Abstract
Background:
Oxidative stress is a driver of multiple sclerosis (MS) pathology. We evaluated the effect of coenzyme Q10 (CoQ10) on laboratory markers of oxidative stress and inflammation, and on MS clinical severity.
Methods:
We included 60 relapsing–remitting patients with MS treated with interferon beta1a 44μg (IFN-β1a) with CoQ10 for 3 months, and with IFN-β1a 44μg alone for 3 more months (in an open-label crossover design). At baseline and at the 3 and 6-month visits, we measured markers of scavenging activity, oxidative damage and inflammation in the peripheral blood, and collected data on disease severity.
Results:
After 3 months, CoQ10 supplementation was associated with improved scavenging activity (as mediated by uric acid), reduced intracellular reactive oxygen species production, reduced oxidative DNA damage, and a shift towards a more anti-inflammatory milieu in the peripheral blood [with higher interleukin (IL)-4 and IL-13, and lower eotaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), interferon (IFN)-γ, IL-1α, IL-2R, IL-9, IL-17F, macrophage inflammatory proteins (MIP)-1α, regulated on activation-normal T cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF). Also, CoQ10 supplementation was associated with lower Expanded Disability Status Scale, fatigue severity scale, Beck’s depression inventory, and the visual analogue scale for pain.
Conclusions:
CoQ10 supplementation improved scavenging activity, reduced oxidative damage, and induced a shift towards a more anti-inflammatory milieu, in the peripheral blood of relapsing–remitting MS patients treated with 44μg IFN-β1a 44μg. A possible clinical effect was noted but deserves to be confirmed over longer follow ups.
Keywords: antioxidant, coenzyme Q10, inflammation, multiple sclerosis, oxidative stress
Introduction
Chronic inflammation in multiple sclerosis (MS) is one of the processes responsible for increased oxidative stress.1–3 Products of oxidative damage are widespread in MS brains, and have been associated with development of inflammation, demyelination and neurodegeneration.2,3 As such, the use of exogenous antioxidants looks particularly promising to treat MS with a multimodal approach, also including conventional disease modifying treatments (DMTs).1,2 Different antioxidant therapies have been studied in MS, with the strongest evidence coming from animal models for alpha-lipoic acid and epigallocatechin-3-gallate,4 and showed anti-inflammatory properties, along with neuroprotective and neuroregenerative effects.4,5 Thus, targeting oxidative stress could represent a valuable therapeutic target for both relapsing–remitting MS (RRMS) and progressive MS.6
Coenzyme Q10 (CoQ10) is a cofactor of the mitochondrial oxidative respiratory chain and acts as a powerful antioxidant and anti-inflammatory compound and, when administered peripherally (e.g. oral or intravenous), is able to cross the blood–brain barrier.7,8 MS patients, in particular those with more severe disease, presented with lower blood levels of CoQ10 and, more in general, with higher levels of oxidative stress, when compared with controls.9,10 When supplementing MS patients with CoQ10 during a 12-week period, a reduction in peripheral markers of oxidative stress,11 and inflammation was noted,12 along with improvement of fatigue and depressive symptoms.13
However, most protocols on antioxidant therapies in MS included a limited set of laboratory and clinical outcomes of MS.4 Thus, we utilized a wide set of laboratory and clinical measures to explore: (1) the effect of CoQ10 supplementation along with interferon beta1a 44μg (IFN-β1a) treatment on variations of markers of oxidative stress and inflammation in the peripheral blood (primary endpoint); (2) the effect of CoQ10 supplementation along with IFN-β1a treatment on variations of clinical measures of MS severity (secondary endpoint); and (3) the associations between variations of laboratory measures as for CoQ10 supplementation, and clinical outcomes (tertiary endpoint).
Methods
Study design
This is a retrospective analysis on prospectively collected data, recorded at the MS Clinical Care and Research Centre of the ‘Federico II’ University (Naples, Italy). Biological materials and clinical data were collected during clinical visits performed according to clinical practice. The study was approved by the ‘Federico II’ University Ethics Committee (n. 137/16), and patients gave informed consent to the study.
Patients having received IFN-β1a alone or with CoQ10 were extracted and assigned to either Group1 (CoQ10 supplementation along with IFN-β1a over the first 3 months, followed by IFN-β1a alone for 3 months) or Group 2 (IFN-β1a alone over the first 3 months, followed by CoQ10 supplementation along with IFN-β1a for 3 months), with a crossover design, in order to obtain groups with similar demographic and clinical features (Table 1). This design used within-patients comparison of treatments and, so, minimized confounding effects by removing any natural biological variation that may have occurred in the measurement of the outcome measures.14
Table 1.
Baseline demographic and clinical features.
Demographic and clinical features of treatment groups at baseline. Group 1 received CoQ10 supplementation along with IFN-β1a over the first 3 months, followed by IFN-β1a alone for 3 months; Group 2 received IFN-β1a alone over the first 3 months, followed by CoQ10 supplementation along with IFN-β1a for 3 months. p values are reported from a Chi-square test, Fisher’s exact test or Student’s t test.
|
Group 1
(n = 30) |
Group 2
(n = 30) |
p values | |
|---|---|---|---|
| Age, years | 42.1 ± 10.5 | 40.9 ± 9.0 | 0.639 |
| Sex, female (%) | 21 (70%) | 21 (70%) | 0.999 |
| Disease duration, years | 10.9 ± 2.0 | 11.1 ± 1.5 | 0.662 |
| Baseline EDSS | 2.7 ± 1.0 | 2.6 ± 1.0 | 0.943 |
| Naïve patients, number (%) | 15 (50%) | 15 (50%) | 0.999 |
| Duration of IFN-β1a, years | 5.2 ± 4.2 | 4.5 ± 4.7 | 0.912 |
CoQ10, coenzyme Q10; EDSS, Expanded Disability Status Scale; IFN-β1a, interferon-beta1a 44μg.
The use of CoQ10 was open label, with patients being aware if they were on treatment, and no washout between the two periods was considered. To minimize any possible bias, our primary outcomes were laboratory-based (not affected by the open-label design) and were recorded at the end of the treatment periods; also, a near-immediate switch of treatments was considered (3 months).14
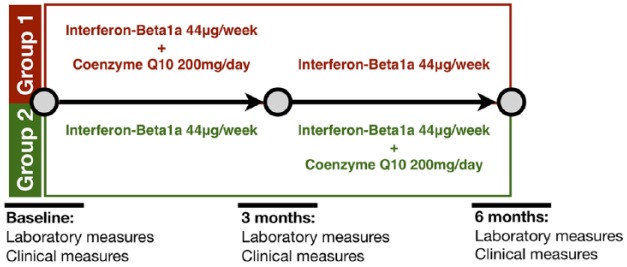
Details of the study design are reported in Figure 1.
Figure 1.
Study design.
A crossover design was considered. Group1 received CoQ10 supplementation along with IFN-β1a over the first 3 months, followed by IFN-β1a alone for 3 months; Group 2 received IFN-β1a alone over the first 3 months, followed by CoQ10 supplementation along with IFN-β1a for 3 months.
CoQ10, coenzyme Q10; IFN-β1a, interferon-beta1a.
Population and CoQ10 supplementation
Patients had dietary supplementation with 200 mg/day CoQ10 during a 3-month period (Figure 1). Ubidecarenone formulation was used in compliance with indications for clinical practice (Skatto®, 100 mg/ml, Chiesi Farmaceutici SpA, Parma, Italy).
Inclusion criteria were: (1) clinical and radiological diagnosis of RRMS with 2010 revisions to the McDonald criteria;15,16 (2) age >18 years old; and (3) treatment with IFN-β1a 44μg. In particular, patients were required to be steadily on treatment with IFN-β1a 44μg for at least 6 months before inclusion in the study and, then, for the whole study period (3 + 3 months); patients were either drug-naïve or previously treated with medications other than IFN-β1a 44μg (1:1) .
Exclusion criteria were: (1) recent relapse or corticosteroid treatment (<6 months); (2) exposure at any time to azathioprine, cladribine, cyclophosphamide, cyclosporine A, methotrexate, or any other immunosuppressive agent; (3) history of malignancy, major systemic disease or other illness that would, in our opinion, interfere with the interpretation of study results; (4) use of contraceptive drugs; (5) use of any vitamins, minerals or other over-the-counter compounds; and (6) concomitant inclusion in any other observational or interventional study.
Laboratory outcomes
Blood samples have been collected in fasting conditions in lithium heparin tubes. Blood was immediately centrifuged, and plasma samples were stored at −80oC for a maximum period of 6 months. We processed 4 cc of plasma in order to analyse:
- Markers of free radical scavenging activity: uric acid (UA) and bilirubin were measured by using the UA2 and the BILTS enzymatic methods, respectively, with the COBAS® c501 analyser (Roche Diagnostic, Mannheim, Germany);
- Markers of serum oxidative damage: 8-hydroxy-2-deoxyguanosine (8-OHdG, an end product of oxidative DNA damage) and protein carbonyls (an end product of oxidative protein damage) were measured by using the OxiSelect™ Oxidative DNA Damage enzyme-linked immunosorbent assay (ELISA) kit, and the OxiSelect™ Protein Carbonyl ELISA Kit, respectively (Cell Biolabs, San Diego, CA, USA);
- Markers of inflammation: the Human Cytokine Magnetic 35-Plex Panel (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used for the quantitative detection of epidermal growth factor (EGF), eotaxin, basic-fibroblast growth factor (FGF), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), interferon (IFN)-α, IFN-γ, interleukin (IL)-1α, IL-1β, IL-1RA, IL-2, IL-2R, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17A, IL-17F, IL-22, IFN-γ-inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, monokine induced by IFN-γ (MIG), macrophage inflammatory proteins (MIP)-1α, MIP-1β, regulated on activation-normal T cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF), in compliance with manufacturer’s instructions; samples were analysed with a Luminex® 200™.
CellROX® Orange Reagent (Life Technologies, Carlsbad, California, USA) was used for measuring intracellular reactive oxygen species (ROS) production. Peripheral blood mononuclear cells (PBMCs) were isolated by stratifying heparinized whole blood on Ficoll-Hypaque (GE Healthcare, Chicago, Illinois, USA). Freshly isolated PBMCs were incubated with 5 µM CellROX® Orange Reagent for 30 min in the dark at 37°C, washed three times and re-suspended in phosphate-buffered saline solution (PBS)). The fluorescence was quantified using FACScanto II analyser (Becton-Dickinson, San Diego, CA, USA) and Flow-Jo software (Tree Star Inc., Ashland, OR, USA); intracellular ROS production (CellROX) was measured as percent positive cells (%) and mean fluorescence intensity (MFI).
Clinical outcomes
Demographic characteristics (age, sex), concomitant diseases and treatments, and MS clinical features (disease duration, occurrence of relapses, Expanded Disability Status Scale [EDSS]) were recorded. Examiners were certified EDSS-raters. Patients were classified as drug-naïve or previously treated, in relation to the use of IFN-β1a; duration of IFN-β1a treatment was calculated.
Patient-reported outcomes
At baseline, and at the 3 and 6-month visits, patients were required to fill in the following questionnaires:
- MS neuropsychological questionnaire (MSNQ) is a 15-item questionnaire for self-reporting everyday life functioning, neuropsychological functioning, and mood, with higher scores indicating a more preserved cognitive function; this is a quick screening tool for cognitive impairement;17,18 a preliminary Italian version of the questionnaire was administered to a subset of patients and then used for this study;
- Visual analogue scale (VAS) is made of a 10 cm straight line with equally distant numbers from 0 to 10, where the individual must indicate severity of pain and headache; this scale is easy to answer and is particularly suitable for exploratory analyses;19
- Fatigue severity scale (FSS) is composed of nine questions, with higher scores indicating a more severe impact of fatigue on daily life activities; this is an easy to answer questionnaire that can be quickly administered in clinical practice;19
- Beck’s depression inventory (BDI) II consists of 21 items assessing the severity of common depressive symptoms; for each item, participants are required to choose the point scale (from 0 to 3) that best describes how they felt in the last 2 weeks, with higher scores indicating the presence of depressive symptoms.20
For each scale, we preferred to use absolute values rather than cut-off points. This is because patient-reported outcomes were a secondary endpoint of the study and, accordingly, our inclusion criteria did not select a population where such cut-off points would necessarily define homogenous groups. On the contrary, absolute values of these scales would better depict variations over time and in relation to treatment.
Sample size calculation
Considering the main outcome of the present study (variations of laboratory markers in RRMS patients evaluated at three different time points by using mixed-effect linear regression models), and the results obtained in our previous longitudinal study on UA in RRMS,21 a sample of 60 patients for a total of 180 records was considered suitable to obtain an acceptable estimate (effect size = 30%; α = 0.05; power = 0.9), accepting 20% missing data. The present sample is larger than previous studies investigating CoQ10 effects in MS.11–13
Statistical analyses
Preliminary comparisons between treatment groups were performed with a Chi-squared test, Fisher’s exact test or Student’s t test, as appropriate.
To evaluate associations between CoQ10 supplementation and variations of each laboratory (primary endpoint) and clinical outcome (secondary endpoint), mixed-effect linear regression models were employed to account for multiple measures repeated within each individual (laboratory and clinical measures collected at baseline and after 3 and 6 months). The crossover model included random effects for patient ID, and fixed-effects for time (baseline, 3 and 6 months) and for the visit after CoQ10 exposure (post-CoQ10 supplementation measures were collected at 3 months in Group 1, and at 6 months in Group 2), overall accounting for possible carry-over effects. An interaction term between treatment and time (continuous) was set and marginal effects were calculated, to estimate treatment-related variations of laboratory and clinical outcomes over time in both Group 1 and 2.
To evaluate associations between CoQ10-related variations of laboratory and clinical outcomes (tertiary endpoint), we selected laboratory and clinical measures being affected by CoQ10 supplementation (p < 0.05 in previous models). Mixed-effect linear regression models were employed to account for multiple measures repeated within each individual. An interaction term between treatment group and each laboratory measure was set and marginal effects were calculated, to estimate possible associations between CoQ10-related variations of laboratory measures and clinical outcomes.
Covariates included in the statistical models were age, sex, disease duration, duration of IFN-β1a treatment, baseline EDSS and, for analysis of UA levels, creatinine.
Results are presented as coefficient (Coeff), 95% confidence interval (CI) and p values. All the variables included in the model were tested for multicollinearity (variance inflation factor < 2.5).
Laboratory analyses, clinical assessments, and patient-reported scales were run blind to each other. The statistician matched the datasets and was blind to treatment codes.
Stata 15.0 and Microsoft Excel were used for data processing and analysis. Results have been considered statistically significant if p < 0.05.
Results
A total of 60 patients with RRMS were included in the study. Treatment groups were similar in age, sex, disease duration, baseline EDSS, and distribution of naïve/on-treatment patients (Table 1). When considering laboratory and clinical measures, missing data were less than 20%, as preliminarily accounted by sample size calculation.
During the study period, four patients presented with a clinical relapse (6.6%), being equally distributed in CoQ10-treated and un-treated groups.
CoQ10 supplementation and variations of laboratory measures
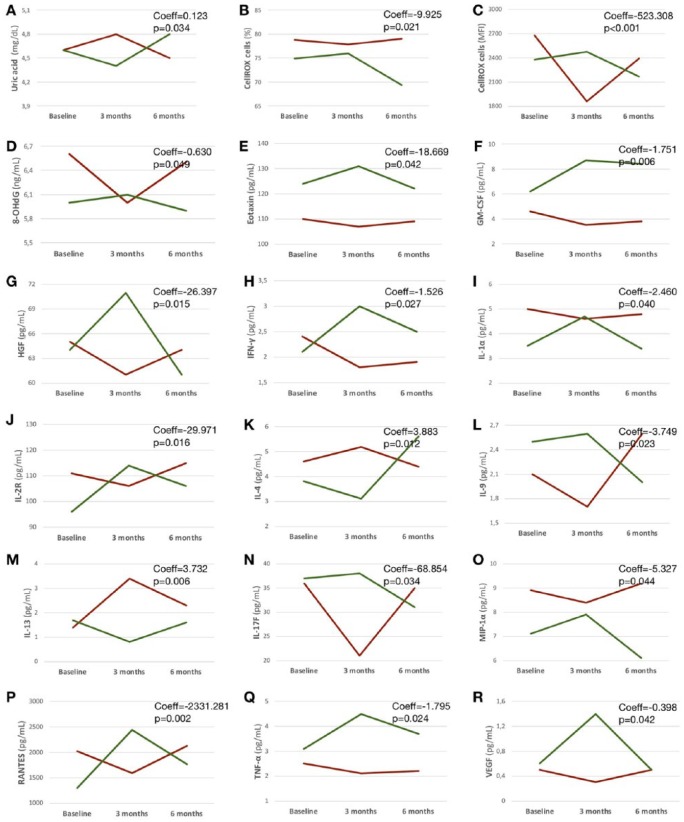
After 3 months, CoQ10 supplementation was associated with increased scavenging activity (UA, Coeff = 0.123; p = 0.034), and with reduced intracellular ROS production (%, Coeff = −9.925; p = 0.021; and MFI, Coeff = −523.308; p < 0.001), and DNA damage (8-OHdG, Coeff = −0.630; p = 0.049).
Also, after 3 months, CoQ10 supplementation was associated with increased IL-4 (Coeff = −3.883; p = 0.012) and IL-13 (Coeff = 3.732; p = 0.006), and with reduced eotaxin (Coeff = −18.669; p = 0.042), GM-CSF (Coeff = −1.751; p = 0.006), HGF (Coeff = −26.397; p = 0.015), IFN-γ (Coeff = −1.526; p = 0.027), IL-1α (Coeff = −2.460; p = 0.040), IL-2R (Coeff = −29.971; p = 0.016), IL-9 (Coeff = −3.749; p = 0.023), IL-17F (Coeff = −68.854; p = 0.034), MIP-1α (Coeff = −5.327; p = 0.044), RANTES (Coeff = −2331.281; p = 0.002), TNF-α (Coeff = −1.795; p = 0.024), and VEGF (Coeff = −0.398; p = 0.042; Table 2; Figure 2).
Table 2.
Variations of laboratory outcomes in relation to CoQ10 supplementation.
Variations of different laboratory outcomes after coenzyme Q10 supplementation along with IFN-β1a, compared with IFN-β1a alone. Laboratory measures repeated within each individual at baseline and after 3 and 6 months were included in mixed-effect linear regression models where we set an interaction term between time and treatment period (post-CoQ10 supplementation visit was at 3 months in Group 1, and at 6 months in Group 2). Coefficients (Coeff), 95% CI and p values are reported (p < 0.05 is presented as *).
| Primary endpoints | Coeff | 95% CI |
p values | |
|---|---|---|---|---|
| Lower | Upper | |||
| Markers of scavenging activity | ||||
| Uric acid, mg/dl | 0.123 | 0.009 | 0.237 | 0.034* |
| Bilirubin, mg/dl | 0.066 | −0.042 | 0.174 | 0.232 |
| Markers of oxidative damage | ||||
| CellROX cells, % | −9.925 | 018.353 | −1.497 | 0.021* |
| CellROX cells, MFI | −523.308 | −758.793 | −287.822 | <0.001* |
| Protein carbonyls, nmol/mg | −0.266 | −1.320 | 0.787 | 0.620 |
| 8-OHdG, ng/ml | −0.630 | −1.294 | −0.034 | 0.049* |
| Markers of inflammation | ||||
| EGF, pg/ml | −3.637 | −17.291 | 10.016 | 0.602 |
| Eotaxin, pg/ml | −18.669 | −36.643 | −0.695 | 0.042* |
| Basic-FGF, pg/ml | −2.736 | −24.007 | 18.535 | 0.801 |
| G-CSF, pg/ml | −4.692 | −16.508 | 7.124 | 0.436 |
| GM-CSF, pg/ml | −1.751 | −2.959 | −0.455 | 0.006* |
| HGF, pg/ml | −26.397 | −56.213 | −13.418 | 0.015* |
| IFN-α, pg/ml | 1.780 | −22.515 | 26.077 | 0.886 |
| IFN-γ, pg/ml | −1.526 | −2.878 | −0.175 | 0.027* |
| IL-1α, pg/ml | −2.460 | −5.313 | −0.591 | 0.040* |
| IL-1β, pg/ml | −1.188 | −3.531 | 1.153 | 0.320 |
| IL-1RA, pg/ml | −10.464 | −38.999 | 18.070 | 0.472 |
| IL-2, pg/ml | 5.099 | −11.619 | 21.817 | 0.550 |
| IL-2R, pg/ml | −29.971 | −54.330 | −5.612 | 0.016* |
| IL-3, pg/ml | 28.661 | −68.832 | 126.155 | 0.564 |
| IL-4, pg/ml | 3.883 | 0.843 | 6.923 | 0.012* |
| IL-5, pg/ml | −12.890 | −34.403 | 8.621 | 0.240 |
| IL-6, pg/ml | 5.559 | −46.568 | 57.687 | 0.834 |
| IL-7, pg/ml | −16.428 | −42.050 | 9.193 | 0.209 |
| IL-8, pg/ml | −11.418 | −23.830 | 0.993 | 0.071 |
| IL-9, pg/ml | −3.749 | −8.057 | −1.557 | 0.023* |
| IL-10, pg/ml | 1615.546 | −1399.093 | 4630.185 | 0.294 |
| IL-12, pg/ml | 2.498 | −11.569 | 16.566 | 0.728 |
| IL-13, pg/ml | 3.732 | 1.045 | 6.419 | 0.006* |
| IL-15, pg/ml | 21.693 | −37.211 | 80.597 | 0.470 |
| IL-17A, pg/ml | −0.453 | −1.508 | 0.602 | 0.400 |
| IL-17F, pg/ml | −68.854 | −140.017 | −12.307 | 0.034* |
| IL-22, pg/ml | −8.406 | −68.069 | 51.255 | 0.782 |
| IP-10, pg/ml | 5.699 | −44.344 | 55.743 | 0.823 |
| MCP-1, pg/ml | 39.540 | −45.290 | 124.371 | 0.361 |
| MIG, pg/ml | −5.409 | −19.197 | 8.379 | 0.442 |
| MIP-1α, pg/ml | −5.327 | −10.515 | −0.138 | 0.044* |
| MIP-1β, pg/ml | 17.125 | −49.443 | 83.695 | 0.614 |
| RANTES, pg/ml | −2331.281 | −3772.510 | 890.052 | 0.002* |
| TNF-α, pg/ml | −1.795 | −3.595 | −0.468 | 0.024* |
| VEGF, pg/ml | −0.398 | −0.821 | −0.052 | 0.042* |
CI, confidence interval; CoQ10, coenzyme Q10; IFN-β1a, interferon-beta1a 44μg; 8-hydroxy-2-deoxyguanosine (8-OHdG), epidermal growth factor (EGF), eotaxin, basic-fibroblast growth factor (FGF), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), hepatocyte growth factor (HGF), interferon (IFN)-α, IFN-γ, interleukin (IL)-1α, IL-1β, IL-1RA, IL-2, IL-2R, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17A, IL-17F, IL-22, IFN-γ-inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, monokine induced by IFN-γ (MIG), macrophage inflammatory proteins (MIP)-1α, MIP-1β, regulated on activation-normal T cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF).
Figure 2.
Laboratory outcomes.
Profile plots show variations of laboratory outcomes over time in relation to the use of IFN-β1a alone or in combination with Coenzyme Q10 (Group 1: group receiving Coenzyme Q10 from baseline to 3-month follow up is in red; Group 2: group receiving Coenzyme Q10 from 3- to 6-month follow up is in green). Coefficients (Coeff) and p values are shown from mixed-effect linear regression models where an interaction term between treatment and time was set and marginal effects were calculated.
IFN-β1a, interferon-beta1a.
CoQ10 supplementation and variations of clinical outcomes
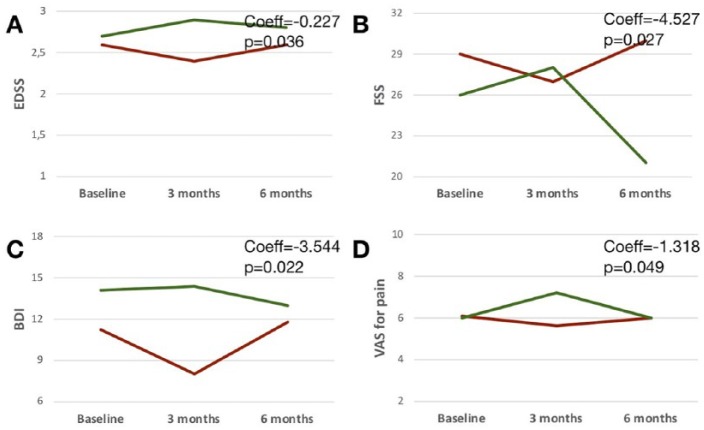
After 3 months, CoQ10 supplementation was associated with reduction of EDSS (Coeff = −0.227; p = 0.036), FSS (Coeff = −4.527; p = 0.027), BDI (Coeff = −3.544; p = 0.022), and VAS for pain (Coeff = −1.318; p = 0.049; Table 3; Figure 3).
Table 3.
Variations of clinical and patient-reported outcomes in relation to CoQ10 supplementation.
Variations of different clinical and patient-reported outcomes (range of scales is reported) after CoQ10 supplementation along with IFN-β1a, compared with IFN-β1a alone. Clinical measures repeated within each individual at baseline and after 3 and 6 months were included in mixed-effect linear regression models where we set an interaction term between time and treatment period (post-CoQ10 supplementation visit was at 3 months in Group 1, and at 6 months in Group 2). Coefficients (Coeff), 95% CI and p values are reported (p < 0.05 is presented as *).
| Secondary endpoints | Coeff | 95% CI |
p values | |
|---|---|---|---|---|
| Lower | Upper | |||
| Clinical outcomes | ||||
| EDSS (0–10) | −0.227 | −0.438 | 0.015 | 0.036* |
| Naïve patients | 0.014 | −0.251 | 0.222 | 0.915 |
| Patient-reported outcomes | ||||
| MSNQ (0–60) | 1.946 | −3.655 | 7.548 | 0.496 |
| FSS (9–63) | −4.527 | −9.424 | −1.368 | 0.027* |
| BDI (0–63) | −3.544 | −7.212 | −1.124 | 0.022* |
| VAS for pain (0–10) | −1.318 | −2.691 | −0.054 | 0.049* |
| VAS for headache (0–10) | −0.909 | −2.359 | 0.541 | 0.219 |
BDI, Beck’s depression inventory; CI, confidence interval; CoQ10, coenzyme Q10; EDSS, Expanded Disability Status Scale; FSS, fatigue severity scale; IFN-β1a, interferon-beta1a; MSNQ, multiple sclerosis neuropsychological questionnaire; VAS, visual analogue scale.
Figure 3.
Clinical outcomes.
Profile plots show variations of clinical outcomes over time in relation to the use of IFN-β1a alone or in combination with Coenzyme Q10 (Group 1: group receiving Coenzyme Q10 from baseline to 3-month follow up is in red; Group 2: group receiving Coenzyme Q10 from 3- to 6-month follow up is in green). Coefficients (Coeff) and p values are shown from mixed-effect linear regression models where an interaction term between treatment and time was set and marginal effects were calculated.
IFN-β1a, interferon-beta1a.
Variations of laboratory and clinical outcomes
Reduction in EDSS was associated with CoQ10 effect on reduced intracellular ROS production (MFI, Coeff = 0.001; p = 0.001), and IFN-γ (Coeff = 0.126; p = 0.009), and on increased IL-13 (Coeff = −0.057; p = 0.028).
Reduction in FSS was associated with CoQ10 effect on reduced intracellular ROS production (%, Coeff = 0.232; p = 0.023).
Reduction in BDI was associated with CoQ10 effect on increased UA (Coeff = −1.665; p = 0.049).
Reduction in VAS for pain was associated with CoQ10 effect on reduced IL-1α (Coeff = 0.127; p = 0.043), and RANTES (Coeff = 0.001; p = 0.009), and on increased IL4 (Coeff = −0.075; p = 0.024).
Full results are reported in the Supplementary Table 1.
Discussion
Supplementation with CoQ10 in RRMS patients treated with IFN-β1a 44μg was associated with improved scavenging activity, reduced oxidative damage and anti-inflammatory changes in the peripheral blood, and with clinical improvement in depressive symptoms, disability, pain and fatigue. When compared with previous investigations on the same topic, the present study included a wider set of laboratory and clinical outcomes, and attempted to relate variations in clinical measures to laboratory changes, in order to shed light on relationships between oxidative stress, inflammation and MS clinical features.
The use of CoQ10 was associated with increased levels of UA in the peripheral blood, a natural antioxidant responsible for a large amount of serum scavenging activity. UA levels are generally lower in MS, when compared with controls, possibly as a consequence of chronic oxidative stimuli.22 During INF-β treatment, UA is expected to progressively decrease, in particular in patients presenting with clinical relapses, disability progression or cognitive worsening.21 In our population, we might argue that antioxidant effects of CoQ10 contributed to restoring serum scavenging activity, ultimately leading to reduction of depressive symptoms, as measured by BDI. Of note, this is the first report of association between UA and depression in MS, whereas this has been shown already for other neurological disorders (i.e. Parkinson’s disease).23
After 3-month CoQ10 supplementation, we observed an improved oxidative balance, with reduction of intracellular ROS production and of oxidative DNA damage in the peripheral blood. Oxidative damage in inflammatory cells and in DNA is a main driver of MS pathology,2,3 and, accordingly, we found reduction of intracellular ROS production being associated with improvement in fatigue and disability. The possibility to reduce oxidative damage and its clinical consequences by using CoQ10 along with DMTs looks particularly promising and deserves to be investigated in future studies with dedicated design.
CoQ10 supplementation reduced proinflammatory cytokines towards a more anti-inflammatory environment in the peripheral blood. We observed a reduction of cytokines determining chronic inflammation within the central nervous system (i.e. GM-CSF, IFN-γ, IL1-α, IL-2R, IL-9, IL-17F, TNF-α),24–28 of chemokines suppressing the activity of microglia towards brain repair (i.e. MIP-1α, RANTES),29,30 and of molecules enhancing lymphocyte activity and subsequent brain damage (i.e. HGF, VEGF).31–33 At the same time, we showed an increase of IL-4 and IL-13, that exert a neuroprotective role through suppression of pathologically active macrophages and microglia.28,34 Previous studies associated modifications towards an anti-inflammatory environment with improved clinical and radiological outcomes of MS.35,36 However, we have to acknowledge that these molecules are highly related to each other,24–27 and direct effects of CoQ10 are hard to distinguish from its indirect, exerted through a general improvement of the oxidative balance.
The present study also included clinical outcomes as exploratory secondary endpoints. After CoQ10 supplementation along with IFN-β1a 44μg, patients presented with fewer depressive symptoms, disability, fatigue and pain. The association between CoQ10 and improvement in depression has already been described in MS,37 and could be related to CoQ10 effects on serotonin pathways.38 Improvement in disability as measured by EDSS is hard to explain considering that baseline EDSS values were relatively low, study duration was 6 months and sustained changes would require longer observation time.39 Thus, it is possible that short-term variations in EDSS could reflect physiological fluctuations or were at least in part related to improvement of fatigue, as already described during CoQ10 supplementation in MS,37 rather than a sustained improvement of disability. CoQ10 looks effective in reducing painful symptoms in other conditions,40 and its use in MS should be further explored with more appropriate scales.
The main limitation of the present study is the open-label design, considering that patients had CoQ10 supplementation according to clinical practice. However, the primary outcome of the study was the measurement of laboratory variations that cannot be influenced by the open-label design and that were associated with clinical changes. Previous studies in MS used higher CoQ10 dosage;11,12,37 however, at 200 mg/day, we were able to detect significant changes on both laboratory and clinical measures in a relatively short time. Additional markers of MS severity could have been included (e.g. magnetic resonance imaging data) but follow up should have been longer than a 3-month treatment duration to observe meaningful changes. Some of the effects we described for CoQ10 could be attributable to IFN-β1a,31,41 and their separate contribution is hard to analyse; we included duration of IFN-β1a in the statistical models but this did not apparently affect study results. We only collected peripheral blood but, considering that CoQ10 is able to penetrate the central nervous system,7,8 we might hypothesize that similar effects could be observed centrally.
In conclusion, the present study showed that the use of CoQ10 in RRMS patients treated with IFN-β1a 44μg improved the oxidative balance and reduced the inflammatory environment in the peripheral blood, along with clinical benefits. In the future, studies should consider combining peripheral measures of oxidative stress and inflammation with central (i.e. cerebrospinal fluid, magnetic resonance imaging), and should be run on larger samples with longer follow ups, in order to detect how CoQ10 can modify the course of MS in the long term.
Supplemental Material
Supplemental material, Supplemental_PDF for Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis by Marcello Moccia, Antonio Capacchione, Roberta Lanzillo, Fortunata Carbone, Teresa Micillo, Francesco Perna, Anna De Rosa, Antonio Carotenuto, Roberto Albero, Giuseppe Matarese, Raffaele Palladino and Vincenzo Brescia Morra in Therapeutic Advances in Neurological Disorders
Footnotes
Funding: This research was partially supported by Merck Serono S.p.A. (Italy), an affiliate of Merck KGaA, Darmstadt, Germany.
GM was supported by the Fondazione Italiana Sclerosi Multipla (grant no. 2016/R/18).
FC was supported by the Ministero della Salute (grant no GR-2016-02363725).
Conflict of interest statement: MM received research grants from MAGNIMS-ECTRIMS and Merck.
GM received research grants from Merck, Biogen Idec and Novartis.
VBM and RL received honoraria from Almirall, Bayer, Biogen Idec, Genzyme, Merck, Novartis, and Roche.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Marcello Moccia  https://orcid.org/0000-0003-2613-3090
https://orcid.org/0000-0003-2613-3090
Contributor Information
Marcello Moccia, Multiple Sclerosis Clinical Care and Research Centre, Department of Neuroscience, Reproductive Science and Odontostomatology, Federico II University, Via Sergio Pansini, 5 - Building 17, Ground floor, Naples, Italy.
Antonio Capacchione, Medical Affairs Department, Merck, Rome, Italy.
Roberta Lanzillo, Department of Neuroscience, Reproductive Science and Odontostomatology, Multiple Sclerosis Clinical Care and Research Center, Federico II University, Naples, Italy.
Fortunata Carbone, Neuroimmunology Unit, Fondazione Santa Lucia, Rome, Italy.
Teresa Micillo, Department of Biology, Federico II University, Naples, Italy.
Francesco Perna, Department of Clinical Medicine and Surgery, Federico II University, Naples, Italy.
Anna De Rosa, Department of Neuroscience, Reproductive Science and Odontostomatology, Multiple Sclerosis Clinical Care and Research Center, Federico II University, Naples, Italy.
Antonio Carotenuto, Department of Neuroscience, Reproductive Science and Odontostomatology, Multiple Sclerosis Clinical Care and Research Center, Federico II University, Naples, Italy.
Roberto Albero, Department of Neuroscience, Reproductive Science and Odontostomatology, Multiple Sclerosis Clinical Care and Research Center, Federico II University, Naples, Italy.
Giuseppe Matarese, Laboratory of Immunology, Institute of Experimental Endocrinology and Oncology, National Research Council (IEOS-CNR), Naples, Italy; Treg Cell Lab, Department of Molecular Medicine and Medical Biotechnologies, Federico II University, Naples, Italy.
Raffaele Palladino, Department of Primary Care and Public Health, Imperial College, London, UK; Department of Public Health, Federico II University, Naples, Italy.
Vincenzo Brescia Morra, Department of Neuroscience, Reproductive Science and Odontostomatology, Multiple Sclerosis Clinical Care and Research Center, Federico II University, Naples, Italy.
References
- 1. Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol 2014; 10: 225–238. [DOI] [PubMed] [Google Scholar]
- 2. van Horssen J, Witte M, Ciccarelli O. The role of mitochondria in axonal degeneration and tissue repair in MS. Mult Scler 2012; 18: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 3. Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain 2011; 134: 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plemel JR, Juzwik CA, Benson CA, et al. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: a systematic review. Mult Scler 2015; 21: 1485–1495. [DOI] [PubMed] [Google Scholar]
- 5. Adamczyk B, Adamczyk-Sowa M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid Med Cell Longev 2016; 2016: 1973834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Angelis F, Plantone D, Chataway J. Pharmacotherapy in secondary progressive multiple sclerosis: an overview. CNS Drugs 2018; 32: 499–526. [DOI] [PubMed] [Google Scholar]
- 7. Spindler M, Flint Beal M, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat 2009; 5: 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belousova M, Tokareva OG, Gorodetskaya E, et al. Intravenous treatment with coenzyme Q10 improves neurological outcome and reduces infarct volume after transient focal brain ischemia in rats. J Cardiovasc Pharmacol 2016; 67: 103–109. [DOI] [PubMed] [Google Scholar]
- 9. Gironi M, Borgiani B, Mariani E, et al. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J Immunol Res 2014; 2014: 961863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi I, Lee P, Adany P, et al. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult Scler 2018; 24: 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanoobar M, Eghtesadi S, Azimi A, et al. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Int J Neurosci 2013; 123: 776–782. [DOI] [PubMed] [Google Scholar]
- 12. Sanoobar M, Eghtesadi S, Azimi A, et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutr Neurosci 2015; 18: 169–176. [DOI] [PubMed] [Google Scholar]
- 13. Sanoobar M, Dehghan P, Khalil M, et al. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: a double blind randomized clinical trial. Nutr Neurosci 2016; 19: 138–143. [DOI] [PubMed] [Google Scholar]
- 14. Sedgwick P. What is a crossover trial? BMJ 2014; 348: 9–10. [DOI] [PubMed] [Google Scholar]
- 15. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis : the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benedict RHB, Munschauer F, Linn R, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler 2003; 9: 95–101. [DOI] [PubMed] [Google Scholar]
- 18. O’Brien A, Gaudino-Goering E, Shawaryn M, et al. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol 2007; 22: 933–948. [DOI] [PubMed] [Google Scholar]
- 19. Tur C. Fatigue management in multiple sclerosis. Curr Treat Options Neurol 2016; 18: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beck A, Steer R, Brown G. Beck depression inventory: second edition manual. San Antonio, TX: The Psychological Corporation, 1996. [Google Scholar]
- 21. Moccia M, Lanzillo R, Costabile T, et al. Uric acid in relapsing–remitting multiple sclerosis: a 2-year longitudinal study. J Neurol 2015; 262: 961–967. [DOI] [PubMed] [Google Scholar]
- 22. Moccia M, Lanzillo R, Palladino R, et al. Uric acid: a potential biomarker of multiple sclerosis and of its disability. Clincial Chem Lab Med 2015; 53: 753–759. [DOI] [PubMed] [Google Scholar]
- 23. Moccia M, Picillo M, Erro R, et al. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson‘s disease. Eur J Neurol 2015; 22: 93–98. [DOI] [PubMed] [Google Scholar]
- 24. Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol 2017; 17: 49–59. [DOI] [PubMed] [Google Scholar]
- 25. Elyaman W, Khoury SJ. Th9 cells in the pathogenesis of EAE and multiple sclerosis. Semin Immunopathol 2017; 39: 79–87. [DOI] [PubMed] [Google Scholar]
- 26. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015; 15: 545–558. [DOI] [PubMed] [Google Scholar]
- 27. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactinve astroctes are induced by activated microglia. Nature 2017; 541: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Göbel K, Ruck T, Meuth SG. Cytokine signaling in multiple sclerosis: lost in translation. Mult Scler J 2018; 24: 432–439. [DOI] [PubMed] [Google Scholar]
- 29. Huber AK, Giles DA, Segal BM, et al. An emerging role for eotaxins in neurodegenerative disease. Clin Immunol 2018; 189: 29–33. [DOI] [PubMed] [Google Scholar]
- 30. Pittaluga A. CCL5-glutamate cross-talk in astrocyte-neuron communication in multiple sclerosis. Front Immunol 2017; 8: 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molnarfi N, Benkhoucha M, Bjarnadóttir K, et al. Interferon-β induces hepatocyte growth factor in monocytes of multiple sclerosis patients. PLoS One 2012; 7: e49882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benkhoucha M, Molnarfi N, Schneiter G, et al. The neurotrophic hepatocyte growth factor attenuates CD8+ cytotoxic T-lymphocyte activity. J Neuroinflammation 2013; 10: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Girolamo F, Coppola C, Ribatti D, et al. Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 2014; 2: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guglielmetti C, Le Blon D, Santermans E, et al. Interleukin-13 immune gene therapy prevents CNS inflammation and demyelination via alternative activation of microglia and macrophages. Glia 2016; 64: 2181–2200. [DOI] [PubMed] [Google Scholar]
- 35. Rossi S, Studer V, Motta C, et al. Neuroinflammation drives anxiety and depression in relapsing–remitting multiple sclerosis. Neurology 2017; 89: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 36. Magliozzi R, Howell O, Nicholas R, et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 2018; 83: 739–755. [DOI] [PubMed] [Google Scholar]
- 37. Sanoobar M, Dehghan P, Khalili M, et al. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: a double blind randomized clinical trial. Nutr Neurosci 2016; 19: 138–143. [DOI] [PubMed] [Google Scholar]
- 38. Abuelezz SA, Hendawy N, Magdy Y. Targeting oxidative stress, cytokines and serotonin interactions via indoleamine 2,3 dioxygenase by coenzyme Q10: role in suppressing depressive like behavior in rats. J Neuroimmune Pharmacol 2017; 12: 277–291. [DOI] [PubMed] [Google Scholar]
- 39. Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain 2015; 138: 3287–3298. [DOI] [PubMed] [Google Scholar]
- 40. Cordero MD, Cotán D, del-Pozo-Martín Y, et al. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 2012; 28: 1200–1203. [DOI] [PubMed] [Google Scholar]
- 41. Zoghi S, Amirghofran Z, Nikseresht A, et al. Cytokine secretion pattern in treatment of lymphocytes of multiple sclerosis patients with fumaric acid esters. Immunol Invest 2011; 40: 581–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_PDF for Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis by Marcello Moccia, Antonio Capacchione, Roberta Lanzillo, Fortunata Carbone, Teresa Micillo, Francesco Perna, Anna De Rosa, Antonio Carotenuto, Roberto Albero, Giuseppe Matarese, Raffaele Palladino and Vincenzo Brescia Morra in Therapeutic Advances in Neurological Disorders