Abstract
Pulmonary arterial hypertension (PAH) is characterized by progressive dyspnea and exercise limitation and is associated with reduced health-related quality of life. Few clinical studies have evaluated the primary effects of treatment of PAH from the patient perspective. Here, we present the impact of riociguat on patient-reported outcomes (PROs) in treatment-naïve patients with PAH. MOTION (NCT02191137) was an open-label, phase 4 trial of riociguat monotherapy in treatment-naïve patients with PAH. The primary endpoint was the change in total score from baseline to Week 24 in the Living with Pulmonary Hypertension (LPH) questionnaire. The Short Form-12 Health Survey and Work Limitations Questionnaire 8 were also utilized to assess PROs. Other secondary endpoints included change from baseline in World Health Organization functional class (WHO FC), 6-min walk distance (6MWD), Modified Borg Dyspnea Scale, and safety. At week 24 (n = 66), the mean (standard deviation [SD]) total LPH score was 37.17 (24.61), for a mean (SD) change from baseline of −10.99 (22.51). At last visit, with week 24 imputed, the mean (SD) total score was 40.63 (28.38), for a mean (SD) change from baseline of −5.40 (27.8) (n = 75; P = 0.0484). Improvement in LPH questionnaire total score was observed by week 4 and was maintained through week 24. Improvements were observed in WHO FC, Modified Borg Dyspnea Scale, and accelerometer-measured 6MWD at week 24. Treatment with riociguat had a positive impact on PROs in treatment-naïve patients with PAH and was well tolerated, with a similar safety profile to that observed in placebo-controlled phase 3 trials.
Keywords: health-related quality of life, pulmonary arterial hypertension, riociguat
Background
Pulmonary arterial hypertension (PAH) is a rare and complex disease, characterized by restricted flow through the pulmonary arterial circulation, increased pulmonary vascular resistance (PVR), and remodeling that can ultimately lead to right heart failure and death.1Progressive dyspnea and exercise limitation are associated with reduced physical and emotional health-related quality of life (HRQoL), often to a severe degree.2Advances in treatment options for patients with PAH have allowed a shift in focus from short-term functional changes to improvements in symptom impact, as well as in longer-term outcomes,3such as increased survival and improved quality of life.4It has been demonstrated that objective measures of functional capacity, such as a decrease in 6-min walking distance (6MWD), a decline in World Health Organization functional class (WHO FC), and a prolonged time to clinical worsening correlate with long-term outcomes; these are commonly used as clinically meaningful trial endpoints.5The importance of incorporating patient-reported outcomes (PROs) into clinical studies of therapies for PAH is increasingly recognized6,7and reflective of the growing importance given to the patient voice in the drug development and approval process.8Although trials have incorporated PRO measures as secondary outcomes,9few, if any, have evaluated PROs as a primary endpoint in the pharmacological treatment of PAH.
Riociguat (Adempas; Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA) is a direct stimulator of soluble guanylate cyclase indicated for the treatment of adult patients with PAH to improve exercise capacity and WHO FC and to delay time to clinical worsening.9It is also the only medical therapy indicated to treat persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH) after surgical treatment or inoperable CTEPH. Riociguat is both efficacious and well tolerated and is associated with sustained improvements in exercise and functional capacity for up to two years.10–12Here, we present the results of the MOTION study, which evaluated the impact of riociguat in treatment-naïve patients with PAH, utilizing PROs as the primary efficacy endpoint.
Materials and methods
Study design
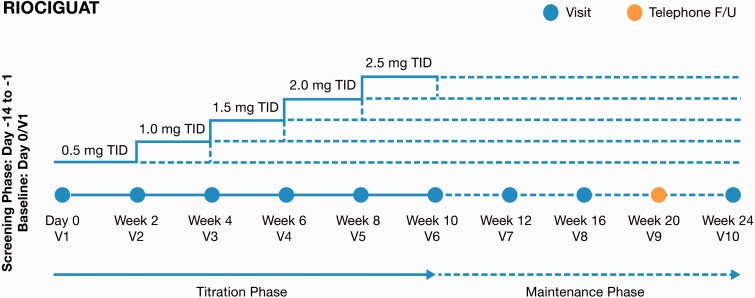
MOTION (NCT02191137) was a prospective, multicenter, single-arm, open-label, phase 4 trial of riociguat monotherapy in patients with PAH who were not on active treatment (Fig. 1). While monotherapy was the standard of care for treatment-naïve patients with PAH when MOTION was initiated, dual therapy has now been established as efficacious.13
Fig. 1.
MOTION study design.
The primary efficacy endpoint of MOTION, modeled after the exploratory endpoints in the phase 3 PATENT-1 study,9was defined as the change from baseline to week 24 in the Living with Pulmonary Hypertension (LPH) questionnaire, a validated, disease-specific HRQoL assessment tool that comprises a physical and emotional dimension.14Secondary variables included change from baseline in each of the following: LPH score after week 16; Work Limitations Questionnaire 8 (WLQ-8); Short Form-12 Health Survey (SF-12); WHO FC, modified Borg Dyspnea Scale; 6MWD after weeks 16 and 24; and safety data.
An exploratory variable consisting of accelerometer wristband activity (number of steps taken using a Fitbit One®) was recorded during the 6MWD test at screening, baseline, week 16, and week 24. These data were then converted into distance covered, allowing a direct comparison to the results of the 6MWD test.
The study design has been previously described in detail.15Briefly, following a screening period of up to 14 days, eligible individuals received therapy with riociguat during a 10-week titration phase (starting dose of 0.5 mg three times daily, with adjustments in 0.5-mg increments at two-week intervals, to a maximum total daily dose of 7.5 mg) and a subsequent 14-week maintenance phase at the optimal individual dose (Fig. 1). A safety follow-up was performed 30 days after the last dose of study medication for all patients.
The study was carried out according to Good Clinical Practice guidelines and under the guiding principles detailed in the Declaration of Helsinki and applicable local laws and regulations. Documented approval from appropriate Institutional Review Boards and Independent Ethics Committees was obtained for all participating centers before the start of the study (IRB Tracking #INC1-14-266).
Patients
Patients eligible for inclusion met all of the following criteria: aged 18–80 years with the diagnosis of symptomatic PAH (WHO Group 1) with PVR > 300 dyn•s•cm−5, mean pulmonary artery pressure ≥ 25 mmHg, pulmonary capillary wedge pressure ≤ 15 mmHg as assessed by right heart catheterization within six months before screening (Visit 0), and 6MWD of 150–450 m with relative difference (i.e. absolute difference/mean) ≤ 15% between the screening and the baseline test. Idiopathic and familial PAH were included, as well as PAH associated with connective tissue disease, congenital heart disease > 1 year after surgical repair, anorexigen or amphetamine use, and portal hypertension with liver cirrhosis. All patients provided written, informed consent before study entry; women of childbearing potential were screened for and took appropriate measures to avoid pregnancy.
Concomitant treatment with nitrate or nitric oxide donor therapy, phosphodiesterase (PDE) type 5 inhibitors (e.g. sildenafil, tadalafil, vardenafil), and non-specific PDE inhibitors (e.g. theophylline, dipyridamole) was not allowed. Patients unable to perform a valid 6MWD were excluded.
HRQoL outcome measures
The LPH, SF-12, and WLQ-8 questionnaires were self-administered at day 0 and weeks 4, 16, and 24. WHO FC was assessed at screening, day 0, and weeks 4, 16, and 24. Modified Borg Dyspnea Scale, 6MWD, and accelerometer wristband activity were assessed at screening, day 0, and weeks 16 and 24.
The assessment tools have been previously reviewed in detail.15The LPH questionnaire was adapted from the Minnesota Living with Heart Failure questionnaire (MLHFQ) to assess PAH patients. Strong content and psychometric validity established the LPH questionnaire as a reliable measure of symptoms and HRQoL for PAH.14The survey consists of 21 items, responded to on a 0 (“No”) to 5 (“Very much”) scale, allowing a total score in the range of 0–105; a higher score indicates that the patient is more affected by his/her medical condition.14The SF-12 was chosen to assess general HRQoL and the WLQ-8 to assess work performance and productivity, both yielding information complementary to that obtained by the LPH questionnaire.15
Statistical analysis
Categorical variables were analyzed by frequency tables, and continuous variables by sample summary statistics, including mean, standard deviation (SD), median, interquartile range, and minimum/maximum. Statistical tests were performed with a Type I, two-sided significance level (alpha = 5%). Mean change in LPH total score from baseline to week 24 was tested for a difference from 0 using a one-sample t-test. Summary statistics for the absolute values and changes from baseline include the standard error and 95% confidence interval. Secondary efficacy variables were analyzed descriptively. Sample size was determined based on results of the PATENT-1 study for riociguat 1.0–2.5 mg. The study was planned with 80% power to detect a difference in using a paired t-test (5% one-sided alpha). To achieve the calculated sample size of 56, enrollment was planned for 61 individuals, which led to finding 56 evaluable patients.
Results
Patients
Study recruitment began in September 2014. The safety and intent-to-treat (ITT) analysis sets were synonymous by definition and included all patients who received at least one dose of riociguat. The completers analysis set included patients who received at least one dose of riociguat and completed an LPH assessment at baseline and at weeks 4, 16, and 24. Of 97 incident patients screened, 22 (22.7%) were excluded and did not receive riociguat (20 patients were screen failures and two patients withdrew from the study); 75 (77.3%) were enrolled by 21 December 2015 and formed the ITT group. Seventeen patients (17.5%) from the ITT group missed ≥ 1 LPH scores and were therefore excluded from the completer analysis set; 58 patients (59.8%) were included in the completers analysis set.
Baseline characteristics
The study population was predominantly female (88%) and white (78.7%), with a mean age of 61.7 years. Baseline clinical characteristics included a most common primary diagnosis of idiopathic PAH (65.3%) and WHO FC III (58.7%), with a mean (SD) LPH total score at baseline of 46.03 (24.57) (Table 1).
Table 1.
Study population (safety analysis set).
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 9 (12.0) |
| Female | 66 (88.0) |
| Race | |
| White | 59 (78.7) |
| Black | 10 (13.3) |
| American Indian or Alaska Native | 1 (1.3) |
| Not reported | 5 (6.7) |
| Mean (SD) |
|
| Age (years) | 61.7 (11.4) |
| Baseline body mass index (kg/m2) | 32.08 (7.19) |
| Select concomitant medications (n (%)) | |
| Antithrombotics | 48 (64.0) |
| Calcium channel blocker | 22 (29.3) |
| Diuretics | 50 (66.7) |
| Primary diagnosis at baseline (n (%)) | |
| Idiopathic PAH (IPAH) | 49 (65.3) |
| Familial PAH (FPAH) | 1 (1.3) |
| Drug- and toxin-induced PAH | 1 (1.3) |
| Associated PAH (APAH) conditions | 1 (1.3) |
| Connective tissue disease | 21 (28.0) |
| Portal hypertension | 2 (2.7) |
| WHO FC at baseline (n (%)) | |
| I | 7 (9.3) |
| II | 23 (30.7) |
| III | 44 (58.7) |
| IV | 1 (1.3) |
| LPH score at baseline (mean (SD)) | |
| Total score | 46.03 (24.57) |
| Physical dimension score | 22.83 (10.70) |
| Emotional dimension score | 10.2 (7.8) |
| WLQ-8 score at baseline (mean (SD)) | |
| Time Management Score | 36.42 (30.82) |
| Mental Interpersonal Demands Score | 28.66 (25.22) |
| Output Demands Score | 36.52 (31.51) |
| Physical Demands Score | 33.47 (28.25) |
Efficacy
The effect of riociguat treatment on change from baseline for LPH questionnaire total scores is shown in Table 2. The ITT analysis set was used for the primary efficacy analysis (n = 75). The majority of these patients (53; 70.7%) had an overall treatment compliance of 90–110%. Overall mean (SD) treatment compliance was 94.7% (11.4).
Table 2.
Mean (SD) change from baseline for Living with Pulmonary Hypertension questionnaire total scores (ITT analysis set).
| Visit | n | Total score at visit (mean (SD)) | Change from baseline (mean (SD)) |
|---|---|---|---|
| Baseline* | 75 | 46.03 (24.57) | |
| Week 4 | 72 | 39.78 (22.72) | −7.65 (18.30) |
| Week 16 | 68 | 39.48 (22.57) | −6.98 (20.85) |
| Week 16 or Last Before† | 75 | 39.83 (23.90) | −6.20 (21.43) |
| Week 24 | 66 | 37.17 (24.61) | −10.99 (22.51) |
| Last visit‡ | 75 | 40.63 (28.38) | −5.40 (27.8) |
Last observed value before start of test drug.
Last observed value up to Visit 8 (week 16). If a patient died before week 16, the worst value of 105 was used.
Last observed value (not including follow-up). If a patient died before week 24 or during follow-up after termination before week 24, the worst value of 105 was used.
ITT, intent to treat; SD, standard deviation.
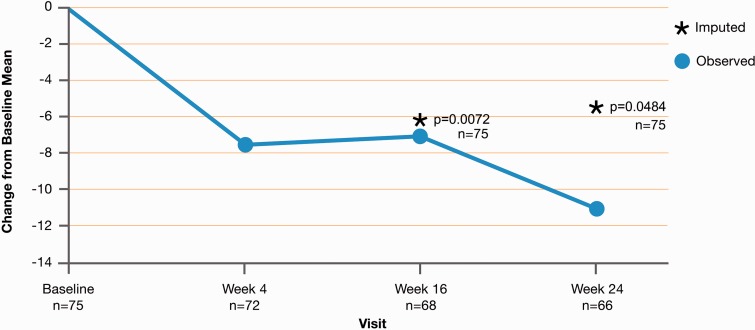
Figure 2demonstrates the change from baseline for LPH total scores for the ITT analysis set over time. At week 24 (n = 66), the mean (SD) total score was 37.17 (24.61), for a mean (SD) change from baseline of −10.99 (22.51). At last visit, with week 24 imputed, the mean (SD) total score was 40.63 (28.38), for a mean (SD) change from baseline of −5.40 (27.8) (n = 75; P = 0.0484). Improvement in LPH questionnaire total score was observed by week 4 and was maintained through week 24 (Fig. 2). At week 24, the improvement from baseline in the LPH physical dimension score (n = 65; −6.10 units) was of greater magnitude than the improvement of the emotional dimension score (n = 66, −2.33 units); both scores showed the greatest improvement at week 24.
Fig. 2.
The mean change from baseline for LPH total scores for the ITT analysis set. Baseline is defined as the last observed value before the start of the test drug. Imputed values are weeks 16 and 24. If a patient died before week 16 or week 24, the worst value of 105 was used.
Results were similar for the completers set (patients who had received at least one dose of riociguat and completed an LPH assessment at baseline and at weeks 4, 16, and 24); at week 24, the observed mean (SD) score was 36.23 (24.38), for a mean (SD) change from baseline of −10.77 (21.89) (n = 58, P = 0.0002).
WLQ-8 scores measure the reported amount of time in the previous two weeks that respondents were limited in performance at the workplace. Only 25 patients (33%) were employed either full-time or part-time at the time of enrollment and most patients who were not working did not complete the WLQ-8 questionnaire. At week 24, improvements from baseline were observed in the WLQ-8 Time Management Score (−2.68 units), Mental Interpersonal Demands Score (−2.13 units), and Output Demands Score (−2.57 units). The Physical Demands Score increased from baseline by 2.33 units. Positive correlations were found between LPH and WLQ scores, but results should be interpreted with caution due to missing data.
SF-12 Health Survey scores for the ITT analysis set revealed mean (SD) changes from baseline to weeks 4, 16, and 24 in Physical Component Summary score of 1.009 (6.474), 0.036 (7.059), and 1.230 (8.401), respectively, and Mental Component Summary score of 1.955 (9.262), 1.869 (7.605), and 2.833 (10.073), respectively.
Most patients in the ITT group were WHO FC III (n = 44; 58.7%) and II (n = 23; 30.7%) at baseline. At week 24, most patients remained in WHO FC II (n = 32; 47.8%) and III (n = 29; 43.3%), with 28.4% improved by at least one level, 64.2% unchanged, and 7.5% worsened. For the Modified Borg Dyspnea Scale, the mean value (SD) at baseline for the ITT analysis set was 4.47 (2.74) and improved to 3.82 (2.65) at week 16, for a mean (SD) change from baseline of −0.68 (3.05). At week 24, the mean (SD) value was 3.82 (2.75), for a mean (SD) change from baseline of −0.66 (3.45). No correlation was observed between the LPH scores and the Modified Borg Dyspnea Scale. In the ITT analysis set, the 6MWD at baseline was a mean (SD) of 310.3 (89.6) and improved a mean (SD) of 2.4 (75.5) m from baseline to week 16 but declined at week 24 with a mean (SD) change from baseline of −2.9 (59.2) m. No correlation was observed between the LPH scores and the 6MWD.
Exploratory efficacy analysis
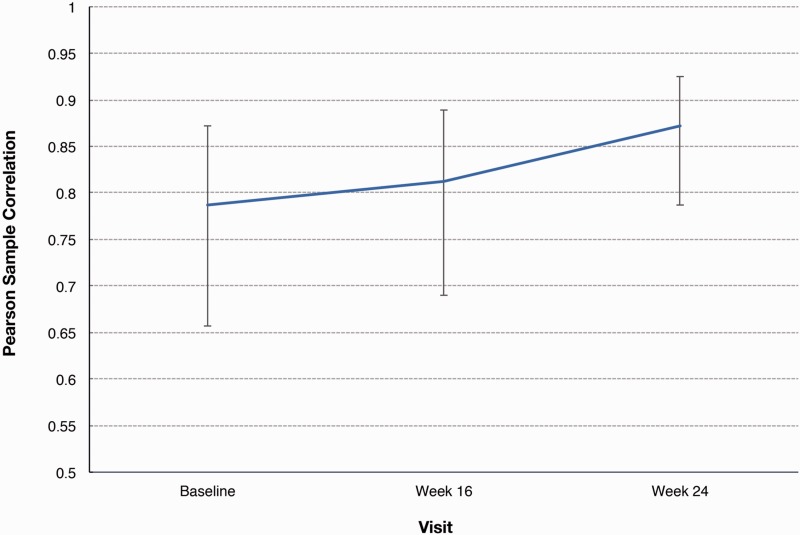
Accelerometer wristband (Fitbit One®) activity was recorded for the ITT analysis set during the 6MWD test at screening, baseline, and at weeks 16 and 24, as an alternative measure of distance. The mean (SD) 6MWD recorded by an accelerometer wristband was 361.4 (95.4) m at baseline and 378.7 (115.0) m at week 24, for a change of 17.5 (61.6) m. At each time point, paired measurements of 6MWD, made directly using the accelerometer wristband, exhibited a strong correlation, with r-values by Pearson correlations in the range of 0.79–0.87 (P < 0.0001) (Fig. 3).
Fig. 3.
Pearson correlation between 6MWD direct and accelerometer wristband measures (ITT analysis set). Note: Baseline = last observed value before start of study treatment. Week 16 or Last Before = last observed value up to Visit 8 (week 16). If a patient died before week 16, the worst value of 0 was used. Last Visit = last observed value (not including follow-up). If a patient died before week 24 or during follow-up after termination before week 24, the worst value of 0 was used.
Safety
There were no new safety signals identified in this study. Table 3presents the number of patients with treatment-emergent adverse events (TEAEs). A total of 46 patients (61.3%) had a TEAE considered related to riociguat. Four patients (5.3%) had an AE with an outcome of death not considered related to riociguat treatment. The most frequently reported TEAEs were dizziness (22.7%), nausea (21.3%), and dyspepsia (20.0%). No significant change in mean systolic blood pressure was noted. The mean (SD) change from baseline to week 24 in SaO2in the 6MWD (difference between pre-walk and post-walk) was −5.79 (6.14) and the corresponding mean (SD) change in heart rate was 24.18 (16.79) bpm.
Table 3.
Number of patients with treatment-emergent adverse events (TEAEs; ≥ 10% patients) (safety analysis set).
| TEAEs (≥ 10% patients) | n (%) |
|---|---|
| Dizziness | 17 (22.7) |
| Nausea | 16 (21.3) |
| Dyspepsia | 15 (20.0) |
| Diarrhea | 14 (18.7) |
| Headache | 13 (17.3) |
| Dyspnea | 11 (14.7) |
| Fatigue | 10 (13.3) |
| Hypotension | 9 (12.0) |
| Edema peripheral | 9 (12.0) |
| Nasopharyngitis | 9 (12.0) |
| Vomiting | 9 (12.0) |
| Chest pain | 9 (12.0) |
| Constipation | 8 (10.7) |
| Gastroesophageal reflux disease | 8 (10.7) |
| Back pain | 8 (10.7) |
Discussion
Treatment with riociguat had a positive impact on PROs using the LPH questionnaire for treatment-naïve patients with PAH. Improvement in LPH scores was observed by week 4 and maintained to week 24. With respect to secondary endpoints, improvement was observed in the WHO FC by week 24 and in the Modified Borg Dyspnea Scale at weeks 16 and 24. These improvements were consistent with those observed in the PATENT-1 trial. Riociguat was well tolerated and the safety profile was similar to that observed in phase 3 studies.
The value of PRO instruments specific for patients with PAH, as a direct measure of treatment benefit in research trials and clinical practice, is acknowledged by regulators and experts in the field.16While studies have looked at improvement in HRQoL as a secondary endpoint,9to our knowledge, the MOTION study is the first clinical trial to utilize PROs in the primary efficacy endpoint evaluation of a treatment for PAH. Compared to baseline, significant improvement in HRQoL with riociguat monotherapy in treatment-naïve patients was demonstrated. In the pivotal PATENT study, where LPH was used as an exploratory endpoint, the data did not show a significant improvement in LPH between the Adempas-treated group and the placebo group.9However, 44% of patients in that study were on background therapy, which may explain the difference in outcomes. A post-hoc analysis of the patients from PATENT who were not on background therapy could be informative.
There appears to be a potential disconnect between objective outcomes and HRQoL outcomes in studies that include both measures. For example, in the AMBITION trial—which demonstrated that upfront combination therapy with ambrisentan and tadalafil in treatment-naïve patients with PAH significantly decreased the risk of clinical failure versus monotherapy—no significant improvement in HRQoL for combination therapy over monotherapy was observed.13,17There were no statistically significant differences between combination and pooled monotherapy in the Short-Form 36 Health Survey (SF-36) physical and mental summary scores, nor in the three summary scores of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) from baseline to week 24. Combination therapy over 24 weeks did result in a statistically significant improvement in the Health Transition score of the SF-36 compared with pooled monotherapy.17In the current study, there was overall improvement in the LPH scores and 6MWD improved at 16 weeks. The patients continued to report an improvement in the LPH scores at 24 weeks, despite a decrease in overall 6MWD. Similarly, in the SERAPHIN and GRIPHON trials—which showed a significant reduction in morbidity endpoints—the improvement in 6MWD was modest.18,19We cannot completely explain this finding, but this may provide further support to the notion that HRQoL measurement may add value over and above the benefit shown by objective clinical assessment methods, such as 6MWD, for some patients. As we move forward, it would help to look at HRQoL as one of the primary endpoints to help us better understand the significance of this discrepancy.
The efficacy of new telemetric technology was explored in the MOTION study through measurement of 6MWD in patients with PAH using an accelerometer wristband (Fitbit One®). Correlation between accelerometer and direct measurement of 6MWD has been previously demonstrated in patients with chronic heart failure.20Even though there was minimal change in 6MWD, the correlation between accelerometer and direct measurement in the current study illustrates the potential for accelerometers to be used as a means for remote measurement of patient function at home. This could translate into new paradigms for assessment in clinical trials and, potentially, treatment guidance for patients with PAH.
There are several limitations to the MOTION study that are common to all single-arm studies. The lack of a placebo group means that it is impossible to unequivocally attribute all efficacy findings to riociguat. Responses could be due to a placebo effect, improvement from focused care, or a spontaneous improvement in natural history.
Treatment with riociguat had a positive impact on LPH scores, WHO FC, and the Modified Borg Dyspnea Scale for treatment-naïve patients with PAH. Riociguat was well tolerated, with no new safety concerns identified.
Acknowledgments
These data were presented in part at the American Thoracic Society 2017 International Conference, Washington, DC, May 19–24, 2017. Medical writing support was provided by Pamela M. Thomas, MD, on behalf of Nascent Medical, LLC. Writing, editorial support, and formatting assistance were provided by Simpson Healthcare Executives.
Conflict of interest
NS: Research support from Actelion, Bayer, Gilead, Arena, United Therapeutics, Reata; AA: Research support from Actelion, Bayer, United Therapeutics, Novartis, GSK; Speaker for Actelion, Gilead, Bayer, United Therapeutics, Pfizer, Genentech; DP: Employee of Bayer HealthCare Pharmaceuticals, Inc.; AL: Employee of Bayer HealthCare Pharmaceuticals, Inc.; FK: Employee of Bayer HealthCare Pharmaceuticals, Inc.; GO: Employee of Bayer HealthCare Pharmaceuticals, Inc.
Funding
This work was supported by Bayer HealthCare Pharmaceuticals, Inc.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53(17): 1573–1619. [DOI] [PubMed] [Google Scholar]
- 2.Taichman DB, Shin J, Hud L, et al. Health-related quality of life in patients with pulmonary arterial hypertension. Respir Res 2005; 6: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013; 62(Suppl. 25): D73–D81. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Taichman DB, Doyle RL. Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proc Am Thorac Soc 2008; 5: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divers C, Platt D, Wang E, et al. A review of clinical trial endpoints of patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension and how they relate to patient outcomes in the United States. J Manag Care Spec Pharm 2017; 23(1): 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(Suppl. 1): S97–S107. [DOI] [PubMed] [Google Scholar]
- 7.Gomberg-Maitland M, Bull TM, Saggar R, et al. New trial designs and potential therapies for pulmonary artery hypertension. J Am Coll Cardiol 2013; 62(Suppl. 25): D82–D91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perfetto EM, Burke L, Oehrlein EM, et al. Patient-focused drug development: a new direction for collaboration. Med Care 2015; 53(1): 9–17. [DOI] [PubMed] [Google Scholar]
- 9.Ghofrani H-A, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 10.Bayer HealthCare Pharmaceuticals, Inc. Adempas (riociguat) Prescribing Information, Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc., 2014. [Google Scholar]
- 11.Rubin LJ, Galie N, Friedrich G, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study [PATENT-2]. Eur Respir J 2015; 45: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 12.Rubin LJ, Galie N, Grimminger F, et al. Presented at: European Respiratory Society International Congress; Munich, Germany; September 2014.
- 13.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 14.Bonner N, Abetz L, Meunier J, et al. Development and validation of the living with pulmonary hypertension questionnaire in pulmonary arterial hypertension patients. Health Qual Life Outcomes 2013; 11: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathai SC, Minai O, Sullivan SD, et al. Rationale and study design of MOTION: a phase 4, prospective, single-arm, open-label study to measure outcomes in patients with pulmonary arterial hypertension not on active treatment. Respir Med 2017; 122: S23–S27. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. The Voice of the Patient: Pulmonary Arterial Hypertension. Washington, DC: U.S. FDA, 2014. Available at: https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM429382.pdf(accessed 5 July 2017).
- 17.Peacock A, Galie N, Barbera J, et al. Initial Combination Therapy of Ambrisentan and Tadalafil and Quality of Life: SF-36 & CAMPHOR Analyses from the AMBITION Trial. Presented at: American Thoracic Society, San Diego, CA; May 18–23, 2017. Poster A6905.
- 18.Pulido T, Adzerikho I, Channick R, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 19.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary artery hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 20.Jehn M, Schmidt-Trucksäess A, Schuster T, et al. Accelerometer-based quantification of 6-minute walk test performance in patients with chronic heart failure: applicability in telemedicine. J Card Fail 2009; 15(4): 334–340. [DOI] [PubMed] [Google Scholar]