Short abstract
We report a case of a 69-year-old patient with esophageal cancer and severe upper gastrointestinal bleeding during neoadjuvant radiochemotherapy who required mass transfusion followed by complex emergency procedures. Despite endoscopic stenting, the bleeding recurred, and thus emergency open surgery was required. Gastric wedge resection of the minor curvature necessitated by perforation caused by the endoscopic stent maneuver and duodenotomy with ligation of the gastroduodenal artery, as the cause of persistent intraluminal bleeding, were performed. The already prepared gastric conduit during the emergency operation did not become ischemic, even though the gastroduodenal artery, left gastric artery, and short gastric arteries were ligated during emergency surgery. After 2 months of recovery, a computed tomographic scan showed collateral perfusion of the conduit via the superior mesenteric artery. Therefore, a fully robotic (abdominal and thoracic) esophagectomy with pull-up of the gastric conduit was performed, with no post-surgical complications. The patient was discharged 10 days after the robotic esophagectomy. Six months after esophagectomy, the patient is in a good condition.
Keywords: Robotic surgery, esophageal cancer, Ivor–Lewis esophagectomy, gastric conduit, gastrointestinal bleeding, real-time fluoroscopy
Introduction
Open emergency surgery after gastric perforation during neoadjuvant radiochemotherapy in esophageal cancer with ligation of the arterial supply is a severe and complex condition. This condition often entails death or later inoperability for esophageal surgery. We present a patient who underwent fully robotic four-arm esophagectomy (RAMIE4)1 after previous open emergency surgery. This surgery included gastric wedge resection of the minor curvature necessitated by gastric perforation and ligation of the gastroduodenal artery owing to uncontrollable postpyloric ulcer bleeding. During the RAMIE4 procedure after the emergency operation, real-time fluoroscopy with rapid zooming and panning was extremely useful for performing controlled robotic re-dissection in the abdomen, while preserving the collateral blood supply.
Case report
A 69-year-old patient with recently diagnosed locally advanced adenocarcinoma of the distal esophagus (cT3N+), which was 30 to 35 cm distal from the upper incisor, received neoadjuvant radiochemotherapy (CROSS protocol).2 Enteral nutrition was ensured by an implanted jejunostomy catheter. After only 1 month of treatment with three cycles of chemotherapy and 12 fractions of radiation with 1.8 Gy each (total of 21.6 Gy), the patient was admitted to our hospital with upper gastrointestinal bleeding leading to cardiac circulatory arrest. Emergency endoscopy was performed after successful mechanical reanimation and mass transfusion. With the assumption of bleeding of the tumor, an esophageal stent was placed during endoscopy to stop acute hemorrhage of the vulnerable esophageal tumor. However, because of recurrence of bleeding, an emergency surgery was necessary. The surgery revealed perforation of the minor curvature caused by the stent maneuver (Figure 1a). The open surgery further showed two acute complications. First, perforation of the smaller curvature of the stomach was observed through which the inserted esophageal stent was dislocated. The stent was removed from the stomach through the perforation and perforation of the stomach was closed via wedge resection, including ligation of the left gastric artery. This procedure required opening of the omentum majus and dissection of the short gastric and left gastric arteries including the lymph nodes to reach the perforation. After resection, the generated gastric sleeve was persistently filling with blood, which was the second major complication. A spurting arterial hemorrhage from a branch of the pancreaticoduodenal artery in the postpyloric antrum was located endoscopically during surgery. This required an additional duodenotomy with ligation of the gastroduodenal artery. Because the gastric sleeve showed no signs of hypoperfusion, total gastrectomy was not performed to allow for the possibility of a later gastric pull-up. During these procedures, the patient needed 28 units of packed red blood cells, 12 units of fresh frozen plasma, and five units of thrombocyte concentrate.
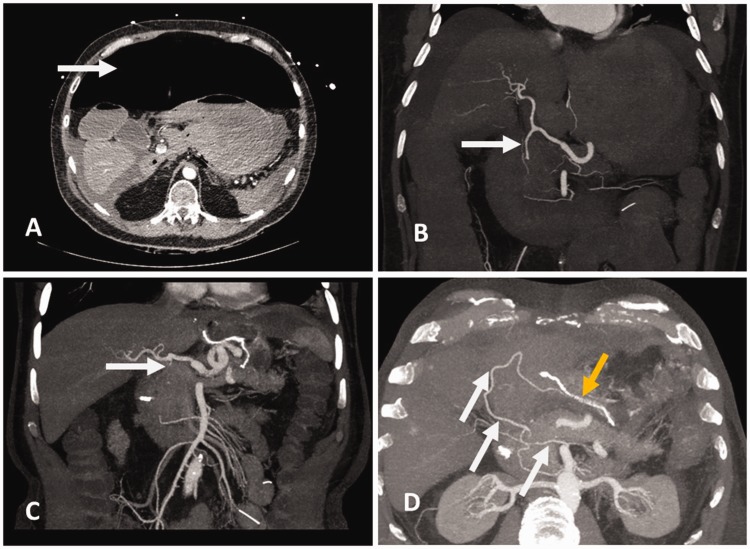
Figure 1.
Computed tomography before (a, b) and after the emergency operation (c, d). (a) Massive free abdominal air due to gastric perforation (white arrow) can be seen. (b) Before the emergency operation, the gastroduodenal artery showed good perfusion (white arrow). However, the patients’ anatomy was altered because of the massive amount of air and liquid in the abdomen causing the hepatic artery to travel a steep course. (c) After the surgery, the gastroduodenal artery was ligated (white arrow). (d) However, the gastric sleeve was supplied by collaterals coming from the superior mesenteric artery (white arrows). The yellow arrow indicates the stapler line from the emergency wedge resection.
In the postoperative course, the patient was monitored under intensive care conditions. On day 10 after surgery, symptomatic re-bleeding from the duodenal ulcer needed to be treated via endoscopic Hemospray application. The patient was still suffering from declining hemoglobin levels and continued to require catecholamines 17 days after surgery. Therefore, two metal clips were placed distal to the known ulcer at the location of oozing bleeding. The patient was discharged 34 days after surgery in a good condition, on an oral diet, and without any further bleeding signs. The patient received no additional neoadjuvant therapy.
A total of 40 days after the emergency intervention, computed tomography was performed. Computed tomography showed a missing gastroduodenal artery with contrasting of the right gastroepiploic artery by collaterals from the superior mesenteric artery (Figure 1b–d). Additionally, the left gastric artery was ligated when treating the gastric perforation. Preoperative endoscopy showed esophageal cancer, which was located 30 to 35 cm distal from the upper incisor.
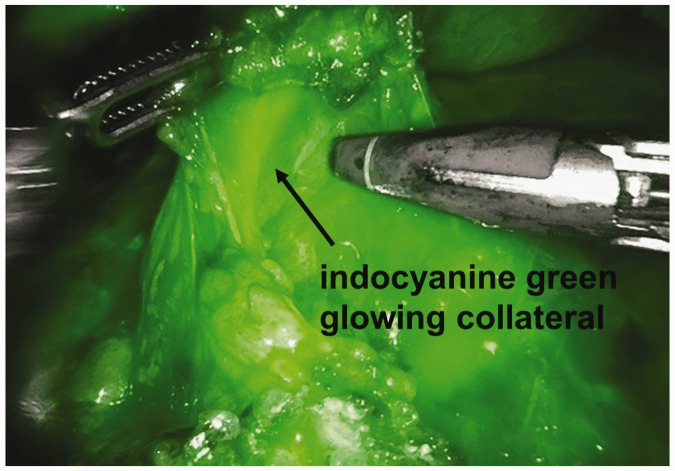
A total of 53 days after the emergency operation, we performed a fully robotic RAMIE4 with chronic ischemia-conditioned gastric sleeve pull-up and an intrathoracic circular stapled anastomosis that reached over the azygos vein. Precise dissection as a result of good magnification and real-time fluorescence imaging during RAMIE4 was of great benefit for better identification of the greater arcade, which was supplied mainly by collaterals. We were able to spare these collaterals when lysing the postoperative adhesions (Figure 2). By preserving the arterial support, obligatory perfusion of the gastric sleeve and the anastomosis was ensured (Figure 3).
Figure 2.
Identified collateral blood supply during the robotic abdominal part of fully robotic four-arm esophagectomy using real-time fluoroscopy.
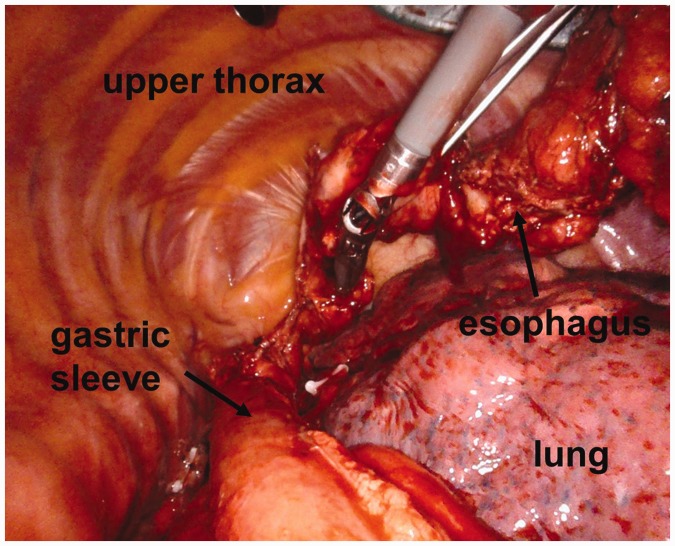
Figure 3.
Thoracoscopic view during fully robotic four-arm esophagectomy after gastric conduit pull-up.
The operation was tolerated well. At day 4, oral feeding was initiated and fully functioning on day 10. On the same day, 63 days after the emergency procedure, the patient was finally discharged with the histopathological stage of ypT3, ypN0 (0/28), L1, V0, pN0, R0 (stage IIB in the seventh International Union Against Cancer).
Ethical permission was not applied for because no study or treatment decision was involved. The patient provided written consent to publish this case report.
Discussion
To the best of our knowledge, this is the first report of performance of RAMIE4 after the complications of bleeding, emergency wedge resection, and loss of arterial blood supply. We showed that fully robotic minimally invasive esophagectomy is a preferable method for re-operating on complex cases. Despite the patient’s advanced age, extensive adhesion from the first surgery, and the gravity of the intervention, the patient showed no more complications after RAMIE4. In fact, the combination of precise and careful dissection with radical oncological resection enabled achievement of this outcome. Robotic-assisted surgery provides greater vision for the surgeon with a three-dimensional view and the possibility of panning and rapid zooming, and it also shows enormous precision of movement with seven grades of freedom. These features, in combination with the live three-dimensional fluorescence showing small vessels, improve handling in a narrow space with delicate structures, such as the collateral arteries. Furthermore, stabilization of the instruments reduces the surgeon’s tremor and imprecision.
However, whether these technical improvements in RAMIE4 are advantageous in complex cases is unknown. To date, there is no evidence for an improved long-term survival rate for patients who are operated on robotically. More data from more experienced surgeons are required to evaluate the benefit of RAMIE4. There have been some reports of fewer complications after several robotic-assisted operations, and these suggested a reduced learning curve for the surgeon.3,4 Apart from these considerations, there are probable risks of neoadjuvant therapy.5 One case report described gastric perforation as a rare complication during neoadjuvant chemoradiotherapy with 5-fluorouracil and oxaliplatin for gastric cancer.6 In the MAGIC trial, 250 patients received perioperative chemotherapy and no incidence of gastrointestinal perforation was reported.7 In the CROSS trial, one of 171 patients died, probably owing to an esophageal perforation, accompanied by major hemorrhage.8
Finally, the possibility of total gastrectomy because of the emergency nature of the situation with a following colonic interposition should be discussed. However, in our patient, the location of the gastric perforation was ideal for developing a tubularized conduit, and despite the vast gastric mobilization, its viability was clearly intact. Colonic interposition is still an operation with higher risk and lower postoperative quality of life, and therefore, was not the first choice of treatment.
Conclusion
In this report, we were able show that, despite major complications during neoadjuvant radiochemotherapy and the high risk of postoperative complications, fully robotic assisted minimally invasive Ivor–Lewis esophagectomy could be performed safely and successfully in our patient. Complex patients, who already have an increased risk for postoperative complications, can benefit from robotic surgery. The possibility of full control and higher surgical precision under real-time-fluoroscopy are helpful in re-operations with unclear or variant blood supply of the stomach, as reported in our case. However, a well-trained surgeon may still be required to manage these highly complex cases.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Grimminger PP, Hadzijusufovic E, Ruurda JP, et al. The daVinci Xi robotic 4-arm approach for robotic-assisted minimally invasive esophagectomy (RAMIE4). Thorac Cardiovasc Surg 2018; 66: 407–409. [DOI] [PubMed] [Google Scholar]
- 2.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev 2013; 5: Cd008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013; 27: 3339–3347. [DOI] [PubMed] [Google Scholar]
- 4.Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013; 26: 159–166. [DOI] [PubMed] [Google Scholar]
- 5.Leibl BJ, Vitz S, Schäfer W, et al. Adenocarcinoma of the esophagogastric junction: neoadjuvant radiochemotherapy and radical surgery: early results and toxicity. Strahlenther Onkol 2011; 187: 231–237. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Wu S, Kundra A, et al. Gastric perforation in a patient receiving neoadjuvant chemoradiotherapy. World J Oncol 2015; 6: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]