Abstract
Neoadjuvant therapy, where patients receive systemic therapy before surgical removal of the tumour, can downstage tumours allowing breast-conserving surgery, rather than mastectomy. In addition to its impact on surgery, the neoadjuvant setting offers a valuable opportunity to monitor individual tumour response. The effectiveness of standard and/or potential new therapies can be tested in the neoadjuvant pre-surgical setting. It can potentially help to identify markers differentiating patients that will potentially benefit from continuing with the same or a different adjuvant treatment enabling personalised treatment. Characterising the molecular response to treatment over time can more accurately identify the significant differences between baseline samples that would not be identified without post-treatment samples. In this review, we discuss the potential and challenges of using the neoadjuvant setting in translational breast cancer research, considering the implications for improving our understanding of response to treatment, predicting therapy benefit, modelling breast cancer dormancy, and the development of drug resistance.
Keywords: Breast cancer dormancy, drug resistance, clinical model, neoadjuvant therapy
Introduction
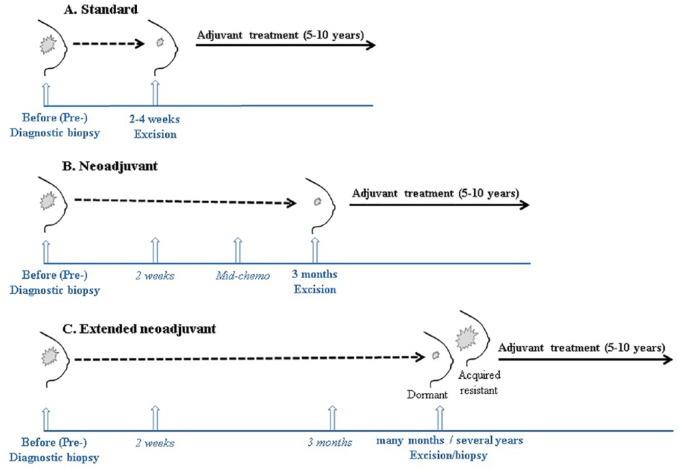
Neoadjuvant therapy is the pre-operative treatment of tumours with chemotherapy, radiation therapy, and endocrine therapy. It was originally used for its impact on surgery, downstaging tumours, and allowing breast-conserving surgery rather than mastectomy. In addition, neoadjuvant therapy offers potential opportunities for response prediction1 and relatively quick assessment for drug development and approval in breast cancer2 by monitoring benefit from the intervention at early stages of disease. Neoadjuvant chemotherapy and endocrine therapy are widely used and studied in breast cancer.3 Development of better markers of response is required for more accurate stratification of patients to neoadjuvant chemotherapy and endocrine therapy. Analysis of gene expression changes in patient-matched sequential samples collected before and on treatment may be a promising way to consider the molecular changes that occur during treatment that are required for response. These samples could be the diagnostic and surgical samples, or additional biopsies taken during treatment (Figure 1). Furthermore, comparing samples from patients who have a non-complete response to neoadjuvant treatment to samples from those do have a complete response can provide better understanding of innate/acquired resistance mechanisms, allowing better therapy management.
Figure 1.
Opportunities for using sequential patient-matched samples for studying response to therapy and predicting therapy benefit in breast cancer. Vertical arrows represent the normal and potential additional (italics) opportunities when sequential patient-matched samples can be taken during a standard treatment regimen (A), during neoadjuvant therapy (B), and during extended neoadjuvant therapy, which could serve as a novel clinical model to investigate treatment-induced dormancy and acquired resistance (C).
In this review, we summarise recent literature focusing on the impact of neoadjuvant chemotherapy and neoadjuvant endocrine therapy for translational research in breast cancer, emphasising opportunities for predicting response and improving our understanding of resistance to therapy.
Neoadjuvant Chemotherapy in Breast Cancer
Neoadjuvant chemotherapy has been established in downstaging large or locally advanced tumours allowing breast-conserving surgery, thereby avoiding mastectomy since the 1970s.4 Patients with tumours that achieve a pathological complete response (pCR) to neoadjuvant chemotherapy have been shown to have lower recurrence rates compared with those with partial response.5 However, pCR is achieved only in 20% to 30% of patients,6 and its predictive value depends on the tumour biology. Patients with human epidermal growth factor receptor (HER)2-positive and triple-negative tumours are good candidates for neoadjuvant chemotherapy as they have higher probability of achieving pCR.7 Residual cancer burden (RCB) which combines pathologic measurements of size and cellularity of primary tumour and number and size of nodal metastases8 provides a standardised procedure for the prospective evaluation of specimens to report response to neoadjuvant chemotherapy. In a recent prospective study, RCB has been shown to be prognostic for long-term survival following neoadjuvant chemotherapy in all 3 subsets of breast cancer, oestrogen receptor alpha positive (ER+), HER2-negative, and triple-negative disease.9
A study of 32 patients across a wide variety of tumour stage, sizes, and hormone receptor status before and after 4 cycles of epirubicin and cyclophosphamide followed by 4 cycles of docetaxel found that prediction of response to neoadjuvant chemotherapy was achievable with a 21-gene list.10 They particularly noted both initial low expression and upregulation of HER4 was found in 26 of 32 (81%; P = .002) and in 23 of 25 (92%; P < .001) responders, respectively. They showed classifying patients by their initial molecular subtype into basal or non-basal like significantly correlated with attainment of pCR to neoadjuvant chemotherapy. Specifically, 7 of 10 basal-like tumours responded to neoadjuvant chemotherapy, whereas 19 of 22 non-basal-like tumours did not respond. Change from basal-like to non-basal like subtype after 4 chemotherapy cycles was also predictive of responder status.
In another study comparing gene expression profiles in 21 patients before and 14 patients after 1 cycle of neoadjuvant docetaxel and capecitabine,11 the focus was on identifying pathways rather than individual genes associated with response and resistance. The study found expression of DNA repair genes was higher in responding tumours, whereas non-responders had significantly higher levels of microtubule-associated protein 2 (MAP2). However, the study’s definition of non-response included tumours with partial response after 4 cycles of treatment and some tumours with up to 80% decrease in size, conflicting with definitions of response in other studies. Analysis of the largest data set of sequential-matched samples, from patients who received neoadjuvant chemotherapy in the I-SPY 1 trial, identified candidate immune and proliferation pathways associated with response and recurrence.12 We have recently conducted a patient-matched sequentially sampled neoadjuvant chemotherapy study, which suggests that on-treatment biomarkers may out-perform pre-treatment biomarkers for predicting response and outcome.13 Data from the I-SPY 1 trial provided the essential validation data set.
Although definitive molecular markers that can predict response to neoadjuvant chemotherapy have not been identified yet, multigene expression assays for prognosis prediction for relapse and risk stratification in the adjuvant setting are already established. Multigene tests EndoPredictClin and Oncotype DX predict patients who can avoid chemotherapy, thereby reducing over-treatment and the associated side-effects. The clinical utility of Oncotype DX in the identification of low-risk patients who will not benefit from adjuvant chemotherapy has also been validated.14 Recently, a PAM50-based chemoendocrine score has been developed based on the finding that most genes associated with endocrine sensitivity were also found to be associated with chemotherapy resistance.15
Despite literature showing efficacy of neoadjuvant chemotherapy, its use varies widely. In addition, its value has recently been questioned16 and, in turn, defended by a number of prominent advocates in the field. The main concern raised by Vaidya et al16 was the controversial value of pCR in predicting survival benefit, questioning the beneficial effect of neoadjuvant chemotherapy on patients. They pointed out EBCTCG meta-analysis showing no significant overall survival advantage with an increase in local recurrences with neoadjuvant chemotherapy compared with adjuvant chemotherapy (21.4% vs 15.9%).17 The reported increase in loco-regional recurrence risk associated with neoadjuvant chemotherapy in some trials have previously been attributed to the inclusion of patients who did not receive surgery as they have undergone pCR following neoadjuvant chemotherapy. However, recent EBCTCG individual patient data meta-analysis showed that higher local recurrence with neoadjuvant chemotherapy compared with adjuvant chemotherapy was not confined to trials where surgery was omitted.17
Second, Vaidya et al questioned the original impact of neoadjuvant chemotherapy in downstaging tumours allowing breast-conserving surgery, by highlighting that it makes the tumour and lymph nodes less palpable and the surgery more difficult and less precise in some patients. The authors concluded by suggesting limiting widespread use of neoadjuvant chemotherapy and considering its use only in patients whose tumours are large and not suitable for breast conservation but will potentially become suitable following neoadjuvant chemotherapy. This actually summarises the current approach where patients are managed by the multidisciplinary teams to decide the most appropriate treatment plan and markers of neoadjuvant therapy response are being investigated to better stratify patients. An informed discussion with patients to inform them about the up-to-date evidence and the possibility of mastectomy in the presence of no/partial response would be appropriate. Discussions on the use of neoadjuvant therapy such as that ignited by Vaidya et al will contribute to improving future precision in breast cancer practice while protecting any potential harm on patients in the meantime.
Pre-surgical Treatment to Improve Accuracy of Predicting Response to Endocrine Therapy
It is well established that oestrogen receptor alpha (ERα) levels correlate with endocrine treatment outcome and ERα presence at diagnosis is the best current predictor for endocrine therapy response. However, its predictive value is limited, as only 50% to 70% of ER+ patients respond to neoadjuvant endocrine therapy,18 which highlights the heterogeneous patient response and the urgent need for biomarkers that more accurately predict those likely to respond, from those who are innately or acquire resistance to endocrine treatment.
Expression profiling of pre- and on-treatment clinical samples under neoadjuvant therapy to predict response to treatment has recently become viable (Figure 1). Dowsett et al19 showed that reduction in proliferation (measured by Ki-67 level) within the first 12 weeks of neoadjuvant therapy predicts recurrence-free survival during adjuvant treatment. Currently, there is no consensus on the standard analytical and scoring methods, and cut-off value for Ki-67. Studies are underway to standardise Ki-67 measurement in clinical practice ensuring its reproducibility as an early marker of treatment efficiency.20 In addition, The Preoperative Endocrine Prognostic Index (PEPI) was developed to identify extreme responders to endocrine therapy such that subsequent management could be modified to avoid chemotherapy in a population at low risk of relapse post-neoadjuvant endocrine therapy. The PEPI was derived as an arithmetic sum of risk points weighted by the size of the hazard ratio (HR) assigned to each statistically significant factor (tumour size, node status, ER status, and Ki-67 natural log intervals, all derived from the surgical specimen).21 Ellis et al21 validated PEPI independently in IMPACT trial with 203 postmenopausal women showing that it significantly predicted recurrence-free survival. In another clinical trial, PEPI status was significantly predictive of recurrence-free survival for both neoadjuvant anastrozole and fulvestrant.22 The currently ongoing ALTERNATE trial will prospectively determine the validity of PEPI score.
In addition, gene expression changes in sequential patient-matched samples have been studied for 2 weeks to 3 months23-25 to determine treatment-induced dynamic changes in tumours. Mello-Grand et al23 proposed a 54-gene signature predictive of response in all the patients involved in the study (n = 17) treated with neoadjuvant anastrozole for 3 months. The results were further validated externally on a different data set where it correctly classified 26 of 37 responders and 11 of 15 non-responders. The differentially expressed genes were linked to downregulation of cell cycle and to stimulation of the immune response in responding tumours, whereas non-responding tumours demonstrated increased immune response and androgen receptor (AR) nuclear signalling suggesting tumour cells may divert androgens to escape aromatase inhibitor-mediated cell cycle inhibition.23
More recently, a 4-gene signature was identified to accurately predict which ER+ patients will clinically respond to short-term neoadjuvant aromatase inhibitor treatment.24 Proliferation genes were able to accurately predict letrozole response at 2 weeks, but not before treatment, suggesting that characterising the molecular response to treatment using early (2 weeks) on-treatment samples rather than relying on pre-treatment markers can more accurately predict responding patients.24 These results suggest that a full standard course of several months of neoadjuvant treatment (Figure 1B) may not be required and a short 2-week pre-surgical therapy (Figure 1A) might be sufficient to improve the accuracy of long-term prediction of outcomes. The POETIC (Peri-Operative Endocrine Therapy for Individualising Care) trial, in which more than 4000 postmenopausal ER+ patients were randomised to receive 2-week treatment of letrozole both before and after surgery, is currently in its follow-up period to assess the clinical predictive value of on-treatment proliferation biomarker Ki-67 for long-term outcome.26 The results will guide the design of future clinical trials particularly for the group of patients predicted to have poorer long-term outcome following neoadjuvant endocrine therapy requiring alternative interventions.
The transcriptional response to the ESR1 degrader fulvestrant was found to have much in common with oestrogen deprivation, but to be stronger with distinctions potentially attributable to arrest of oestrogen-independent ER activity and involvement of AR signalling.25 In NEWEST trial, high-dose fulvestrant (500 mg) resulted in a greater reduction of Ki-67 and ER levels compared with 250 mg after 4 weeks of neoadjuvant therapy. In CARMINA 02 trial, a homogeneous cohort of postmenopausal women with ER+, HER2-negative breast cancer, no significant differences between anastrozole and fulvestrant for pathological response and breast-conserving surgery rates were observed with higher clinical response rates (approximately 15%) for anastrozole.22 Although, it should be noted that the duration of neoadjuvant therapy was different for anastrozole and fulvestrant (5.7 and 4.8 months, respectively). Also, CARMINA trial showed that reduction in ER levels was greater with fulvestrant compared with anastrozole with similar reductions in Ki-67 expression, which are comparable to the results of a previous trial.27 The better biological activity/transcriptional response of fulvestrant does not necessarily result in better clinical response.22
Profiling of axillary lymph nodes can also contribute to optimal treatment of breast cancer. Histopathologic assessment of immune and stromal features of axillary lymph nodes combined with primary tumour have been recently shown to predict which node-positive patients will develop distant metastasis more accurately.28 Studies investigating gene expression profiling of primary breast cancers and matched metastases have been summarised by Kroigard et al.29 Studying axillary nodes with matched primary tumours before and after neoadjuvant treatment can provide further insight into tumour evolution and metastasis.
Extended Neoadjuvant Endocrine Therapy as a Clinical Model of Dormancy
The benefits of adjuvant endocrine therapy for breast cancer have been demonstrated in meta-analysis of large randomised trials.30 However, 20% to 50% of clinically disease-free breast cancer patients relapse 7 to 25 years following an apparently successful endocrine treatment.31,32 This period of years or decades of cancer-free survival between the removal of primary tumour and relapse is termed clinical cancer dormancy. Molecular studies have demonstrated that node and distant metastasis are highly similar to their matched primary tumours,29,33,34 suggesting that the original tumour cells reside undetected at distant sites in the body before reawakening.
Dormant tumour cells are thought to persist either by transitioning to a quiescence state or by continuing to proliferate that is counterbalanced by cell death.32 Reawakening dormant cells have been suggested to be detectable after reaching a detection threshold or reactivated via increased angiogenesis, and/or escape from inhibitory effects of microenvironment or immune system.35,36 Dormant cells survive despite endocrine treatment, and therefore, dormancy is believed to be a major mechanism underlying resistance to therapy. Although several mechanisms underlying metastasis dormancy have been suggested,35,37 our knowledge about clinical breast cancer dormancy is still limited. This is largely due to the limited availability of dormant cells from patients and lack of appropriate dormancy models.
Previous efforts to identify genes predictive of dormancy have failed to be translated into clinic. Kim et al38 previously showed that ER+ cell lines and clinical samples are more dormant than ER-negatives by calculating a dormancy score based on expression levels of 49 genes that have previously been associated with cell quiescence and angiogenic failure. In addition, in ER+ breast cancers treated with adjuvant tamoxifen, a gene classifier has been proposed that can discriminate between early (⩽3 years) and late (⩾5 years) distant recurrences, which may represent emergence from dormancy.39 However, this signature was based on pre-treatment samples only and has not been translated into clinical practice.
There is no consensus on optimal duration of neoadjuvant endocrine therapy and most patients receive it for 3 to 6 months. Median time to maximum response to neoadjuvant letrozole was shown to be 4.2 months, whereas 37.1% of patients achieve that response within 6–12 months.40 A small proportion of patients receiving neoadjuvant endocrine therapy do not have their tumours excised after 3 months for a number of reasons such as being unfit for the surgery. These patients continue to receive neoadjuvant endocrine therapy for longer duration, and we have suggested that these tumours represent a unique circumstance, which can be used to study how tumours respond to extended oestrogen deprivation in situ.41,42 Tumours that initially shrink in size and continue to respond to neoadjuvant therapy model dormancy, whereas those that subsequently begin to regrow under neoadjuvant treatment, represent acquired resistance (Figure 1C).
Using this novel clinical model, we have identified extended (>4 months) letrozole-treatment-induced changes in dormant and resistant tumours by comparing pre- and on-treatment samples.41,42 Some of the identified pathways were dormancy-related such as cell cycle arrest and senescence with established roles in metastasis dormancy,43 further supporting the relevance of extended neoadjuvant therapy as a clinical model.
Neoadjuvant Therapy as a Drug Development Platform
Neoadjuvant pre-surgical therapy also provides an opportunity for novel drugs such as cyclin-dependent kinase (CDK) 4/6 inhibitors to be tested, either alone or in combination2 along with assessing the potential for repurposing approved drugs, such as metformin originally intended for type II diabetes for improved outcomes. PALLET trial, evaluating the effects of combination of CDK4/6 inhibitor palbociclib and aromatase inhibitor letrozole as neoadjuvant therapy in ER+ breast cancer, showed that adding palbociclib significantly enhances Ki-67 suppression without any increase in the clinical response rate in 14 weeks.44 In addition, NeoPalAna study, assessing the antiproliferative effect of neoadjuvant palbociclib and aromatase inhibitor anastrozole for clinical Stage 2/3 ER+ breast cancer, showed significantly higher cell cycle arrest rate after adding palbociclib to anastrozole.45 A recent review by Guerrero-Zotano et al46 provides detailed information regarding the role of neoadjuvant endocrine therapy in drug development and discovery in ER+ breast cancer. More information on neoadjuvant therapy as a platform for new therapies in triple-negative breast cancer, including immunotherapy, and poly ADP ribose polymerase (PARP) inhibitors can be found in another recent review by Escriva-de-Romani et al47
Assessment of therapy response using traditional clinical trial design requires long-term follow-up data. The neoadjuvant setting offers a potential solution to this problem by allowing relatively quick detection of patient subpopulations that benefit from an intervention.1 Pertuzumab received an accelerated US Food and Drug Administration (FDA) approval in 2013 for HER2-positive patients based on pCR criteria, in the absence of long-term data in the neoadjuvant setting.2 In 2017, the FDA granted a regular approval to adjuvant pertuzumab for use in combination with trastuzumab and chemotherapy for HER2-positive early breast cancer at high risk of recurrence.
Conventional drug development approaches utilise heterogeneous patient populations, which have often been extensively pre-treated with other agents and the new candidate is simply a last resort. Neoadjuvant trials can maximise information gathered from individual patients with less confounding. Short-term responses can be monitored in real time, and predictive biomarkers of response can be identified using neoadjuvant approach.
Some studies define pCR as disappearance of all target lesions in the breast and associated axillary lymph nodes at the time of surgery,48 whereas others define it regardless of nodal involvement. The lack of a uniform definition makes interpretation of data from neoadjuvant trials challenging. But this challenge is evident for both neoadjuvant and traditional clinical trials where different endpoints are assessed, making direct comparison of clinical evidence from different trials complicated. Efforts have been done to standardise the endpoint criteria such as The Response Evaluation Criteria In Solid Tumours (RECIST) guidelines.49 Disease progression and tumour burden are currently accepted endpoints for clinical assessment of cancer chemotherapeutics. Disease progression can be assessed by different approaches including time-to-progression, progression-free/overall survival, whereas tumour burden is commonly assessed by mammography and ultrasonography. In the neoadjuvant setting, functional imaging tool magnetic resonance imaging (MRI) is recommended over mammography and computerised tomography to follow tumour burden according to RECIST.49
The value of pCR as an endpoint for long-term outcome remains controversial. It has been shown to correlate with long-term clinical benefit; however, the correlation was strongest in triple-negative breast cancer patients, but not in lower grade patients.7 Based on the variability of pCR among different breast cancer subtypes, enrolment of only high-risk patients in neoadjuvant clinical trials utilising pCR as a surrogate survival endpoint has been suggested.50
NeoALTTO, the neoadjuvant trial on HER2-positive tumours, found a statistical improvement in pCR rate for the lapatinib and trastuzumab suggesting dual inhibition of HER2 for the treatment of HER2-positive breast cancer in the neoadjuvant setting.51 This trial measured pCR response in the breast only, rather than the breast and axillary lymph nodes, which is the currently accepted standard according to FDA’s draft guidance for accelerated drug approval. In contrast to NeoALTTO results, adjuvant ALTTO trial in HER2-positive breast cancer showed a nonsignificant reduction in disease-free survival for adding lapatinib to trastuzumab and chemotherapy.52 The observed pCR difference in NeoALTTO and the observed hazard ratio in ALTTO were later shown to be concordant.53 The 5-year follow-up analysis of NeoSphere, a phase 2 randomised neoadjuvant trial with treatment-naive locally advanced, inflammatory, or early-stage HER2-positive breast cancer patients, reported beneficial role of pertuzumab when combined with trastuzumab and docetaxel, as well as suggesting that pCR can be an early indicator of long-term outcome.54 Another neoadjuvant trial of trastuzumab in early-stage HER2-positive breast cancer patients demonstrated that immune signatures evaluated after a single dose of trastuzumab can predict response suggesting these signatures may provide early evaluation of trastuzumab response.55
The inconsistent results do not mean that neoadjuvant trials should be abandoned, but rather emphasise the importance of standardising the trial protocols, appropriate use of endpoints, and focusing on target patient populations such as those with a particular biomarker or higher risk in adjuvant trials testing promising new drugs. Neoadjuvant trials can provide reliable evidence even when performed on a small cohort of target patient population.56
Challenges Facing the Use of Neoadjuvant Treatment in Breast Cancer
The use of the neoadjuvant setting to identify potential molecular markers of response has its own challenges. One major obstacle to characterising response to treatments is the unavailability of patient-matched tumour material for molecular evaluation following pCR. This is a limitation especially in the context of neoadjuvant chemotherapy as it leads to the complete disappearance of the primary tumour in 20% to 30% of ER-negative patients.6 Samples taken after 2 weeks or mid-chemo may be an appropriate alternative (see Figure 1), to discover early on-treatment whether an approach will be effective.
Most gene expression profiling studies to date have attempted to identify candidate predictive markers for chemotherapeutic response using pre-treatment only biopsies. Aside from the existing potential for improving stratification, this approach does not account for therapy-induced perturbations. Similarly, post-treatment-only clinical samples investigated at the molecular level57 have limited value without an appropriate reference.
Comparing matched pre- and post-treatment samples from the same patient removes much individual-specific variability. Therefore, gene expression analysis of serial tumours in the neoadjuvant setting may identify predictors with better sensitivity than baseline samples alone.58 Although the potential of these studies is high, this approach has its own technical limitations as identifying and analysing matched samples is challenging. Attempts to characterise patient-matched samples following neoadjuvant endocrine therapy or chemotherapy have often been limited to relatively small numbers of patients.10,23,59
Variability due to tissue heterogeneity can also have an impact; however, a recent study profiling expression in multiple samples of the same tumours suggested that macroscopic intra-tumour heterogeneity is unlikely to affect molecular profiling of breast tumours.60 We recently reported the significant impact of the sampling method and tumour heterogeneity on gene expression profiles in neoadjuvant studies.61 Analysis of dynamic changes in gene expression from 37 paired pre-treatment samples (core biopsies) and surgically excised breast cancer biopsies from women receiving no neoadjuvant therapy revealed sampling method as a potential confounding factor.61 Expression of early growth response genes (dual specificity phosphatase 1 [DUSP1], early growth response 1 [EGR1], Finkel-Biskis-Jinkins osteosarcoma [FOS], and FOS homolog B [FOSB]) was significantly higher in surgically excised samples compared with their core biopsied counterparts.61 Another study also demonstrated significant changes in early-response gene expression within an hour following surgery,62 confirming our findings. The effect of sampling method and systematic changes that occur in the absence of treatment (no intervening treatment) should, therefore, be considered for valid interpretation of the results.
Conclusions
Neoadjuvant therapy is a valuable clinical and research tool with great potential for advancing breast cancer treatments. In clinical practice, neoadjuvant therapy is commonly continued for 3 months to achieve a certain tumour volume reduction response that allows breast-conserving surgery. Therefore, determining molecular changes during the early weeks of neoadjuvant therapy may help to identify potential biomarkers of response. The lack of validated surrogate biomarkers remains the major challenge for neoadjuvant studies. Furthermore, utilising samples from patients treated with extended neoadjuvant therapy where available could significantly contribute to in situ identification of the molecular changes under extended therapy. In summary, the neoadjuvant setting is a promising approach for rapid introduction of efficient and individualised novel therapies as well as biomarkers for precision oncology to get treatments for breast cancer into the clinic.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: This work was funded by European Commission H2020 Marie Skłodowska Curie Action Individual Fellowship (H2020-MSCA-IF, 658170) to C.S. A.H.S. is very grateful for funding provided by Breast Cancer Now. The work was partly supported by the Wellcome Trust Institutional Fund (ISSF3) to C.S. and A.H.S.
Author Contributions: CS drafted the manuscript. AHS contributed to writing and editing the manuscript. All authors read and approved the final manuscript.
References
- 1. Sims AH, Bartlett JMS. Approaches towards expression profiling the response to treatment. Breast Cancer Res. 2008;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bardia A, Baselga J. Neoadjuvant therapy as a platform for drug development and approval in breast cancer. Clin Cancer Res. 2013;19:6360–6370. [DOI] [PubMed] [Google Scholar]
- 3. Poleszczuk J, Luddy K, Chen L, et al. Neoadjuvant radiotherapy of early-stage breast cancer and long-term disease-free survival. Breast Cancer Res. 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer. 1980;16:351–356. [DOI] [PubMed] [Google Scholar]
- 5. Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10:6622–6628. [DOI] [PubMed] [Google Scholar]
- 7. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 8. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 9. Symmans WF, Wei CM, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stickeler E, Pils D, Klar M, et al. Basal-like molecular subtype and HER4 up-regulation and response to neoadjuvant chemotherapy in breast cancer. Oncol Rep. 2011;26:1037–1045. [DOI] [PubMed] [Google Scholar]
- 11. Korde LA, Lusa L, McShane L, et al. Gene expression pathway analysis to predict response to neoadjuvant docetaxel and capecitabine for breast cancer. Breast Cancer Res Treat. 2010;119:685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magbanua MJ, Wolf DM, Yau C, et al. Serial expression analysis of breast tumors during neoadjuvant chemotherapy reveals changes in cell cycle and immune pathways associated with recurrence and response. Breast Cancer Res. 2015;17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bownes RJ, Turnbull AK, Martinez-Perez C, Cameron DA, Sims AH, Oikonomidou O. On-treatment biomarkers can improve prediction of response to neoadjuvant chemotherapy in breast cancer. Paper presented at: San Antonio Breast Cancer Symposium; December 4-8, 2018; San Antonio, TX: https://www.sabcs.org/2018-SABCS-sup-sup. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prat A, Lluch A, Turnbull AK, et al. A PAM50-based chemoendocrine score for hormone receptor-positive breast cancer with an intermediate risk of relapse. Clin Cancer Res. 2017;23:3035–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaidya JS, Massarut S, Vaidya HJ, et al. Rethinking neoadjuvant chemotherapy for breast cancer. BMJ. 2018;360:j5913. [DOI] [PubMed] [Google Scholar]
- 17. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller WR, Larionov A, Renshaw L, et al. Gene expression profiles differentiating between breast cancers clinically responsive or resistant to letrozole. J Clin Oncol. 2009;27:1382–1387. [DOI] [PubMed] [Google Scholar]
- 19. Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s-958s. [PubMed] [Google Scholar]
- 20. Goncalves R, De Schryver K, Ma C, et al. Development of a Ki-67-based clinical trial assay for neoadjuvant endocrine therapy response monitoring in breast cancer. Breast Cancer Res Treat. 2017;165:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lerebours F, Rivera S, Mouret-Reynier MA, et al. Randomized phase 2 neoadjuvant trial evaluating anastrozole and fulvestrant efficacy for postmenopausal, estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients: results of the UNICANCER CARMINA 02 French trial (UCBG 0609). Cancer. 2016;122:3032–3040. [DOI] [PubMed] [Google Scholar]
- 23. Mello-Grand M, Singh V, Ghimenti C, et al. Gene expression profiling and prediction of response to hormonal neoadjuvant treatment with anastrozole in surgically resectable breast cancer. Breast Cancer Res Treat. 2010;121:399–411. [DOI] [PubMed] [Google Scholar]
- 24. Turnbull AK, Arthur LM, Renshaw L, et al. Accurate prediction and validation of response to endocrine therapy in breast cancer. J Clin Oncol. 2015;33:2270–2278. [DOI] [PubMed] [Google Scholar]
- 25. Patani N, Dunbier AK, Anderson H, et al. Differences in the transcriptional response to fulvestrant and estrogen deprivation in ER-positive breast cancer. Clin Cancer Res. 2014;20:3962–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robertson JFR, Dowsett M, Bliss JM, et al. Peri-operative aromatase inhibitor treatment in determining or predicting longterm outcome in early breast cancer – the POETIC trial (CRUK/07/015). Cancer Res. 2018;78(4). [Google Scholar]
- 27. Robertson JFR, Dixon JM, Sibbering DM, et al. A randomized trial to assess the biological activity of short-term (pre-surgical) fulvestrant 500 mg plus anastrozole versus fulvestrant 500 mg alone or anastrozole alone on primary breast cancer. Breast Cancer Res. 2013;15:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grigoriadis A, Gazinska P, Pai T, et al. Histological scoring of immune and stromal features in breast and axillary lymph nodes is prognostic for distant metastasis in lymph node-positive breast cancers. J Pathol Clin Res. 2018;4:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kroigard AB, Larsen MJ, Thomassen M, Kruse TA. Molecular concordance between primary breast cancer and matched metastases. Breast J. 2016;22:420–430. [DOI] [PubMed] [Google Scholar]
- 30. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. [DOI] [PubMed] [Google Scholar]
- 31. Ma CX, Sanchez CG, Ellis MJ. Predicting endocrine therapy responsiveness in breast cancer. Oncology. 2009;23:133–142. [PubMed] [Google Scholar]
- 32. Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108:12396–12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, van’t Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A. 2003;100:15901–15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang MH, Dahlgren M, Brueffer C, et al. Remarkable similarities of chromosomal rearrangements between primary human breast cancers and matched distant metastases as revealed by whole-genome sequencing. Oncotarget. 2015;6:37169–37184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dittmer J. Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol. 2017;44:72–82. [DOI] [PubMed] [Google Scholar]
- 37. Zhang LX, Ridgway LD, Wetzel MD, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim RS, Avivar-Valderas A, Estrada Y, et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS ONE. 2012;7:e35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loi S, Haibe-Kains B, Desmedt C, et al. Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genom. 2008;9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Llombart-Cussac A, Guerrero A, Galan A, et al. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol. 2012;14:125–131. [DOI] [PubMed] [Google Scholar]
- 41. Selli C, Turnbull AK, Renshaw L, Thomas JS, Dixon JM, Sims AH. Long-term neoadjuvant endocrine treatment as a clinical model of breast cancer dormancy. Paper presented at: UK Breast Cancer Research Symposium; July 22-23, 2016; London, England. [Google Scholar]
- 42. Selli C, Turnbull AK, Pearce DA, et al. Molecular changes during extended neoadjuvant letrozole treatment of breast cancer: distinguishing acquired resistance from dormant tumours. Breast Cancer Res. 2019;21:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang XHF, Giuliano M, Trivedi MV, Schiff R, Osborne CK. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res. 2013;19:6389–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnston S, Puhalla S, Wheatley D, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J Clin Oncol. 2019;37:178–189. [DOI] [PubMed] [Google Scholar]
- 45. Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23:4055–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guerrero-Zotano AL, Arteaga CL. Neoadjuvant trials in ER(+) breast cancer: a tool for acceleration of drug development and discovery. Cancer Discov. 2017;7:561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Escriva-de-Romani S, Arumi M, Zamora E, Bellet M. Neoadjuvant model as a platform for research in breast cancer and novel targets under development in this field. Breast Care. 2018;13:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. [DOI] [PubMed] [Google Scholar]
- 49. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 50. Pennisi A, Kieber-Emmons T, Makhoul I, Hutchins L. Relevance of pathological complete response after neoadjuvant therapy for breast cancer. Breast Cancer (Auckl). 2016;10:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Piccart-Gebhart MJ, Holmes AP, Baselga J, et al. First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T -> L), or their combination (T plus L) in the adjuvant treatment of HER2-positive early breast cancer (EBC). J Clin Oncol. 2014;32(18). [Google Scholar]
- 53. DeMichele A, Yee D, Berry DA, et al. The neoadjuvant model is still the future for drug development in breast cancer. Clin Cancer Res. 2015;21:2911–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. [DOI] [PubMed] [Google Scholar]
- 55. Varadan V, Gilmore H, Miskimen KL, et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperative trastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res. 2016;22:3249–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. [DOI] [PubMed] [Google Scholar]
- 57. Vendrell JA, Robertson KE, Ravel P, et al. A candidate molecular signature associated with tamoxifen failure in primary breast cancer. Breast Cancer Res. 2008;10:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Selli C, Dixon JM, Sims AH. Accurate prediction of response to endocrine therapy in breast cancer patients: current and future biomarkers. Breast Cancer Res. 2016;18:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gonzalez-Angulo AM, Iwamoto T, Liu S, et al. Gene expression, molecular class changes, and pathway analysis after neoadjuvant systemic therapy for breast cancer. Clin Cancer Res. 2012;18:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karthik GM, Rantalainen M, Stalhammar G, et al. Intra-tumor heterogeneity in breast cancer has limited impact on transcriptomic-based molecular profiling. BMC Cancer. 2017;17:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pearce DA, Arthur LM, Turnbull AK, et al. Tumour sampling method can significantly influence gene expression profiles derived from neoadjuvant window studies. Sci Rep. 2016;6:29434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lopez-Knowles E, Gao Q, Cheang MCU, et al. Heterogeneity in global gene expression profiles between biopsy specimens taken peri-surgically from primary ER-positive breast carcinomas. Breast Cancer Res. 2016;18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]