Abstract
Purpose: Systemic malignancies may have ocular manifestations in the form of metastatic tumors, carcinoma associated retinopathy or central retinal vein occlusion (CRVO). Although CRVO has been mentioned in association with renal, lung, prostatic and ovarian malignancies, the association of CRVO with breast carcinoma is unreported. We report a patient with one such rare association.
Methods: We describe a patient with breast carcinoma who was diagnosed to have CRVO. The history, ocular examination, retinal optical coherence tomography scan, ocular ultrasound scan, hematological profile, mammogram details and aspiration cytology description of the malignant breast lesion are reported in this article.
Results: The retina showed extensive hemorrhages and dilated retinal veins. Complete hematologic evaluation revealed the presence of microcytic hypochromic anemia and increased hematocrit. These changes are possibly related to the malignant disease and might contribute to the pathogenesis of CRVO.
Coclusion: This case report demonstrates the rare association between breast malignancy and CRVO.
Keywords: breast carcinoma, central retinal vein occlusion, microcytic hypochromic anemia
Introduction
Any systemic condition that causes altered blood flow, abnormal blood viscosity, abnormalities in blood coagulability or induces changes in blood vessel structure can lead to central retinal vein occlusion (CRVO) [1]. It stands to reason that diabetes mellitus, hypertension, cardiovascular disease, blood dyscrasias (leukemia, lymphoma and polycythemia), paraproteinemias, systemic vasculitis or autoimmune diseases are often associated with CRVO [2]. Besides these common associations, anemia and systemic cancers have shown to be independently associated with CRVO.
Case description
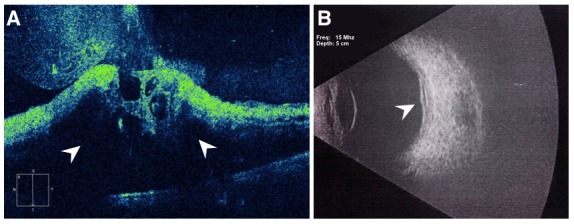
A 59-year-old female presented with decreased vision in the left eye (OS) for 15 days. She was recently diagnosed with left breast carcinoma. She had been advised mastectomy for the breast malignancy 1 month ago and had not followed up with her oncologist since then. The patient did not have diabetes, hypertension or hyperlipidemia. There was no history of coronary artery disease or stroke. The blood pressure recorded in the clinic was 128/90 mm Hg. Visual acuity in OS was 1/60 and VA in right eye (OD) was 6/6. Intraocular pressure in both eyes was 14 mm Hg by non-contact tonometry. The anterior segment examination in both eyes was normal and the pupil in OS did not show a relative afferent pupillary defect. Fundus exam of OS revealed mild pallor of the optic disc (arrowhead, Figure 1 (Fig. 1)). Multiple superficial and deep retinal hemorrhages were striking (arrows, Figure 1 (Fig. 1)). Few cotton wool spots were noted supero-temporal to the optic disc (asterisk, Figure 1 (Fig. 1)). Preretinal hemorrhage was present at the macula with macular edema. Retinal exam of the OD was normal. Optical coherence tomography of OS showed macular edema (arrowhead, Figure 2A (Fig. 2)) with a central foveal thickness of 1,162 microns (normal: 220–240 microns). Backshadowing was present due to retinal hemorrhages. Ultrasound scan of OS showed thickened retina at the posterior pole (arrowhead, Figure 2B (Fig. 2)) of the eye suggesting macular edema. There were no apparent ocular, optic nerve or orbital mass lesions.
Figure 1. Montage image of the left retina shows optic disc pallor (arrowhead). Extensive superficial retinal hemorrhages are seen (arrows) and the retinal veins are dilated and tortuous consistent with central retinal vein occlusion. Few cotton wool spots are seen superotemporal to the optic disc (asterisk). Macular edema can also be made out.
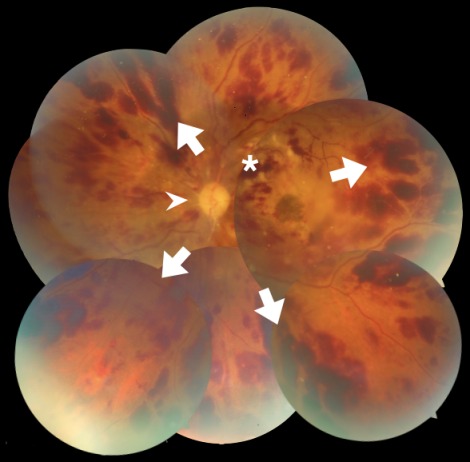
Figure 2. The optical coherence tomography image shows extensive macular edema seen as hyporeflective regions within the retina (arrowhead, 2A). Back shadowing due to superficial hemorrhages is also seen. Ultrasound imaging shows thickening of the retina over the posterior pole (arrowhead, 2B).
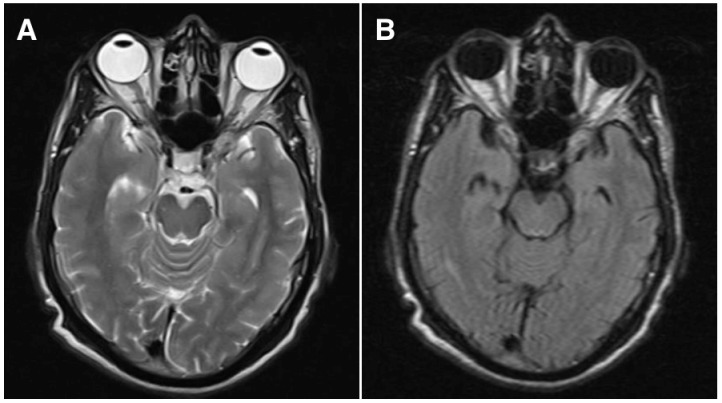
Hematological investigations showed that the hemoglobin was 9 gm% (normal: 12–15), platelet count was 255,000/mm3 (normal: 150,000–400,000), fasting plasma glucose was 106 mg% (normal: 80–120), blood urea was 34 mg% (normal: 15–40), serum Creatinine was 0.8 mg% (normal: 0.5–0.9), erythrocyte sedimentation rate was 28 mm at one hour (normal: 0–15), hematocrit was 89.8% (normal: 36–46), bleeding time was 2 minutes 05 seconds (normal: 0–7 minutes) and clotting time was 4 minutes 15 seconds (normal: 4–9 minutes). The lipid profile was normal. Peripheral blood smear showed microcytic hypochromic anemia with tear drop cells and Rouleaux formation. Total leucocyte count was normal with differential count showing eosinophilia (19%). The blood sample was negative for human immunodeficiency virus and hepatitis B virus. X-ray mammogram, sonomammogram and elastomammogram of the left breast in the craniocaudal and mediolateral oblique views showed a 2x 2.7 cm ill defined hypoechoeic and hypolucent lesion with irregular margins in the retro areolar region with architectural alterations and nipple retraction. The axillary lymph nodes were enlarged with the largest measuring 1x 0.9 cm. Ultrasound abdomen and magnetic resonance imaging of the head and orbit (axial T2-weighted sequence, Figure 3A (Fig. 3); fluid-attenuated inversion recovery sequence (FLAIR), Figure 3B (Fig. 3)) did not show any secondary mass lesions.
Figure 3. Magnetic resonance imaging of the brain and orbit by axial T2-weighted sequence (A) and fluid-attenuated recovery (FLAIR) sequence images (B) do not show any lesions in the choroid, optic nerve or orbit on both sides.
Discussion
Of the many risk factors for CRVO, the metabolic syndrome (hyperglycemia, hypertension and hyperlipidemia) and past vascular events are considered most important [3]. In our patient, CRVO occurred in the absence of any of these risk factors. However, she had microcytic hypochromic anemia and breast carcinoma. The association of CRVO with anemia and few systemic carcinomas (renal, lung, prostate and ovarian carcinoma) has been described separately. In our patient, both anemia and carcinoma coexist and appear to contribute to the pathogenesis of CRVO. There are no previous reports of CRVO in association with breast carcinoma.
Cancer is a cause of hypercoagulability and an association of CRVO with systemic malignancies is well known [4], [5], [6], [7]. In malignancies, CRVO is considered a paraneoplastic process where dynamic interactions between the tumor cells, blood coagulation system, vascular endothelium, leucocytes and platelets contribute to the pathogenesis of CRVO. Rhone and colleagues have shown that the concentration of plasminogen activator inhibitor type 1 is increased and activity of tissue factor pathway inhibitor is reduced in patients with breast carcinoma [8]. These hematologic changes promote thrombosis. Therapeutic agents used in cancer treatment may also contribute to the process of CRVO [9], [10], [11]. Besides, when a metastatic tumor in the optic nerve causes compression, venous outflow channels are impeded and CRVO could occur [12]. The other rare paraneoplastic syndrome in carcinoma that can cause vision loss is cancer-associated retinopathy (CAR) [13], [14]. Unlike CRVO, which presents with striking retinal hemorrhages, the clinical picture in CAR is rather subtle. An immunological process directed at the tumor antigens is implicated in CAR. These antibodies cross-react with retinal antigens to produce symptoms that resemble those with retinitis pigmentosa.
Anemic retinopathy presents with extravasation of blood into the retina due to retinal vasodilation as a response to relative retinal ischemia in the setting of acute blood loss [15], [16]. Retinal hemorrhages in anemic retinopathy are usually located in the superficial retinal layers and are white centered. Occasionally, hemorrhages are also encountered anterior to the retina in the sub internal limiting membrane (ILM) space, in the sub hyaloid space or in the vitreous [17]. Among the above, sub ILM hemorrhage tends to remain localized, as the potential space beneath the ILM is limited. A sub ILM hemorrhage at the fovea obliterates the foveal contour on OCT [18]. Retinal veins may become dilated and tortuous and if associated with the widespread hemorrhages described above, anemic retinopathy may mimic CRVO [19], [20]. Besides the hemorrhages described; anemic retinopathy may also exhibit cotton wool spots, retinal edema and venous tortuosity [21].
In young patients who have no other recognizable risk factors for CRVO, hyperviscosity syndromes have to be ruled out. Evaluation of homocysteine, activated protein C resistance, protein C activity, protein S activity, antithrombin III activity, antiphospholipid antibodies, and anticardiolipin antibodies may be performed [22]. However, in our patient, the systemic malignancy is likely to be responsible for hyperviscosity and increased hematocrit. Elevated blood viscosity contributes to vein occlusion by influencing the blood coagulability.
Visual function in patients with CRVO depends on the extent of macular involvement. If there were minimal macular edema without any hemorrhages in front of the macula, the patient would retain good vision. With extensive ischemia and macular edema as seen in this patient, vision is significantly reduced. Treatment is aimed at reducing the macular edema with use of intravitreal anti-vascular endothelial growth factor agents (Ranibizumab or Aflibercept) and treating the underlying systemic abnormality.
In our patient, we postulate that rheological changes in blood due to breast carcinoma, anemia and hyperviscosity were responsible for the onset of CRVO.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gutman FA. Evaluation of a patient with central retinal vein occlusion. Ophthalmology. 1983 May;90(5):481–483. doi: 10.1016/S0161-6420(83)34528-0. [DOI] [PubMed] [Google Scholar]
- 2.Kolar P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol. 2014;2014:724780. doi: 10.1155/2014/724780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013 Feb;120(2):362–370. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrean SD, Schwab IR. Central retinal vein occlusion and renal cell carcinoma. Am J Ophthalmol. 2003;136(6):1185–1186. doi: 10.1016/S0002-9394(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 5.Ronchetto F. Occlusion of a branch of the central retinal vein as a manifestation of hypercoagulability in a patient with lung cancer. A possible paraneoplastic event. Recenti Prog Med. 1994 Feb;85(2):108–112. [PubMed] [Google Scholar]
- 6.Castro-Navarro V, Odaibo SG, Ghodasra DH, Besirli CG. Bilateral BRVO in a patient with recurrent prostate cancer. BMJ Case Rep. 2015 Oct 21; doi: 10.1136/bcr-2015-212463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asensio-Sánchez VM, Hernaez-Ortega MC, Castresana-Jauregui I. Oclusión de vena central de la retina como primera manifestación de cáncer de ovario. [Central retinal vein occlusion as the first symptom of ovarian cancer]. Arch Soc Esp Oftalmol. 2013 Dec;88(12):482–484. doi: 10.1016/j.oftal.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Rhone P, Ruszkowska-Ciastek B, Bielawski K, Brkic A, Zarychta E, Góralczyk B, Roszkowski K, Rość D. Comprehensive analysis of haemostatic profile depending on clinicopathological determinants in breast cancer patients. Biosci Rep. 2018 Apr;38(2):BSR20171657. doi: 10.1042/BSR20171657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczepanik S, Kęcik D. Bilateral central retinal vein occlusion in a patient with disseminated metastatic renal cell carcinoma treated with sorafenib. Retin Cases Brief Rep. 2012;6(2):148–150. doi: 10.1097/ICB.0b013e3182160965. [DOI] [PubMed] [Google Scholar]
- 10.Federici TJ. Leuprolide acetate and central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2007 Nov-Dec;38(6):497–499. doi: 10.3928/15428877-20071101-09. [DOI] [PubMed] [Google Scholar]
- 11.Li ZY, Fan XX, Wang YJ, Yao K, Liu ZW, Pan WT, Ye YL, Yang P, Huang YC, Wu ZM, Zhou FJ. Metastatic renal cell carcinoma: the first report of unilateral fundus hemorrhage induced by sorafenib. Oncotarget. 2016 Jun;7(23):35181–35187. doi: 10.18632/oncotarget.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backhouse O, Simmons I, Frank A, Cassels-Brown A. Optic nerve breast metastasis mimicking meningioma. Aust N Z J Ophthalmol. 1998 Aug;26(3):247–249. doi: 10.1111/j.1442-9071.1998.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 13.Sawyer RA, John MD, Selhorst JB, Lorenz MD, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81(5):606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 14.Kamei M, Fujitomi Y, Kondo Y, Adachi T, Shibata K, Takumi Y, Abe M, Sugio K. Cancer-associated retinopathy after surgery for breast cancer: a case report and review of the literature. Surg Case Rep. 2018 Jan;4(1):10. doi: 10.1186/s40792-018-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carraro MC, Rossetti L, Gerli GC. Prevalence of retinopathy in patients with anemia or thrombocytopenia. Eur J Haematol. 2001 Oct;67(4):238–244. doi: 10.1034/j.1600-0609.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 16.Turco CD, La Spina C, Mantovani E, Gagliardi M, Lattanzio R, Pierro L. Natural history of premacular hemorrhage due to severe acute anemia: clinical and anatomical features in two untreated patients. Ophthalmic Surg Lasers Imaging Retina. 2014 Mar 1;45 Online:E5–E7. doi: 10.3928/23258160-20140131-01. [DOI] [PubMed] [Google Scholar]
- 17.Holt JM, Gordon-Smith EC. Retinal abnormalities in diseases of the blood. Br J Ophthalmol. 1969 Mar;53(3):145–160. doi: 10.1136/bjo.53.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lekha T, Safiya P, Narasimham D. Fundus autofluorescence imaging of subinternal limiting membrane hemorrhage in anemic retinopathy. Indian J Ophthalmol. 2017 Oct;65(10):1021–1022. doi: 10.4103/ijo.IJO_340_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesen MR, Bakri SJ. Blood Dyscrasias. In: Agarwal A, editor. Fundus Fluorescein and Indocyanine Green Angiography. A Textbook and Atlas. Thorofare: Slack Incorporated; 2008. pp. 235–239. [Google Scholar]
- 20.Nagai T, Komatsu N, Sakata Y, Miura Y, Ozawa K. Iron deficiency anemia with marked thrombocytosis complicated by central retinal vein occlusion. Intern Med. 2005 Oct;44(10):1090–1092. doi: 10.2169/internalmedicine.44.1090. [DOI] [PubMed] [Google Scholar]
- 21.Aisen ML, Bacon BR, Goodman AM, Chester EM. Retinal abnormalities associated with anemia. Arch Ophthalmol. 1983 Jul;101(7):1049–1052. doi: 10.1001/archopht.1983.01040020051008. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Lahey JM, Karlen R, Stewart JM. Laboratory evaluation of hypercoagilable states in patients with central retinal vein occlusion who are less than 56 years of age. Retina. 2018 Jun;38(6):1175–1179. doi: 10.1097/IAE.0000000000001661. [DOI] [PubMed] [Google Scholar]


