Summary
Objectives
Lipodystrophies are characterized by regional or generalized loss of adipose tissue and severe metabolic complications. The role of visceral adipose tissue (VAT) in the development of metabolic derangements in lipodystrophy is unknown. The study aim was to investigate VAT contribution to metabolic disease in lipodystrophy versus healthy controls.
Methods
Analysis of correlations between VAT volume and biomarkers of metabolic disease in 93 patients and 93 age/sex‐matched healthy controls.
Results
Patients with generalized lipodystrophy (n = 43) had lower VAT compared with matched controls, while those with partial lipodystrophy (n = 50) had higher VAT versus controls. Both groups with lipodystrophy had lower leg fat mass versus controls (p < 0.05 for all; unpaired t‐test). In both generalized and partial lipodystrophy, there was no correlation between VAT and glucose, triglycerides or high‐density lipoprotein cholesterol (p > 0.05 for all; Spearman correlation). In controls matched to patients with generalized or partial lipodystrophy, VAT correlated with glucose (R = 0.42 and 0.36), triglycerides (R = 0.36 and 0.60) and high‐density lipoprotein cholesterol (R = −0.34 and −0.64) (p < 0.05 for all; Spearman correlation).
Conclusions
In contrast to healthy controls, metabolic derangements in lipodystrophy did not correlate with VAT volume. These data suggest that, in lipodystrophy, impaired peripheral subcutaneous fat deposition may exert a larger effect than VAT accumulation on the development of metabolic complications. Interventions aimed at increasing functional subcutaneous adipose tissue may provide metabolic benefit.
Keywords: Lipodystrophy, subcutaneous adipose tissue, visceral adipose tissue
Introduction
Visceral adipose tissue (VAT) is one of the major white fat depots, together with lower body and upper body subcutaneous adipose tissue (SAT) 1. Opposite associations with metabolic disease have been reported for VAT and lower body SAT. In the general population, increased VAT is associated with higher prevalence of insulin resistance, diabetes, dyslipidaemia and the metabolic syndrome 2, 3. On the other hand, increased lower body SAT is associated with a favourable metabolic profile and decreased risk of metabolic syndrome 4, 5, 6. Similar to VAT, increased upper body SAT, especially the abdominal SAT, has been associated with increased metabolic disease, although this association is weaker compared with VAT 2, 3.
Generalized and partial lipodystrophies are rare genetic or acquired disorders characterized by deficient adipose tissue. Patients with generalized lipodystrophy have loss of essentially all SAT depots, while those with partial lipodystrophy usually have loss of gluteal and leg SAT, with variable loss of arm and trunk SAT. Patients with lipodystrophy develop ectopic triglyceride deposition in liver and muscle, leading to metabolic complications analogous to the obesity‐associated metabolic syndrome, including extreme insulin resistance and diabetes, and severe hypertriglyceridaemia 7, 8.
Preliminary data based on visual analysis of abdominal magnetic resonance imaging suggest that patients with generalized lipodystrophy usually have decreased VAT, while those with partial lipodystrophy may have decreased, preserved or increased VAT 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20; however, a quantification of VAT volume in lipodystrophy has not been reported in previous studies. Unlike in the general population, little is known about the role of VAT in the development of metabolic complications in lipodystrophy. Due to the unusual fat distribution in patients with lipodystrophy, they may serve as models to help understand the roles of VAT versus lower body SAT in the development of metabolic disease.
The primary aim of the current study was to understand the contribution of VAT to metabolic disease in patients with lipodystrophy compared with the general population. To achieve this aim, the relationship of VAT with metabolic parameters in patients with lipodystrophy and in healthy controls was analysed. The hypothesis was that, as in the general population, there would be a positive relationship between VAT and biomarkers of metabolic disease in patients with lipodystrophy.
Material and methods
Study design
This was a cross‐sectional study including patients with lipodystrophy and healthy controls. Patients with lipodystrophy participated in any of five protocols conducted at the National Institutes of Health (NIH), registered on clinicaltrials.gov as NCT00001987, NCT00025883, NCT01778556, NCT02262806 and NCT02639286. All protocols were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Written informed consent was obtained from patients or their legal guardians. Assent was obtained from participants under age 18 years.
Retrospective de‐identified data for healthy volunteers were collected through the NIH Biomedical Translational Research Information System 21. Use of individual subject data was exempted from informed consent by the NIH Office of Human Subjects Research Protection (OHSR_BTRIS_2017_1306_MALANDRINO_N_NIDDK).
Study subjects and variables
Subjects with lipodystrophy had a diagnosis of genetic or acquired, non‐HIV‐related lipodystrophy. Forty‐three metreleptin‐naïve patients with generalized lipodystrophy and 50 metreleptin‐naïve patients with partial lipodystrophy, with available VAT volume by Hologic dual‐energy X‐ray absorptiometry (DXA) and metabolic data during the same visit, were included.
Controls were selected among individuals enrolled as healthy volunteers in several NIH clinical trials between September 2014 and October 2017 who had DXA data available. A total of 376 healthy volunteers received at least one DXA scan during this time. Among these subjects, 11 were excluded from the present study due to concomitant medical diagnoses and/or possible or documented treatment with medications that might have affected their metabolic status, i.e. diagnosis of attention deficit disorder, bipolar disorder, alcohol use disorder, precocious puberty, HIV or treatment with HIV‐protease inhibitors and steroid treatment. One subject with a concomitant diagnosis of hypoparathyroidism and another subject erroneously included as healthy volunteer were also excluded. One subject was excluded due to large discrepancy in VAT volume measurements on three different DXA scans performed between 2014 and 2016, despite similar body weight. Three subjects were excluded due to unavailable VAT volume data. The remaining population comprised 359 healthy volunteers. Forty‐three sex‐matched paediatric subjects and 50 age‐matched and sex‐matched adult subjects, with available VAT volume by Hologic DXA and metabolic data within a 60‐d time frame, were selected from the cohort of 359 healthy volunteers.
Study procedures
Demographic, anthropometric and metabolic measurements
Age, sex, race and body mass index were recorded for all patients and controls. After an 8‐12 hour fast, blood glucose, HbA1c, triglycerides, and HDL‐C were collected and measured at the NIH Clinical Center laboratory, using standard methodology.
Dual‐energy X‐ray absorptiometry measurements
Total body fat percentage (FM%) and leg fat mass were assessed using a Hologic QDR 4500 (Hologic, Bedford, MA) scanner.
Visceral adipose tissue volume (cm3) was estimated by Hologic Apex 4.0 software as previously described 22, 23. In brief, the total abdominal adipose tissue contains both visceral and subcutaneous fat. DXA can directly measure a portion of the SAT localized lateral to the abdominal cavity. Hologic software uses a specific, proprietary algorithm to estimate the total amount of abdominal SAT volume from the portion directly assessed by DXA. VAT is estimated by subtracting total abdominal SAT from total abdominal adipose tissue. Hologic software measures VAT in a 5‐cm high region above the iliac crest, approximately at the level of the 4th lumbar vertebrae 22. This region of interest does not include the liver.
Statistical analysis
Visceral adipose tissue volume, leg fat mass and triglycerides were log transformed for analyses due to non‐normal distribution. VAT volume of zero was coded as 1 to permit log transformation for analysis.
The relationship between VAT and metabolic parameters was analysed separately in patients with generalized versus partial lipodystrophy and their matched controls. Differences in baseline characteristics and VAT volume between lipodystrophy and controls were analysed by χ2 test for categorical variables and unpaired t‐test, Welch's t‐test or Mann–Whitney U‐test for continuous variables. The relationship between VAT volume and metabolic parameters was assessed by Spearman correlation in patients with generalized and partial lipodystrophy and their corresponding controls.
Statistical analyses were performed using GraphPad Prism (version 7.00 GraphPad Software, La Jolla, CA, www.graphpad.com), Microsoft Excel and SAS (version 9.4, Cary, NC). Results are presented as mean ± standard deviation or median (25th and 75th percentile), based on distribution. A p value <0.05 was considered statistically significant.
Results
Baseline characteristics
Demographics and metabolic characteristics of the subjects with and without lipodystrophy are reported in Table 1. As expected, patients with lipodystrophy had significant metabolic disease compared with their matched controls, including higher fasting glucose, triglycerides and haemoglobin A1c and lower HDL‐C. VAT volume (Figure 1a,b) was lower in patients with generalized compared with partial lipodystrophy (93 [57, 153] vs. 534 [281, 810] cm3, p < 0.0001). However, VAT was detectable (greater than zero) in all but one patient with generalized lipodystrophy. VAT volume was significantly lower in patients with generalized lipodystrophy versus matched controls and significantly higher in patients with partial lipodystrophy versus matched controls.
Table 1.
Demographics and metabolic characteristics of subjects included in analyses of visceral adipose tissue volume versus metabolic parameters
| Partial lipodystrophy + matched controls | Generalized lipodystrophy + matched controls | |||||
|---|---|---|---|---|---|---|
| Lipodystrophy (N = 50) | Healthy controls (N = 50) | p value | Lipodystrophy (N = 43) | Healthy controls (N = 43) | p value | |
| Sex | 48F, 2M | 47F, 3M | >0.99 | 34F, 9M | 35F, 8M | >0.99 |
| Age (years) | 34.5 (17.0, 46.5) | 33.5 (15.8, 46.0) | 0.72 | 15.0 (10.0, 18.0) | 12.0 (9.0, 20.0) | 0.4 |
| Race (n) | 40 WNH, 2 B, 1 WH, 7 O | 37 WNH, 5 B, 4 WH, 4 O | 0.3 | 22 WNH, 9 B, 9 WH, 3 O | 23 WNH, 7 B, 5 WH, 8 O | 0.3 |
| Height (cm) | 163 (157, 170) | 165 (161, 171) | 0.3 | 160 (148, 167) | 155 (137, 175) | 0.08 |
| Weight (kg) | 68.1 ± 17.5 | 70.7 ± 23.0 | 0.5 | 50.9 ± 16.3 | 51.1 ± 20.3 | 0.9 |
| BMI (kg m−2) | 25.7 ± 5.2 | 26.0 ± 6.8 | 0.78 | 20.2 ± 3.6 | 21.5 ± 5.3 | 0.19 |
| Total body fat (%) | 27.5 ± 5.6 | 35.0 ± 7.6 | <0.0001 | 14.5 ± 2.9 | 31.2 ± 8.0 | <0.0001 |
| VAT volume (cm3) | 534 (281, 810) | 277 (138, 443) | <0.0001 | 93 (57, 153) | 153 (101, 212) | 0.02 |
| Leg fat mass (g) | 4,017 (3,371, 5,281) | 10,631 (6,466, 14,081) | <0.0001 | 1,794 (1,371, 2,856) | 6,298 (3,762, 10,983) | <0.0001 |
| Glucose (mmol L−1) | 6.4 (5.0, 10.1) | 5.1 (4.8, 5.3) | <0.0001 | 10.0 ± 4.6 | 4.9 (4.6, 5.2) | <0.0001 |
| HbA1c (%) | 7.6 ± 2.0 | 5.3 ± 0.4 (N = 32) | <0.0001 | 8.7 ± 2.5 | 4.9 ± 0.3 (N = 9) | <0.0001 |
| Triglycerides (mmol L−1) | 3.6 (2.4, 6.3) | 0.8 (0.6, 1.3) | <0.0001 | 5.3 (2.9, 13.8) | 0.68 (0.49, 0.90) | <0.0001 |
| HDL‐C (mmol L−1) | 0.8 ± 0.3 (N = 45) | 1.6 ± 0.4 | <0.0001 | 0.7 ± 0.2 (N = 39) | 1.5 ± 0.3 | <0.0001 |
Data are presented as mean ± standard deviation for normally distributed variables and median (25th and 75th percentile) for non‐normally distributed variables. B, African–American; BMI, body mass index; HbA1c, haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; O, other; VAT, visceral adipose tissue; WH, White Hispanic; WNH, White non‐Hispanic.
Figure 1.
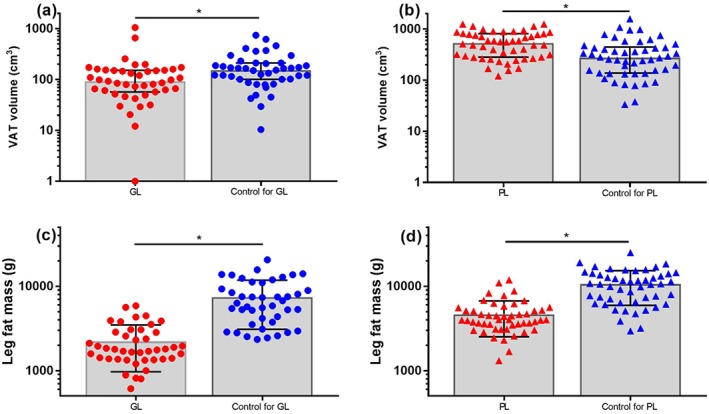
Visceral adipose tissue (VAT) volume (a and b) and leg fat mass (c and d) in patients with generalized lipodystrophy (GL, red circles) and partial lipodystrophy (PL, red triangles) versus age‐matched and sex‐matched controls (blue circles and triangles). VAT volume was zero in one patient with GL, coded as 1 to permit display on a logarithmic scale. * p < 0.05.
Total per cent body fat and leg fat mass (Table 1, Figure 1c,d) were lower in patients with both generalized and partial lipodystrophy compared with controls and were lower in patients with generalized lipodystrophy compared with partial lipodystrophy (p < 0.0001).
Relationship of visceral adipose tissue volume with metabolic parameters in lipodystrophy and controls
In analyses of patients with generalized lipodystrophy versus controls, control subjects demonstrated significant positive correlations between VAT volume and both glucose and triglycerides and a significant negative correlation between VAT volume and HDL‐C. By contrast, none of these relationships were significant in patients with generalized lipodystrophy (Figure 2, Table 2). In patients with generalized lipodystrophy, there was no correlation between VAT volume and haemoglobin A1c. Haemoglobin A1c was not available in matched controls (Table 2).
Figure 2.
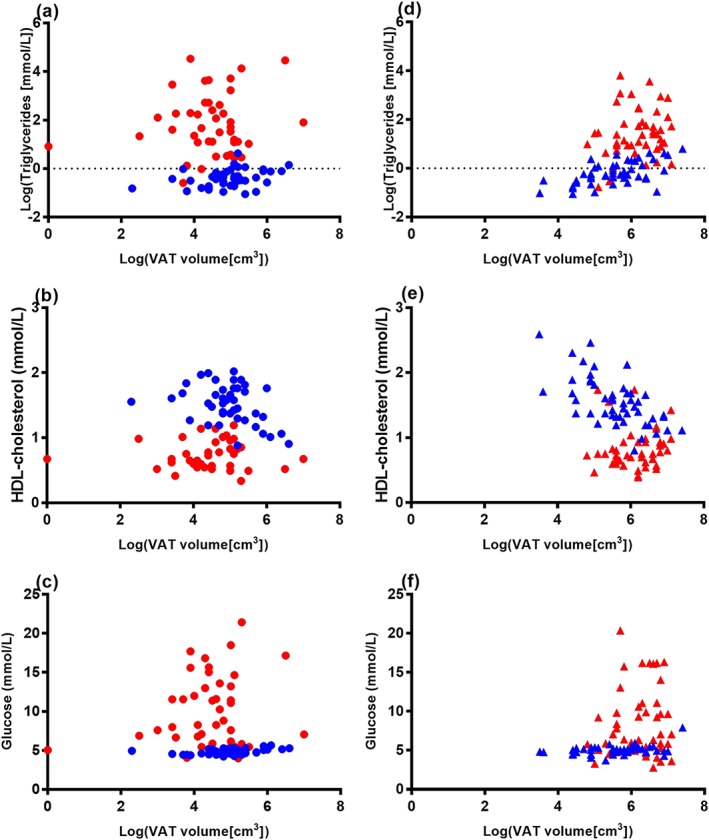
Correlation between visceral adipose tissue (VAT) volume and biomarkers of metabolic disease in patients with generalized and partial lipodystrophy (red circles and triangles, respectively) and age‐matched and sex‐matched controls (blue circles and triangles, respectively). Log‐transformed VAT volume was significantly correlated with log‐transformed triglycerides, high‐density lipoprotein (HDL) cholesterol and fasting plasma glucose, in healthy control subjects but not in patients with generalized lipodystrophy (a–c) or partial lipodystrophy (d–f).
Table 2.
Correlation between visceral adipose tissue and metabolic parameters in patients with lipodystrophy versus matched controls
| Generalized lipodystrophy (N = 43) | Control (N = 43) | Partial lipodystrophy (N = 50) | Control (N = 50) | |||||
|---|---|---|---|---|---|---|---|---|
| Spearman correlation coefficient | p value | Spearman correlation coefficient | p value | Spearman correlation coefficient | p value | Spearman correlation coefficient | p value | |
| Glucose | −0.01 | 0.9 | 0.42 | 0.005 | 0.14 | 0.3 | 0.36 | 0.013 |
| Haemoglobin A1c | −0.07 | 0.7 | n/a | n/a | 0.03 | 0.9 | 0.30 | 0.09 |
| Triglycerides | −0.01 | 0.9 | 0.36 | 0.02 | 0.18 | 0.2 | 0.60 | <0.0001 |
| HDL‐C | 0.05 (N = 39) | 0.8 | −0.34 | 0.03 | 0.22 (N = 45) | 0.1 | −0.64 | <0.0001 |
HDL‐C, high‐density lipoprotein cholesterol; n/a, not applicable.
In analyses of patients with partial lipodystrophy versus controls, control subjects again demonstrated significant positive correlations between VAT volume and both glucose and triglycerides and a significant negative correlation between VAT volume and HDL‐C. By contrast, correlations between VAT volume and these metabolic parameters were not significant in patients with partial lipodystrophy (Figure 2, Table 2). Control subjects had a trend towards positive correlation between VAT volume and haemoglobin A1c; this was not observed in patients with partial lipodystrophy (Table 2).
Discussion
The present study investigated the association of VAT with metabolic disease in metreleptin‐naïve patients with generalized and partial lipodystrophy compared with healthy controls. Although the expected associations were observed in healthy controls in this study, there were no significant associations between VAT and metabolic parameters in patients with lipodystrophy. This finding suggests that VAT may not be causally related to metabolic disease in patients with lipodystrophy. Lower body subcutaneous fat was markedly lower in patients with both generalized and partial lipodystrophy compared with control subjects. Thus, lipodystrophy serves as unique model to demonstrate that decreased lower body fat may be a major contributor to metabolic disease, without a required contribution from VAT.
Visceral adipose tissue is proposed to play a causal role in metabolic disease due to its higher sensitivity to lipolytic effects of catecholamines and lower suppression of lipolysis in response to insulin, compared with other white adipose depots 24. Direct release of free fatty acids (FFAs) from VAT lipolysis to the liver, via the portal vein, may contribute to ectopic fat deposition and development of hepatic insulin resistance 25. The contribution of VAT lipolysis to hepatic FFA delivery ranges from 5–10% in lean individuals to 20–25% in individuals with visceral obesity 26. Supporting this mechanism, numerous studies in the general population have shown significant correlations between VAT volume and metabolic parameters including fasting glucose, triglycerides, HOMA‐IR and HDL‐C 2, 3, 27, 28.
Despite adipose tissue deficiency, patients with lipodystrophy show increased rates of lipolysis, possibly due to increased lipolysis in the residual adipose tissue and/or increased intrahepatic or intravascular lipolysis 29, 30. The hypothesis of this study was that there would be a similar positive relationship between VAT and metabolic disease in patients with lipodystrophy and controls, resulting from contributions of VAT lipolysis to hepatic FFA delivery in both groups. Surprisingly, there was no correlation between VAT volume and metabolic parameters in patients with lipodystrophy. One possible explanation for the absence of a relationship between VAT and metabolic parameters in patients with lipodystrophy might have been related to a narrow range of VAT volume compared with controls, making it difficult to detect such a relationship. While this may be relevant for patients with generalized lipodystrophy, who typically had low VAT volume, patients with partial lipodystrophy had wide variability in VAT volume and actually had higher mean VAT volume compared with controls. Thus, if VAT was contributing to metabolic disease in partial lipodystrophy in the same manner as controls, an association between VAT volume and metabolic parameters should have been observed. In addition, while the patients were metreleptin naïve, most were receiving diabetes and/or lipid‐lowering agents, which might have affected the results. However, due to their severe metabolic disease, it is difficult to enrol patients who are not already treated with such medications.
The lack of correlation between VAT volume and metabolic parameters in subjects with lipodystrophy suggests that alternate pathophysiologic mechanisms may be leading to metabolic disease in this patient population. Patients with lipodystrophy show marked deficiency of lower body SAT and severe fatty liver disease. One hypothesis to explain these observations is that, in patients with lipodystrophy, metabolic disease is mainly mediated by the reduced lower body SAT, resulting in failure to store very low‐density lipoprotein‐derived (VLDL‐TG) and/or chylomicron‐derived triglycerides, which in turn leads to increased FFA spillover, hepatic FFA delivery, ectopic lipid deposition and insulin resistance.
Lower body SAT has a major role in long‐term storage of lipids, especially FFAs derived from VLDL‐TG, and plays a protective role against ectopic fat deposition 31, 32. During the fasting state, women with lower body obesity store a larger amount of VLDL‐TG in subcutaneous femoral fat compared with subcutaneous abdominal fat 33. This is particularly relevant given that this subgroup of individuals generally have a more favourable metabolic profile than those with upper body obesity. In addition, both in the fasting and post‐prandial state, women with increased femoral fat have greater efficiency of fatty acid uptake 34, 35.
Interestingly, Lotta and colleagues have recently identified genetic loci that may link decreased lower body SAT with insulin resistance both in the general population and in patients with familial partial lipodystrophy type 1 36. Furthermore, in a case study of patient with partial lipodystrophy caused by peroxisome proliferator‐activated receptor gamma mutation, the co‐existence of active lipolysis and inability to store meal‐derived FFAs has been observed, with increased FFA spillover suggested to occur in peripheral SAT. The authors hypothesize that the resulting FFA spillover would increase hepatic FFA delivery, leading to greater secretion of VLDL‐TG 37. These studies support the notion that absent lower body SAT in lipodystrophy results in inability to store surplus energy, leading to increased FFA delivery to the liver, ectopic lipid deposition, followed by insulin resistance and metabolic disease. Although the current data support this model, they cannot prove causality. Arteriovenous and stable isotope techniques could provide more accurate information on regional FFA kinetics, including lipolysis and FFA spillover. However, these techniques are invasive and difficult to perform in large numbers of individuals.
In conclusion, this study investigated the relationship between VAT and metabolic disease in patients with lipodystrophy and healthy controls. In contrast to controls, there were no significant correlations between VAT and metabolic parameters in lipodystrophy. These findings suggest that VAT does not play a major role in the development of metabolic disease in lipodystrophy. Instead, the authors speculate that limited expandability of lower body subcutaneous fat and increased liver fat deposition may exert a larger effect than VAT on the development of insulin resistance and metabolic complications in patients with lipodystrophy. While this study cannot distinguish the relative contributions of lower body subcutaneous fat versus VAT in the development of metabolic disease in the general population, it supports a growing body of evidence on the importance of deficient lower body subcutaneous fat in metabolic disease.
Conflict of Interest Statement
The authors declared no conflict of interest.
Funding
This study was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Acknowledgements
Noemi Malandrino and Rebecca J. Brown developed the rationale and designed the study. Noemi Malandrino, Sungyoung Auh and Rebecca J. Brown developed the data analysis plan and performed the statistical analysis. All authors contributed to the interpretation of the data and results and provided critical revision for important intellectual content. Noemi Malandrino and Rebecca J. Brown wrote the first draft of the manuscript. All authors provided final approval of the manuscript and agreed to be accountable for all aspects of the work. We are grateful to Mala Prasad for support in the collection and analysis of the DXA scans and Andrea Beri and Austin Layne, on the BTRIS Support & Analytics Team, for the collection of healthy volunteer data. Finally, we would like to thank our patients and their families.
Malandrino, N. , Reynolds, J. C. , Brychta, R. J. , Chen, K. Y. , Auh, S. , Gharib, A. M. , Startzell, M. , Cochran, E. K. , and Brown, R. J. (2019) Visceral fat does not contribute to metabolic disease in lipodystrophy. Obesity Science & Practice, 5: 75–82. 10.1002/osp4.319.
Clinical Trial Registration: This study included cross‐sectional baseline data obtained from clinical trials but did not itself constitute a clinical trial. Clinicaltrials.gov registration numbers were NCT00001987, NCT00025883, NCT01778556, NCT02262806 and NCT02639286.
References
- 1. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013; 17: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116: 39–48. [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 2010; 95: 5419–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab 2002; 282: E1023–E1028. [DOI] [PubMed] [Google Scholar]
- 5. Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005; 48: 301–308. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Hu EA, Wu H, Malik V, Sun Q. Associations of leg fat accumulation with adiposity‐related biological factors and risk of metabolic syndrome. Obesity (Silver Spring) 2013; 21: 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg A. Acquired and inherited lipodystrophies. N Engl J Med 2004; 350: 1220–1234. [DOI] [PubMed] [Google Scholar]
- 8. Joseph J, Shamburek RD, Cochran EK, Gorden P, Brown RJ. Lipid regulation in lipodystrophy versus the obesity‐associated metabolic syndrome: the dissociation of HDL‐C and triglycerides. J Clin Endocrinol Metab 2014; 99: E1676–E1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003; 82: 129–146. [DOI] [PubMed] [Google Scholar]
- 10. Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab 2003; 88: 5433–5437. [DOI] [PubMed] [Google Scholar]
- 11. Akinci B, Onay H, Demir T, et al. Natural history of congenital generalized lipodystrophy: a nationwide study from Turkey. J Clin Endocrinol Metab 2016; 101: 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al‐Attar SA, Pollex RL, Robinson JF, et al. Quantitative and qualitative differences in subcutaneous adipose tissue stores across lipodystrophy types shown by magnetic resonance imaging. BMC Med Imaging 2007; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akinci B, Onay H, Demir T, et al. Clinical presentations, metabolic abnormalities and end‐organ complications in patients with familial partial lipodystrophy. Metab Clin Exp 2017; 72: 109–119. [DOI] [PubMed] [Google Scholar]
- 14. Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 1999; 84: 170–174. [DOI] [PubMed] [Google Scholar]
- 15. Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore) 2004; 83: 18–34. [DOI] [PubMed] [Google Scholar]
- 16. Robertson DA, Wright R. Cirrhosis in partial lipodystrophy. Postgrad Med J 1989; 65: 318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Premkumar A, Chow C, Bhandarkar P, et al. Lipoatrophic‐lipodystrophic syndromes: the spectrum of findings on MR imaging. AJR Am J Roentgenol 2002; 178: 311–318. [DOI] [PubMed] [Google Scholar]
- 18. Yuh WT, Collison JS, Sickels WJ, Barloon TJ, Brennan DC, Flanigan MJ. Partial lipodystrophy. Magnetic resonance findings in one case. J Comput Tomogr 1988; 12: 287–291. [DOI] [PubMed] [Google Scholar]
- 19. Singhal PC, Sakhuja V, Jain SK, Chugh KS. Partial lipodystrophy, hypocomplementaemia and dense deposit disease. J Indian Med Assoc 1983; 81: 201–202. [PubMed] [Google Scholar]
- 20. McNeill AD. Progressive lipodystrophy. Br J Surg 1966; 53: 216–218. [DOI] [PubMed] [Google Scholar]
- 21. Cimino JJ, Ayres EJ, Remennik L, et al. The National Institutes of Health's Biomedical Translational Research Information System (BTRIS): design, contents, functionality and experience to date. J Biomed Inform 2014; 52: 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual‐energy X‐ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012; 20: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaul S, Rothney MP, Peters DM, et al. Dual‐energy X‐ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012; 20: 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poulain‐Godefroy O, Lecoeur C, Pattou F, Fruhbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1–R7. [DOI] [PubMed] [Google Scholar]
- 25. Svedberg J, Stromblad G, Wirth A, Smith U, Bjorntorp P. Fatty acids in the portal vein of the rat regulate hepatic insulin clearance. J Clin Invest 1991; 88: 2054–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113: 1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vega GL, Adams‐Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 2006; 91: 4459–4466. [DOI] [PubMed] [Google Scholar]
- 28. Sasai H, Brychta RJ, Wood RP, et al. Does visceral fat estimated by dual‐energy X‐ray absorptiometry independently predict cardiometabolic risks in adults? J Diabetes Sci Technol 2015; 9: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 2002; 109: 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown RJ, Valencia A, Startzell M, et al. Metreleptin improves insulin sensitivity independent of food intake in humans with lipodystrophy. J Clin Invest 2018; 128: 3504–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karpe F, Pinnick KE. Biology of upper‐body and lower‐body adipose tissue – link to whole‐body phenotypes. Nat Rev Endocrinol 2015; 11: 90–100. [DOI] [PubMed] [Google Scholar]
- 32. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010; 34: 949–959. [DOI] [PubMed] [Google Scholar]
- 33. Gormsen LC, Nellemann B, Sorensen LP, Jensen MD, Christiansen JS, Nielsen S. Impact of body composition on very‐low‐density lipoprotein‐triglycerides kinetics. Am J Physiol Endocrinol Metab 2009; 296: E165–E173. [DOI] [PubMed] [Google Scholar]
- 34. Nellemann B, Gormsen LC, Christiansen JS, Jensen MD, Nielsen S. Postabsorptive VLDL‐TG fatty acid storage in adipose tissue in lean and obese women. Obesity (Silver Spring) 2010; 18: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes 2007; 56: 2589–2597. [DOI] [PubMed] [Google Scholar]
- 36. Lotta LA, Gulati P, Day FR, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017; 49: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan GD, Savage DB, Fielding BA, et al. Fatty acid metabolism in patients with PPARgamma mutations. J Clin Endocrinol Metab 2008; 93: 4462–4470. [DOI] [PubMed] [Google Scholar]


