Short abstract
Objective
To investigate the effects of parenteral nutrition (PN) including ω-3 fish-oil emulsion on nutritional state, inflammatory response, and prognosis in patients with acute paraquat poisoning.
Methods
Patients randomized to receive medium chain triglycerides (MCT)/long chain triglycerides (LCT)-based PN (control group) or MCT/LCT-based PN containing ω-3 fish-oil emulsion (intervention group) were compared for 90-day survival and short-term treatment efficacy.
Results
Tumour necrosis factor-α levels were significantly lower in the intervention group (n = 101) versus controls (n = 73) on treatment days 4 and 7. Intervention group C-reactive protein (CRP) levels were significantly increased on day 4, decreased to baseline (day 1) levels on day 7, and were significantly lower than baseline on day 10. Control group CRP levels were significantly increased on days 4 and 7 versus baseline, and returned to baseline levels on day 10. On day 7, retinol binding protein had recovered to baseline levels in the intervention group only. Intervention group mortality rate (36.6%) was significantly lower than controls (57.5%). ω-3 fish-oil PN was associated with reduced risk of death (hazard ratio 0.52; 95% confidence interval 0.33, 0.82).
Conclusion
In patients with acute paraquat poisoning, MCT/LCT with ω-3 fish-oil emulsion PN plus combination treatment advantageously attenuated the inflammatory response, modified the nutritional state, and was associated with significantly improved 90-day survival versus treatment without ω-3 fish oil.
Keywords: Paraquat poisoning, ω-3 fish oil fat emulsion, combination treatment, inflammatory factors
Introduction
Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) is an effective and safe nonselective herbicide when used appropriately, however, it is highly toxic to humans.1 Paraquat poisoning, which is mainly caused by intentional ingestion for suicide, is characterized by multiple organ failure due to excessive production of reactive oxygen species (ROS) and the subsequent inflammatory response.2,3 Additionally, paraquat has been shown to increase the expression levels of inflammatory response genes, such as interleukin (IL)-1, IL-6, IL-8, tumour necrosis factor (TNF)-α, TNF-β, interferon-1, transforming growth factor (TGF)-β, and nuclear factor (NF)-kβ.4 Pro-inflammatory cytokines, such as TNF-α and others, can induce ROS formation.5,6 There is no targeted antidote for paraquat poisoning, and standard treatment includes a combination of cyclophosphamide, steroids, antioxidants, and haemperfusion, which has been demonstrated to be effective in preventing respiratory failure and significantly improves survival rates in moderate to severe cases.7–10 Cyclophosphamide and glucocorticoid treatments have been associated with increased ROS production, however, which subsequently leads to oxidative stress, lipid peroxidation, glutathione consumption, increased glutathione peroxidase and superoxide dismutase activities, and glucocorticoid resistance accompanied by immunosuppression.11–15
Clinically, most patients with paraquat poisoning who have gastrointestinal tract injury have been found to develop feeding difficulties, and total parenteral nutrition (PN) is needed to reduce nutritional risks.1,11,16–19 The most common parameters measured to evaluate nutritional status include body weight, triceps skinfold thickness, mid-arm circumference, and mid-arm muscle circumference,20 however, these parameters are insensitive to early changes in the nutritional status of patients. Conversely, prealbumin (PA) and retinol binding protein (RBP) measurements are sensitive to early changes in nutritional status.21,22 Thus, PA and RBP were used in the present study to evaluate short-term changes in nutrition status following treatment for acute paraquat poisoning.
Clinical nutrition has evolved and is considered to be a joint pharmacological/nutritional therapy in treating pro-inflammatory states, such as sepsis and acute respiratory distress syndrome.23,24 Studies have revealed that PN containing an ω-3 fish-oil emulsion exhibits anti-inflammatory properties and can reduce the development of lung oedema and improve pulmonary function through decreasing the 4-series leukotrienes and increasing release of the anti-inflammatory cytokines TGF-β, IL-10 and IL-13.24–31 Intravenous ω-3 fish-oil emulsion administration also inhibits the 5-lipoxygenase pathway and the leukotriene B4-mediated function in inflammatory cells, and increases the production of specialized pro-resolving mediators that counter regulate pro-inflammatory mediators.32–35
Based on the above findings, the aim of the present study was to test the hypothesis that addition of PN containing an ω-3 fish-oil emulsion to combination therapy would beneficially ameliorate the altered nutritional state, decrease the inflammatory response, and potentially decrease the mortality rate in patients with paraquat poisoning.
Patients and methods
Study population
This prospective, randomized, controlled single-centre clinical trial was conducted at the Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China between January 2014 and December 2016, and included patients who conformed fully to the following criteria: aged 14–75 years with a clear past medical history and a normal nutrition state; a clear history of paraquat ingestion; admission to the Second Hospital of Hebei Medical University within 48 h of paraquat ingestion; and positive paraquat test in plasma. Patients who met any of the following criteria were excluded: aged < 14 years, pregnant, transcutaneous or intravenous paraquat exposure and undetectable levels of paraquat in the blood, combined drug exposure, transfer to another hospital during treatment or treatment abandonment, a history of chronic obstructive pulmonary disease, psychosis, or diabetes with severely impaired liver or renal function, hospital exit, or missing study data.
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Review Board and Ethics Committee of the Second Hospital of Hebei Medical University. Written informed consent was obtained from patients or their legal guardians. All patient information was available to the clinicians only, and all the research data were analysed anonymously.
Treatment protocols
Consecutive, eligible participants were randomly allocated to the control group or the intervention group following baseline assessments. Randomization was conducted using a computer-generated randomization scheme.
Immediately upon baseline assessment and diagnosis, all patients were administered 100 g activated carbon tablets plus Fuller's earth via a gastric tube to minimize further absorption.36 All patients also received a femoral venous dual lumen catheter with a blood flow rate of 150–200 ml/min × 2 h for a 6-h interval with HA330 resin haemperfusion (Jafron Biomedical Co. Ltd., Zhuhai City, China). Haemperfusion was discontinued in patients whose plasma paraquat concentration was < 50 ng/ml.
In addition, all patients received daily pulse methylprednisolone therapy (1 g; Pfizer Manufacturing Belgium NV, Rijksweg, Belgium) via intravenous infusion for three days and 15 mg/kg cyclophosphamide (Jiangsu Sheng Di Medicine Co., Ltd, Lianyungang, Jiangsu, China) for three days. The calorie intake for all patients was maintained at 20 kcal/kg/day. Novamin 8.5% (Huarui Pharmaceutical Co., Ltd., Wuxi, Jiangsu, China) was administered to provide 35–50% of the total calories and 0.15–0.20 g/kg/day nitrogen. Glucose and exogenous insulin (6:1 ratio), vitamins, electrolytes, trace elements, and water were administered via central venous infusion for 10–12 h immediately following diagnosis. Both groups of patients received combination therapy, however, the control group received 0.6 g/kg Lipofundin 20%, a medium chain triglycerides (MCT)/long chain triglycerides (LCT)-based emulsion (Braun Melsungen AG, Melsungen, Germany), while the intervention group received 0.4 g/kg Lipofundin 20% plus 0.2 g/kg ω-3 fish-oil emulsion (Fresenius Kabi Huarui Pharmaceutical Co., Ltd., Sub packaging enterprises, Wuxi, Jiangsu, China). Treatment was continued for 10 days depending on survival. The two patient groups were given the same nutritional meals provided by the Nutrition Department of the Second Hospital of Hebei Medical University.
Outcome measures
The primary endpoint was overall 90-day mortality. The secondary endpoints included changes in inflammatory status (measured by total lymphocyte count [TLC], TNF-α and C-reactive protein [CRP] levels) and nutritional status (measured by serum RBP and prealbumin levels) following the administration of PN plus combination treatment.
Data collection
Sociodemographic and clinical characteristics were collected by systematically reviewing the patients’ medical records. The Acute Physiology And Chronic Health Evaluation (APACHE) II score was calculated to classify the severity of disease following patient admission. Venous blood samples for biochemistry analyses were collected at 2 h following lipid infusion on days 1, 4, 7 and 10. Biochemical tests were performed mainly on fresh blood samples (a very small number of samples were centrifuged and stored at –80 °C prior to testing) according to standard hospital protocols, as patients with paraquat poisoning have priority to blood tests at the Second Hospital of Hebei Medical University. RBP and serum TNF-α levels were measured using commercially available kits for use with the DPC IMMULITE 1000 system (all Siemens Healthcare Ltd., Erlangen, Germany) according to the manufacturer’s operating instructions. Levels of CRP, TLC and prealbumin were quantified using a Cobas Integra 700 chemistry analyser and reagents (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. These data were collected by postgraduate students unfamiliar with the study to avoid bias.
Statistical analyses
Data for continuous variables are presented as mean ± SD, and categorical variables are presented as n (%) prevalence. Between-group differences were statistically analysed using Student’s t-test, paired t-test, or χ2-test, as appropriate. Survival curves were estimated according to the Kaplan-Meier method, and treatments were compared using log-rank tests. Multivariable Cox proportional hazard models were applied to the outcome while controlling for possible confounding baseline characteristics, and results are presented as hazard ratios (HR) and 95% confidence intervals (CI). Statistical analyses were performed using SPSS software, version 18.0 (IBM Corporation, New York, NY, USA), and a P value <0.05 was considered statistically significant.
Results
A total of 238 patients who fulfilled the criteria for acute oral poisoning between January 2014 and December 2016 were included for enrolment into the study (Figure 1). Of these, 15 patients were excluded either because they declined to participate (n = 10) or due to other reasons (n = 5). Thus, a total of 223 patients were enrolled and randomized to the two treatment groups: 113 patients in the intervention group and 110 patients in the control group. Twenty-one patients withdrew from the study and 28 patients were lost to follow-up, leaving 174 patients included in the final analyses (Figure 1). The final study population comprised 101 patients in the intervention group and 73 patients in the control group (mean age, 32.60 ± 12.03 years, range, 14–70 years; with 90 [51.7%] male patients and 84 [48.3%] female patients; Table 1). The mean time from paraquat ingestion to treatment was 3.20 ± 4.71 h (range, 25 min to 47 h). During hospitalization, there were no significant side effects associated with parenteral nutritional support, and there were no deaths.
Figure 1.
Study flow diagram showing selection and randomization to treatment with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group) in patients with acute paraquat poisoning; MCT, medium chain triglycerides; LCT, long chain triglycerides
Table 1.
Demographic and baseline characteristics in patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control)
| Study groupa |
||
|---|---|---|
| Variable | Intervention(n = 101) | Control(n = 73) |
| Sex, male: female | 54:47 | 36:37 |
| Age, years | 32.46 ± 12.76 | 32.79 ± 11.01 |
| Body mass index, kg/m2 | 22.3 ± 16.8 | 22.4 ± 15.7 |
| Baseline serum paraquat level, mg/l | 6.92 ± 3.99 | 6.14 ± 3.97 |
| Time to treatment, h | 2.99 ± 3.71 | 3.49 ± 5.82 |
| Baseline total protein, g/l | 72.61 ± 7.31 | 72.56 ± 5.62 |
| Baseline ALB, g/l | 46.50 ± 5.27 | 46.09 ± 5.15 |
| Baseline TC, mmol/l | 4.42 ± 1.03 | 4.11 ± 0.99 |
| Baseline haemoglobin, g/l | 139.80 ± 22.13 | 136.78 ± 19.81 |
| Baseline serum K+ levels, mmol/l | 3.86 ± 0.39 | 3.82 ± 0.43 |
| Pleural effusion (yes/no) | 34/67 (33.66%) | 29/44 (39.73%) |
| Height, cm | 167.5 (147–181) | 169.2 (150–186) |
| APACHE II score | 21.40 ± 3.48 | 21.7 ± 6.32 |
Data presented as n (%) prevalence, mean ± SD or median (range).
MCT, medium chain triglycerides; LCT, long chain triglycerides; ALB, albumin; TC, total cholesterol; APACHE, Acute Physiology And Chronic Health Evaluation.
aNo statistically significant between-group differences (P > 0.05; Student’s t-test, paired t-test, or χ2-test).
There were no statistically significant between-group differences regarding demographics and baseline characteristics, time to treatment, nutritional (except RBP levels) and inflammatory indicators or APACHE II scores (all P > 0.05 except RBP, P = 0.02; Tables 1 and 2).
Table 2.
Comparison of variables in patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control)
| Study group |
|||
|---|---|---|---|
| Analysis variable | Intervention, n = 101 | Control, n = 73 | Statistical significance |
| TLC, × 109/l | |||
| Baseline (day 1) | 1.26 ± 0.63 | 1.24 ± 0.70 | NS |
| Day 4 | 0.62 ± 0.76 | 0.46 ± 0.25 | NS |
| Day 7 | 0.36 ± 0.40 | 0.34 ± 0.53 | NS |
| Day10 | 0.24 ± 0.51 | 0.38 ± 0.36 | NS |
| TNF-α, pg/ml | |||
| Baseline (day 1) | 31.89 ± 6.21 | 33.47 ± 6.92 | NS |
| Day4 | 30.32 ± 5.51 | 34.43 ± 5.61 | P < 0.001 |
| Day7 | 27.81 ± 6.98 | 32.37 ± 6.82 | P < 0.001 |
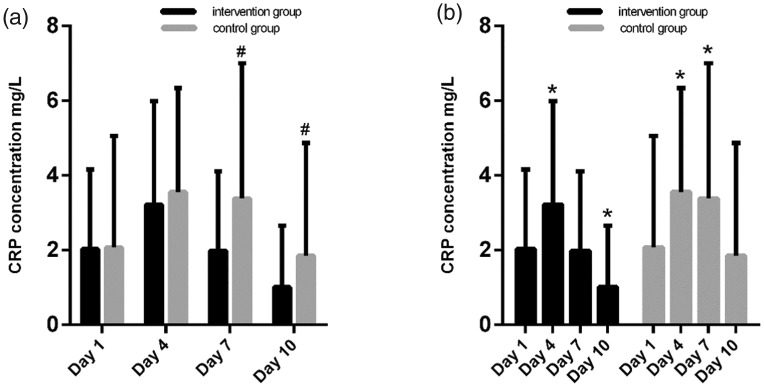
| CRP, mg/l | |||
| Baseline (day 1) | 2.03 ± 2.13 | 2.08 ± 2.98 | NS |
| Day4 | 3.22 ± 2.77 | 3.56 ± 2.78 | NS |
| Day7 | 1.98 ± 2.13 | 3.38 ± 3.62 | P < 0.001 |
| Day10 | 1.01 ± 1.65 | 1.85 ± 3.02 | P = 0.02 |
| RBP, mg/l | |||
| Baseline (day 1) | 32.26 ± 6.73 | 29.72 ± 6.77 | P = 0.02 |
| Day 4 | 28.50 ± 6.68 | 27.24 ± 7.18 | NS |
| Day 7 | 33.75 ± 7.80 | 27.05 ± 7.45 | P < 0.001 |
| PA, g/l | |||
| Baseline (day 1) | 0.25 ± 0.07 | 0.23 ± 0.07 | NS |
| Day4 | 0.27 ± 0.16 | 0.24 ± 0.06 | NS |
| Day7 | 0.30 ± 0.25 | 0.28 ± 0.26 | NS |
| Day10 | 0.31 ± 0.31 | 0.29 ± 0.27 | NS |
Data presented as mean ± SD.
MCT, medium chain triglycerides; LCT, long chain triglycerides; TLC, total lymphocyte count; TNF, tumour necrosis factor; CRP, C-reactive protein; RBP, retinol binding protein; PA, prelabumin.
NS, no statistically significant between-group difference (P > 0.05; Student’s t-test)
In terms of TLC, there were no statistically significant between-group differences on days 1 (baseline), 4, 7 or 10 (all P > 0.05; Table 2, Figure 2a). Within-group analyses showed that TLC was consistently reduced on days 4, 7, and 10 after initiation of treatment compared with baseline in both treatment groups (intervention group, baseline versus day 4, P = 0.02; baseline versus day 7, P < 0.001; baseline versus day 10, P < 0.001; control group, baseline versus day 4, 7 and 10, all P < 0.001; Figure 2b).
Figure 2.
Comparison of total lymphocyte count (TLC) between patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group), and within-group comparisons on each day following initiation of treatment: *P < 0.05 versus baseline values (intragroup comparison using Student's t-test); No statistically significant differences between the two groups on each treatment day; MCT, medium chain triglycerides; LCT, long chain triglycerides
There was no statistically significant between-group difference in TNF-α levels at baseline (day 1 of admission; intervention versus control group, P = 0.12), however, there were statistically significant between-group differences on days 4 and 7 (P < 0.001; Table 2 and Figure 3a). This difference was due to a statistically significant decreasing trend in the intervention group on days 4 and 7 versus baseline (both P <0.001; Figure 3b).
Figure 3.
Comparison of tumour necrosis factor (TNF)-α levels between patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group), and within-group comparisons on each day following initiation of treatment: #P < 0.05, between-group differences at days 4 and 7; *P < 0.05, versus baseline (all Student's t-test); MCT, medium chain triglycerides; LCT, long chain triglycerides
There were statistically significant between-group differences in CRP levels on days 7 and 10 (P < 0.05; Table 2 and Figure 4a). In both the intervention and control groups, CRP levels increased from the baseline reading to day 4 (both P < 0.001). In the intervention group, CRP levels then decreased to baseline levels on day 7 (baseline versus day 7, P = 0.505), and decreased to below baseline levels on day 10 (baseline versus day 10, P < 0.001). In the control group, CRP levels remained fairly stable at day 7 (day 7 versus baseline P = 0.008), but decreased back to baseline levels on day 10 (day 10 versus baseline, P = 0.512; Table 2 and Figure 4b).
Figure 4.
Comparison of C-reactive protein (CRP) levels between patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group), and within-group comparisons on each day following initiation of treatment: #P < 0.05, between-group differences at days 7 and 10; *P < 0.05, versus baseline (all Student's t-test); MCT, medium chain triglycerides; LCT, long chain triglycerides
In the intervention group, serum RBP levels were significantly higher than the control group on day 1 (baseline) and day 7 (P = 0.02 and P < 0.001, respectively; Figure 5a). In both groups, serum RBP levels were decreased on day 4 (intervention group baseline versus day 4, P < 0.001; control group baseline versus day 4, P = 0.01). On day 7, serum RBP levels in the intervention group were higher than those on day 4 (P < 0.001 day 7 versus day 4), and had recovered to baseline levels (P = 0.126, baseline versus day 7). Control group RBP levels failed to return to baseline levels on day 7 (P = 0.001; Table 2 and Figure 5b).
Figure 5.
Comparison of retinol binding protein (RBP) levels between patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group), and within-group comparisons on each day following initiation of treatment: #P < 0.05, between-group differences at days 1 and 7; *P < 0.05, versus baseline; †P < 0.05, day 4 versus day 7 (all Student’s t-test); MCT, medium chain triglycerides; LCT, long chain triglycerides
Prealbumin levels were not significantly different between the two treatment groups at any time-point, and did not markedly change in either group following initiation of treatment (Table 2 and Figure 6a and b).
Figure 6.
Comparison of prealbumin (PA) levels between patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group), and within-group comparisons on each day following initiation of treatment: There were no statistically significant between-group or within-group differences (Student's t-test)
Sub-analyses of indices in the survivor and non-survivor groups (Table 3) revealed statistically significant between-group differences in baseline prealbumin levels (0.25 versus 0.23 g/l), the proportion of patients with pleural effusion (25.3% versus 49.4%), changes in RBP, CRP and TNF-α levels, and the proportion of patients who had received ω-3 fish-oil emulsion (67.4% versus 46.8%, survivor versus non-survivor, respectively).
Table 3.
Comparison of indices in survivor versus non-survivor patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control)
| Study group |
|||
|---|---|---|---|
| Variable | Non-survivors(n = 79) | Survivors(n = 95) | Statistical significance |
| Sex, male: female | 42:37 | 48:47 | NS |
| Age, years | 34.33 ± 12.45 | 31.16 ± 11.53 | NS |
| Body mass index, kg/m2 | 22.4 ± 15.8 | 22.5 ± 12.7 | NS |
| Baseline serum paraquat level, mg/l | 6.81 ± 3.96 | 6.42 ± 4.02 | NS |
| Time to treatment, h | 3.71 ± 6.31 | 2.77 ± 2.71 | NS |
| Baseline haemoglobin, g/l | 138.99 ± 21.31 | 138.16 ± 21.17 | NS |
| Total protein, g/l | 72.80 ± 6.09 | 72.42 ± 7.08 | NS |
| Baseline PA, g/l | 0.23 ± 0.07 | 0.25 ± 0.07 | P = 0.02 |
| ALB | 46.71 ± 4.23 | 46.01 ± 5.91 | NS |
| TC, mmol/l | 4.32 ± 1.07 | 4.27 ± 0.98 | NS |
| Baseline serum K+ level, mmol/l | 3.85 ± 0.38 | 3.84 ± 0.43 | NS |
| Pleural effusion, yes/no | 39/40 (49.37%) | 24/71 (25.26%) | P < 0.001 |
| Change in RBP, day 7–day 1 | 48+ /31– (60.76%) | 76+/19– (80%) | P = 0.01 |
| Change in CRP, day 7– day 1 | 26+/53– (32.91%) | 12+/83– (12.63%) | P < 0.001 |
| ω-3 fish oil PN (yes/no) | 37/42 (46.84%) | 64/31 (67.37%) | P = 0.01 |
| Change in TNF-α, day 7–day 1 | 26+/53– (32.91%) | 12+/83– (12.63%) | P < 0.001 |
MCT, medium chain triglycerides; LCT, long chain triglycerides; PA, prealbumin; ALB, albumin; TC, total cholesterol; RBP, retinol binding protein; CRP, C-reactive protein; PN, parenteral nutrition; TNF, tumour necrosis factor.
+ increase or no decrease; –decrease.
NS, no statistically significant between-group difference (P > 0.05; Student’s t-test or χ2-test).
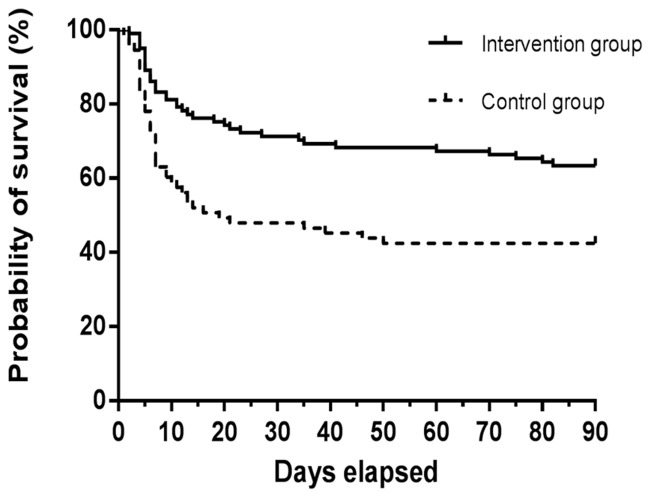
During the 90-day follow-up period, 95 patients had survived following oral ingestion of paraquat and 79 patients had died. The 90-day mortality rates were as follows: 37/101 patients in the intervention group (36.6%) and 42/73 patients in the control group (57.5%; P = 0.006). In multivariable Cox proportional hazard regression models, ω-3 fish-oil PN was associated with a reduced risk of death after adjusting for baseline variables (HR 0.52; 95% CI 0.33, 0.82; P = 0.005; Table 4 and Figure 7). Existing pleural effusion, RBP (day 7–day 1) decreases and TNF-α (day 7–day 1) increases were associated with an increased risk of death (HR 2.985, 95% CI 1.849, 4.704 [P <0.001]; HR 2.015, 95% CI 1.271, 3.196 [P = 0.003]; and HR 2.308, 95% CI, 1.438, 3.706 [P = 0.001], respectively; Table 4).
Table 4.
Regression analyses using cox proportional hazards models summarising the clinical indices predicting mortality in patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control)
| Index | Univariate Cox Model |
Multivariate Cox Model |
||
|---|---|---|---|---|
| HR (95% CI) | Statistical significance | Adjusted HR (95% CI) | Statistical significance | |
| Pleural effusion | 2.907 (1.862, 4.539) | P < 0.001 | 2.985 (1.849, 4.704) | P < 0.001 |
| Change in RBP↓ (day 7–day 1) | 1.874 (1.191, 2.950) | P = 0.007 | 2.015 (1.271, 3.196) | P = 0.003 |
| Change in TNF↑ (day 7–day 1) | 2.306 (1.438, 3.698) | P = 0.001 | 2.308 (1.438, 3.706) | P = 0.001 |
| ω-3 fish oil PN | 0.509 (0.327, 0.793) | P = 0.003 | 0.522 (0.332, 0.820) | P = 0.005 |
| Change in PA↓ (day 7–day 1) | 1.683 (1.077, 2.629) | P = 0.022 | 1.323 (0.836, 2.094) | NS |
MCT, medium chain triglycerides; LCT, long chain triglycerides; HR, hazard ratio; CI, confidence interval; RBP, retinol binding protein; TNF, tumour necrosis factor; PN, parenteral nutrition, PA, prealbumin.
NS, no statistically significant correlation (P > 0.05).
Figure 7.
Kaplan-Meier estimates of the probability of survival at 90 days between patients with acute paraquat poisoning treated with 0.4 g/kg lipofundin 20% MCT/LCT-based emulsion plus 0.2 g/kg ω-3 fish-oil emulsion (intervention group) or 0.6 g/kg lipofundin 20% MCT/LCT-based emulsion (control group): Hazard ratio 0.522, 95% confidence interval 0.332, 0.820 (P = 0.005)
Discussion
The high mortality rates associated with paraquat poisoning are due largely to a lack of effective treatments.10,37,38 Paraquat ingestion induces ROS generation, leading to lipid peroxidation-induced cellular damage and the activation of many types of inflammatory cytokines. The activation of this cascade may lead to subsequent damage to the mitochondria and cell apoptosis at the organ level. These effects may result in conditions such as pneumonitis and fibrosis of the lungs, and renal and liver damage.11,28,39–41 TNF-α is considered a promising inflammatory marker in the early stages of acute lung injury induced by paraquat poisoning. CRP, with a half-life of 4–6 h, is a key acute-phase protein, and CRP levels generally return to normal within 3–7 days of the cessation of inflammation.42 In the present study, following the initiation of ω-3 fish-oil emulsion treatment, TNF-α levels in the intervention group gradually decreased and were significantly lower than those in the control group at days 4 and 7. In contrast, TNF-α levels showed no significant decrease in the control group. Intervention-group CRP levels were increased at day 4, then returned to baseline levels on day 7 and fell below baseline levels on day 10. In the control group, however, CRP levels remained increased on day 7, and had not decreased below baseline levels by day 10. The initial increased levels of TNF-α and CRP were normalized by the infusion of ω-3 fish-oil emulsion-based PN treatment in the intervention group, suggesting protective effects of ω-3 fish-oil emulsion-based PN against paraquat-induced inflammation in cases of acute paraquat poisoning. Lipid emulsions containing ω-3 fatty acids have been shown to attenuate inflammatory activity via inhibiting the toll-like receptor (TLR)4 and nucleotide-binding oligomerization domain (NOD) signalling pathways, which subsequently activate NF-ĸβ signalling, downregulate the secretion of pro-inflammatory cytokines, such as TNF-a, IL-6, and CRP, and positively affect patient prognosis.43,44 Other research has demonstrated that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) can scavenge intracellular ROS and attenuate oxidative stress through upregulating the nuclear factor erythroid 2-related factor 2 (NRF2)-mediated antioxidant response.45,46
The recent discovery of specialized pro-resolving mediators (SPMs), including lipoxins, resolvins, protectins and maresins, has indicated the anti-inflammatory mechanism of ω-3 fatty acids.32 SPMs in the host govern the extent and duration of the inflammatory response, which is related to the patient’s prognosis.47 Intravenous administration of ω-3 fatty acids affects SPM levels in plasma, and this may be associated with better outcomes in acutely ill patients.33
Plasma PA and RBP have short half-lives (1/2 day) and are often used to measure changes in the nutritional state over short periods of time.21,22 The negative association between serum PA levels and clinical outcomes has been demonstrated in many types of diseases.48 In the current study, according to nutritional status based on RBP and PA levels, PA was not markedly increased in the two groups following initiation of treatment, which may be partly explained by the relatively small dosage of ω-3 fatty acids or the short period of administration. However, hepatic protein synthesis is also influenced by acute inflammation, which decreases PA production.49
In the present study, RBP levels in the intervention group had recovered to baseline levels on day 7, but remained low in the control group. A previous study revealed that patients receiving a combination emulsion had better liver tolerance than those receiving a soybean oil-based lipid emulsion alone.50 This finding may be due to the role of ω-3 fatty acids in increasing liver blood perfusion and reversing steatosis and cholestasis,51,52 and these effects may have contributed to reducing the hepatic damage from acute paraquat poisoning. These findings support the hypothesis that ω-3 fish-oil emulsion-based PN advantageously modifies the nutritional state compared with an MCT/LCT-based fat emulsion.
A total of 174 patients were included in the present study, with 95 survivors and 79 deaths equating to a total mortality rate of 45.4% during the 90-day follow-up. The mortality rate was lower in the intervention group than in the control group, and this decreased mortality may be due to the anti-inflammatory and protective hepatic effects of ω-3 fish-oil PN.
Paraquat concentration-time data are often used as tools to predict patient prognosis in cases of paraquat poisoning, however, this tool appears only to be useful within a relatively limited time window.53 For instance, when the time from ingestion to treatment is <4 h, this tool incorrectly predicts death or survival.54 In the present study, 66.67% of patients had a time to treatment of < 4 h, which may explain why baseline serum paraquat levels and time to treatment were not found to be correlated with paraquat prognosis.
Through Cox proportional hazards models analyses, ω-3 fish oil PN was found to be associated with a reduced risk of death following paraquat ingestion. Existing pleural effusion, RBP (day 7– day 1) decreases and TNF-α (day 7–day 1) increases were associated with an increased risk of death.
The present results may be limited by a number of factors. The cases included in the present study were obtained from a relatively small, single-centre sample, and thus, may not be truly representative of the wider population. In addition, there were potential sampling biases, as only 73% of those who met inclusion criteria were included in the final analysis. No placebo group was used due to ethical concerns of non-treatment.
In conclusion, the current study showed that, compared with MCT/LCT-based fat emulsion treatment, ω-3 fish-oil emulsion-based PN plus combination treatment is advantageous in attenuating the inflammatory response, modifying the nutritional state, and in significantly improving 90-day survival rates in patients with acute paraquat poisoning. Future well-designed prospective, multicentre, randomized-controlled trials are essential to build on the results of the current study in establishing the use of PN containing ω-3-rich fish oil as an effective paraquat poisoning therapy.
Acknowledgements
The authors thank the archive staff of the Second Hospital of Hebei Medical University for their assistance in collecting the data for this study. The authors also thank Rui Zhang, Liang Liu and Yingli Jin for their counsel regarding sample preparation and for their analytical support, and Yaqing An and Hao Xiao for their assistance in data collection.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008; 38: 13–71. [DOI] [PubMed] [Google Scholar]
- 2.Suntres Z.E. Role of antioxidants in paraquat toxicity. Toxicology 2002; 180: 65–77. [DOI] [PubMed] [Google Scholar]
- 3.Yu G, Kan B, Jian Xet al. A case report of acute severe paraquat poisoning and long-term follow-up. Exp Ther Med 2014; 8: 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Wu Q, Chu Tet al. High-dose acute exposure of paraquat induces injuries of swim bladder, gastrointestinal tract and liver via neutrophil-mediated ROS in zebrafish and their relevance for human health risk assessment. Chemosphere 2018; 205: 662–673. [DOI] [PubMed] [Google Scholar]
- 5.Esrefoglu M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon 2012; 12: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev 2004; 9: 43–51. [DOI] [PubMed] [Google Scholar]
- 7.He F, Xu P, Zhang J, et al. Efficacy and safety of pulse immunosuppressive therapy with glucocorticoid and cyclophosphamide in patients with paraquat poisoning: A meta-analysis. Int Immunopharmacol 2015; 27: 1–7. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CW, Lin JL, Lin-Tan DTet al. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One 2012; 7: e48397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med 2008. 11: 387–391. [PubMed] [Google Scholar]
- 10.Sun IO, Lee KY. Cyclophosphamide dose: how much is needed to win the war against paraquat poisoning? Korean J Intern Med 2013; 28: 410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol 2011; 72: 745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol 2014; 140: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 13.Keski-Nisula J, Pesonen E, Olkkola KTet al. High-dose methylprednisolone has no benefit over moderate dose for the correction of Tetralogy of Fallot. Ann Thorac Surg 2016. 102: 870–876. [DOI] [PubMed] [Google Scholar]
- 14.Varan B, Tokel K, Mercan Set al. Systemic inflammatory response related to cardiopulmonary bypass and its modification by methyl prednisolone: high dose versus low dose. Pediatr Cardiol 2002; 23: 437–441. [DOI] [PubMed] [Google Scholar]
- 15.Dejager L, Vandevyver S, Petta Iet al. Dominance of the strongest: inflammatory cytokines versus glucocorticoids. Cytokine Growth Factor Rev 2014; 25: 21–33. [DOI] [PubMed] [Google Scholar]
- 16.Chen HH, Lin JL, Huang WHet al. Spectrum of corrosive esophageal injury after intentional paraquat or glyphosate-surfactant herbicide ingestion. Int J Gen Med 2013; 6: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubenoff R, Roubenoff RA, Preto Jet al. Malnutrition among hospitalized patients. A problem of physician awareness. Arch Intern Med 1987; 147: 1462–1465. [PubMed] [Google Scholar]
- 18.Seo JY, Kang KJ, Kang HSet al. Corrosive esophagitis caused by ingestion of picosulfate. Clin Endosc 2015; 48: 66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherukuri H, Pramoda K, Rohini D, et al. Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicol Int 2014; 21: 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn GL, Bistrian BR, Maini BSet al. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr 1977; 1: 11–22. [DOI] [PubMed] [Google Scholar]
- 21.Helms RA, Dickerson RN, Ebbert MLet al. Retinol-binding protein and prealbumin: useful measures of protein repletion in critically ill, malnourished infants. J Pediatr Gastroenterol Nutr 1986; 5: 586–592. [PubMed] [Google Scholar]
- 22.Devoto G, Gallo F, Marchello Cet al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem 2006; 52: 2281–2285. [DOI] [PubMed] [Google Scholar]
- 23.Bharadwaj S, Gohel T, Deen OJet al. Fish oil-based lipid emulsion: current updates on a promising novel therapy for the management of parenteral nutrition-associated liver disease. Gastroenterol Rep (Oxf) 2015; 3: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khor BS, Liaw SJ, Shih HCet al. Randomized, double blind, placebo-controlled trial of fish-oil-based lipid emulsion infusion for treatment of critically ill patients with severe sepsis. Asian J Surg 2011; 34: 1–10. [DOI] [PubMed] [Google Scholar]
- 25.Teitelbaum DH, Guenter P, Griebel D, et al. Proceedings from FDA/A.S.P.E.N. Public workshop: clinical trial design for intravenous fat emulsion products, October 29, 2013. JPEN J Parenter Enteral Nutr 2015; 39: 768–786. [DOI] [PubMed] [Google Scholar]
- 26.Heller AR. Intravenous fish oil in adult intensive care unit patients. World Rev Nutr Diet 2015; 112: 127–140. [DOI] [PubMed] [Google Scholar]
- 27.Heller AR, Rössler S, Litz RJet al. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med 2006; 34: 972–979. [DOI] [PubMed] [Google Scholar]
- 28.Berger MM, Delodder F, Liaudet Let al. Three short perioperative infusions of n-3 PUFAs reduce systemic inflammation induced by cardiopulmonary bypass surgery: a randomized controlled trial. Am J Clin Nutr 2013; 97: 246–254. [DOI] [PubMed] [Google Scholar]
- 29.Manzanares W, Langlois PL, Lemieux Met al. Fish oil-containing emulsions: when fat seems to improve clinical outcomes in the critically ill. JPEN J Parenter Enteral Nutr 2016; 40: 305–307. [DOI] [PubMed] [Google Scholar]
- 30.Vollmar B, Bauer C, Menger MD. n-3 polyunsaturated fatty acid-enriched diet does not protect from liver injury but attenuates mortality rate in a rat model of systemic endotoxemia. Crit Care Med 2002; 30: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 31.Mayer K, Gokorsch S, Fegbeutel Cet al. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J Respir Crit Care Med 2003; 167: 1321–1328. [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014; 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molfino A, Amabile MI, Monti M, et al. Omega-3 polyunsaturated fatty acids in critical illness: anti-inflammatory, proresolving, or both? Oxid Med Cell Longev 2017; 2017: 5987082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martindale RG, Warren MM, McClave SA. Does the use of specialized proresolving molecules in critical care offer a more focused approach to controlling inflammation than that of fish oils? Curr Opin Clin Nutr Metab Care 2016; 19: 151–154. [DOI] [PubMed] [Google Scholar]
- 35.Lee TH, Hoover RL, Williams JDet al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med 1985; 312: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Kawasaki N, Tamura Tet al. In vitro adsorption characteristics of paraquat and diquat with activated carbon varying in particle size. Bull Environ Contam Toxicol 2000; 64: 377–382. [DOI] [PubMed] [Google Scholar]
- 37.Addo E, Poon-King T. Leucocyte suppression in treatment of 72 patients with paraquat poisoning. Lancet 1986; 1: 1117–1120. [DOI] [PubMed] [Google Scholar]
- 38.Wu WP, Lai MN, Lin CHet al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One 2014; 9: e87568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson DL, Cox MM. Lehninger principles of biochemistry. 6th ed New York: W H Freeman and Co, 2013, pp.744–745. [Google Scholar]
- 40.Giustarini D, Dalle-Donne I, Tsikas Det al. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci 2009; 46: 241–281. [DOI] [PubMed] [Google Scholar]
- 41.Ranjbar A, Pasalar P, Sedighi Aet al. Induction of oxidative stress in paraquat formulating workers. Toxicol Lett 2002; 131: 191–194. [DOI] [PubMed] [Google Scholar]
- 42.Dellinger RP, Levy MM, Carlet JMet al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008; 36: 296–327. [DOI] [PubMed] [Google Scholar]
- 43.Honda KL, Lamon-Fava S, Matthan NRet al. EPA and DHA exposure alters the inflammatory response but not the surface expression of Toll-like receptor 4 in macrophages. Lipids 2015; 50: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen F, Liu Y, Zhu Het al. Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun 2013; 19: 504–515. [DOI] [PubMed] [Google Scholar]
- 45.Sakai C, Ishida M, Ohba Het al. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One 2017; 12: e0187934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimazawa M, Nakajima Y, Mashima Yet al. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res 2009; 1251: 269–275. [DOI] [PubMed] [Google Scholar]
- 47.Dalli J, Colas RA, Quintana Cet al. Human sepsis eicosanoid and proresolving lipid mediator temporal profiles: correlations with survival and clinical outcomes. Crit Care Med 2017; 45: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JD, Xu XF, Han Jet al. Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study. HPB (Oxford) 2018. Epub ahead of print 3 August 2018. doi: 10.1016/j.hpb.2018.06.1803. [DOI] [PubMed]
- 49.Fuhrman MP. The albumin-nutrition connection: separating myth from fact. Nutrition 2002; 18: 199–200. [DOI] [PubMed] [Google Scholar]
- 50.Mertes N, Grimm H, Fürst Pet al. Safety and efficacy of a new parenteral lipid emulsion (SMOFlipid) in surgical patients: a randomized, double-blind, multicenter study. Ann Nutr Metab 2006; 50: 253–259. [DOI] [PubMed] [Google Scholar]
- 51.Wei Z, Wang W, Chen Jet al. A prospective, randomized, controlled study of ω-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumors. Nutr J 2014; 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meisel JA, Le HD, de Meijer VEet al. Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J Pediatr Surg 2011; 46: 666–673. [DOI] [PubMed] [Google Scholar]
- 53.Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet 1984; 2: 1222–1223. [DOI] [PubMed] [Google Scholar]
- 54.Senarathna L, Eddleston M, Wilks MFet al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM 2009; 102: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]