Abstract
Pulmonary hypertension (PH) occurs when the pulmonary vasculature is itself diseased or becomes affected secondarily by comorbid conditions, commonly left heart or lung disease. The high prevalence of chronic cardiopulmonary conditions among patients served by Veterans Health Administration (VHA) suggests this population may be particularly susceptible to PH. We sought to identify clinical features and outcomes in veterans diagnosed with PH. We utilized the VHA Corporate Data Warehouse to identify veterans diagnosed between January 1, 2003 and September 30, 2015, assess relevant patient characteristics and their survival time. The effects of PH subtype and baseline factors on outcome were estimated by Cox modeling. There were 110,564 veterans diagnosed with PH during the study period. These veterans were predominantly male, had median age 70.2, and had a high burden of comorbid conditions. PH was frequently due to left heart and/or lung disease. Average survival after PH diagnosis was 3.88 years. Compared with other types, PH due to left heart disease, lung disease or both had shorter survival. This large retrospective study of veterans demonstrates the significance of PH due to left heart and/or lung disease which was common and had high risk of death. Multi-comorbidity was common and added to risk. These findings underscore the need for risk assessment tools for subjects with non-Group 1 PH and novel management strategies to improve their outcome. This study details the largest retrospective cohort assembled for evaluation of secondary PH and allows hypothesis-generating inquiries into these common conditions that are rarely prospectively studied.
Keywords: pulmonary hypertension, comorbidity, retrospective cohort study, survival, risk factors
Pulmonary hypertension (PH) is an abnormal state of elevated blood pressure in the pulmonary circulation that presents with non-specific symptoms including shortness of breath, edema, poor exertion tolerance, palpitations, syncope, and rarely sudden death. PH is uncommon as an isolated condition; it most frequently occurs secondary to a separate underlying disorder. The World Symposium on PH categorizes PH into five clinically recognizable groups based on the underlying cause of the disorder.1Pulmonary arterial hypertension (PAH; Group 1) is an intrinsic pulmonary vascular disease with an estimated prevalence of 10–25 cases per million adults in US and European registry studies.1,2PAH differs from other types of PH which may be secondary to left heart disease (PH-LHD; Group 2), parenchymal lung diseases and/or hypoxemia (Group 3), chronic thromboembolism (CTEPH; Group 4), or other unclear or multifactorial mechanisms (Group 5).1
The evaluation and management of Group 1 PAH has evolved substantially over the last 25 years. Iteratively revised evidence-based guidelines have been developed for assessment, diagnosis, management, and serial follow-up of patients with PAH.1,3Prospectively enrolled registries form much of the basis for guideline recommendations of evaluation and serial monitoring of PAH, and randomized clinical trials have led to the treatment recommendations. While pulmonary vascular diseases included in Group 1 PAH have been traditionally considered the most lethal,4recent data suggest that PH secondary to underlying heart or lung diseases has equal or higher risk of death.5–8This risk is compounded by the fact that PH due to underlying heart or lung disease—Groups 2 and 3, respectively—are the most common forms of PH.2,9–12Further, recognition of PH in those with underlying cardiac or pulmonary disease is important, as the occurrence of PH is a known independent risk factor for morbidity and mortality in various disease populations such as chronic obstructive pulmonary disease (COPD), interstitial lung diseases, and chronic systolic or diastolic heart failure (CHF).9,12While existing registries have improved our understanding of Group 1 PAH, fewer registries are dedicated to understanding the epidemiology, risk factors, and outcome of subjects with these other more prevalent forms of PH.2
For patients with Group 2 and 3 PH, evaluation and treatment guidelines recommend expert assessment and management of the underlying disorder.1However, due to insufficient supporting evidence from clinical trials, targeted pulmonary vascular therapies are not recommended. To date, only small studies that are often underpowered to detect clinically meaningful improvements have examined PAH-targeted therapies in patients with Group 2 or 3 PH. It is unclear whether a beneficial effect of those therapies should be expected, as the mechanisms of increased mortality risk due to PH are unknown. For example, it may be due to increased CHF severity, a specific pulmonary vascular or right ventricular component of the disease, coexisting non-cardiopulmonary comorbid diseases, or other factors. Nonetheless, without information regarding motive, and lacking supporting evidence, PAH-targeted therapies are often pragmatically used in subjects with PH caused by lung or left heart disease.11,13,14
In an effort to define the relative prevalence of PH subtypes and associated comorbidities, this study employed data from the Veterans Health Administration (VHA). VHA has one of the largest resources of administrative and healthcare data available.15Veterans have increased incidence of chronic left-sided heart disease and lung disease and higher prevalence of risk factors for other cardiopulmonary diseases compared with the general US population.16–18We designed a retrospective longitudinal cohort study utilizing nationwide VHA data from January 1, 2003, to September 30, 2016 to evaluate non-PAH forms of PH. We identified veterans newly diagnosed with PH, assessed the subtypes of PH present, and evaluated the influence of PH subtype and other comorbid conditions on mortality. We hypothesized that veterans with PH would commonly have comorbid heart or lung disease as the underlying cause, and that outcome would depend on PH subtype, with better outcome in WHO Groups 1 and 4 PH as a result of the availability of targeted therapies and more effective management strategies for these groups.
Methods
We performed a retrospective cohort study of veterans receiving medical care in the VHA system and diagnosed with PH between January 1, 2003 and September 30, 2015. The study was approved by the Emory University Institutional Review Board and the Atlanta VA Medical Center Research & Development Committee. The need for informed consent was waived for this study.
Data sources
Data for the analyses were from the national VHA Corporate Data Warehouse (CDW) accessed via Veterans Administrations Informatics and Computing Infrastructure (VINCI). VINCI is a secure computing environment enabling researcher access to broad datasets including CDW that facilitates data analysis. The CDW includes >16 years of longitudinal data on >22.3 million veterans.15Data include medical and administrative information from within the VHA system as well as data on selected aspects of medical care obtained by enrolled veterans outside the VHA system.
Study population
Adult veterans with data available in the CDW were eligible for inclusion. Subjects were included if a diagnosis of PH by International Classification of Diseases, Ninth Revision(ICD-9) diagnosis code—including 416.0, 416.2, 416.8, 416.9—was first entered during the inclusion period, defined as January 1, 2003 through September 30, 2015. The end of the inclusion period was chosen to coincide with the major change from using ICD-9diagnosis codes to the more recent International Classification of Diseases, Tenth Revision, ICD-10. Patients were excluded if they were not a veteran, were less than 18 years of age at the time of possible cohort entry, or if an ICD-9code for PH was used and stored in CDW at any point prior to January 1, 2003. Data are available in CDW beginning with fiscal year 1999. Follow-up data including dates of hospitalizations and death were extracted for the duration of the inclusion period and through one additional year (through September 30, 2016) constituting the full study period. Follow-up was administratively censored at the end of this study period. Censoring was also performed prior to death or the end of the study period by using data stored in CDW that record veterans’ last healthcare utilization date (e.g. clinic visit, pharmacy event, physical therapy appointment, radiology exam).
Definition of the cohort and measured covariates
PH was identified by ICD-9diagnosis code as described above. Because diagnosis codes may be improperly used to “rule out” a disorder, patients were excluded if the ICD-9code for PH did not: (1) appear at least once on an inpatient claim, or (2) appear on at least two outpatient records/claims separated by at least 30 days during the study period.19For each veteran in the cohort, the subtype of PH was classified based on comorbid diagnoses utilizing a modification of the currently accepted clinical classification of PH (Table 1).1The presence of those relevant comorbid conditions was assessed at baseline, defined as the period of time prior to PH diagnosis and up to 6 months after diagnosis. The use of diagnosis codes to classify subtypes of PH for epidemiologic study has been applied in the past14,20and the method used in this study was minimally adapted. In the method used by Kim et al. to analyze appropriateness of phosphodiesterase type 5 inhibitor prescribing for PH, the classification algorithm was developed with a preference for Group 1 PAH.14In this study, veterans are excluded from being classified as Group 1 PAH if they have any underlying comorbid condition that may relate to a non-Group 1 PH classification. For instance, if an ICD-9code indicating systolic heart failure (428.8×) was present, the patient was categorized as Group 2 PH (PH-LHD), while if an ICD-9code for idiopathic pulmonary fibrosis (516.31) was present, the patient was categorized as Group 3 PH (PH due to lung diseases and/or hypoxemia). Because some patients have ICD-9codes spanning more than one clinical classification grouping (e.g. ICD-9codes for both systolic heart failure and idiopathic pulmonary fibrosis), we classified such patients as “Multiple causes, unclassifiable PH,” which is not part of the clinical classification of PH.1Selected covariates available within the national CDW were collected based on review of existing literature and suspicion of playing a role in the development, progression, and/or outcome of PH-LHD. Covariates included demographics, comorbid conditions, medication use, and measures of PH disease severity, as well as the outcomes of interest. Comorbid conditions included in the extended Elixhauser comorbidity index were assessed at PH diagnosis.21
Table 1.
Schema for classification of pulmonary hypertension in veterans by ICD-9 diagnosis code usage.
| PH Grouping Designation | Included ICD-9Codes |
|---|---|
| Group 1, PAH | 416.0, 416.2, 416.8, 416.9 (Excluded if meeting any of the below) |
| Group 2, PH due to left heart disease | 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx |
| Group 3, PH due to lung diseases and/or hypoxemia | 490.xx–496.xx, 500.xx–508.xx, 515.xx, 516.xx, 517.1, 517.2, 327.23 |
| Group 4, chronic thromboembolic PH | 416.2 |
| Group 5, PH with unclear multifactorial mechanisms | 282.0x, 282.4x, 282.6x, 271.xx, 272.7 |
| Multiple causes, unclassifiable | Patients with ICD-9 diagnosis codes included in more than 1 of Groups 2, 3, 4, and/or 5 above |
ICD-9 codes of 416.0, 416.2, 416.8, 416.9 were required to be present for inclusion. PAH, pulmonary arterial hypertension; PH, pulmonary hypertension.
Outcome
The relevant outcome for this study was time to death from any cause. Death data are available within CDW’s Vital Status Files which collate death data from several sources including deaths in VA facilities, VA benefits claims, Social Security Administration, and Centers for Medicare & Medicaid Services. The CDW Vital Status Files have been validated against the National Death Index with a sensitivity of 98.3% and specificity of 99.8%.22The beginning of the risk period for time to outcome analysis was the date of initial PH diagnosis code use (index date). Survival after PH diagnosis was compared with estimated average life expectancy for the US population from the Center from Disease Control National Center for Health Statistics and for US veterans from the National Center for Veterans Analysis and Statistics.23,24
Statistical analyses
All statistical analyses were performed utilizing SAS Enterprise Guide software, version 7.1 (SAS Institute, Cary, NC). Continuous variables are reported as means and standard deviation or median with interquartile ranges (IQRs). Categorical variables are reported as percentages unless otherwise noted. Variables displaying strongly non-normal distribution, such as levels of brain natriuretic peptide (BNP), were transformed and parametric tests were utilized for multivariable analysis. We utilized date of initial PH diagnosis and date of death with censoring to estimate survival in veterans with PH which was our primary aim. Univariate and multivariable Cox proportional hazards regression analysis were used to generate unadjusted and adjusted hazard ratios for death with 95% confidence intervals (CI). P-values were calculated as two-sided and considered statistically significant when P < 0.05.
Missing data
Data were missing for some variables. No variable used in the analysis had a high degree of missingness (>10%). With variables with modest rate of missingness (3–10%), we chose to categorize data and include a category for missing data. For variables with <3% missing values, we used complete case analysis.
Results
From an estimated 22 million unique veterans with data available in the VHA CDW,15205,147 were selected for evaluation through an automated query performed on 09/08/2017 based on having an ICD-9diagnosis code for PH used between 01/01/2003 and 09/30/2015. Of these, 94,583 were excluded (Fig. 1) and did not contribute to analyses or results. The most frequent exclusion was because the ICD-9diagnosis code was not utilized for ≥ 30 days as an outpatient or ever used as an inpatient. The remaining 110,564 patients constituted the cohort utilized for all reported analyses. Significant longitudinal VHA data were available, over 12 years on average, in these veterans who were predominantly male (n = 106,629, 96.4%), overweight or obese (68.2%) and had a median age of 70.2 years (IQR 62.1–79.6) (Table 2).
Fig. 1.
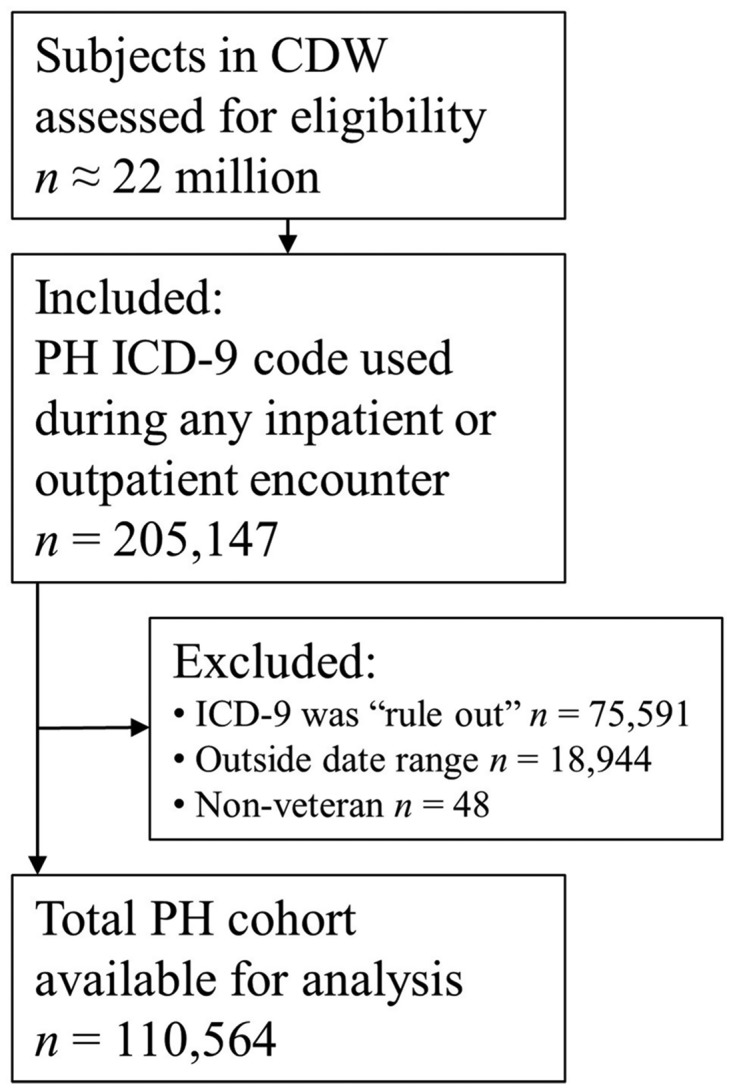
Flow diagram for cohort development. Cohort generation query was performed 09/08/2017. Note that if a subject had the diagnosis code used as an outpatient only and for a duration <30 days, the diagnosis code use was considered a “Rule Out” use and the subject was excluded (see reference 19). CDW: corporate data warehouse; ICD-9: International Classification of Diseases 9th version; PH: pulmonary hypertension.
Table 2.
Characteristics of US veterans diagnosed with pulmonary hypertension in the Veteran Health Administration system, 01/01/2003-09/30/2015.
| Clinical variable | Full cohort |
|---|---|
| N = 110,564 | |
| Age, median [IQR] | 70.2 [62.1, 79.6] |
| Gender, N(%) | |
| Male | 106,629 (96.4%) |
| Female | 3,933 (3.6%) |
| Unknown/Missing | 2 (0.0%) |
| Race, N(%) | |
| Black | 18,211 (16.5%) |
| White | 81,265 (73.5%) |
| Other race | 2,119 (1.9%) |
| Race missing | 8,969 (8.1%) |
| BMI, median [IQR], kg/m2 | 29.1 [24.8, 34.8] |
| BMI classification, N(%) | |
| Underweight | 2,834 (2.6%) |
| Normal | 23,632 (21.4%) |
| Overweight | 29,247 (26.5%) |
| Obese | 46,086 (41.7%) |
| Missing | 8,765 (7.9%) |
| Studies within 1 year of diagnosis, N(%) | |
| Echocardiogram | 95,636 (86.5%) |
| Chest CT | 60,717 (54.9%) |
| V/Q imaging scan | 12,667 (11.5%) |
| Pulmonary function testing | 57,136 (51.68%) |
| Left heart catheterization | 8,646 (7.8%) |
| Right heart catheterization | 8,341 (7.5%) |
| Six-minute walk test | 6,489 (5.9%) |
| Workup with echocardiogram, V/Q, RHC, CT Chest, and PFT, N(%) | |
| Within 1 year of PH diagnosis | 1,241 (1.1%) |
| Ever | 2,613 (2.4%) |
| Workup with echocardiogram, V/Q, and RHC, plus either CT chest or PFT, N(%) | |
| Within 1 year of PH diagnosis | 1,630 (1.5%) |
| Ever | 3,171 (2.9%) |
| Workup with echocardiogram, V/Q, plus either CT chest or PFT (no RHC required), N(%) | |
| Within 1 year of PH diagnosis | 10,843 (9.8%) |
| Ever | 14,925 (13.5%) |
| Rurality, center of initial care | |
| Highly rural | 10 (0.0%) |
| Rural | 8,082 (7.3%) |
| Urban | 101,706 (92.0%) |
| Missing | 766 (0.7%) |
| Census region, patient | |
| Northeast | 15,963 (14.4%) |
| South | 40,693 (36.8%) |
| Midwest | 26,342 (23.8%) |
| West | 26,176 (23.7%) |
| Data Missing | 1,390 (1.3%) |
| Lab data, median [IQR] | |
| BNP (pg/mL) | 368 [127, 886] |
| N terminal Pro-BNP (pg/mL) | 2,180 [568, 6076] |
| Creatinine (mg/dL) | 1.2 [0.9, 1.5] |
| Sodium (mEq/L) | 139 [136, 141] |
| PH subtype, N(%) | |
| Group 1 PAH | 8,839 (8.0%) |
| Group 2 PH-LHD | 17,831 (16.1%) |
| Group 3 PH, lung disease | 18,382 (16.6%) |
| Group 4 CTEPH | 1,562 (1.4%) |
| Group 5 miscellaneous PH | 309 (0.3%) |
| PH with multiple causes | 63,641 (57.6%) |
| With left heart disease contributing | 78,191 (70.7%) |
| With lung disease contributing | 80,154 (72.5%) |
| Person time available, median [IQR] | |
| Total data availability, person-years | 12.3 [8.6, 15.5] |
| Time before PH diagnosis, person-years | 8.0 [5.0, 11.1] |
| Follow-up time after PH diagnosis, person-years | 2.9 [1.2, 5.5] |
BMI: body mass index; IQR: interquartile range; V/Q: ventilation-perfusion nuclear scintigraphy; COPD: chronic obstructive pulmonary disease; BNP: brain natriuretic peptide; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; PH-LHD: pulmonary hypertension due to left heart disease; CTEPH: chronic thromboembolic pulmonary hypertension. BMI is reported as kg/m2and classified by accepted standards: Underweight BMI is less than 18.5, normal BMI is 18.5 to 24.9, overweight BMI is 25 to 29.9, and obese is BMI 30 or higher.
Relevant PH evaluation studies performed within the VHA frequently included echocardiogram and computed tomography (CT) chest imaging (Table 2). However, right heart catheterization (RHC) procedures occurred in less than 10% of patients. Up to 1 year after PH diagnosis, 8.4% of patients had echocardiogram, ventilation/perfusion (V/Q) scan, chest CT and RHC. Very few subjects had PH evaluation that included RHC or completed the following elements of a comprehensive evaluation for pulmonary parenchymal disease (pulmonary function tests (PFTs) and/or CT chest), chronic thromboembolism (V/Q scan), and left-sided cardiac disease (echocardiogram). Omitting RHC, about 9.8% of subjects had evaluation for lung disease, left heart disease, and chronic thromboembolism within 1 year of PH diagnosis.
We utilized the presence of diagnosed comorbid conditions to identify factors contributing to PH etiology. If a patient had comorbid conditions that would cause PH of two different subtypes (e.g. Group 2 and 3 PH), then they were considered unclassifiable. Of the 110,564 patients in our cohort, 8.0% (n = 8,839) had only conditions that would be classified in Group 1 PAH. Underlying left heart disease or underlying lung disease were identified as the only contributor in 16.1% (n = 17,831) and 16.6% (n = 18,382), respectively, representing those with presumed Group 2 and Group 3 PH (Table 2). The majority of patients (57.6%, n = 63,641) had comorbid conditions that could indicate inclusion in more than one PH subgroup and thus were not able to be classified into one of the traditional WHO PH Groups. These patients frequently had comorbid left heart disease, comorbid lung disease or both.
Given the frequency of left heart disease and lung disease in veterans with PH, we assessed the presence of other comorbidities using the extended Elixhauser comorbidity index which includes diseases classified into 31 separate organ systems or processes including pulmonary vascular disease which all patients had (Table 3).21On average, at or before baseline, these veterans with PH had 10 (IQR 7, 13) Elixhauser conditions present. The most frequently represented conditions were chronic pulmonary disease, essential hypertension, and congestive heart failure. Several non-cardiopulmonary comorbidities were present in a large proportion including diabetes (46.4%), depression (41.3%), and obesity (40.9%). HIV was uncommon in our cohort (<1%).
Table 3.
Comorbid conditions in US veterans diagnosed with pulmonary hypertension, 01/01/2003-09/30/2015.
| Full cohort | |
|---|---|
| Comorbidity | N = 110,564 |
| Elixhauser21extended comorbidity count present at baseline (range 0-31 conditions), median [IQR] | 10.0 [7.0, 13.0] |
| Specific Elixhauser components, N(%) | – |
| Congestive heart failure | 73,771 (66.7%) |
| Cardiac arrhythmias | 69,724 (63.1%) |
| Valvular disease | 55,544 (50.2%) |
| Peripheral vascular disorders | 38,734 (35.0%) |
| Hypertension (any) | 99,569 (90.1%) |
| Hypertension, uncomplicated | 98,968 (89.5%) |
| Hypertension, complicated | 33,100 (29.9%) |
| Paralysis | 4,124 (3.7%) |
| Other neurological disorders | 13,307 (12.0%) |
| Chronic pulmonary disease | 105,850 (95.7%) |
| Diabetes, uncomplicated | 53,250 (48.2%) |
| Diabetes, complicated | 32,594 (29.5%) |
| Hypothyroidism | 16,388 (14.8%) |
| Renal failure | 36,989 (33.5%) |
| Liver disease | 17,777 (16.1%) |
| Peptic ulcer disease excluding bleeding | 8,701 (7.9%) |
| AIDS/HIV | 797 (0.7%) |
| Lymphoma | 2,661 (2.4%) |
| Metastatic cancer | 4,863 (4.4%) |
| Solid tumor without metastasis | 26,869 (24.3%) |
| Rheumatoid arthritis/collagen vascular diseases | 39,331 (35.6%) |
| Coagulopathy | 19,151 (17.3%) |
| Obesity | 47,099 (42.6%) |
| Weight loss | 18,373 (16.6%) |
| Fluid and electrolyte disorders | 51,534 (46.6%) |
| Blood loss anemia | 4,567 (4.1%) |
| Deficiency anemia | 24,822 (22.5%) |
| Alcohol abuse | 23,078 (20.9%) |
| Drug abuse | 16,155 (14.6%) |
| Psychoses | 14,642 (13.2%) |
| Depression | 48,656 (44.0%) |
| Selected comorbidities (ever documented), N(%) | |
| Left heart disease | 86,444 (78.2%) |
| Diabetes | 61,309 (55.5%) |
| COPD | 72,501 (65.6%) |
| Interstitial lung disease | 13,032 (11.8%) |
| HIV | 861 (0.8%) |
| Liver cirrhosis | 21,307 (19.3%) |
| Chronic kidney disease | 63,033 (57.0%) |
| Connective tissue disease | 8,117 (7.3%) |
Abbreviations same as in Table 2.
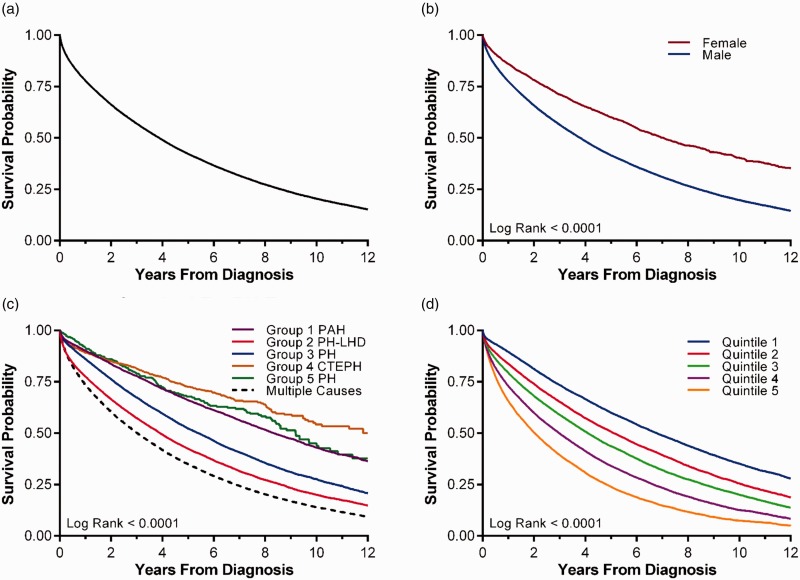
Veterans with PH were shown to have poor overall survival after PH diagnosis (Fig. 2, Tables 4and 5). Overall survival at 1, 3, and 5 years was 77.9%, 57.0%, and 42.3%, respectively, with the median overall survival being 3.88 years (95% CI 3.85, 3.92) (Fig. 2a). The life expectancy after PH diagnosis was reduced compared with the general population of the US and with US veterans. While the average life expectancy of a 70-year-old US male and US veteran is 14.5 and 13.0 years, respectively, the average survival in this cohort for a male veteran diagnosed with PH at age 70 was 4.2 years. Women were underrepresented in this cohort, but had better overall survival compared with men (median 7.05 years vs. 3.81 years) (Fig. 2b). With each decade increase in age at PH diagnosis, there was a 46% increase in hazard for mortality (95% CI 45%, 47%). The presumed underlying cause of PH was also predictive of outcome (Fig. 2c). Patients with more than one cause of PH had poorest survival. Likewise, increasing burden of comorbid conditions was associated with shorter survival (Fig. 2d).
Fig. 2.
Kaplan–Meier survival curve for veterans with pulmonary hypertension receiving care in the Veteran Health Administration system, 01/01/2003-09/30/2015. (a) Overall survival. Median overall survival after diagnosis of PH is 3.88 years. (b) Overall survival stratified by gender. Median survival for females is 7.05 years compared with 3.81 years for males. (c) Overall survival stratified by PH subtype. There is lower survival in those with PH due to left heart disease (Group 2), lung disease (Group 3) and multiple causes, when compared with patients with presumed Group 1 PAH. (d) Overall survival stratified by Elixhauser comorbidity deciles. Higher comorbidity burden is associated with lower survival from time of PH diagnosis.
Table 4.
Mortality in veterans with PH, effect of baseline factors.
| Survival |
Death during follow-up |
||||||
|---|---|---|---|---|---|---|---|
| Covariate | Median [IQR] | 1-year | 3-year | 5-year | HR* | 95% CI | P-value |
| Overall cohort | 3.88 [3.85, 3.92] | 77.9% | 57.0% | 42.3% | |||
| Female gender | 7.05 [6.60, 7.46] | 86.1% | 71.2% | 60.1% | Ref | ||
| Male gender | 3.81 [3.76, 3.85] | 77.6% | 56.5% | 41.6% | 1.73 | 1.65, 1.82 | <.0001 |
| Age at diagnosis (each 10 y) | 1.46 | 1.45, 1.47 | <.0001 | ||||
| PH Group | |||||||
| Group 1 PAH | 8.35 [8.10, 8.65] | 90.2% | 77.9% | 66.1% | Ref | ||
| Group 2 PH-LHD | 3.92 [3.82, 4.02] | 77.6% | 57.4% | 42.4% | 2.02 | 1.95, 2.09 | <.0001 |
| Group 3 PH, lung disease | 5.41 [5.27, 5.55] | 86.2% | 67.4% | 52.3% | 1.53 | 1.47, 1.58 | <.0001 |
| Group 4 CTEPH | 13.08 [9.89, –] | 89.2% | 81.4% | 72.7% | 0.75 | 0.68, 0.83 | <.0001 |
| Group 5 miscellaneous PH | 9.19 [8.28, 10.54] | 91.6% | 79.3% | 67.8% | 0.91 | 0.76, 1.08 | 0.28 |
| PH with multiple causes | 3.03 [2.98, 3.07] | 73.6% | 50.2% | 34.9% | 2.49 | 2.41, 2.57 | <.0001 |
| Elixhauser comorbidity index, quintile | |||||||
| 1st quintile (lowest) | 6.80 [6.64, 6.93] | 89.6% | 73.5% | 60.0% | Ref | ||
| 2nd quintile | 5.12 [5.00, 5.25] | 83.8% | 65.3% | 50.8% | 1.33 | 1.30, 1.37 | <.0001 |
| 3rd quintile | 4.10 [4.02, 4.19] | 79.4% | 59.0% | 43.7% | 1.62 | 1.58, 1.66 | <.0001 |
| 4th quintile | 2.99 [2.93, 3.05] | 73.0% | 49.9% | 34.1% | 2.11 | 2.06, 2.16 | <.0001 |
| 5th quintile (highest) | 2.03 [1.97, 2.09] | 65.7% | 39.5% | 24.0% | 2.80 | 2.73, 2.87 | <.0001 |
Abbreviations same as in Table 2. HR: hazard ratio. *Unadjusted HR given.
Table 5.
Multivariable analyses of risk of death in veterans with PH.
| Risk of death |
|||
|---|---|---|---|
| Covariate | HR | 95% CI | P-value |
| Effect of PH Group, adjusted for age at PH diagnosis, gender, race | |||
| Age at PH Dx (each 10 y) | 1.46 | 1.45, 1.47 | <.0001 |
| Sex (M vs. F) | 1.36 | 1.30, 1.43 | <.0001 |
| White race | Ref | ||
| Black race | 1.05 | 1.02, 1.07 | <.0001 |
| Other race | 0.98 | 0.93, 1.04 | 0.48 |
| PH Group | |||
| Group 1 PAH | Ref | ||
| Group 2 PH-LHD | 1.97 | 1.89, 2.04 | <.0001 |
| Group 3 PH, lung disease | 1.58 | 1.52, 1.65 | <.0001 |
| Group 4 CTEPH | 0.88 | 0.79, 0.97 | 0.01 |
| Group 5 miscellaneous PH | 1.06 | 0.88, 1.27 | 0.53 |
| PH with multiple causes | 2.54 | 2.45, 2.63 | <.0001 |
| Effect of comorbidity, adjusted for age at PH diagnosis, gender, race | |||
| Age at PH Dx (each 10 y) | 1.42 | 1.41, 1.44 | <.0001 |
| Sex (M vs. F) | 1.31 | 1.25, 1.38 | <.0001 |
| White race | Ref | ||
| Black race | 0.92 | 0.90, 0.94 | <.0001 |
| Other race | 0.91 | 0.86, 0.96 | 0.0012 |
| Elixhauser condition | |||
| Congestive Heart Failure | 1.59 | 1.56, 1.62 | <.0001 |
| Cardiac Arrhythmia | 0.98 | 0.97, 1.00 | 0.08 |
| Valvular Disease | 0.91 | 0.89, 0.92 | <.0001 |
| Peripheral Vascular Disease | 1.11 | 1.09, 1.13 | <.0001 |
| Hypertension, Uncomplicated | 0.79 | 0.76, 0.81 | <.0001 |
| Hypertension, Complicated | 1.01 | 0.99, 1.03 | 0.52 |
| Paralysis | 1.07 | 1.03, 1.11 | 0.0009 |
| Other Neurological Disorders | 1.10 | 1.07, 1.13 | <.0001 |
| Chronic Pulmonary Disease | 1.40 | 1.34, 1.47 | <.0001 |
| Diabetes, Uncomplicated | 1.06 | 1.04, 1.08 | <.0001 |
| Diabetes, Complicated | 1.10 | 1.07, 1.12 | <.0001 |
| Hypothyroidism | 1.00 | 0.98, 1.02 | 0.99 |
| Renal Failure | 1.23 | 1.20, 1.26 | <.0001 |
| Liver Disease | 1.21 | 1.19, 1.24 | <.0001 |
| Peptic Ulcer Disease | 0.98 | 0.95, 1.01 | 0.15 |
| AIDS/HIV | 0.98 | 0.89, 1.08 | 0.71 |
| Lymphoma | 1.21 | 1.16, 1.27 | <.0001 |
| Cancer, Metastatic | 1.81 | 1.74, 1.87 | <.0001 |
| Solid Tumor, Without Metastasis | 1.09 | 1.07, 1.11 | <.0001 |
| Collagen Vascular Disease | 0.97 | 0.95, 0.98 | 0.0001 |
| Coagulopathy | 1.15 | 1.13, 1.17 | <.0001 |
| Obesity | 0.79 | 0.78, 0.80 | <.0001 |
| Weight Loss | 1.32 | 1.30, 1.35 | <.0001 |
| Fluid and Electrolyte Disorders | 1.36 | 1.34, 1.38 | <.0001 |
| Blood Loss Anemia | 1.10 | 1.06, 1.14 | <.0001 |
| Deficiency Anemia | 1.08 | 1.06, 1.10 | <.0001 |
| Alcohol Abuse | 1.15 | 1.12, 1.17 | <.0001 |
| Drug Abuse | 1.03 | 1.00, 1.05 | 0.06 |
| Psychoses | 1.17 | 1.14, 1.19 | <.0001 |
| Depression | 1.01 | 0.99, 1.03 | 0.40 |
Abbreviations same as in Table 4.
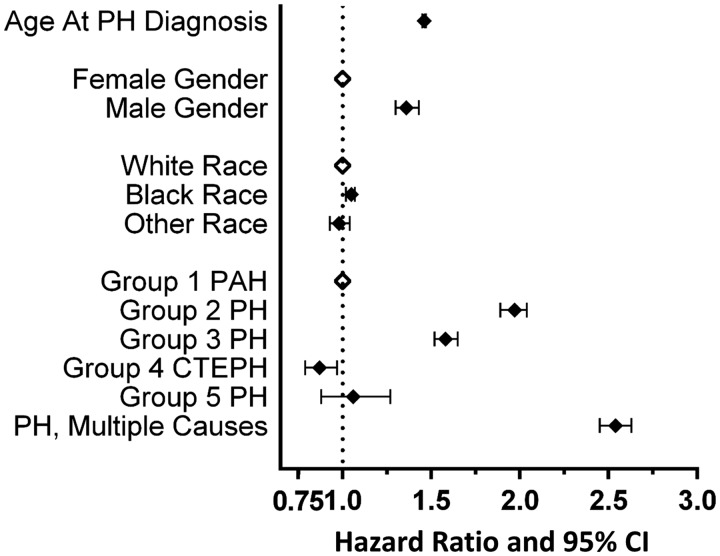
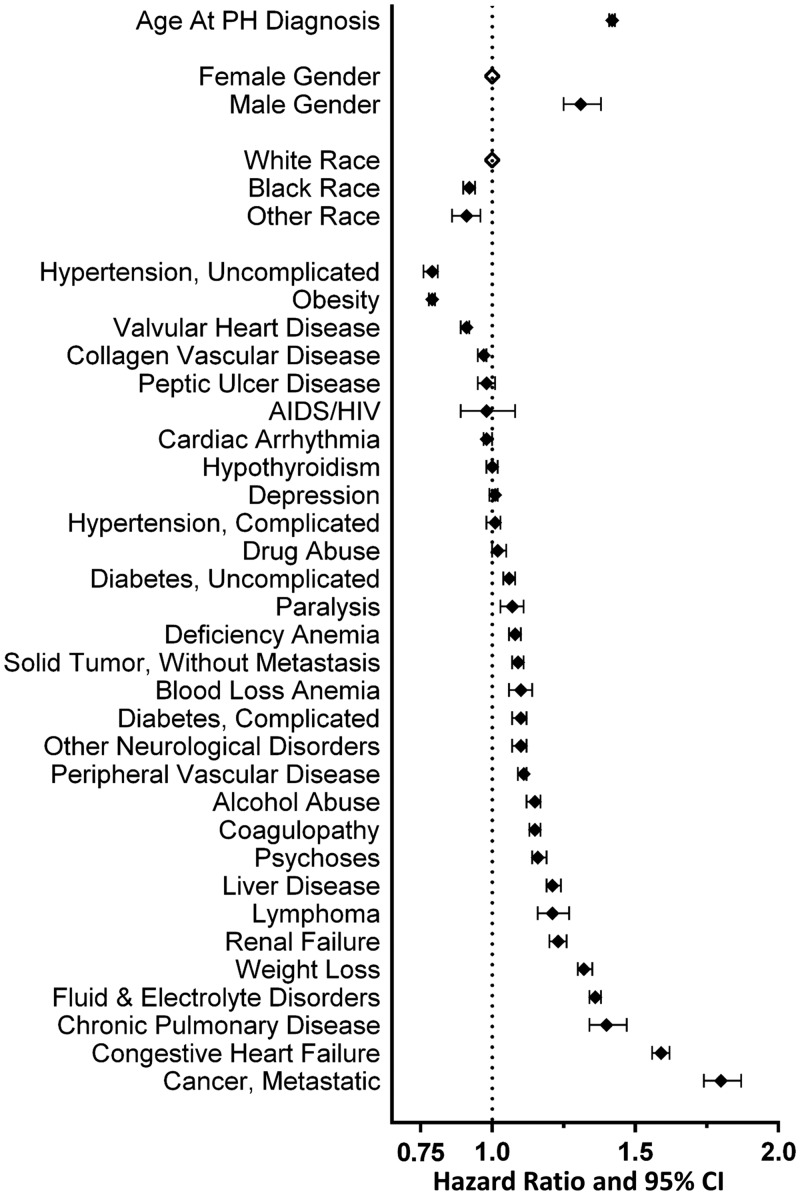
Both underlying PH etiology and Elixhauser comorbidities had relevant influence on risk of death, both statistically and in magnitude, after correcting for age at PH diagnosis, race and gender (Table 5, Fig. 3and 4). Of the traditional PH Groups, Group 2 was associated with highest risk of death with an adjusted hazard ratio (HR) of 1.97 (95% CI 1.89, 2.04) compared with that for PAH and median survival under 4 years. Group 3 PH had the second highest risk with adjusted HR of 1.58 (95% CI 1.52, 1.65). Those patients with comorbid conditions that would be associated with more than one form of PH had the highest risk of death (Fig. 3). Non-cardiopulmonary comorbid conditions were also associated with outcome (Fig. 4). Notably, obesity, often observed in this cohort, was protective. Uncomplicated essential hypertension and valvular heart disease were also protective. Not unexpectedly, metastatic malignancy was associated with a very high risk of death. The presence of renal failure, liver disease, alcohol abuse, and diabetes were independently associated with poor outcome (Table 5).
Fig. 3.
Effect of PH subtype on risk of death. In a multivariable model, male gender, increasing age at PH diagnosis, and PH due to lung disease, heart disease or multiple causes are each associated with increasing risk of death. Open diamonds indicate reference categories. PH: pulmonary hypertension; PAH: pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; CI: confidence interval.
Fig. 4.
Effect of Elixhauser comorbidity categories on outcome of veterans with pulmonary hypertension. A multivariable regression model of the influence of age, gender, race and comorbidities on risk of death from time of PH diagnosis. Increased age and male gender significantly increase risk of death. Notably, weight loss, renal failure, liver disease, and diabetes increase risk of death while obesity is protective. Open diamonds indicate reference categories. PH: pulmonary hypertension; CI: confidence interval.
Discussion
In this retrospective analysis, we identified a large cohort of veterans diagnosed with PH, the majority presumed due to underlying comorbid left heart and/or lung disease. These veterans with PH are predominantly male, an expected finding given the cohort’s source population. However, they are older and have substantially more comorbidities than the limited prior reports that evaluate PH in veterans. Longitudinal follow-up from the time of PH diagnosis demonstrates high risk of death with median survival of 3.88 years and 22.1% dying within 1 year. The recognition of this high-risk population has significant potential implications for healthcare systems that manage subjects with increasing age and comorbidities, including VHA as well as the aging Medicare population, as it is known that a large proportion of healthcare spending is in the final few years of life.25,26
Data on race were known for the majority of this cohort. The cohort was majority white, but in an unadjusted analysis, black race was associated with lower risk of death. About 8% of the cohort had no identifiable race. These subjects had poorer outcome. This is postulated to be due to reverse causality; for example, subjects that have poor survival have lower likelihood of having race entered into their medical record.
Our study is the largest report of veterans diagnosed with PH and extends prior reports of PH in veterans by identifying the distribution of PH subtypes clinically encountered in the VHA, the frequency with which diagnostic procedures are used, and prevalence of comorbid conditions. Compared with earlier reports in non-veterans, patients in this study with PH are generally older and more frequently male, but are similarly more likely to have non-Group 1 causes of PH.20Survival of subjects with presumed Group 2 and 3 PH was significantly worse than those with presumed PAH, a finding consistent with recent reports from other single centers.5,8Maron et al. identified veterans who had RHC, the gold standard diagnostic test for PH.27It should be noted that there is no recommendation that RHC be universally used in all subjects with or subtypes of PH, nor would all who had RHC be expected to have PH (e.g. subjects who have RHC prior to high-risk procedures).1The study by Maron et al. included fewer subjects than our cohort, likely because our cohort arose from diagnoses ascribed in clinical practice and not all patients would be expected to benefit from invasive hemodynamic measurement. This consideration, and the possibility of selection bias for invasive procedures such as RHC, is supported by the fact that the earlier-reported cohort is younger (the median age of the Maron cohort was 65 versus 70 years in this cohort). Also noteworthy was that our cohort was less frequently of minority race (20.0% versus 24.1% reported by Maron and colleagues).27Thus, our cohort may provide a more comprehensive assessment of the population of veterans with PH that are encountered in clinical care within VHA. While Maron et al. identified that PH and even borderline elevation of PA pressures are associated with increased risk of death, the current study further emphasizes that the comorbidities that subjects have, especially those that are associated with PH, further increase a given patients risk.
Veterans in this study were frequently lacking components of the diagnostic evaluation of suspected PH.1While studies may have been obtained outside the VHA system, veterans in this cohort received medical care in the VHA system for an average of 8 years prior to the diagnosis of PH, demonstrating that they consistently accessed medical care via the VHA system. We acknowledge that this cohort was frequently of Medicare-eligible age, and therefore may have also obtained care outside VHA. Guidelines recommend referral of subjects with PH to centers with expertise, and part of such a center’s evaluation includes guideline-recommended studies to ascertain correctly the underlying etiology of PH. This can be a challenging task and requires methodical evaluation and critical review of diagnostic testing. Whether patients included in our cohort were evaluated by providers with expertise in PH cannot be known, but would at most be infrequent given the cohort’s size. RHC, the gold standard diagnostic test to confirm PH, was obtained in only 7.5% of subjects. In our cohort, diagnostic studies that are widely available and/or less invasive were used more frequently than RHC. At least one echocardiogram was obtained in 86.5% of subjects, pulmonary function testing was performed in 51.7%, and chest CT was performed in 54.9%. Yet, these diagnostic testing procedures were used less commonly than reported in a cohort of subjects with suspected PH referred to specialty centers (the RePHerral study11), who had the above diagnostic tests in 100%, 70.7% and 79.3%, respectively. Ventilation/perfusion (V/Q) lung scanning was obtained in 11.5% of our cohort. Although recommended by guidelines for evaluation of possible CTEPH, use of V/Q scans is generally felt to be underutilized.1,28,29In our study, the proportion of patients having a V/Q scan was lower than in patients in the RePHerral study (11.5% versus 23.6% before expert referral in the RePHerral study and 51.4% post-referral).11Because of low utilization of V/Q scan, the proportion with presumed CTEPH may be underestimated in our cohort. Differences in the frequency with which diagnostic testing was performed may be explained by a clinician’s identification of a clear cause of secondary PH and decision that further evaluation of other causes was not indicated. This retrospective cohort study cannot identify whether the diagnostic testing performed was sufficient, or whether the determination of PH subtype was correct.
Acknowledging this study’s reliance on comorbid conditions for PH subtype determination, some noteworthy findings regarding those subtypes are evident. Similar to reports including fewer subjects, our study identified that subjects with PH due to left heart disease or lung disease (Groups 2 and 3 PH) have poorer outcome than other groups, including presumed PAH.5–7Compared with presumed PAH, patients with PH due to left heart disease or lung disease were shown to have 1.97 and 1.58 fold increased risk of death, respectively, accounting for differences in age, race, and gender between groups. This finding underscores the importance of recognizing PH in subjects with chronic left heart and/or lung disease as they may be at especially high risk of death. However, this association could be due to differences in other comorbidities. For this reason, we utilized the Elixhauser comorbidity index adapted by Quan et al.,21which is commonly used to predict outcome in administrative datasets like ours and constitutes 31 separate disease categories.
Comorbid conditions were common in this cohort. At the time of PH diagnosis (within 6 months after to ever beforehand), the average patient had 10 of the 31 possible Elixhauser comorbidity categories. While methods for assessing comorbid conditions may vary between other administrative studies and ours, this frequency is higher than those reported in other studies of similar patients, including veterans in primary care, with heart failure, and with stroke.30–32In the study from Bates et al.32which evaluated mortality after stroke, veterans were marginally younger (mean 68.3 years) but had significantly lower prevalence of cardiopulmonary and non-cardiopulmonary comorbidities. Chronic liver disease, renal disease, and depression were all substantially more prevalent in veterans with PH compared with stroke (16% vs. 3%, 34% vs. 24%, and 44% vs. 20%, respectively). The minor difference in age between these cohorts would not be expected to cause such differences in comorbid conditions. Rather, we hypothesize that the PH diagnosed in veterans in our cohort is often the result of a chronic disease milieu frequently including cardiac, pulmonary, metabolic, and other chronic conditions. In addition to being more frequent, these comorbid conditions have a large impact on patient outcome. The Elixhauser index, which is the sum of the 31 categories present in any single patient, and most individual Elixhauser categories were associated with increased risk of death. These findings further compound the dilemma facing physicians caring for PH of how best to address this high-risk condition and suggesting that a monotonic intervention may underestimate the complexity of PH.
Notably, diabetes mellitus (both with and without diabetic complications) was common in veterans with PH and was associated with increased risk of death, even when accounting for the presence of all other comorbidity categories, race, age, and gender. Multiple prior investigators—using varied methods including cell and animal models, translational research, and epidemiologic studies—have shown that metabolic conditions such as glucose intolerance, diabetes mellitus, and the metabolic syndrome are closely linked to pulmonary vascular conditions such as PAH and hypoxia-mediated PH.33–40One prior report on the impact of diabetes mellitus on PAH survival showed increased risk for death, consistent with our findings.40Whether intervention on these metabolic processes would mitigate the increase in risk of disease is unknown; although a prior study had been posed (NCT00825266 at clinicaltrials.gov41), no study has been completed.
In contrast to the presence of diabetes, a diagnosis of obesity was shown to be protective consistent with the “obesity paradox” described in patients with various cardiovascular disease, including Group 1 PAH.42,43Baseline body mass index (BMI) and the BMI category were associated with survival with improved outcome as BMI increased. Compared with those with a normal BMI, overweight and obese veterans had 27% and 41% lower risk of mortality, respectively, and those who were underweight had 44% higher risk of mortality. Similar findings were seen when using ICD-9diagnosis codes via the Elixhauser categories which include both obesity and weight loss. A subject having a diagnosis in those categories was associated with 21% lower and 32% higher risk of death, respectively.
Our retrospective analysis of clinical and administrative data has several important limitations that merit consideration. First, our study relied on ICD-9codes for determining whether patients had PH and to which subtype they best fit. While this is an inherent limitation that could result in misclassification bias, it allowed us to capture a larger cohort for analysis. We are reassured that the captured cohort resembled those in previous small single-center studies.5,27The same concern applies to other covariates assessed at baseline, importantly the presence of comorbid conditions. The cohort assembled is generally older and may seek care outside the VHA system, as a majority of them would be Medicare eligible. Indeed, others have observed that analysis of external Medicare data adds to the understanding of comorbidities in veterans.44In addition, we report here the presence of diagnostic testing obtained within the VHA system on patients with PH, but these studies could also have been obtained elsewhere. An important part of PH patient assessment is that of disease severity (e.g. NYHA/WHO Functional Classification).1We were unable to account for disease severity in our models of outcome as this information is not recorded in CDW. In addition, RHC is a means to confirm and measure the severity of PH. This invasive test is often performed at VHA centers and we were able to assess for whether it had been performed, but were unable to include data from the study itself, which may have confirmed or refuted the presence of PH, and provided data on PH severity. Manual chart review to include that data is impractical in a dataset the size presented here. Lastly, because our study included only veterans receiving care in the VHA system our findings may not be generalizable to other populations with PH, including veterans outside the VHA system or non-veterans. Similarly, our cohort was predominantly male, so interpretation and application of our findings to females should be done with caution. Despite limitations, this cohort is a valuable tool for ongoing study of PH, especially that due to comorbid heart or lung disease.
In conclusion, we have developed a large cohort of veterans diagnosed with PH, commonly due to underlying heart or lung disease. These underlying commonly acknowledged causes of PH are deterministic for outcome of these veterans, with those having PH presumed resulting from lung disease, heart disease, or a combination of the two having worsened survival compared with all other subtypes of PH. Diabetes, neurologic/psychiatric, renal and hepatic disease were predictors of poor outcome, while higher BMI or a diagnosis of obesity was protective.
Acknowledgements
The authors would like to acknowledge the assistance provided in data acquisition by Ms. Christine Jasien.
Contributorship
All authors contributed significantly to the study design and interpretation of data and results. AWT acquired all data and performed all statistical analyses. All authors assisted in drafting the manuscript or critically revising it for intellectual content and have approved the final version.
Declaration of conflicting interests
Dr. Phillips reports that he has served on Scientific Advisory Boards for Janssen, and the Profil Institute for Clinical Research, and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, Abbvie, Vascular Pharmaceuticals, Janssen, Glaxo SmithKline, Pfizer, Kowa, and the Cystic Fibrosis Foundation. In the past, he was a speaker for Novartis and Merck, but not for the last five years. Dr. Phillips is also a cofounder and Officer and Board member and stockholder of a company, DIASYST, Inc., which is developing software aimed to help improve diabetes management. All other authors report no conflicts of interests are present with regard to the work reported.
Ethical approval
Approval for the study was approved by the Emory Institutional Review Board and the Atlanta VA Research and Development Committee.
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR002378. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported in part by funding from the Department of Veterans Affairs, Biomedical Laboratory Research and Development Office, Merit Review Award (1I01BX001910 to CMH), by NIH NHLBI R01 (HL102167 to CMH), and with resources and the use of facilities at the Atlanta Veterans Affairs Medical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Guarantor
All authors take responsibility for the validity of the work and the study results reported.
References
- 1.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: Epidemiology and registries. J Am Coll Cardiol 2013; 62: D51–D59. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- 4.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107: 216–223. [DOI] [PubMed] [Google Scholar]
- 5.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36: 957–967. [DOI] [PubMed] [Google Scholar]
- 6.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 7.Mueller-Mottet S, Stricker H, Domeninghetti G, et al. Long-term data from the Swiss Pulmonary Hypertension Registry. Respiration 2015; 89: 127–140. [DOI] [PubMed] [Google Scholar]
- 8.Prins KW, Rose L, Archer SL, et al. Disproportionate right ventricular dysfunction and poor survival in Group 3 pulmonary hypertension. Am J Respir Crit Care Med 2018; 197: 1496–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–D116. [DOI] [PubMed] [Google Scholar]
- 10.Pugh ME, Sivarajan L, Wang L, et al. Causes of pulmonary hypertension in the elderly. Chest 2014; 146: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deano RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: The multicenter RePHerral study. JAMA Intern Med 2013; 173: 887–893. [DOI] [PubMed] [Google Scholar]
- 12.Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013; 62: D100–D108. [DOI] [PubMed] [Google Scholar]
- 13.Trammell AW, Pugh ME, Newman JH, et al. Use of pulmonary arterial hypertension-approved therapy in the treatment of non-group 1 pulmonary hypertension at US referral centers. Pulm Circ 2015; 5: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D, Lee KM, Freiman MR, et al. Phosphodiesterase-5-inhibitor therapy for pulmonary hypertension in the US: Actual vs recommended use. Ann Am Thorac Soc 2018; 15: 693–701. [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan D, Gonsoulin ME. CDW statistical snapshot: Patient demographics. US Dept of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center 2016. [Google Scholar]
- 16.Assari S. Veterans and risk of heart disease in the United States: A cohort with 20 years of follow up. Int J Prevent Med 2014; 5: 703–709. [PMC free article] [PubMed] [Google Scholar]
- 17.Hoerster KD, Lehavot K, Simpson T, et al. Health and health behavior differences: U.S. Military, veteran, and civilian men. Am J Prev Med 2012; 43: 483–489. [DOI] [PubMed] [Google Scholar]
- 18.Fryar CD, Herrick K, Afful J, et al. Cardiovascular disease risk factors among male veterans, U.S., 2009-2012. Am J Prev Med 2016; 50: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: A comparison of hospital records and Medicare claims for cancer patients. Med Care 2006; 44: 921–928. [DOI] [PubMed] [Google Scholar]
- 20.Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: Prevalence and mortality in the Armadale echocardiography cohort. Heart 2012; 98: 1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 22.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat Rep 2017; 66: 1–64. [PubMed] [Google Scholar]
- 24.National Center for Veterans Analysis and Statistics. USVETS: Mortality Rates and Life Expectancy of Veterans. https://www.va.gov/vetdata/report.asp: Department of Veterans Affairs, 2017.
- 25.Davis MA, Nallamothu BK, Banerjee M, et al. Identification of four unique spending patterns among older adults in the last year of life challenges standard assumptions. Health Aff (Millwood) 2016; 35: 1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French EB, McCauley J, Aragon M, et al. End-of-life medical spending in last twelve months of life is lower than previously reported. Health Aff (Millwood) 2017; 36: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 27.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. Circulation 2016; 133: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins IM, Pugh ME, Hemnes AR. Update on chronic thromboembolic pulmonary hypertension. Trends Cardiovasc Med 2017; 27: 29–37. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S, Helmersen D, Provencher S, et al. Diagnostic evaluation and management of chronic thromboembolic pulmonary hypertension: A clinical practice guideline. Can Respir J 2010; 17: 301–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients with heart failure in the veterans health administration. Am J Cardiol 2012; 110: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care 2013; 51: 368–373. [DOI] [PubMed] [Google Scholar]
- 32.Bates BE, Xie D, Kwong PL, et al. One-year all-cause mortality after stroke: A prediction model. PM&R 2014; 6: 473–483. [DOI] [PubMed] [Google Scholar]
- 33.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009; 33: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assad TR, Hemnes AR. Metabolic dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep 2015; 17: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugh ME, Hemnes AR, Trammell A, et al. Variability in hemodynamic evaluation of pulmonary hypertension at large referral centers. Pulm Circ 2014; 4: 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeligar SM, Kang BY, Bijli KM, et al. PPARgamma regulates mitochondrial structure and function and human pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 2018; 58: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green DE, Sutliff RL, Hart CM. Is peroxisome proliferator-activated receptor gamma (PPARgamma) a therapeutic target for the treatment of pulmonary hypertension? Pulm Circ 2011; 1: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson L, Brittain EL, Pugh ME, et al. Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ 2014; 4: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Insulin Resistance in Pulmonary Arterial Hypertension. https://ClinicalTrials.gov/show/NCT00825266.
- 42.Lavie CJ, McAuley PA, Church TS, et al. Obesity and cardiovascular diseases. Implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol 2014; 63: 1345–1354. [DOI] [PubMed] [Google Scholar]
- 43.Mazimba S, Holland E, Nagarajan V, et al. Obesity paradox in group 1 pulmonary hypertension: Analysis of the NIH-Pulmonary Hypertension registry. Int J Obes 2017; 41: 1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axon RN, Gebregziabher M, Everett CJ, et al. Dual health care system use is associated with higher rates of hospitalization and hospital readmission among veterans with heart failure. Am Heart J 2016; 174: 157–163. [DOI] [PubMed] [Google Scholar]