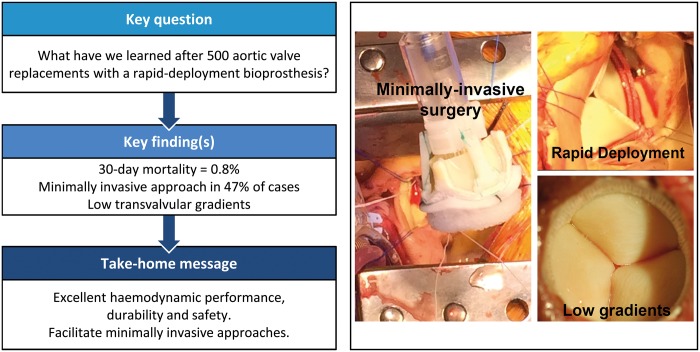
Abstract
OBJECTIVES
The Edwards INTUITY Valve System is a balloon-expandable bioprosthesis, inspired from the Edwards Magna valve and transcatheter technology, with a subvalvular stent frame to enable rapid deployment. We report a single-centre experience of aortic valve replacement with this novel bioprosthesis.
METHODS
Five hundred consecutive patients, of whom 45.6% were female with a mean age of 73.5 [standard deviation (SD) 7.9 years], with severe aortic stenosis who received a rapid deployment aortic valve between May 2010 and July 2017 were included in a prospective and ongoing database. The median follow-up time was 12 months, and the total accumulated follow-up time was 818 patient years. Preoperative characteristics, operative parameters, survival, valve-related adverse events and valve haemodynamics were assessed.
RESULTS
Thirty-day mortality was 0.8% (4/500), and overall survival at 1, 3 and 5 years was 94%, 89% and 81%, respectively. A minimally invasive surgical approach was chosen in 236 patients (47%), of which 122 (24%) were operated on through an anterior right thoracotomy. Cross-clamp and cardiopulmonary bypass times for isolated aortic valve replacement were 53 (SD 17) and 89 (SD 29) min for full sternotomy as well as 75 (SD 23) and 110 (SD 31) min for minimally invasive surgery approaches (P < 0.001). Mean gradients at discharge, 1, 3 and 5 years were 13 (SD 5), 11 (SD 4), 12 (SD 5) and 11 (SD 3) mmHg, respectively. New pacemaker implantation was necessary in 8.6% of patients. A single case (0.2%) of structural degeneration was registered after 6 years. Valve explantation for non-structural dysfunction or endocarditis occurred in 9 patients (1.8%).
CONCLUSIONS
This rapid deployment aortic valve has shown excellent results concerning haemodynamic performance, durability and safety. Implantation requires specific training, and the rate of pacemaker implantation remains a matter of concern. This novel valve also facilitates minimally invasive approaches and may be beneficial in complex combined procedures.
Keywords: Aortic valve replacement , Rapid-deployment , Transvalvular gradient , Minimally invasive surgery
INTRODUCTION
Surgical aortic valve replacement (SAVR) is the gold standard for treatment of severe aortic stenosis. Currently, more than 80% of patients are implanted with a tissue valve [1]. Transcatheter aortic valve implantation (TAVI) was widely accepted as a standard approach in elderly high-risk patients who are not eligible for standard cardiac surgery. However, routine TAVI use in lower risk patient populations is still controversial and long-term results are required [2]. TAVI displaces the native valve and anchors itself into the calcified area, while SAVR assumes the removal of the modified valve prior to implantation; this fact may contribute to a higher incidence of paravalvular regurgitation, increased rates of conduction disturbances and a higher risk of subclinical leaflet thrombosis in TAVI patients [3]. Therefore, this technical burden of TAVI technology may lead to an ongoing need for SAVR in several patients.
A major step forward in surgical techniques was the introduction of minimally invasive approaches for valve surgery [4]. To overcome the increased technical complexity, novel surgical rapid-deployment aortic bioprostheses have been developed [5]. These systems facilitate minimally invasive procedures and may provide better haemodynamics [6–8]. We report herein a comprehensive 6-year single-centre experience of 13 different surgeons with the Edwards INTUITY Valve System with regard to survival, reoperation rate, valve-related adverse events and haemodynamic performance.
MATERIALS AND METHODS
Study population
All 500 consecutive patients who underwent successful isolated or combined SAVR with an Edwards INTUITY rapid deployment aortic valve (RD-AV) system (all generations—G1A, G1B and G2) between May 2010 and July 2017 at the Department of Cardiac Surgery at the Medical University of Vienna were included in a prospective ongoing database with longitudinal end point assessment (Vienna Intuity Comprehensive Evaluation—VICE Registry), and are reported herein. The institutional review board approved this registry (1861/2016), and patients signed the informed consent postoperatively. Comprehensive safety and effectiveness of this novel bioprosthesis was assessed. Furthermore, the number of failed implantations was collected, but patients were not further analysed in this registry if another valve type was implanted (‘as treated’ analysis).
Patients receiving the RD valve were initially included in the TRITON (n = 83) market-release trial (Surgical Treatment of Aortic Stenosis with a Next-Generation Surgical Aortic Valve, clinical trial number: NCT01445171 on http://clinicaltrials.gov) and thereafter part of the FOUNDATION (n = 63) post-market release registry (Assessing Standard of Care and Clinical Outcomes Using the EDWARDS INTUITY Valve System in an European Multicentre, Active, Post‐market Surveillance Study, clinical trial number: NCT02338154 on http://clinicaltrials.gov) or MISSION (n = 44) Registry (Assessing Clinical Outcomes Using the EDWARDS INTUITY Elite Valve System in Isolated AVR Using Minimally Invasive Surgery in a European Multi-center, Active, Post-market Registry, clinical trial number: NCT02907463 on http://clinicaltrials.gov). Furthermore, all patients who received the rapid-deployment valve outside these trials were included in this analysis and followed up according to the VICE protocol. This comprises (i) clinical follow-up at discharge, 3 months, 1, 3, 5 and 10 years, which evaluates the clinical status on the basis of the functional classification of the New York Heart Association (NYHA) and occurrence of any adverse events, (ii) haemodynamic performance by transthoracic echocardiography and (iii) electrocardiogram during the follow-up visits. At 2, 4 and 7 years postoperatively, a telephone follow-up interview was performed. The follow-up time was up to 7 years, with a mean of 19 [standard deviation (SD) 21] months and a median of 12 (3–34) months. For the sake of this study, the database was locked at the end of August 2017.
Surgical techniques
A detailed description of the procedure was reported previously. Technical success was defined as successful implantation of the INTUITY valve within 2 attempts [6].
Study end points
The primary end points of the registry are assessment of short- and long-term survival, structural-/non-structural valve dysfunction and haemodynamic valve performance by transthoracic echocardiography [9, 10]. As secondary end points, we assessed the occurrence of valve-related events according to the guidelines for reporting mortality and morbidity after heart valve surgery [9].
Mortality
We included all deaths after valve implantation regardless of the cause for the calculation of overall mortality. Early mortality was defined as mortality within the first 30 postoperative days, in-hospital mortality was defined as any event occurring between surgery and first discharge, and perioperative mortality was calculated as a cumulative value of early and in-hospital mortality. The risk of early postoperative mortality was assessed at baseline by means of the EuroSCORE II and logistic EuroSCORE (European system for cardiac operative risk evaluation) and retrospectively using the STS score (Society of Thoracic Surgeons Score). Patients at intermediate and high preoperative risk were defined as patients with an STS score of 4–8% and above 8%, respectively.
Morbidity
Valve-related adverse events, including structural valve deterioration, non-structural valve deterioration, endocarditis, major bleeding events, valve thrombosis, stroke, transient ischaemic attack, peripheral emboli, pacemaker implantation and myocardial infarction, were assessed during the follow-up according to the current guidelines [9]. Reoperations were categorized according to the underlying pathology into reoperations for structural valve disease, non-structural valve disease, valve thrombosis and endocarditis. Early surgical revision for bleeding (intrathoracic bleeding or haematoma requiring re-entry or subxiphoidal drainage) was separated from the major bleeding events and reported as revisions.
Statistical analysis
Descriptive statistical methods were applied to depict the study population. Continuous variables were presented as mean and SDs or median (25th–75th interval). Total numbers and proportions were reported for categorical outcomes. The Kaplan–Meier and life table methods were performed to assess survival and valve-related events. The safety end points of this trial are reported as early (≤30 postoperative day) or late (>30 postoperative day) events. Early events are reported as numbers and percentages and late events as linearized event rates per patient year (%ppy) of follow-up and calculated as a cumulative number of late events divided by the total patient-years. Statistical calculations comparing continuous variables were made using the t-test or the Wilcoxon–Mann–Whitney test (non-parametric variables), and comparisons of categorical variables were made using the Pearson’s χ2 test. The IBM SPSS Statistics 24 (IBM Corp. Released 2016, IBM SPSS Statistics for Mac, Version 24.0. Armonk, NY: IBM Corp.) was used for statistical analysis. A P-value of <0.05 was considered as significant.
RESULTS
Demographics and baseline characteristics
Between May 2010 and July 2017, a total of 500 patients underwent SAVR with the INTUITY Valve System at our institution. The mean age was 73.5 (SD 7.9) years (range 41–93), and 228 (45.6%) were women. Sixty-four percent of patients presented with NYHA functional class III or IV symptoms. The predicted operative risk was estimated preoperatively by means of EuroSCORE II and logistic EuroSCORE, revealing a median risk of early mortality of 2.3 (1.4–4.2) and 6.7 (4.3–11.7), respectively. We also estimated the risk of mortality retrospectively by means of the STS score, revealing 87 (17.4%) patients at intermediate risk and 21 (4.2%) patients at high preoperative risk. Baseline characteristics and cardiovascular comorbidities are listed in Table 1.
Table 1:
Baseline patient characteristics
| Factors | |
|---|---|
| Age (years) | 73.5 (7.9) |
| Female gender | 228 (46) |
| Height (cm) | 169 (9) |
| Weight (kg) | 80 (16) |
| Body mass index (kg/m2) | 27.7 (5.3) |
| Body surface area (m2) | 1.91 (0.2) |
| Logistic EuroSCORE (25th–75th interval) | 6.7 (4.3–11.7) |
| EuroSCORE II (25th–75th interval) | 2.3 (1.4–4.2) |
| STS score (25th–75th interval) | 2.3 (1.5–3.7) |
| Low risk (<4%) | 392 (78) |
| Intermediate risk (4–8%) | 87 (17) |
| High risk (>8%) | 21 (4) |
| Diabetes | 140 (28) |
| Dyslipidaemia | 292 (58) |
| Coronary artery disease | 193 (39) |
| Cerebrovascular disease | 78 (16) |
| Renal insufficiency | 68 (14) |
| Creatinine | 1.1 (0.6) |
| Peripheral vascular disease | 35 (7) |
| Chronic lung disease | 113 (23) |
| Previous cardiovascular interventions | 72 (14) |
| Previous valve surgery | 11 (2) |
| Previous pacemaker implantation | 19 (4) |
| Previous rhythm abnormalities | 178 (36) |
| Previous atrial fibrillation | 101 (20) |
| Paroxysmal | 53 (11) |
| Persistent | 48 (10) |
Continuous data are presented as mean (standard deviation) and categorical data as n (%). The STS score, EuroSCORE II and logistic EuroSCORE are reported as medians (25th–75th interval).
EuroSCORE: European system for cardiac operative risk evaluation.
Procedural aspects
Thirteen different surgeons, comprising 100% of adult cardiac staff surgeons, performed the implants. Concomitant procedures were carried out in 235 (47%) patients, coronary bypass grafting was performed in 142 (28.4%) patients, and other procedures are detailed in Table 2.
Table 2:
Intraoperative characteristics and early follow-up
| Factors | |||
|---|---|---|---|
| Elective procedure | 457 (91.4) | ||
| Access | |||
| Full sternotomy | 264 (52.8) | ||
| Hemi-sternotomy | 114 (22.8) | ||
| Thoracotomy | 122 (24.4) | ||
| Access conversion | 3 (0.6) | ||
| ART | 2 (0.4) | ||
| UHS | 1 (0.2) | ||
| Concomitant procedures | 235 (47) | ||
| CABG | 142 (28.4) | ||
| Aortic reduction plasty | 37 (7.4) | ||
| MVR/MVr | 33 (6.6) | ||
| TVr | 23 (4.6) | ||
| Atrial fibrillation surgery | 42 (8.4) | ||
| Aortic valve condition | |||
| Stenosis | 219 (44) | ||
| Stenosis-insufficiency | 281 (56) | ||
| Implanted valve sizes (mm) | |||
| 19 | 53 (11) | ||
| 21 | 121 (24) | ||
| 23 | 169 (34) | ||
| 25 | 113 (23) | ||
| 27 | 44 (9) | ||
| Cross-clamp time (min) | Isolated AVR | Combined procedures | |
| Full sternotomy | 53 (17) | 99 (36) | |
| Hemi-sternotomy | 62 (14) | 71 (21) | P < 0.001 |
| Thoracotomy | 84 (24) | 98 (16) | |
| CPB time (min) | |||
| Full sternotomy | 89 (29) | 145 (54) | |
| Hemi-sternotomy | 97 (18) | 108 (30) | P < 0.001 |
| Thoracotomy | 120 (34) | 126 (20) | |
| Revision for bleeding | 18 (3.6) | ||
| Revision for myocardial ischaemia | 0 (0) | ||
| New PMI (early and long-term) | 58 (11.6) | ||
| Early pacemaker implantation (<14 days) | 43 (8.6) | ||
| New onset of atrial fibrillation | 137 (27) | ||
Continuous data are presented as mean (standard deviation) and categorical data as n (%).
ART: anterior right thoracotomy; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; MVR: mitral valve replacement; MVr: mitral valve repair; PMI: pacemaker implantation; TVr: tricuspid valve repair; UHS: upper hemi-sternotomy.
Full sternotomy was used in 264 patients (52.8%) and upper hemi-sternotomy or anterior right thoracotomy in 114 (22.8%) and 122 (24.4%) patients, respectively, and 3 patients required conversion to FS (0.6%). Overall cardiopulmonary bypass and cross-clamp times were 121 (SD 46) and 82 (SD 32) min, including patients with combined procedures (Table 2). Cannulation was performed mainly through direct access (aorta/right atrial appendage or superior vena cava) even in minimally invasive approaches (n = 222/236, 94.1%). A subgroup analysis of isolated AVR patients operated on through a full sternotomy revealed reduced aortic cross-clamp time and perfusion time compared with minimally invasive approaches (Table 2). Other subgroups, periprocedural specifications and outcomes are also reported in Table 2. A second and successful deployment attempt to place the valve was necessary in 14 patients (2.8%).
Switch to conventional valve
Fourteen patients (2.7% based on 514 patients for this specific analysis) could not receive a RD-AV, and they were switched to a conventional valve. The RD-AV could not be placed in the annulus in 2 of these patients (1 was a reoperation after a calcified stentless bioprosthesis), 10 patients had a pop-out, 1 had severe paravalvular regurgitation after clamp removal and 1 suffered from an atrioventricular dehiscence after extensive decalcification of the mitral-aortic continuity. These patients were excluded from further analysis.
Clinical outcomes
Mortality
Four patients died during the first 30 days (0.8%). Perioperative mortality occurred in none (0%) of the isolated AVR and in 6 (1.2%) patients after AVR with concomitant procedures (P = 0.003). The reasons for these 6 in-hospital deaths were as follows: cardiac arrest due to asystole, refractory to re-animation on 6th postoperative day (n = 1, 0.2%), cardiogenic shock with cardiac arrest (n = 1, 0.2%) on Day 53, multiorgan failure (n = 2, 0.4%) on Days 4 and 92, acute respiratory distress syndrome (n = 1, 0.2%) on Day 34 and acute kidney failure (n = 1, 0.2%) on Day 149. Overall, 40 patients died (8%). Long-term survival was 94%, 89% and 81% at 1, 3 and 5 years after surgery, respectively (Fig. 1), and comparable for minimally invasive surgery and full sternotomy (Fig. 2). Overall median logistic EuroSCORE for full sternotomy (including combined procedures) and minimally invasive approaches were 8.7 (4.9–16.1) and 5.8 (3.5–9.7) (P < 0.001), respectively, revealing a significant lower operative risk in the minimally invasive population.
Figure 1:
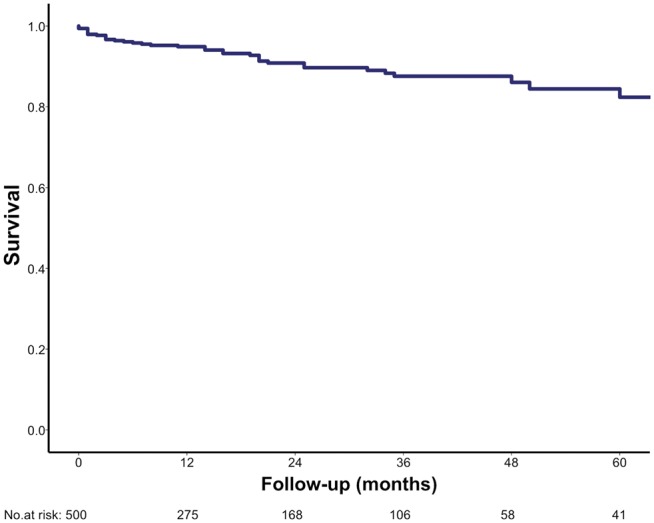
The Kaplan–Meier survival estimate (overall survival).
The mortality risk was also retrospectively analysed by means of the STS score (Fig. 3), and the overall 1-year survival in the intermediate- and high-risk cohort was 87% and 78%, respectively.
Figure 3:
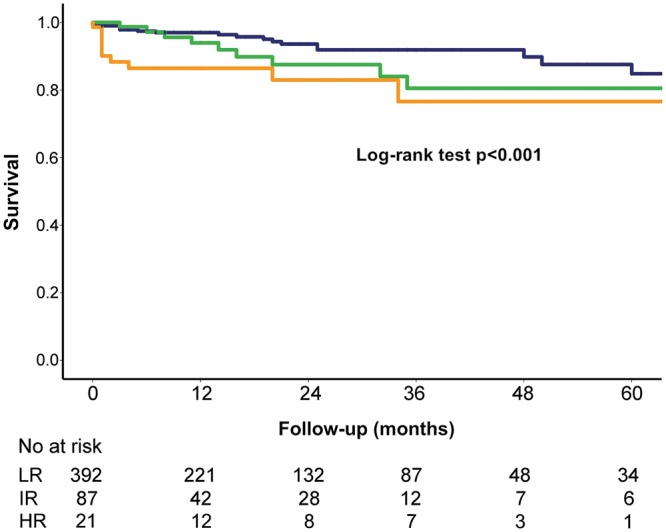
The Kaplan–Meier survival estimate. Blue = survival LR (STS score <4), green = survival IR (STS score 4–8), orange = survival HR (STS score >8) (log-rank test P < 0.001). HR: high risk; IR: intermediate risk; LR: low risk.
Stroke
Perioperative stroke occurred in 13 (2.6%) patients, and 3 (0.36 %ppy) late events were reported, of which only 1 was haemorrhagic. Other events are presented in Table 3.
Table 3:
Postoperative events
| Factors | ≤30 days (%) | >30 days (%ppy) |
|---|---|---|
| Structural valve dysfunction (reintervention) | 0 (0) | 1 (0.12) |
| Non-structural valve dysfunction (reoperation) | 5 (1) | 3 (0.36) |
| >Mild paravalvular leak | 4 (0.8) | 2 (0.24) |
| Other | 1 (0.2) | 1 (0.12) |
| Major bleeding | 1 (0.2) | 5 (0.61) |
| Stroke | 13 (2.6) | 3 (0.36) |
| TIA | 5 (1) | 0 (0) |
| Peripheral emboli | 0 (0) | 3 (0.36) |
| Myocardial infarction | 2 (0.4) | 3 (0.36) |
| Valve thrombosis | 0 (0) | 0 (0) |
| Endocarditis | 0 (0) | 2 (0.24) |
| Endocarditis (reoperation) | 0 (0) | 1 (0.12) |
| Acute kidney injury | 12 (2.4) | 0 (0) |
| ECMO | 10 (2) | 0 (0) |
Overall valve-related outcome regarding adverse events [n (%) ≤30 days postoperatively and n (events per patient year) >30 days postoperatively] are reported.
ECMO: extracorporeal membrane oxygenation; TIA: transient ischaemic attack.
Bleeding events
There were 19 (3.8%) early bleeding events identified, of which 18 (3.6%) were perioperative revisions for bleeding, as well as 5 (0.61 %ppy) late major bleeding events, of which 4 (0.48 %ppy) were gastrointestinal and 1 (0.12 %ppy) was cerebral (Table 3).
Structural and non-structural valve dysfunction
One patient (0.12 %ppy) received a valve-in-valve procedure 6 years after the SAVR for structural degeneration. Major paravalvular regurgitation occurred in 13 patients (2.6%), of which 7 (1.4%) were moderate and 6 (1.2%) were severe. Whenever a clinical impact was present (haemodynamics, haemolysis or acute bleeding), the valve was explanted as described below. Cases of coronary ostia obstruction or other causes of non-structural dysfunction were not observed.
Endocarditis
Two patients (0.24 %ppy) had postoperative endocarditis: 1 underwent reoperation and valve explantation 112 days after the primary operation and 1 died 1832 days after the procedure.
Valve explantation (reoperation/intervention)
Five patients (1%) were reoperated on within 30 days of the index operation and additional 4 patients (0.48 %ppy) during the first postoperative year (Table 3).
The early 5 reoperations took place on postoperative days 1, 8, 15, 26 and were necessary due to severe paravalvular regurgitation in 4 cases and due to septal rupture and acute bleeding in a patient by whom an aggressive myectomy was performed (all cases reported as non-structural valve disease, Table 3), and another 3 patients underwent valve explantation for non-structural dysfunction: 2 patients due to progressive paravalvular regurgitation on Days 154 and 186 and 1 patient on Day 49 with structural damage of the mitral valve apparatus (severe mitral regurgitation due to chordae rupture) and an intact aortic valve. All patients who underwent reoperation due to paravalvular leakage had a severely calcified aortic root. Three out of the 6 patients who required reoperation and valve explantation due to significant paravalvular leakage did not present any regurgitation in the intraoperative transoesophageal echocardiography performed immediately after valve implantation; 2 patients presented a light-moderate regurgitation intraoperatively and another patient had severe calcium deposition extending into the anterior mitral valve leaflet and septum; in this particular case, the intraoperative post-implantation transoesophageal echocardiography identified an atypical jet which was not considered to be paravalvular leakage; however, at the time of explantation an incomplete expansion of the subvalvular stent was observed due to residual calcifications.
Another patient (0.12 %ppy) received a valve-in-valve procedure 6 years after SAVR for structural degeneration. The transcatheter procedure was technically feasible with a good haemodynamic outcome. The technical details are described elsewhere [11].
Pacemaker and additional valve related outcomes
A pacemaker implantation was required in 43 patients (8.6%) during the first 14 postoperative days. Other procedure and valve-related events are summarized in Table 3.
Haemodynamic outcome
The mean gradients at discharge, 3-month, 1-, 3- and 5-year follow-up were 13 (SD 5), 11 (SD 4), 11 (SD 4), 12 (SD 5) and 11 (SD 3) mmHg, respectively. The effective orifice area and indexed effective orifice area at discharge were 1.87 (SD 0.53) cm2 and 0.99 (SD 0.27) cm/m2, respectively, and severe prosthesis-patient mismatch (PPM) occurred in 13 patients (2.6%). The implanted valve sizes are reported in Table 2. Paravalvular regurgitation occurred in 43 patients (8.6%), of whom 30 (6%) were only trace-mild, 7 (1.4%) were moderate and 6 (1.2%) were severe.
DISCUSSION
The Edwards INTUITY valve system combines a new rapid-deployment, stent-based fixation system with an established biological valve prosthesis known for proven long-term durability [6, 8, 12, 13]. We report the largest single-centre experience with this valve. Thirteen staff surgeons (all adult cardiac surgeons) were trained and performed the 500 surgical procedures reported herein. A conventional valve was implanted in 14 patients after an attempt at RD-AV deployment, mainly due to valve pop-out. These results reflect the real-world experience with this new technology, which is beyond the scope of a clinical trial. The learning curve of all surgeons is included in the results, which has to be considered for the discussion and interpretation of the results. The novel delivery handle and accurate training may reduce the necessity for switching to another valve.
Minimally invasive isolated surgical aortic valve replacement was commonly performed at our centre. Due to the learning curve, we started implanting this valve through a standard full sternotomy and shifted gradually to minimally invasive approaches, and currently almost all patients with isolated surgical aortic valve replacement referred to our institution are planned for a minimally invasive access by either upper hemi-sternotomy or anterior right thoracotomy: 74.2% between 2013 and 2014 and 85.5% from 2015 until present date. This suggests that the rapid-deployment valve system facilitates a minimally invasive surgical approach as reported previously [14]. Direct arterial and venous cannulation was performed in 94.1% of the minimally invasive cases in order to avoid vascular and groin complications associated with femoral cannulation [15].
We performed a subgroup analysis on patients in whom a TAVI procedure could be indicated according to the STS score. One-year mortality in the high-risk group was 22%, which was slightly lower than previously described in TAVI registries, ranging from 24.3% to 34.9% [16–18]; it is important to notice that concomitant procedures were performed on 16 (76.2%) patients of this high-risk subgroup. In the intermediate risk group, which also consisted of a high number of patients undergoing combined procedures with a higher long-term risk, 1-year all-cause mortality was 13%, comparable with the reported 1-year mortality in transcatheter valves varying from 6.7% to 12.3% [19–21]. Postoperative survival was excellent after RD-AV implantation. No impact of minimally invasive procedures on survival was reported (Fig. 2). Patients with a higher calculated preoperative risk revealed decreased long-term survival (Fig. 3).
Figure 2:
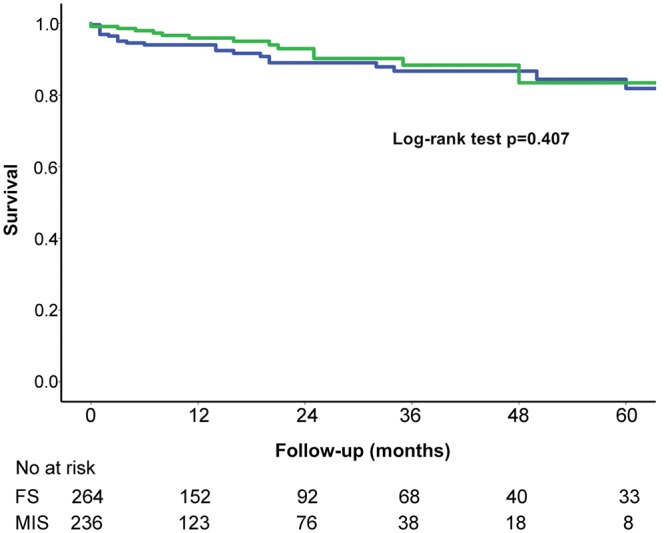
The Kaplan–Meier survival estimate. Blue = survival FS, green = survival MIS (log-rank test P = 0.407). FS: full sternotomy; MIS, minimally invasive approaches.
Conduction disturbances may occur due to mechanical trauma applied by the stent frame to the septum in the region of the atrioventricular conduction system. We report a total rate early new pacemaker implantation of 8.6%, which is significantly lower than previously described in TAVI technologies [16, 17], but slightly higher than in conventional surgical valves [22]. The second sutureless valve currently on the market has a similar pacemaker rate according to a recent meta-analysis [23]. However, our data and the recent meta-analysis are below the pacemaker rate of 10.4% reported from the Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry [24]. No significant influence on overall survival related to pacemaker implantation was observed in this study. We adapted our implantation technique to reduce the risk of pacemaker. Therefore, tension on the right/non-coronary commissural suspension stitch is relaxed prior to valve deployment. Furthermore, correct sizing is crucial for rapid-deployment valves. While the sizer should fit perfectly to the annulus without any extra space, a too-snug fit under pressure should be avoided.
Although the increased pacemaker rate may be attributed to the subvalvular stent fixation, this technology offers a unique re-shaping of the outflow tract. We previously reported that transvalvular gradients are reduced in this rapid-deployment valve compared to the conventional valve of the same manufacturer [8, 25]. The subvalvular stent-based fixation system may be the reason for a reduced transvalvular gradient as it reshapes the left ventricular outflow tract, avoids pledgets and reduces turbulent flow [26]. Furthermore, this valve system does not allow the use of a smaller valve size than indicated, which might occur in conventional valves, as undersizing may lead to paravalvular leakage.
Fallon and colleagues revealed that 54% and 11% of all patients aged above 65 documented in the STS database are suffering from moderate or severe PPM, respectively [27]. This led to an 8% and 32% increase in relative risk of mortality in patients with moderate or severe PPM. We confirm the very low incidence of PPM after RD-AV implantation in this large monocentric, real-world experience. Moderate and severe PPM occurred in 13.2% and 2.6% of all patients, respectively. The low PPM rate is in the line with previous reports for RD-AVs and represents a specific advantage related to the valve design potentially offering a survival benefit [13, 25, 27].
Residual moderate–severe paravalvular regurgitation was observed in 2.6% of the patients. In order to reduce the incidence of paravalvular leakage, we apply an extra stitch in the non-coronary sinus in selected patients and we do not implant this particular type of valve in extensive calcifications of the root with rigid sinuses.
Limitations
This study combines the results of pre-market clinical trials and a post-market registry. Therefore, patient population was not highly selected and our results included the learning curve of 13 surgeons.
CONCLUSION
In conclusion, this RD-AV system has shown excellent results concerning haemodynamic performance, durability and safety. Implantation requires specific training, and the pacemaker rate remains a matter of concern. This novel valve also facilitates minimally invasive approaches, and it may be beneficial in complex combined procedures.
Funding
The patients from multicentre clinical trials funded by Edwards Lifesciences (Irvine, CA, USA) were included in this analysis. Our institution receives financial support from the same company to conduct a long-term follow-up after INTUITY valve implantation and the VICE registry.
Conflict of interest: Alfred Kocher and Dominik Wiedemann received speakers honoraria from Edwards Lifesciences; Martin Andreas is proctor for Edwards Lifesciences and Guenther Laufer received consulting fees from Edwards Lifesciences. Iuliana Coti and Martin Andreas also received travel cost reimbursement.
Footnotes
†Presented at the EACTS Congress 10/2017 in Vienna, Austria (Abstract #1189).
REFERENCES
- 1. Andreas M, Wallner S, Ruetzler K, Wiedemann D, Ehrlich M, Heinze G. et al. Comparable long-term results for porcine and pericardial prostheses after isolated aortic valve replacement. Eur J Cardiothorac Surg 2015;48:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK. et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 3. Hatoum H, Moore BL, Maureira P, Dollery J, Crestanello JA, Dasi LP.. Aortic sinus flow stasis likely in valve-in-valve transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2017;154:32–43.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosgrove DM 3rd, Sabik JF.. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596–7. [PubMed] [Google Scholar]
- 5. Brown ML, McKellar SH, Sundt TM, Schaff HV.. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670–9.e5. [DOI] [PubMed] [Google Scholar]
- 6. Kocher AA, Laufer G, Haverich A, Shrestha M, Walther T, Misfeld M. et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110–16. [DOI] [PubMed] [Google Scholar]
- 7. Shrestha M, Folliguet TA, Pfeiffer S, Meuris B, Carrel T, Bechtel M. et al. Aortic valve replacement and concomitant procedures with the perceval valve: results of European trials. Ann Thorac Surg 2014;98:1294–300. [DOI] [PubMed] [Google Scholar]
- 8. Andreas M, Wallner S, Habertheuer A, Rath C, Schauperl M, Binder T. et al. Conventional versus rapid-deployment aortic valve replacement: a single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact CardioVasc Thorac Surg 2016;22:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL. et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523–8. [DOI] [PubMed] [Google Scholar]
- 10. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA. et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr 2009;22:975–1014. [DOI] [PubMed] [Google Scholar]
- 11. Andreas M, Coti I, Laufer G, Kastner J, Valve-in-valve transcatheter aortic valve implantation into a novel, sutureless bioprosthesis: technical considerations. EuroIntervention. 2018;13:1902–3. [DOI] [PubMed] [Google Scholar]
- 12. Haverich A, Wahlers TC, Borger MA, Shrestha M, Kocher AA, Walther T. et al. Three-year hemodynamic performance, left ventricular mass regression, and prosthetic-patient mismatch after rapid deployment aortic valve replacement in 287 patients. J Thorac Cardiovasc Surg 2014;148:2854–61. [DOI] [PubMed] [Google Scholar]
- 13. Laufer G, Haverich A, Andreas M, Mohr FW, Walther T, Shrestha M. et al. Long-term outcomes of a rapid deployment aortic valve: data up to 5 years. Eur J Cardiothorac Surg 2017;52:281–7. [DOI] [PubMed] [Google Scholar]
- 14. Borger MA, Moustafine V, Conradi L, Knosalla C, Richter M, Merk DR. et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17–25. [DOI] [PubMed] [Google Scholar]
- 15. Andreas M, Mahr S, Kocher A, Laufer G.. Minimalinvasiver Aortenklappenersatz über eine anteriore rechtsseitige Thorakotomie. Z Herz- Thorax- Gefäßchir 2017;31:241–6. [Google Scholar]
- 16. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM. et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–84. [DOI] [PubMed] [Google Scholar]
- 17. Popma JJ, Reardon MJ, Khabbaz K, Harrison JK, Hughes GC, Kodali S. et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery. JACC Cardiovasc Intervent 2017;10:268–75. [DOI] [PubMed] [Google Scholar]
- 18. Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH. et al. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. Ann Thorac Surg 2016;101:789–800. [DOI] [PubMed] [Google Scholar]
- 19. Reardon MJ, Kleiman NS, Adams DH, Yakubov SJ, Coselli JS, Deeb GM. et al. Outcomes in the randomized CoreValve US pivotal high risk trial in patients with a Society of Thoracic Surgeons risk score of 7% or less. JAMA Cardiol 2016;1:945–9. [DOI] [PubMed] [Google Scholar]
- 20. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M. et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 21. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V. et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218–25. [DOI] [PubMed] [Google Scholar]
- 22. Greason KL, Lahr BD, Stulak JM, Cha YM, Rea RF, Schaff HV. et al. Long-term mortality effect of early pacemaker implantation after surgical aortic valve replacement. Ann Thorac Surg 2017;104:1259–64. [DOI] [PubMed] [Google Scholar]
- 23. Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S. et al. Sutureless perceval aortic valve versus conventional stented bioprostheses: meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Assoc 2018;7:e006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Eusanio M, Phan K, Berretta P, Carrel TP, Andreas M, Santarpino G. et al. Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry (SURD-IR): early results from 3343 patients. Eur J Cardiothorac Surg 2018;54:768--73. [DOI] [PubMed] [Google Scholar]
- 25. Wahlers TCW, Andreas M, Rahmanian P, Candolfi P, Zemanova B, Giot C. et al. Outcomes of a rapid deployment aortic valve versus its conventional counterpart: a propensity-matched analysis. Innovations (Phila) 2018;13:177–83. [DOI] [PubMed] [Google Scholar]
- 26. Capelli C, Corsini C, Biscarini D, Ruffini F, Migliavacca F, Kocher A. et al. Pledget-armed sutures affect the haemodynamic performance of biologic aortic valve substitutes: a preliminary experimental and computational study. Cardiovasc Eng Technol 2017;8:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fallon JM, DeSimone JP, Brennan JM, O’Brien S, Thibault DP, DiScipio AW. et al. The incidence and consequence of prosthesis-patient mismatch after surgical aortic valve replacement. Ann Thorac Surg 2018;106:14–22. [DOI] [PubMed] [Google Scholar]