Short abstract
Objectives
This study aimed to directly measure pH in the lungs, determine lactate dehydrogenase (LDH), C-reactive protein (CRP), and glucose levels in serum and bronchoalveolar aspirate, and identify bacterial pathogens from bronchoalveolar fluid during acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Methods
We performed an observational, analytical case–control study from February 2015 to March 2017. We included 84 patients with AECOPD and 42 with stable chronic obstructive pulmonary disease (COPD). All participants underwent detailed medical anamnesis, a clinical examination, chest radiography, spirometry, an arterial blood gas test, bronchoscopy, bacterial culture, and serum/bronchiolar aspirate laboratory testing.
Results
The mean pH of bronchoalveolar fluid was significantly higher in patients with AECOPD than in patients with stable COPD. The mean lung pH value, bronchoalveolar and serum LDH levels, and serum CRP levels in patients with isolated bacteria were higher than those in patients without isolated bacteria in the AECOPD patient group. Lung pH values in patients with AECOPD were significantly correlated with bronchoalveolar LDH and glucose levels.
Conclusions
AECOPD is associated with local cell and tissue injury in the lungs, especially in the presence of bacterial pathogens, which is accompanied by a low systemic inflammatory response.
Keywords: Chronic obstructive pulmonary disease, disease exacerbation, C-reactive protein, lactate dehydrogenase, glucose, bronchoalveolar aspirate
Introduction
Chronic obstructive pulmonary disease (COPD) is an important global health issue that is predicted to be the third cause of death in the world by 2020. COPD is described as a chronic and progressive inflammatory lung disease, which causes persistent respiratory symptoms and airflow limitation.1–3 Development of COPD is related to smoking, air pollution, genetic factors, and infections. Increased levels of inflammatory mediators and cytokines are also observed in COPD as part of systemic inflammation.4
COPD is a progressive disease, and patients experience periods of increased COPD symptoms called exacerbations.5 Acute exacerbation of COPD (AECOPD) are periods of this disease with worsening of respiratory symptoms, according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD).6 Exacerbations are often triggered by respiratory pathogens, such as bacteria or viruses. AECOPD is usually accompanied by normal or slightly elevated blood test results, such as C-reactive protein (CRP) levels, the sedimentation rate, and lactate dehydrogenase (LDH) levels.
Recently, our research group showed that bronchoalveolar pH can also reflect the local inflammatory burden in patients with idiopathic pulmonary fibrosis (IPF) and those with gastroesophageal reflux.7,8 Lung pH has most commonly been investigated using non-invasive sampling methods of the lower respiratory tract in humans. However, direct measurement of lung pH from the peripheral branches of bronchi has not been performed in patients with AECOPD.
In this study, we aimed to examine systemic and local inflammatory processes in patients with AECOPD by determining inflammatory biomarkers, bronchoalveolar pH, and bacterial culture from bronchoalveolar fluid. We hypothesized that the local pH of patients with AECOPD is more alkaline compared with those with stable COPD, as well as more alkaline in bacteria-isolated versus non-isolated bacteria patients with AECOPD.
Methods
The study was performed at the University Hospital Center Split, Department of Pulmonary Diseases, from February 2015 to March 2017. The clinical diagnosis of AECOPD was determined by an experienced pulmonologist according to previously published GOLD criteria as described above.6 COPD was confirmed by spirometry, and it was defined by an forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) < 0.70 after inhalation of nebulized salbutamol (400 mcg), which proves permanent limitation of lung airflow. We included patients in whom exacerbation began no more than 3 days before admission to the Emergency Department. All patients were diagnosed with COPD in the moderate or severe stage (GOLD B, C, D) within 5 years before admission to hospital, by following the recommendations of GOLD.6 All patients who received antibiotics and/or systemic corticosteroids at least 2 months before admission were excluded from the study. Participants underwent detailed medical anamnesis, a clinical examination, chest radiography, spirometry, an arterial blood gas test, bronchoscopy, bacterial culture, and serum/bile acid laboratory testing. The study was granted approval by the University Hospital Center Split Ethics Committee. To be included in this study and for presentation of data in this paper, all of the participants signed an informed consent. All experimental procedures were performed following the Helsinki Declaration.
During admission, FVC and FEV1 values were obtained by a plethysmograph (Masterlab circa 2000; Jaeger, Würzburg, Germany). A blood gas electrolyte analyzer (GEM® Premier™ 3000; Instrumentation Laboratory, Lexington MA, USA) was used to perform arterial blood gas analysis. Blood samples were withdrawn anaerobically from the radial artery. The partial tension of arterial CO2 (PaCO2) and partial tension of arterial O2 (PaO2) were analyzed.
Before the beginning of antibiotic treatment, patients underwent a video-bronchoscopy procedure. For this procedure, we used a video-bronchoscope (Olympus BF 1T160; Olympus Corp., Tokyo, Japan). As preparation for the bronchoscopy procedure, the patients were sedated with 2% lidocaine local anesthesia. We used test strips (Multistix 10 SG; Bayer-Diagnostics, Leverkusen, Germany) to measure lung pH within the range of 4.5 to 8.0 as reported in our previous studies.7,8 Briefly, a piece of test strip was inserted by forceps for 15 to 20 seconds into the right lower lobe peripheral branch (DB9, DB10). The forceps with the strip were retracted from the working channel of the bronchoscope, and a pH value reading was obtained at the same moment. The color of strips changed from orange to green to blue in 0.5 pH value increments. The pH value was always read and verified by the same three medical staff, including one doctor and two assistants. The pH strip was advanced through a biopsy/suction channel of the fiber bronchoscope to avoid any contamination of the pH strip from the bronchial tree and other structures during the procedure. A catheter (18 cm long, 2 mm wide) was used for deep catheter aspiration to obtain aspirate from DB9 or DB10 peripheral bronchial branches. After the lumen of the catheter was completely filled with the aspirated fluid, the assistant used 1.5 mL of distilled water to extract the aspirate from the catheter. A minimum amount of distilled water was used to avoid any effect on inflammation markers, which were analyzed later.
Bronchoalveolar aspirate lactate dehydrogenase (BA-LDH), C-reactive protein (BA-CRP), and glucose (BA-Glu) levels were measured using an Architect c8000 clinical chemistry analyzer (Abbott Laboratories, Abbott Park, IL, USA) within 1 hour. Levels of these parameters were also measured in the serum of participants (S-LDH, S-CRP, and S-Glu, respectively). Finally, identification of microorganisms from the bronchoalveolar aspirate was performed according to standard methods.9 Bacteria were only regarded as clinically significant in case of reaching > 103cfu/mL. One pulmonologist (K.M.) with more than 30 years of bronchoscopic experience conducted all endoscopic procedures in all of the subjects. There were no periprocedural complications or serious adverse effects related to study procedures.
For statistical analysis, the Student’s t-test (analysis of variance) was used to compare group differences in quantitative variables (normally distributed data). The Mann–Whitney test was used for data that were not normally distributed. We also used Pearson’s correlation coefficient (r) to determine the correlation of bronchoalveolar pH with bronchoalveolar LDH levels, bronchoalveolar glucose levels, and FEV1. All analyses were conducted using Statistica 8 software (StatSoft Inc., Tulsa, OK, USA). A p value < 0.05 was considered statistically significant.
Results
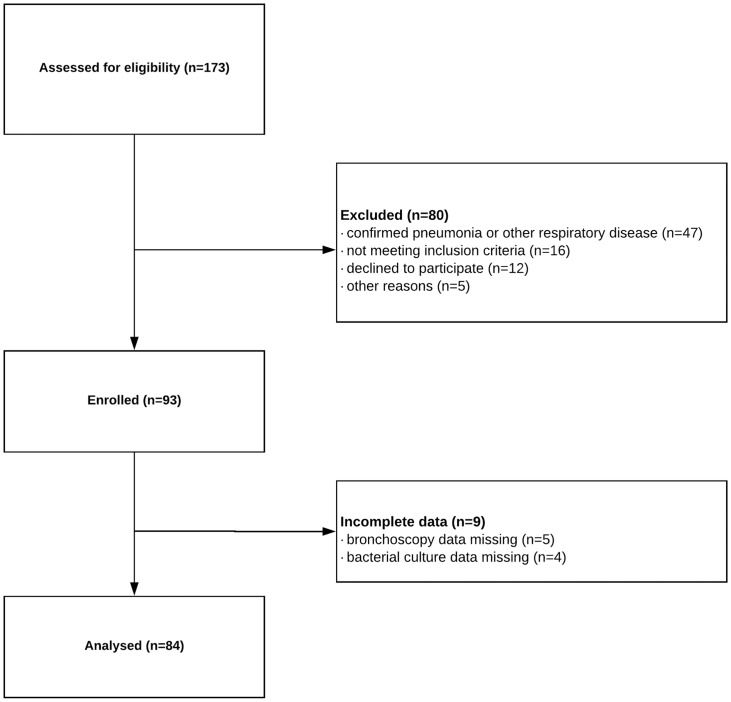
A total of 84 patients were included with AECOPD without concomitant pneumonia or other significant respiratory disease other than COPD (e.g., pulmonary fibrosis, asthma, or bronchiectasis) who were consecutively admitted to hospital. We also included 42 age- and sex-matched patients with stable COPD (Figure 1). Anthropometric data are presented in Table 1. FEV1 predicted was significantly lower in patients with AECOPD compared with those with stable COPD (p = 0.014, Table 1). PaO2 (p = 0.006) was significantly lower in patients with AECOPD, while PaCO2 (p = 0.019) was higher than that in patients with stable COPD (Table 1). The mean pH of bronchoalveolar fluid in patients with AECOPD was 6.89 ± 0.53, which was significantly higher than that of patients with stable COPD (6.21 ± 0.37, p < 0.001). The mean pH of bronchoalveolar fluid in patients without isolated bacteria was significantly lower than that in patients with isolated bacteria in the AECOPD patient group (p < 0.05, Table 2). No significant difference was found between lung pH in AECOPD patients according to smoking status (ex-smokers versus current smokers: 6.67 ± 0.67 and 6.99 ± 0.63, p = 0.238, respectively).
Figure 1.
A flow diagram of all assessed patients (included and excluded). This figure shows the number of patients assessed for eligibility and number of patients completing the study. Only patients with acute exacerbation of chronic obstructive disease, who had completed the whole study protocol, were included in the present analysis.
Table 1.
Anthropometrics, smoking status, pulmonary test function, and arterial blood gas data.
| AECOPD (n = 84) | Stable COPD (n = 42) | p | |
|---|---|---|---|
| Age (years) | 61.9 ± 8.1 | 62.4 ± 11.6 | 0.545 |
| Sex, male | 58 (69%) | 16 (64%) | 0.637 |
| BMI (kg/m2) | 25.4 ± 5.2 | 24.7 ± 3.7 | 0.604 |
| Current smoker | 30 (35.7%) | 14 (33.3%) | 0.625 |
| Pack years of smoking (current smokers) | 38.6 ± 21.4 | 35.2 ± 18.9 | 0.593 |
| Former smoker | 45 (53.6%) | 23 (54.8%) | 0.609 |
| Pack years of smoking (former smokers) | 34.1 ± 23.1 | 32.8 ± 20.4 | 0.534 |
| Non-smoker | 9 (10.7%) | 5 (11.9%) | 0.612 |
| FVC (L) | 2.37 ± 0.73 | 3.14 ± 0.96 | 0.331 |
| FVC (% predicted) | 60.1 ± 15.9 | 85.7 ± 13.9 | 0.104 |
| FEV1 (L) | 1.27 ± 0.41 | 2.07 ± 0.68 | 0.156 |
| FEV1 (% predicted) | 41.02 ± 11.5 | 79.4 ± 11.2 | 0.014 |
| FEV1/FVC (%) | 53.58 ± 14.4 | 66.52 ± 4.92 | 0.215 |
| PaO2 (kPa) | 8.6 ± 1.5 | 12.1 ± 0.61 | 0.006 |
| PaCO2 (kPa) | 5.7 ± 0.9 | 4.52 ± 0.39 | 0.019 |
| Arterial blood pH | 7.29 ± 0.3 | 7.47 ± 0.4 | 0.052 |
Data are presented as mean ± standard deviation or n (%). AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide. Statistical significance for group comparisons was tested using the Student’s t-test or one sample t-test.
Table 2.
Bronchoalveolar pH and other variables in bronchoalveolar fluid and serum in patients with AECOPD versus those with stable COPD.
| Bacteria isolated in AECOPD |
Stable COPD controls (n = 42) | ||
|---|---|---|---|
| No (n = 24) | Yes (n = 60) | ||
| Lung pH | 6.31 ± 0.55 | 7.13 ± 0.52* | 6.21 ± 0.37‡ |
| BA-LDH (U/L) | 361 ± 250 | 1381 ± 1621* | 124 ± 50†,‡ |
| S-LDH (U/L) | 173 ± 36 | 188 ± 24* | 160 ± 25‡ |
| BA-CRP (mg/L) | 0.17 ± 0.34 | 0.14 ± 0.26 | 0.12 ± 0.11 |
| S-CRP (mg/L) | 16.8 ± 11.6 | 29.4 ± 11.2* | 4.3 ± 2.6†,‡ |
| BA-Glu (mmol/L) | 1.6 ± 0.7 | 0.9 ± 0.7* | 1.6 ± 0.6‡ |
| S-Glu (mmol/L) | 5.8 ± 0.7 | 6.1 ± 1.4 | 5.6 ± 1.2‡ |
Data are presented as mean ± standard deviation. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; BA-LDH, bronchoalveolar lactate dehydrogenase; S-LDH, serum lactate dehydrogenase; BA-CRP, bronchoalveolar C-reactive protein; S-CRP, serum C-reactive protein; BA-Glu, bronchoalveolar glucose; S-Glu, serum glucose. *p < 0.05, non-isolated bacteria versus isolated bacteria; †p < 0.05, non-isolated bacteria versus stable COPD controls; ‡p < 0.05, isolated bacteria versus stable COPD controls.
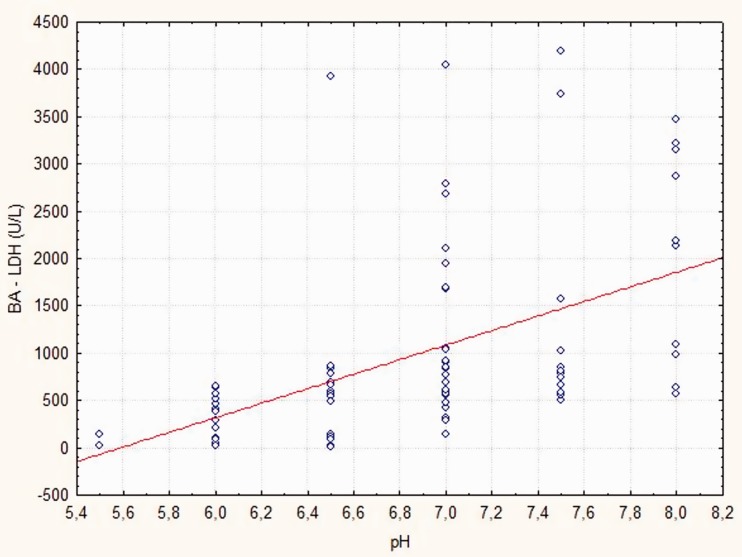
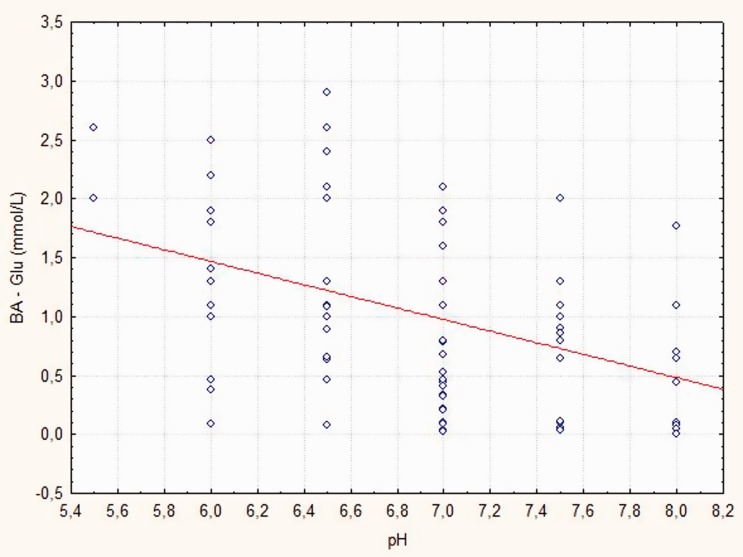
BA-LDH, S-LDH, and S-CRP levels were significantly lower in patients without isolated bacteria compared with those with isolated bacteria in the AECOPD patient group (p < 0.05; p = 0.042; p = 0.028, respectively) (Table 2). BA-Glu levels were significantly higher in patients without isolated bacteria compared with those with isolated bacteria in the AECOPD patient group (p = 0.014). No significant differences were found in BA-CRP and S-Glu levels between the AECOPD groups (Table 2). Patients with AECOPD had higher BA-LDH and S-CRP levels compared with patients with stable COPD, and this difference was more pronounced if a bacterial pathogen was isolated (all p < 0.05, Table 2). Significant differences were found in lung pH, and S-LDH, BA-Glu, and S-Glu levels (p < 0.05, p < 0.05, p = 0.012, and p = 0.032) only in patients with isolated bacteria and AECOPD compared with patients with stable COPD (Table 2). Lung pH values were significantly correlated with BA-LDH levels (r = 0.629, p < 0.001) and BA-Glu levels (r = 0.573, p < 0.001) (Figures 2, 3). Lung pH was not correlated with FEV1 in the AECOPD patient group (r = −0.198, p = 0.070). Furthermore, lung pH was not correlated with FEV1 in the stable COPD patient group (r= −0.093, p = 0.402).
Figure 2.
Relationship between bronchoalveolar lactate dehydrogenase (BA-LDH) and pH at acute exacerbation of chronic obstructive pulmonary disease.
Figure 3.
Relationship between bronchoalveolar glucose (Ba-Glu) levels and pH at acute exacerbation of chronic obstructive pulmonary disease.
In 60 of 84 (71%) patients the following bacteria were isolated: Haemophilus influenzae (n = 15, 25%), Streptococcus pneumoniae (n = 11, 18%), Moraxella catarrhalis (n = 8, 13%), Pseudomonas aeruginosa (n = 6, 10%), Escherichia coli (n = 4, 7%), Klebsiella pneumoniae, Methicillin-sensitive Staphylococcus aureus, Proteus mirabilis, and anaerobic bacteria (n = 3, 5%), Enterobacter cloacae and Serratia marcescens (n = 2, 3%). Lung pH and levels of bronchoalveolar and serum parameters for the five most frequently isolated bacterial pathogens are shown in Table 3.
Table 3.
Lung pH and levels of parameters measured in bronchoalveolar fluid and serum for the five most frequently isolated bacterial pathogens.
| Lung pH | BA-LDH | S-LDH | BA-CRP | S-CRP | BA-Glu | S-Glu | |
|---|---|---|---|---|---|---|---|
| Haemophilus influenzae (n = 15) | 7.37 ± 0.64 | 2912 ± 2505 | 186 ± 19 | 0.03 ± 0.06 | 27.3 ± 13.5 | 0.9 ± 0.7 | 6.3 ± 1.4 |
| Streptococcus pneumoniae (n = 11) | 7.14 ± 0.56 | 959 ± 829 | 197 ± 28 | 0.21 ± 0.33 | 17.8 ± 8.7 | 1.1 ± 0.6 | 6 ± 1.4 |
| Moraxella catarrhalis (n = 8) | 7.25 ± 0.27 | 859 ± 133 | 186 ± 26 | 0.12 ± 0.20 | 15.1 ± 8.6 | 0.8 ± 0.7 | 5.8 ± 1.2 |
| Pseudomonas aeruginosa (n = 6) | 7.01 ± 0.45 | 1457 ± 1256 | 198 ± 21 | 0.09 ± 0.19 | 18.7 ± 9.8 | 0.8 ± 0.3 | 6.1 ± 1 |
| Escherichia coli (n = 4) | 6.75 ± 0.29 | 673 ± 121 | 186 ± 38 | 0.23 ± 0.37 | 27.1 ± 14.7 | 1.1 ± 1.3 | 6.2 ± 0.9 |
Data are presented as mean ± standard deviation. BA-LDH, bronchoalveolar lactate dehydrogenase; S-LDH, serum lactate dehydrogenase; BA-CRP, bronchoalveolar C-reactive protein; S-CRP, serum C-reactive protein; BA-Glu, bronchoalveolar glucose; S-Glu, serum glucose.
Discussion
This study showed that pH of bronchoalveolar fluid in patients with AECOPD was more alkaline compared with that in patients with stable COPD. Furthermore, the mean lung pH value in patients with isolated bacteria was higher than that in patients without isolated bacteria in the AECOPD patient group. These findings confirmed our hypothesis. In patients with stable COPD, the mean bronchoalveolar pH was 6.21 ± 0.37 and in patients with AECOPD it was 6.89 ± 0.53, while pH was even more alkaline in the presence of bacteria. We speculate that the inflammatory process accompanied by bacterial metabolites contributed to the increase in pH. Previous studies have shown that sputum in patients with chronic bronchitis is alkaline (7.6 and 7.8)10 and that the lower airway mucus pH is alkaline in patients with cystic fibrosis.11 Lung pH in patients with pneumonia is approximately 7.3,12 and patients with rhinitis have nasal mucosa pH values ranging from 7.2 to 8.3.13,14 These studies indicate that there is an association between alkaline mucosal pH and inflamed respiratory tract tissue. Our findings of arterial blood pH showed that there was a 3% lower arterial blood pH in patients with AECOPD compared with those with stable COPD, which is consistent with findings by Grumelli.15
Alkalinity in the lungs contributes to local tissue damage by promoting cellular injury, apoptosis, necrosis, and inflammation.10 In our study, amore alkaline bronchoalveolar pH values were significantly correlated with higher BA-LDH values, which indicated more intense tissue damage. Higher lung pH in exacerbation contributes to surfactant structural changes, which can result in ventilatory problems (microatelectasis). This could explain fluctuations in the patient’s state.11 To date, lung pH has most commonly been investigated in exhaled breath condensate (EBC), which represents a sampling method of the lower respiratory tract that is not invasive.12 Several studies have shown that EBC pH is more acidic in patients with AECOPD/COPD compared with the healthy population.13,14 However, there are difficulties encountered in sampling and analysis of EBC. Detection and quantification of biomarkers in EBC, including cytokines, nitric oxide and pH, are compromised by many factors. These factors include the condenser type, cigarette smoking, ambient temperature, contamination of saliva, indoor humidity, liquid intake, volatile oral contaminants, and exhaled carbon dioxide as potential confounders, especially in patients with acute or chronic hypercapnic pulmonary insufficiency, which is often present in AECOPD and even in stable COPD.9,16–20 EBC is only used in experimental conditions and is not validated for use in clinical practice. Based on these findings, we consider that direct bronchoalveolar fluid sampling provides a more accurate assessment of local lung pH and inflammatory biomarkers compared with EBC sampling. Bronchoalveolar fluid sampling thus provides better information for evaluating local lung tissue damage.
To define different phenotypes of COPD and to assess the therapy response, biomarkers of respiratory tract inflammation and oxidative stress could be useful. Our measurements of LDH and CRP levels from serum are not valid local pulmonary inflammation and tissue damage indicators. Obtaining these biomarkers from bronchoalveolar aspirate may represent a more specific alternative.
LDH is a cytoplasmic enzyme that is found in the majority of body cells, including the lungs. Detection of higher than normal range LDH values is used to identify cellular damage or death.21 The highest BA-LDH values in the present study were observed in patients with isolated Haemophilus influenzae and Pseudomonas aeruginosa, which indicated the most active local inflammation, cell injury, and necrosis when the most pathogenic bacteria were present. If a bacterial pathogen was isolated, 7.4 times higher values of LDH were found in bronchoalveolar aspirate compared with serum, while if no bacterial pathogen was isolated, this ratio was only 2.1. In patients with stable COPD, mean S-LDH and BA-LDH levels were 160 ± 25 and 124 ± 50 UL, respectively. Compared with these values, S-LDH and BA-LDH in patients with non-isolated bacteria in the AECOPD patient group were 1.1 and 2.9 times higher, respectively; and if a bacterial pathogen was isolated, this ratio was 1.2 and 11.2, respectively. Higher BA-LDH levels in bronchoalveolar aspirate in patients with AECOPD, especially if a bacterial pathogen was isolated, compared with patients with stable COPD suggests the presence of local inflammation, cell injury, necrosis, and disease activity. Our finding indicates that BA-LDH is a relevant biomarker of tissue damage in the lungs and it may reliably be used to estimate bacterial infection in AECOPD. However, only 9% of patients had S-LDH values ≥220 U/L (above the reference range). This finding indicated that in patients with AECOPD, S-LDH levels were relatively normal, suggesting a low systemic inflammatory response.
CRP is a marker of inflammation, and CRP levels are increased during underlying inflammatory conditions.22 Some studies have shown an association of higher systemic CRP levels and other inflammatory biomarkers in patients with COPD compared with healthy patients.23–25 A study by Bircan et al.26 showed that increased CRP values in serum are an indicator of COPD exacerbation caused by a respiratory infection. In the present study, we found slight, but significantly higher S-CRP levels in patients with AECOPD compared with those with stable COPD, which were even higher if a bacterial pathogen was isolated. Recently, Peng et al.27 showed that, to identify the bacterial origin of AECOPD, the cut-off value of CRP was 19.65 mg/L. S-CRP appears to be a better indicator of a systemic inflammatory response in patients with AECOPD than S-LDH. However, BA-CRP levels were low in our study. Our findings suggest that BA-CRP levels should not be measured because their levels were low or even hardly detectable, with no difference in measured levels among the groups.
While blood glucose levels are not related to mortality, hospital stay duration, or re-hospitalization in patients with AECOPD,28 detection of glucose in bronchoalveolar aspirate is associated with the presence of pathogenic bacteria.29,30 In the present study, if bacteria were isolated in patients with AECOPD, BA-Glu levels were 1.8 times lower than those in patients without bacterial isolation, while no difference was found in S-Glu levels. Therefore, results for BA-Glu levels could also serve as a good predictor of bacterial presence.
We also investigated bacterial pathogens to evaluate the level of inflammation and pH values in relation to bacterial pathogens. This study was based on identification of bacterial etiology directly from bronchoalveolar aspirate, and not from sputum, as performed in previous studies.31,32 Bacterial etiology is predominant as an origin of inflammation in AECOPD. In this study, bacterial pathogens were found in 71% of patients in contrast to 24% in study by Boixeda et al.33 We believe that the reason for this difference between studies was due to direct analysis of bronchoalveolar fluid instead of sputum samples because sputum samples are vulnerable to sampling errors. We isolated 42 (70%) Gram-negative bacteria and 18 (30%) Gram-positive bacteria. Microbiological patterns were similar as shown by Siddiqi et al.34
There are two possible limitations in the present study. First, our study was performed on a relatively low number of subjects and thus the results have to be viewed with caution. Second, a semi-quantitative technique (pH test strips) was used to directly measure pH in the lungs, and ideally, a more precise method to determine pH would be better. However, we believe that the technique that we used is an acceptable substitute. Recently, this technique has been shown many times to be efficient in studies performed by our research group.12,13
In conclusion, this study shows a strong pathological process in the lungs of patients with COPD during AECOPD. This pathological process is present, even in cases with scarce clinical symptoms of deterioration or scant or no pathological findings in peripheral blood, such as CRP and LDH levels, especially in the presence of a bacterial pathogen. More alkaline bronchoalveolar pH values are a clear indicator of more intense tissue damage. There is no correlation between bronchoalveolar pH and FEV1 in patients with AECOPD and those with stable COPD. FEV1 measurement does not adequately express the effect of COPD on the quality of life of patients. Therefore, measurement of local bronchoalveolar pH and proposed inflammatory mediators in bronchoalveolar fluid in some patients could be useful as an additional test for diagnosing the severity of AECOPD. Although somewhat invasive, analyses of lung pH and inflammatory bronchoalveolar and serum biomarkers represent a feasible and easy method for repeated assessment of systemic and local inflammation in AECOPD. Further research is required to fully determine the importance and influence of bacterial culture, bronchoalveolar pH, and inflammatory biomarkers regarding clinical intervention and patient care in patients with AECOPD.
Acknowledgments
The authors wish to thank all volunteers who participated in this study, as well as Ivan Škopljanac and Ivana Šegrt for their knowledge, enthusiasm, and technical support.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Sidhaye VK, Nishida K, Martinez FJ. Precision medicine in COPD: where are we and where do we need to go? Eur Respir Rev 2018; 27: 180022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest 2011; 139: 165–173. [DOI] [PubMed] [Google Scholar]
- 3.Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology 2015; 20: 1160–1171. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138: 16–27. [DOI] [PubMed] [Google Scholar]
- 5.Agusti A, Faner R, Celli B, et al. Precision medicine in COPD exacerbations. Lancet Respir Med 2018; 6: 657–659. [DOI] [PubMed] [Google Scholar]
- 6.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017; 195: 557–582. [DOI] [PubMed] [Google Scholar]
- 7.Mise K, Capkun V, Jurcev-Savicevic A, et al. The influence of gastroesophageal reflux in the lung: a case-control study. Respirology 2010; 15: 837–842. [DOI] [PubMed] [Google Scholar]
- 8.Lozo Vukovac E, Lozo M, Mise K, et al. Bronchoalveolar pH and inflammatory biomarkers in newly diagnosed IPF and GERD patients: a case-control study. Med Sci Monit 2014; 20: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horváth I, Barnes PJ, Loukides S, et al. A European respiratory society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017; 49: 1600965. [DOI] [PubMed] [Google Scholar]
- 10.Adler K, Wooten O, Philippoff W, et al. Physical properties of sputum. 3. Rheologic variability and intrinsic relationships. Am Rev Respir Dis 1972; 106: 86–96. [DOI] [PubMed] [Google Scholar]
- 11.McShane D, Davies JC, Davies MG, et al. Airway surface pH in subjects with cystic fibrosis. Eur Respir J 2003; 21: 37–42. [DOI] [PubMed] [Google Scholar]
- 12.Steinmann E. La secretion bronchique et le pH. Bronches 1956; 6: 126–129. [Google Scholar]
- 13.England R, Homer J, Knight L, et al. Nasal pH measurement: a reliable and repeatable parameter. Clin Otolaryingol Allied Sci 1999; 24: 67–68. [DOI] [PubMed] [Google Scholar]
- 14.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 2006; 211: 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grumelli S. Choline triggers exacerbations of chronic obstructive pulmonary disease in patients infected with pseudomonas aeruginosa. Curr Respir Med Rev 2016; 12: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosias P. Methodological aspects of exhaled breath condensate collection and analysis. J Breath Res 2012; 6: 027102. [DOI] [PubMed] [Google Scholar]
- 17.Hüttmann EM, Greulich T, Hattesohl A, et al. Comparison of two devices and two breathing patterns for exhaled breath condensate sampling. PLoS One 2011; 6: e27467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koczulla AR, Noeske S, Herr C, et al. Ambient temperature impacts on pH of exhaled breath condensate. Respirology 2010; 15: 155–159. [DOI] [PubMed] [Google Scholar]
- 19.Vyas A, Zhang Q, Gunaratne S, et al. The effect of temperature on exhaled breath condensate collection. J Breath Res 2012; 6: 036002. [DOI] [PubMed] [Google Scholar]
- 20.Kullmann T, Barta I, Antus B, et al. Drinking influences exhaled breath condensate acidity. Lung 2008; 186: 263–268. [DOI] [PubMed] [Google Scholar]
- 21.Glick JH., Jr. Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase values in health and disease, and clinical evaluation of these tests by means of discriminantanalysis. Am J ClinPathol 1969; 52: 320–328. [DOI] [PubMed] [Google Scholar]
- 22.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biljak VR, Pancirov D, Cepelak I, et al. Platelet count, mean platelet volume and smoking status in stable chronic obstructive pulmonary disease. Platelets 2011; 22: 466–470. [DOI] [PubMed] [Google Scholar]
- 24.Schols AM, Buurman WA, Staal van den Brekel AJ, et al. Evidence for a relation between metabolic dearangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996; 51: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Torres JP, Cordoba-Lanus E, López-Aguilar C, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. EurRespir J 2006; 27: 902–907. [DOI] [PubMed] [Google Scholar]
- 26.Kawamatawong T, Apiwattanaporn A, Siricharoonwong W. Serum inflammatory biomarkers and clinical outcomes of COPD exacerbation caused by different pathogens. Int J Chron Obstruct Pulmon Dis 2017; 12: 1625–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng C, Tian C, Zhang Y, et al. C-reactive protein levels predict bacterial exacerbation in patients with chronic obstructive pulmonary disease. Am J Med Sci 2013; 345: 190–194. [DOI] [PubMed] [Google Scholar]
- 28.Islam EA, Limsuwat C, Nantsupawat T, et al. The association between glucose levels and hospital outcomes in patients with acute exacerbations of chronic obstructive pulmonary disease. Ann Thorac Med 2015; 10: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alsayed S, Marzouk S, Mousa E, et al. Bronchial aspirates glucose level as indicator for methicillin-resistant Staphylococcus aureus (MRSA) in intubated mechanically ventilated patients. J Egypt Soc Parasitol 2014; 44: 381–388. [DOI] [PubMed] [Google Scholar]
- 30.Mallia P, Webber J, Gill SK, et al. Role of airway glucose in bacterial infections in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 142: 815–823.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gump DW, Phillips CA, Forsyth BR, et al. Role of infection in chronic bronchitis. Am Rev Respir Dis 1976; 113: 465–474. [DOI] [PubMed] [Google Scholar]
- 32.Lambert HP, Stern H. Infective factors in exacerbations of bronchitis and asthma. Br Med J 1972; 3: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boixeda R, Rabella N, Sauca G, et al. Microbiological study of patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) and the usefulness of analytical and clinical parameters in its identification (VIRAE study). Int J Chron Obstruct Pulmon Dis 2012; 7: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqi A, Sethi S. Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis 2008; 3: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]