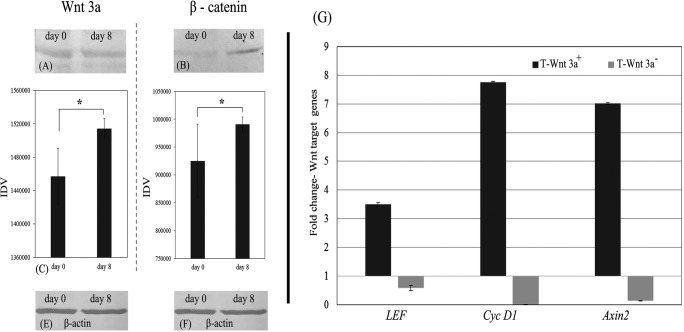
Wnt 3a and β-catenin was expressed in PBMNC intially and after culture for 8d on ‘T’ as shown by the Western blot analysis (Fig. 4a, b). Semi quantitative analysis of the band intensities of Western blot data shows statistically significant increase in the expression of β-catenin and Wnt-3a proteins suggesting triggering of Wnt-3a signals upon differentiation in the matrix (Fig. 4c). There was increase in the β-catenin expression as well and is found to be statistically significant (p = 0.03; Fig. 4d). β-actin was used as the loading control and its expression is shown in Fig. 4e, f.
Fig. 4.
Demonstration of Wnt3a and its effectors in cells: a-f Western blot analysis data; a shows Wnt protein in NPCs on day 0d and 8d of culture on test matrix (‘T’); b shows β-catenin protein in NPC on day 0d and 8d of culture on test matrix (‘T’); c Quantified Western blot band intensity of Wnt (40 kDa) on day 0d & 8d of culture; d Quantified Western Blot band intensity of β-catenin (92 kDa) day 0 & 8d of culture; e & f β-actin in NPC on day 0 and 8d of culture on test matrix (‘T’), respectively; *marks P < 0.05 estimated by t-test; g Quantitative RT-PCR data showing up regulation and down regulation of Wnt target genes - LEF1, Cyc D1 and Axin2 upon adding Wnt3a protein and chemical inhibitor to the test matrix (‘T’), respectively, as compared to the baseline gene expressions in cells grown on test matrix (‘T’). Graphical data is average ± S.D. of 3–6 replicates
In the positive control (T-Wnt 3a+), all three Wnt target genes were upregulated as compared to the effect in the ‘T’ culture (Fig. 4g). In the negative control (T-Wnt 3a−), all three Wnt target genes were downregulated. The increase in the LEF, Cyc D1 and Axin2 expression in the Wnt3a protein-added positive control was about 3.5-fold, 7.5-fold and 7-fold, respectively as compared to ‘T’. In the Wnt3a inhibitor-added negative control, the LEF, Cyc D1 and Axin2 expressions were down-regulated significantly.
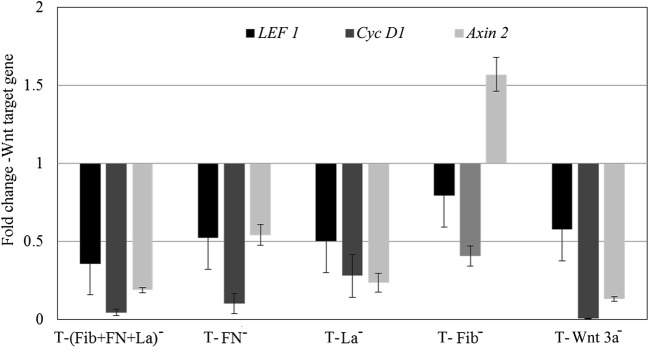
Interestingly, antibody-treated matrices down regulated Wnt target genes in NPCs except in one case i.e. when anti-fibrin was added auxin-2 remained upregulated (Fig. 5). The fold changes of downregulated LEF, Cyc D1 and Axin2 were ~0.3, 0.05 and 0.1, respectively in ‘T-(Fib+FN + La)-’, 0.5, 0.1 and 0.5 fold in ‘T- FN−’, and 0.5, 0.2 and 0.25 in ‘T-La−’. However, in ‘T-Fib−’, while the fold changes of downregulated LEF and Cyc D1 were 0.7 and 0.4 respectively, Axin2 was upregulated 1.5 fold. The down regulation of Wnt genes in the presence of antibodies is comparable to that in the negative control (Fig. 4e).
Fig. 5.
Effect of different antibodies on Wnt gene expressions. The graphical representation of the effect of antibodies against fibronectin (FN), fibrin (Fib), and laminin (La) upon their exposure with test matrix (‘T’) individually and in combination on the regulation of target genes- LEF, Cyc D1 and Axin2, as compared to the baseline value in test matrix (‘T’) is shown. Graphical qRT-PCR data is average ± S.D. of 3 replicate experiment
Effects on proliferation
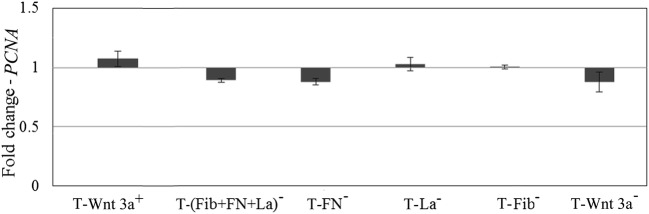
The cells cultured in ‘T’ showed proliferation till 8 days as marked by PCNA expression. The proliferation marker had increased marginally in the positive control; but was down regulated in the negative control. Similar downregulation in the proliferation marker expression was found in ‘T-FN−‘and ‘T-(FN + Fib+La)−also (Fig. 6). Neither ‘T-La−’ nor ‘T-Fib−’ showed a difference from ‘T’ on PCNA expression (Fig. 6).
Fig. 6.
Effect of Wnt signals on NPC proliferation. Quantitative RT-PCR data demonstrating activation of the PCNA gene upon cell culture after adding Wnt 3a protein to ‘T’ matrix (T-Wnt 3a+). Inhibition of PCNA when ‘T’ matrix was treated with chemical inhibitor (T-Wnt 3a−) or with antibodies against fibronectin (FN), fibrin (Fib), and laminin (La) individually or in combination as compared to baseline value in test matrix (‘T’) is also shown. Graphical representation of qRT-PCR data is average ± S.D. of 3 replicate experiment
Effect on neural gene expression
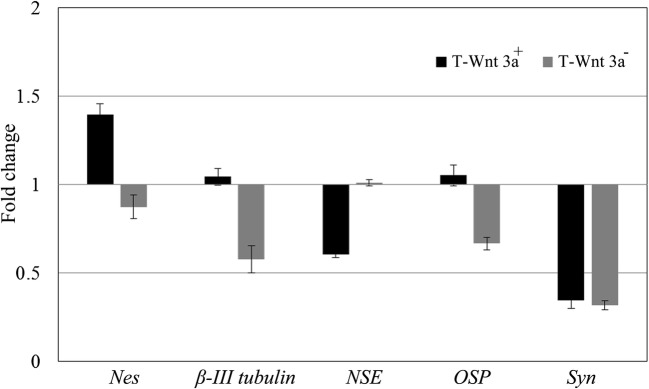
In the positive control, the neural progenitor marker Nes was upregulated whereas the NSE expression was downregulated (Fig. 7). The expression of a functional neuronal marker Syn was also downregulated. Both the intermediary markers of neuron and oligodendrocyte, namely, β-III tub and OSP, were marginally upregulated in the positive control as compared to ‘T’. The astrocyte marker, GFAP was not expressed in any of the cultures. In the negative control, the neuronal lineage markers were downregulated.
Fig. 7.
Effect of signal inducer and inhibitor on neural gene expression in NPC upon culture. Both Wnt 3a added (T-Wnt 3a+) and Inhibitor added (T-Wnt 3a−) cultures showed upregulation and downregulation of neural genes Nes, β-III tubulin, NSE, OSP and Syn, respectively. The values shown in the graph are normalised to baseline value in test matrix (‘T’). Graphical representation of qRT-PCR data is average ± S.D. of 3 replicate experiment
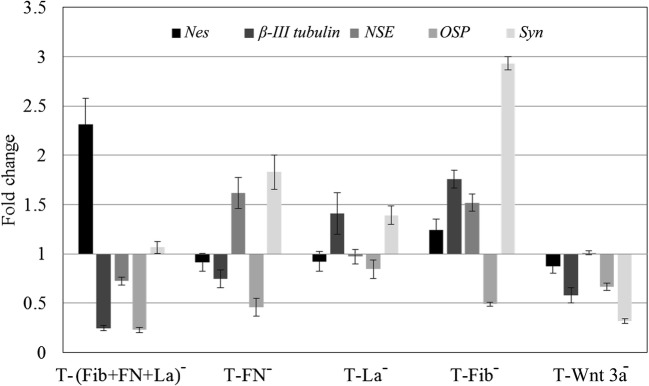
Upon adding antibodies, the signaling response affected each marker distinctly (Fig. 8). In the negative control, all 5 genes studied were downregulated. In ‘T-Fib−’, only one gene was downregulated, i.e., OSP, all the other 4 studied were upregulated. In ‘T-FN−’, involvement of FN in signalling was evident; Nes, β-III tub and OSP were downregulated. However, in the absence of FN, both NSE and Syn were upregulated. In ‘T-(FN + Fib+La)−’, upregulation of only the progenitor marker Nes was observed. All other neuronal markers involved in lineage commitment as well as maturation, including β-III tub and NSE, and OSP were downregulated. The expression of Syn was marginal.
Fig. 8.
Effect of treating test matrix (‘T’) with different antibodies on the neural specific gene expression. The graphical representation of the effect of antibodies against fibronectin (FN), fibrin (Fib), and laminin (La) individually and in combination on the expression of neural genes Nes, β-III tubulin, NSE, OSP and Syn, as compared to the baseline value in ‘test matrix (‘T’) is shown. Graphical representation of qRT-PCR data is average ± S.D. of 3 replicates
Effect on neural-specific proteins
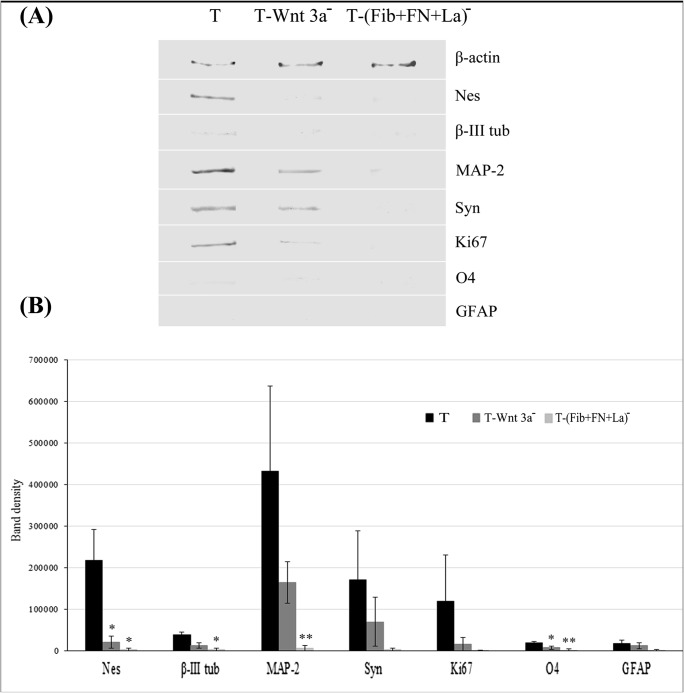
As compared to ‘T’, in the negative control, downregulation of marker proteins was evident (Fig. 9a). Similarly, the expression of all markers studied were down regulated in ‘T-(FN + Fib+La)−’ (Fig. 9a). Quantitative data of protein band intensities obtained from three replicate experiments is presented in graph 9B. Downregulation of all markers was more intense in ‘T-(FN+Fib+La)- as compared to ‘T’ and the negative control in all the donors studied. Statistically significant difference in the expression of Nes (P < 0.05), β-III tubulin (P < 0.05), MAP-2 (P < 0.01), and O4 (P < 0.01) in the matrix blocked cultures as compared to test wasobserved. The effect of supplemented chemical inhibitor and antibodies were similar at the transcriptional and translational levels (Figs. 8 and 9).
Fig. 9.
Effect of antibodies on the neural specific protein expressions in NPC (A) Effect of treating test ‘T’ substrate with chemical inhibitor and antibodies against fibronectin (FN), fibrin (Fib), and laminin (La) in combination on the neural/glial specific proteins and proliferation markers detected using Western Blot analysis is shown. The reduced molecular weights of the specific proteins are: β-actin (42 kDa), Nes (46 kDa), β-III tubulin (55 kDa), MAP-2 (42 kDa), Syn (38 kDa), O4 (22 kDa), GFAP (50 kDa) and Ki67 (55 kDa); (B) Semi quantitative analysis of band intensity of the proteins detected in Western blot is represented in the graph; data is average ± S.D. of 3 replicates. **indicates P < 0.01 and *indicates P < 0.05 estimated using ANOVA
Discussion
The identification of circulating neural progenitors and demonstration of their differentiation into NLC could pose questions regarding their biological significance. The uncertainties include (i) why are the progenitors released into peripheral blood? (ii) Can these progenitors contribute to homeostasis of CNS? It may be possible that the circulating progenitors participate in regeneration of the injured peripheral nerve and if they do so it should follow a mechanism similar to the homeostasis of adult tissue. Even though different signaling mechanisms are known to be involved in CNS homeostasis, the current study explored involvement of Wnt signalling. In the adult brain, Wnt signaling is known to play an important role in homeostasis (Malaterre et al. 2007). Out of different Wnt –linked mechanisms, β-catenin and cadherin pathways are primarily described for cell-cell adhesion, organization and maintenance of stem cells (Dravid et al. 2005). Wnt signaling is also implicated in cell fate determinations (Teo et al. 2006). It regulates the proliferation of NPC and their differentiation to neurons in the neurogenic regions of the brain (Alvarez-Palazuelos et al. 2011). Wnt signaling is also an obligate component involved in NPC differentiation into neurons (Cajánek et al. 2009). Wnt pathway activation by a specific pharmacological inhibitor of glycogen synthase kinase-3 maintains the undifferentiated phenotype in ESCs and sustains expression of the pluripotent, state-specific transcription factors. Similarly, Wnt signaling has been found to promote proliferation in the retina of adult mammals (Osakada et al. 2007). Such literature reports barbed us to explore possible role of Wnt in the NPC system that was standardized earlier (Tara and Krishnan 2015).
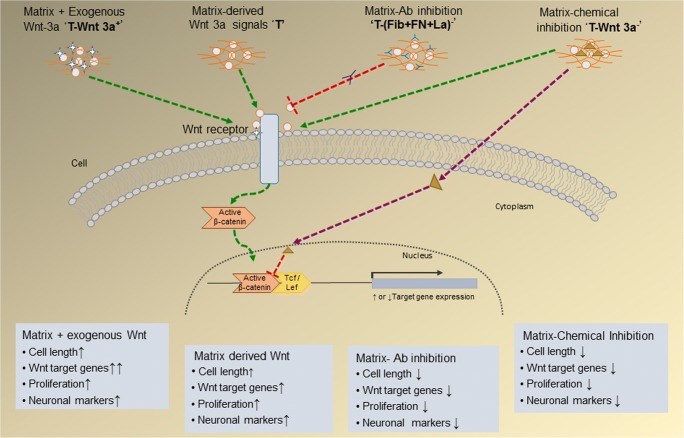
In the NPC system, neural progenitors with proliferation and differentiation potential were obtained from peripheral blood, which showed stage-specific expression of neuronal markers nestin, β-III tubulin and MAP-2. It has been observed that different adhesive proteins are essential constituents of the neuron-specific matrix for NPC survival and differentiation (Jose and Krishnan 2010); (Tara and Krishnan 2015). The matrix proteins FN and La are known to elicit various signals for cell survival, proliferation and differentiation, in addition to their key role in adhesion and spreading of cells. Therefore, this study proposed that specific moieties of these proteins in the matrix may elicit Wnt-like signaling to induce NPC to neurons (Fig.10), which could be inhibited using antibodies without affecting adhesion as seen in the Fig. 3.
Fig. 10.
Schematic representation of proposed Wnt signals in in vitro differentiation of circulating NPCs on the fibrin based matrix (T). The influences demonstrated upon adding Wnt 3a exogeneously on signal activation; inhibition by chemical inhibitor and inhibition by protein specific antibodies individually and in combination on cellular and molecular changes in NPC culture on fibrin-based niche are summarized in the figure
Wnt 3a is used as an activator of canonical Wnt signaling in a variety of cell systems (Lee et al. 2000). In this study the serum was given initially to facilitate cell adhesion and was gradually withdrawn to eliminate the effect of serum constituents on Wnt signaling. After depleting the serum, bFGF was added to the culture medium and later withdrawn to allow the process of differentiation as described earlier (Wexler et al. 2009). Commercially available Wnt protein was supplemented in the niche to accelerate signalling but morphological features were not improved by this. So it may be inferred that Wnt signaling is already elicited by the matrix proteins and caused best possible morphological changes of NPC to NLC in this culture system. The chemical inhibitor selected is known to impede the interaction between β-catenin and T cell factor 4 (Tcf4) leading to disruption of the Wnt signaling pathway. Complete disruption of all morphological features that occurred before adding the inhibitor strongly suggest that Wnt signaling is necessary for cell maintenance, proliferation and progression to NLC. Based on published literature (Kondo et al. 2011); (Demilly et al. 2013) and our own preliminary experiments, non-toxic concentrations of Wnt proteins and inhibitor were optimized for the current signaling experiments. Therefore, the effect of chemical inhibitor on Wnt-specific targets is responsible for all negative results seen in the negative control. Because the inhibitor was added to the culture after NPCs adhered and extended to neuron-like morphology which was lost upon adding inhibitor, the role of Wnt-like signalling could be confirmed.
Attempt to block Wnt-like signals that may be elicited by the adhesive matrix components using polyclonal antibodies is a crude way but since no signalling epitopes are reported so far, specific monoclonal antibodies could not be used. Specific involvement of RGD peptides and other peptide sequences are implicated in adhesion. Since the cell adhesion was not affected much as compared to ‘T’ matrix and inhibition effect was mostly similar to that in the negative control, it may be confirmed that the composed neuron-specific matrix elicit Wnt-like signals. The role of the matrix in activation of Wnt 3a-like signalling pathway for elongation of attached cells is evident from this study. Wnt 3a signals are also reported to cause reorganization of cytoskeleton including F-actin, other actin-associated proteins and villin (Shibamoto et al. 1998), so could probably inhibit NPC elongation to NLC as seen in this study.
Fibronectin is already known to direct cell adhesion, spreading, outgrowth and migration of other cell types (Chen et al. 2012) and has been found to be specifically important for NPC differentiation(Jose and Krishnan 2010). Fibronectin binding to fibrin is required for maximal cell attachment (Corbett et al. 1996) and it is also known to be involved in various signaling pathways. The ability of moieties present in FN to activate Wnt-like signaling is evident from this study. Fibrin and La are also known to have an effect on cell attachment. This study confirmed that La also plays a role in Wnt signaling because all three genes were inhibited when La was blocked with antibodies, even without any impact on morphological changes. In the case of Fib blocking, only two genes in the Wnt-signaling pathway were downregulated, while one was upregulated. So, there is differential regulation of genes in the pathway. Involvement of Wnt-3a signalling in the differentiation process was confirmed by protein expression studies. More extensive study may reveal effect of these proteins on differentiation of NPC to different neural cell types. Current scope was only w.r.t. NPC differentiation to NLC and expression of specific markers for this cell type.
Activation of Wnt/β-catenin signaling increases TCF-mediated transcription and expression of the Wnt target genes Axin2, LEF1 and Cyclin D1 in ReN b5 cell and VM cells has been observed (Hirsch et al. 2007); (Hübner et al. 2010). Also, Wnt 3a activation mediated by β-catenin promoted differentiation in the P19 cell line through Axin downregulation (Lyu et al. 2003). In this study, Axin expression was found to be upregulated in the absence of fibrin (T-Fib−). Therefore, the presence of Fib may have downregulated Axin-2, resulting in the progression of NPC differentiation to NLC as in the case of the P19 cells.
Earlier, FN was identified as an important adhesive protein to elicit neuronal induction in MAPC/NPC (Jose and Krishnan 2010). In this study, all three matrix proteins are seen to be involved in activating Wnt cascade as per the gene expression profile. Individually, each constituent showed specific effect on selected neural gene markers. Depletion of fibrin was found to increase synaptophysin expression and in the fibrin depleted matrix, more cell-cell contact is seen (Fig. 3d) as compared to other surfaces. Therefore, post differentiation, depletion of fibrin might promote synapse formation which needs to be established in future studies. If proven, it becomes a significant observation, because during tissue injury fibrin deposits and promotes cell adhesion and growth but complete fibrinolysis may be necessary for proper neuronal synapse formation. This could have more relevance in the context of neuronal regeneration in stroke patients. The decrease in expression of the neural proteins in the presence of anti-matrix immunoglobulins confirmed the role of Wnt signaling from matrix components in regulating proliferation and enhancing differentiation of neural progenitors. From these results we assume that differentiation of the circulating progenitors to neurons (Tara and Krishnan 2015) is through natural signalling mechanism (Wnt 3a). Therefore, NPC derived from peripheral blood could be a cell source with translational potential for successful regenerative therapies if the matrix composite is available in vivo for post transplantation cell survival and differentiation. It is possible that other similar signals linked to Wnt family also may be operating which is not included under the scope of the current study.
Conclusion
Presence of Wnt3a protein in NPC which is up regulated upon cell culture on fibrin-based niche is demonstrated using Western Blotting. Wnt3a inhibitor alone could completely disrupt NPC to NLC differentiation on fibrin based niche. A similar inhibitory response to Wnt signaling pathway is seen when fibrin-based culture substrate was pretreated with antibodies against FN, Fib and La indicating that these adhesive protein play a role in activation of Wnt 3a signals in NPC. The efficacy of cell-based therapies depends on the ability to control proliferation, and differentiation of NPCs through natural processes. Therefore, involvement of composite matrix in eliciting Wnt-like signaling proposes the standardized niche to be prospective biomimetic matrix for neural engineering to promote neural regeneration. Since post tissue injury angiogenesis and neurogenesis are parallel processes, the results of this study postulate involvement of circulating NPCs in peripheral nerve repair. Tissue injury-associated fibrin may promote harness of NPC, promote neural differentiation and establish synapse formation as and when fibrin is removed through fibrinolysis. Future experiments may focus on proving this hypothesis.
Acknowledgements
The authors acknowledge The Head, Biomedical Technology Wing and The Director of SCTIMST for the encouragement and support throughout this study. We thank Ms. Priyanka S and Mr. Ranjith S of the Thrombosis Research Unit for providing human fibrinogen and thrombin. This work was supported by the project grant received by Department of Science and Technology (DST), Govt of India to LKK; Council of Scientific and Industrial Research and Indian Council of Medical Research fellowships, Govt of India, to Tara S.
Abbreviations
- CNS
Central Nervous system
- NPC
Neural Progenitor Cells
- PBMNC
Peripheral Blood Mononuclear Cells
- FN
Fibronectin
- Fib
Fibrinogen
- La
Laminin
- T
test
References
- Alvarez-Palazuelos LE, Robles-Cervantes MS, Castillo-Velazquez G, et al. Regulation of neural stem cell in the human SVZ by trophic and morphogenic factors. Curr Signal Transduction Ther. 2011;6:320–326. doi: 10.2174/157436211797483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajánek L, Ribeiro D, Liste I, et al. Wnt/beta-catenin signaling blockade promotes neuronal induction and dopaminergic differentiation in embryonic stem cells. Stem Cells Dayt Ohio. 2009;27:2917–2927. doi: 10.1002/stem.210. [DOI] [PubMed] [Google Scholar]
- Chen X, Su Y-D, Ajeti V, et al. Cell adhesion on micro-structured fibronectin gradients fabricated by multiphoton excited photochemistry. Cell Mol Bioeng. 2012;5:307–319. doi: 10.1007/s12195-012-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996;88:158–166. [PubMed] [Google Scholar]
- Demilly A, Steinmetz P, Gazave E, et al. Involvement of the Wnt/β-catenin pathway in neurectoderm architecture in Platynereis dumerilii. Nat Commun. 2013;4:1915. doi: 10.1038/ncomms2915. [DOI] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, et al. Defining the role of Wnt/β-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hirsch C, Campano LM, Wöhrle S, Hecht A. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res. 2007;313:572–587. doi: 10.1016/j.yexcr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Hsu Y-C, Chen S-L, Wang D-Y, Chiu I-M. Stem cell-based therapy in neural repair. Biom J. 2013;36:98–105. doi: 10.4103/2319-4170.113226. [DOI] [PubMed] [Google Scholar]
- Hübner R, Schmöle A-C, Liedmann A, et al. Differentiation of human neural progenitor cells regulated by Wnt-3a. Biochem Biophys Res Commun. 2010;400:358–362. doi: 10.1016/j.bbrc.2010.08.066. [DOI] [PubMed] [Google Scholar]
- Jose A, Krishnan LK. Effect of matrix composition on differentiation of nestin-positive neural progenitors from circulation into neurons. J Neural Eng. 2010;7:036009. doi: 10.1088/1741-2560/7/3/036009. [DOI] [PubMed] [Google Scholar]
- Kondo T, Matsuoka AJ, Shimomura A, et al. Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells Dayt Ohio. 2011;29:836–846. doi: 10.1002/stem.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development (Cambridge, English) 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Lyu J, Costantini F, Jho E, Joo C. Ectopic expression of Axin blocks neuronal differentiation of embryonic carcinoma P19 cells. J Biol Chem. 2003;278:13487–13495. doi: 10.1074/jbc.M300591200. [DOI] [PubMed] [Google Scholar]
- Malaterre J, Ramsay RG, Mantamadiotis T. Wnt-frizzled signalling and the many paths to neural development and adult brain homeostasis. Front Biosci J Virtual Libr. 2007;12:492–506. doi: 10.2741/2077. [DOI] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313:915–921. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27(15):4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat Y, Porozov S, Belkin D, et al. Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial and neural lineages. Br J Haematol. 2006;135:703–714. doi: 10.1111/j.1365-2141.2006.06344.x. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, et al. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells Devoted Mol Cell Mech. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE. Wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345(2):789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Tanabe S. Signaling involved in stem cell reprogramming and differentiation. World J Stem Cells. 2015;7:992–998. doi: 10.4252/wjsc.v7.i7.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tara S, Krishnan LK. Bioengineered fibrin-based niche to direct outgrowth of circulating progenitors into neuron-like cells for potential use in cellular therapy. J Neural Eng. 2015;12:036011. doi: 10.1088/1741-2560/12/3/036011. [DOI] [PubMed] [Google Scholar]
- Teo R, Möhrlen F, Plickert G, et al. An evolutionary conserved role of Wnt signaling in stem cell fate decision. Dev Biol. 2006;289:91–99. doi: 10.1016/j.ydbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Vandervelde S, Vanluyn M, Tio R, Harmsen M. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Paucer A, Kornblum HI, et al. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells Dayt Ohio. 2009;27:1130–1141. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]