Short abstract
The generation of neuropathic pain is a complex dynamic process. Factors involved include one or more dysregulated sensory neural pathways; dysregulated activity of specific neurotransmitters, synapses, receptors and cognitive and emotional neural circuits; and the balance between degenerative and regenerative neural events. Risk factors include age, sex, cognition, emotions, genetic polymorphism, previous or ongoing chronic pain conditions and the use of certain drugs. Intense pain experienced before, during and after surgery is a risk factor for the development of central sensitization with consequent persistent postsurgery neuropathic pain. Blockade of N-methyl-D-aspartate receptors with appropriate drugs during and immediately after surgery may prevent persistent postsurgical pain. Most cancers, but particularly malignant metastases in bone, can induce persistent pain. Local factors including direct damage to sensory nerve fibres, infiltration of nerve roots by cancer cells and algogenic biological agents within the microenvironment of the tumour bring about central sensitization of dorsal horn neurons, characterized by neurochemical reorganization with persistent cancer pain. In this article, the clinical features, pathogenesis and principles of management of persistent postsurgery pain and cancer pain are briefly discussed.
Keywords: Inflammatory pain, neuropathic pain, persistent postsurgery pain, bone cancer pain, metastatic jaw cancer, oral squamous cell carcinoma, metastatic spinal cord compression, procedural pain
Introduction
Pain associated with inflammation or peripheral nerve injury is induced by tonic impulses in primary afferent nociceptors that are generated in response to noxious mechanical or chemical stimuli. In the dorsal horn of the spinal cord, the central terminals of primary afferent nociceptors release excitatory amino acid neurotransmitters including glutamate, aspartate and substance P. These biological agents bring about a long-term increase in the sensitivity of post-synaptic pain-transmitting neurons, with a consequent increase in their responsiveness to mechanical, thermal and chemical stimuli.1,2
A persistent barrage of neural signals, delivered by primary nociceptors, induces changes in the dorsal horn of the spinal cord with the development of neuronal structural and functional plasticity, facilitating hyperalgesia and spontaneous pain. These changes are characterized by central hyperexcitability, expansion of receptive fields, alterations in the expression of genes encoding neuropeptides, changes in the number and functional activity of sodium, calcium and potassium ion channels within the affected sensory neurons, and suppression or loss of pain-inhibitory mechanisms.3–7 Differences in central sensitization and processing of peripheral nociceptive signals, between comparable persons with comparable tissue damage, may partially explain the inter-personal variability in pain experience.8
Activation of postsynaptic N-methyl-D-aspartate (NMDA) receptors by glutamate, released in the dorsal horn from central terminals of primary afferent nociceptors, appears to be an important mechanism in central sensitization.9 Thus, blockade of tonic impulses in primary afferent nociceptors by either local anaesthesia, blockade of NMDA receptors with antagonistic agents, or by inhibition of excitatory inputs with opioid agents, singly or in combination, is likely to prevent central sensitization and reduce the development of neuropathic pain, including postoperative persistent pain and pain related to cancer.9,10 Untreated persistent neuropathic pain may alter neural connectivity and pain processing at various levels of the spinal cord dorsal horn and in various brain regions, causing maladaptive neural plasticity, which may promote the development of abnormal behaviours such as depression and anxiety.11
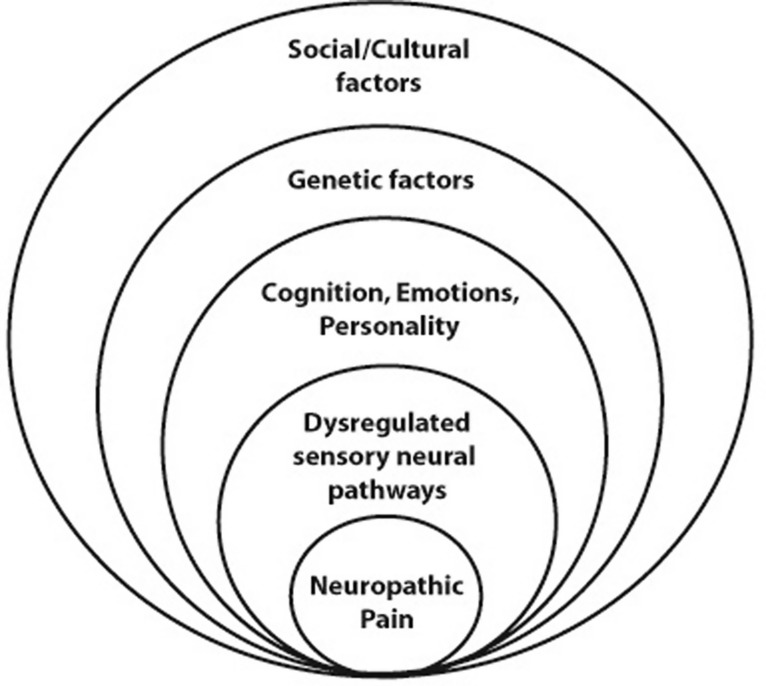
Descending modulatory pathways are neuronal circuits by which social, cultural, cognitive, emotional or attentional factors may moderate pain transmission at the spinal cord level (Figure 1).12,13 Descending inhibitory inputs have the capacity to dampen spinal cord hyperexcitability brought about by a persistent peripheral barrage of neuronal signals during inflammation.3,14–16 Thus, the inter-personal differences in pain processing and experience may also be associated with variable biopsychosocial and genetic factors and processes of pain modulation in the cortex (Figure 1).11,17–19
Figure 1.
Cognition, emotion and personality, which influence the pain experience, are determined by genetic, social and cultural factors; and dysregulation of sensory neural pathways, which promote the conduction of pain, may be the result of genetic factors influencing the functional activity of specific neurotransmitters, synapses, receptors and the balance between degenerative and regenerative neural processes.11,13 The position of factors in the diagram does not imply relative frequency or importance.
C-fibre primary afferent nociceptors can be divided into two subpopulations of neurons: peptide-rich C-fibres that produce calcitonin gene-related peptide (CGRP), substance P, and tyrosine kinase receptor A (trk-A), which upon activation by nerve growth factor (its cognate ligand), mediates the production of neuropeptides; and peptide-poor IB4+ C-fibres that produce Mas-related G protein-coupled receptor member D (encoded by MRGPRD), the P2X3 purinergic receptor and the glial cell line-derived neurotrophic factor (GDNF) receptor complex, which responds to GDNF.20,21
Peptide-poor C-fibres primarily innervate the skin and deeper soft tissues but are largely absent from the skeleton, which is chiefly innervated by peptide-rich C-fibers.21 Furthermore, while the skin is innervated by myelinated A-β sensory nerve fibres, which respond to touch and light pressure stimuli, this class of nerves is largely absent in bone, probably because no function evolved for them under physiological conditions.20
In contrast to those in the skin, sensory nerve fibres in bone are to a great extent silent, and are activated only by injury to the bone. The periosteum, cortical bone and bone marrow are all innervated by A-δ and peptide-rich C fibres, with innervation being richest in the periosteum followed by the bone marrow and then the bone cortex.20,22
Pain related to cancer treatment and diagnostic procedures (procedural pain) may intensify the existing cancer pain, increasing anxiety, depression, and the feeling of vulnerability, and interfering with every-day activity (work, social interactions, eating, and sleeping), thus significantly reducing quality of life.23 Surgery, chemotherapy, radiotherapy, biological therapy, supportive therapy (e.g. bisphosphonates, or granulocyte colony stimulating factor), and hormonal therapy are all known to cause pain; and biopsy, bone marrow aspiration, lumbar puncture, venepuncture, arterial puncture, and percutaneous venous catheter placement, may all be associated with different degrees of severity of pain adding to the patient’s overall pain.24
In the present article, various pathobiological mechanisms associated with persistent postsurgical pain and cancer pain are highlighted, with special reference to pain caused by malignant metastatic bone disease.
Persistent postsurgery pain
Uneventful healing usually occurs within weeks of major surgery, but after certain surgical procedures, including axillary lymph node dissection for breast cancer, limb amputation, lateral thoracotomy or coronary artery bypass surgery, some patients are left with severe persistent pain.25–27 Although the nature of the surgery does have an impact on the incidence of persistent postsurgery pain,11 the incidence and severity of the pain are not directly related to factors such as anaesthesia, duration of surgery, or extent of nerve injury, and it is not known why some patients develop persistent postsurgery pain, while others who have had comparable surgical procedures do not.19
The biopathological mechanisms determining the transition of acute postoperative pain, generated by inflammatory mediators in response to surgical trauma or to persistent postsurgery pain, remain unclear. It appears, however, that intense acute pain experienced immediately before and after surgery, the pain of surgery performed with inadequate analgesia, and severe operative trauma, are all associated with increased risk of persistent postsurgery pain.11,25,26,28,29
Comprehensive management of pre-, intra- and postoperative pain, the minimizing of surgical trauma and blockade of NMDA receptors during and immediately after surgery, are more effective than opioid agents in preventing central sensitization to noxious impulses and reducing such sensitization once it is established, and may significantly reduce the incidence and severity of persistent postsurgery pain.25,26,30,31 Thus, early robust multimodal pharmacological intervention for acute pain immediately following surgery may minimize the risk of persistent postsurgery pain.26 Gabapentinoids and serotonin-noradrenalin reuptake inhibitors are pharmacological agents which have the capacity to reduce central sensitization and to be beneficial in the treatment of persistent postsurgery pain.31
The combined use of an NMDA receptor blocker and an opioid has an additive effect in decreasing the incidence and intensity of postoperative hyperalgesia, but the use of opioids alone does not usually prevent the development of central sensitization and consequent persistent postsurgery pain.10,30 Furthermore, tolerance to opioids characterized by decreasing analgesic effects, and opioid-induced hyperalgesia are not uncommon when opioids alone are used. The biological explanation for this is that activation of µ receptors by opioids potentiates the effect of NMDA receptor activation by glutamate, at the level of the spinal cord dorsal horn, causing increased sensitization of nociceptive pathways with hyperalgesia. Thus, downregulation of NMDA receptor activity by NMDA receptor antagonists may prevent the development of both opioid tolerance and opioid-induced hyperalgesia.9,10,32,33
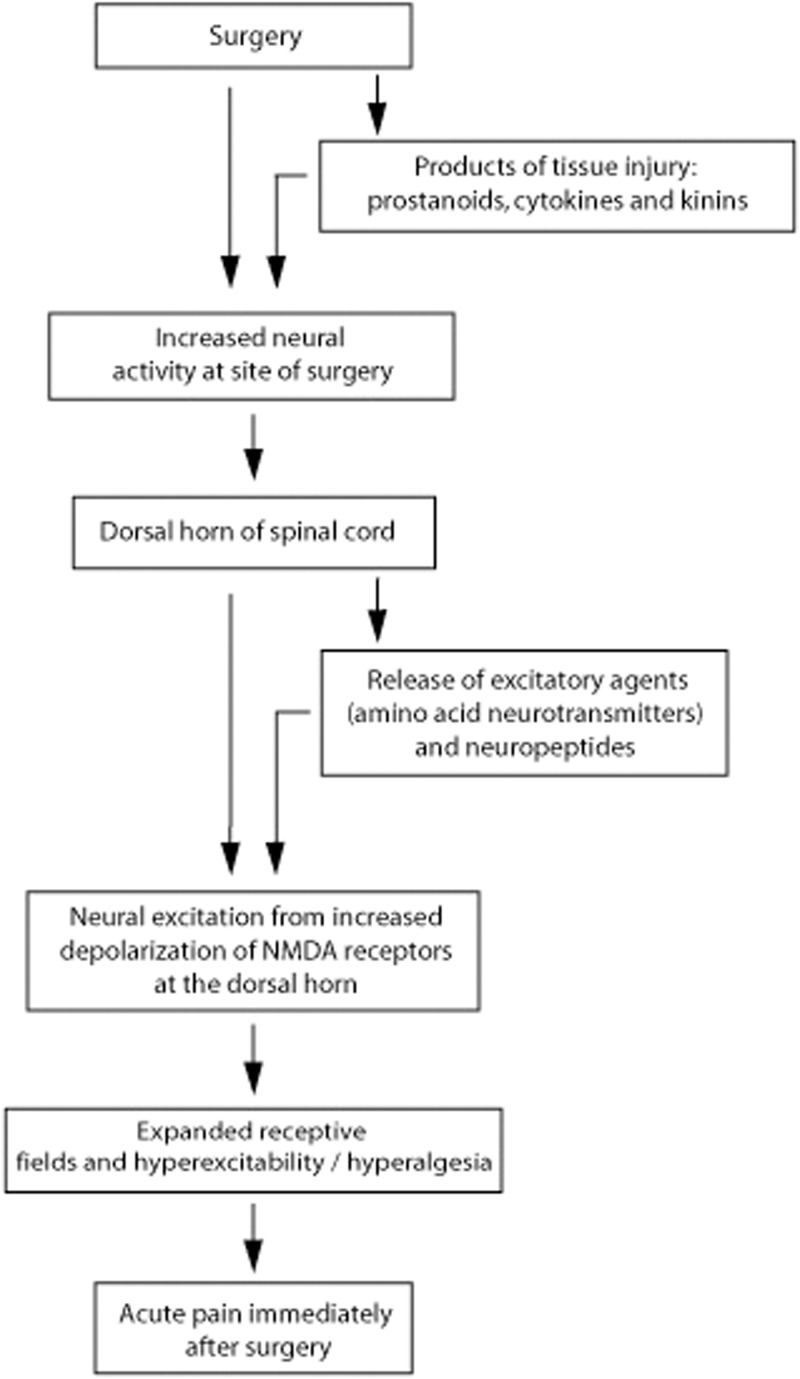
Acute pain immediately following surgery is caused by an acute inflammatory reaction, and this pain resolves with resolution of the inflammation,26 though hyperalgesia of the healed site to touch, pressure or heat may persist for some time.26,30 This relatively short-term acute pain immediately following surgery is caused by a barrage of action potentials generated in the peripheral afferent sensory neurons in response to inflammation (peripheral sensitization), resulting in the release of excitatory transmitters in the dorsal horn (Figure 2). Cyclooxygenase inhibitors and opioids effectively suppress this acute local postoperative inflammatory pain.26,34 However, sometimes after major surgery with significant damage to the peripheral nerves, spontaneous firing of action potentials with central neuronal sensitization may occur, resulting in persistent postsurgical neuropathic pain.26 This central sensitization is characterized by chemical, structural and functional changes, with decreased thresholds to mechanical stimuli and augmental temporal summation (‘wind-up’) associated with tonic impulses received from the surgical field (Figure 3). The temporal summation in the dorsal horn neurons, which represent an excitatory modulating process, is mediated by activation of NMDA receptors, and facilitated by substance P.10,11,27,35 This burning or aching persistent postsurgical pain usually lasts for three to six months after the surgical wound has healed and is not associated with any identifiable local or systemic cause other than the surgery.26,27,36
Figure 2.
Sequence of neural events caused by immediate post-surgery acute inflammatory reaction resulting in pain. Adapted from Dubner, 1997.3 The unavoidable nerve and other tissue damage caused by surgery results in release of prostanoids, cytokines and kinins, with increased neural activity at the site of injury (peripheral sensitization). This induces the release of excitatory agents and neuropeptides in the dorsal horn of the spinal cord causing reduction in neural threshold, hyperexcitability, increased responsiveness and prolonged after-effect. This results in hyperalgesia and expansion of receptive fields.26,31 The acute post-surgery pain is reversible and is maintained until the surgical wound has healed and the inflammation has resolved.26
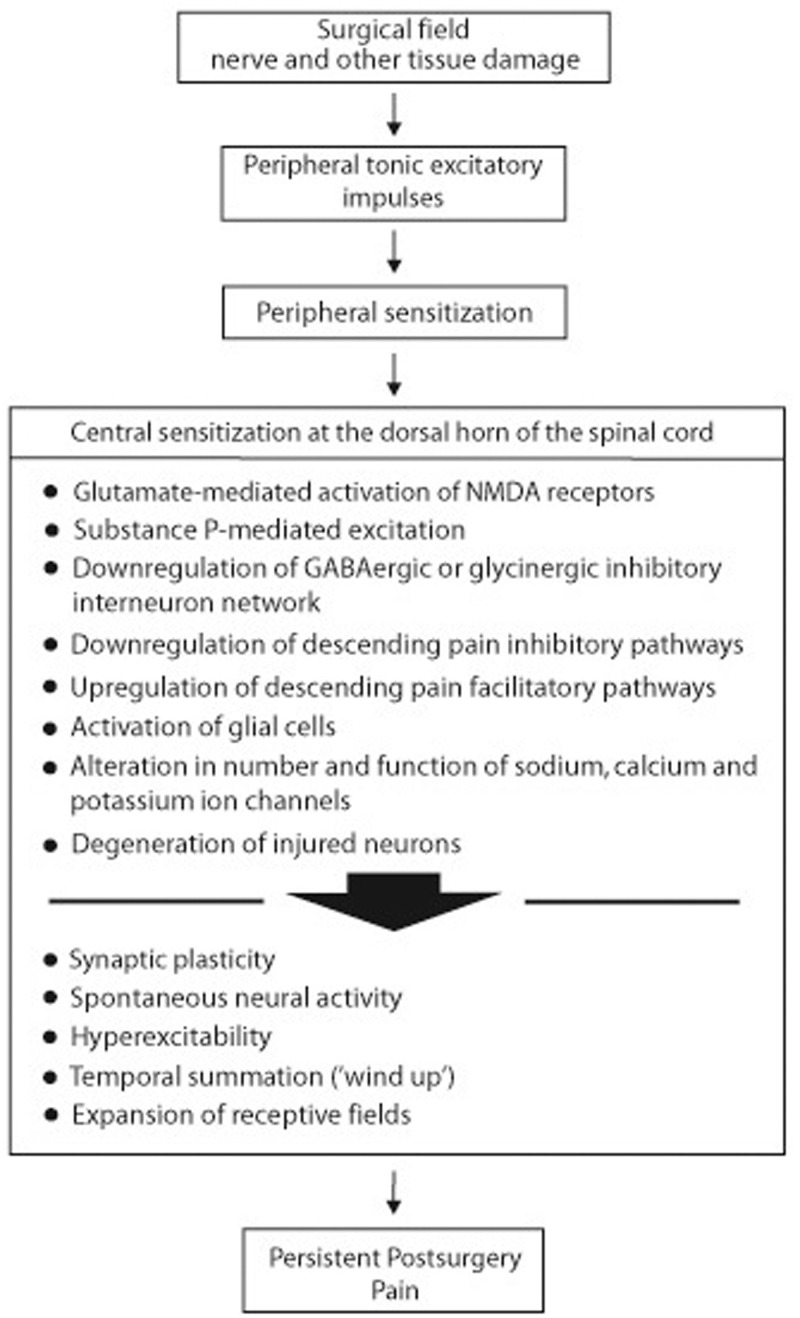
Figure 3.
Sequence of postsurgery neural events that may lead to persistent postsurgical pain. Adapted from Dubner, 1997.3 As a consequence of surgery-induced nerve and other tissue damage, a cascade of neural events is generated that includes increased neural activity at the surgical site, peripheral sensitization, and central sensitization with the mediation of persistent postsurgery neuropathic pain. Social, cultural and genetic factors, and cognition, emotions and personality all influence the pain experience (Figure 1). The combination of glutamate-mediated N-methyl-D-aspartate (NMDA) receptor activation, substance P-mediated excitation, downregulation of γ-aminobutyric acid (GABA)ergic or glycinergic inhibitory interneuron network, nerve-injury-induced downregulation of inhibitory and upregulation of excitatory pain descending circuits, activation of glial cells, alteration in the number and function of ion channels, synaptic plasticity, and failure of injured neurons to fully regenerate, disrupts synaptic circuits and neural connectivity at the level of the dorsal horn, leading to central sensitization. Unlike the reversible central sensitization associated with inflammatory pain that resolves with the resolution of the inflammatory process, central sensitization drives and maintains the persistent postsurgery neuropathic and cancer pain.11,26,31
Poor pain coping strategies, negative cognitive appraisal of pain, and to some extent anxiety and depression are associated with increased frequency of developing persistent postsurgery pain. The biological explanation is that these cognitive-emotional factors can downregulate pain inhibitory pathways and upregulate pain excitatory pathways, thus facilitating central sensitization. Therefore, cognitive-behavioural therapy and provision of relevant information about the neuroscience of pain, before surgery, may improve the centrally mediated cognitive-emotional regulation of pain, and improve pain coping strategies, which may reduce the experience of persistent postsurgery pain.37
For obscure reasons, persistent postsurgery pain is uncommon in the oral and maxillofacial regions.38 As reported by Kehlet et al., 2006,26 only about 10% of patients who have severe intraoperative nerve damage while undergoing mandibular osteotomy go on to develop persistent postsurgery neuropathic pain. This suggests that nerve injury in itself is not sufficient to cause persistent postsurgery pain, but in most cases, other factors such as genetic, cognitive and emotional predisposition are necessary for persistent postsurgery pain to develop.26,34 However, post-traumatic trigeminal neuropathy is not uncommon and is caused by iatrogenic injury to the lingual, mental or inferior alveolar nerves during third molar or implant surgery, or uncommonly, by factors such as needle injury during inferior alveolar nerve anaesthetic block, buried foreign material or broken instruments at the site of surgery, poor wound closure, infection, or by undetected operative bone fracture. Patients with such post-traumatic iatrogenic trigeminal neuropathy may have allodynia to mechanical or cold stimuli, burning or tingling sensations and pain during functional activities.38
Further research is necessary to identify the psychological and physiological risk factors in the pathogenesis of persistent postsurgery pain, and the reason for marked variations in its prevalence between persons of similar age, gender and health status, undergoing comparable surgeries. More information is also needed regarding the roles that socio-cultural and genetic factors, personality, cognition, and emotion play in persistent postsurgery pain; and regarding the elements of the sensory neural pathways that are impaired in subjects with persistent postsurgery pain.8
Taken together, this information would give much-needed insight into the pathogenesis of persistent postsurgery pain and should facilitate the identification of subjects at risk of this neural disorder, and the formulation and implementation of policies and programs for prevention and for developing new treatment modalities.8,11 Logical strategies that should be explored include inhibition of glial cell activation, promotion of regeneration and prevention of degeneration of injured neurons.26
Meanwhile, persons who wish to undergo elective surgery for reasons other than illness or disability must be made aware that persistent postsurgery pain is not uncommon, and may significantly reduce the quality of life for some time; and informed of the psychic and depressive risk factors associated with persistent postsurgery pain.19 Such pre-surgical information will enable the patient to reach a well-balanced decision, and avoid disappointment.
Cancer pain
Around 30–50% of all patients with cancer and almost all patients with advanced cancer, will experience pain during the course of their disease, and pain intensity appears to increase as cancer progesses.20,39–41 Approximately 70% of cases of cancer pain are caused by factors related to the cancer itself, such as invasion of nerves, bone, and mucous membranes; about 20% by surgical, chemotherapy and radiotherapy treatment-related collateral tissue damage; and in 10% of cases, the pain experienced by patients with cancer is unrelated to the cancer or to its treatment.42 Regardless of the cause, cancer-related neuropathic pain is described as lancinating, burning, electric or shock-like or prickling, corresponding to the distribution of neural injury.43 The mechanisms that drive cancer-related neuropathic pain are similar to those of other types of persistent postsurgery neuropathic pain (Figure 3).
Radiotherapy and chemotherapy may cause dry mouth, oral mucositis, and disturbance of taste, with pain and impairment of speech and mastication.44,45 Furthermore, administration of certain chemotherapeutic agents, such as the neurotoxic paclitaxel and vincristine, can cause peripheral neuropathy with sensory symptoms of numbness, tingling and burning pain.46–49 Cancer-associated inflammation, superficial ulceration with secondary infection, compression and/or invasion of sensory nerves by the tumour, may all contribute to cancer pain.50,51
In treated patients with adequately controlled chronic cancer pain that occurs either spontaneously or in relation to movement of the body part affected by cancer, more than 65% experience breakthrough cancer pain that may unpredictably occur 1–4 times a day, with each episode lasting about 30 minutes. Most cases of breakthrough pain are related to metastatic bone disease,52–54 and cause psychological distress and impairment of everyday activities. Such breakthrough pain is difficult to differentiate from fluctuations in pain intensity owing to inadequate management of the pain arising from unsuccessfully eradicated or recurrent cancer.53
Depression, anxiety, a feeling of hopelessness, decreased cognitive function and impaired everyday activity are common to terminal patients with cancer, and cancer related chronic pain only exaggerates this.55 Thus, the management of pain in patients with advanced cancer is complex and the physical, emotional and cognitive aspects of the disease must be addressed.55,56
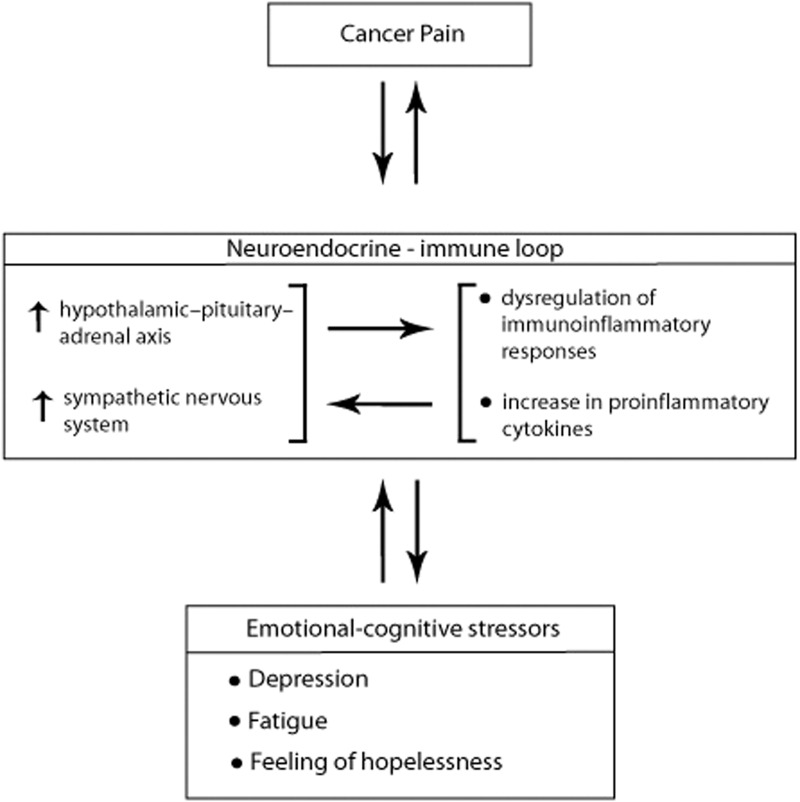
As with any other chronic debilitating pain condition, persistent cancer pain can activate the hypothalamic-pituitary-adrenal (HPA) axis and the central sympathetic nervous system (SNS), and can induce psychological distress. Psychological stressors, either induced by pain, or occurring independently of pain, constitute a feedback system further activating the HPA axis and the SNS, influencing the homeostasis of peripheral tissues and dysregulating immunoinflammatory reactions.57–60 The proinflammatory cytokines arising from this immunoinflammatory dysregulation may accentuate cancer growth,61,62 and may additionally stimulate central neuroendocrine activities.58 Furthermore, the pain/psychological stressor-induced tonic sympathetic input may disturb sleep and cause fatigue, exaggerating the chronic pain59 (Figure 4); can directly sensitize or even excite peripheral nociceptors;63 and can also promote tumour angiogenesis, thus favouring tumour growth with consequent cancer pain.60
Figure 4.
Pain, fatigue and depression often occur concurrently in patients with cancer, and proinflammatory cytokines are thought to play a role in the pathogenesis of this cluster of symptoms. In the context of cancer pain, it appears that inflammation and depression are reciprocal. Psychological therapy may thus indirectly downregulate inflammatory responses.62
Thus, dysregulation of the neuroendocrine-immune system, with elevated levels of neuropeptides and hormones of the SNS and HPA axis, and changes in the metabolism of neurotransmitters such as serotonin, dopamine and noradrenalin, are common factors in pain, depression and fatigue. Improving the function of the neuroendocrine-immune loop, by pharmacologically balancing the central activity of the relevant neurotransmitters, may confer clinical benefits.58
Metastatic bone disease-related cancer pain
In the bone microenvironment, malignant metastases to bone are the most common cause of cancer-related pain.64 Interactions between biological agents secreted by metastatic cells, and osteoblasts, osteoclasts and bone organic matrix, will determine whether the metastatic bone disease will have an osteolytic or an osteoblastic phenotype, and will drive the progressive process of metastatic proliferation and bone destruction.65,66 Many factors may contribute to the pain of malignant metastatic bone disease, including algogenic biological agents within the metastatically cancerous microenvironment, increased pressure upon bone and nerves and stretching of the periosteum from growth of metastases within the bone, microfracture of bone trabeculae, gross pathological fracture owing to progressive reduction of the bone structural integrity as a result of tumour growth, direct tumour-induced injury to sensory nerve fibres within the bone and infiltration of nerve roots by tumour cells.22,40,64,67
In patients with pain related to malignant metastatic bone disease, dorsal horns in the part of the spinal cord that is neurologically related to the site of metastatic bone disease, show neurochemical changes, morphological functional and structural plasticity with sprouting of new nerve terminals, upregulation of expression of receptors and their associated neurotransmitters and reacting glial cells.20,40 These changes are correlated with the degree of the tumour growth and with the bone destruction.67,68
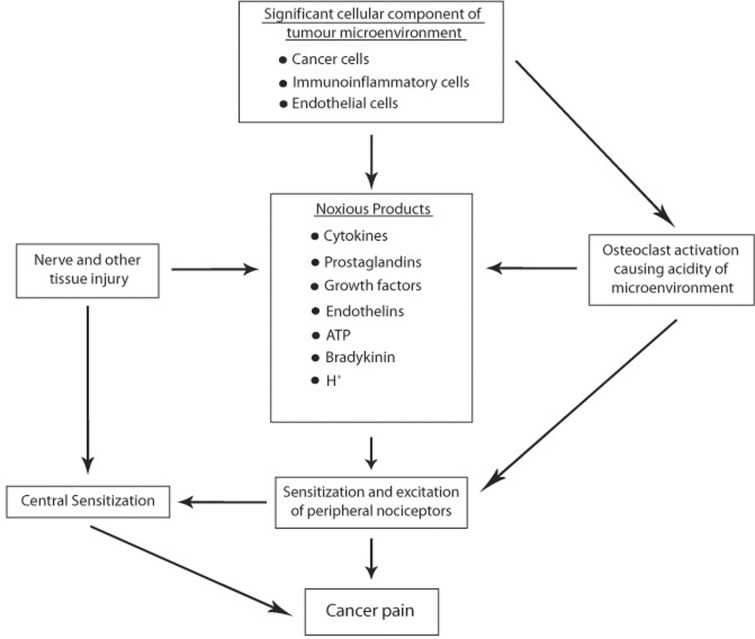
Furthermore, proteases, bradykinin, prostaglandins, endothelins, cytokines, chemokines, growth factors, ATP and H+ are all released by malignant cells and/or immunoinflammatory cells in the tumour microenvironment, and some of these have the capacity to excite primary afferent nociceptors, thus contributing to the generation of cancer pain.20,40,51,67,69,70 Acidic ions generated by osteoclasts at sites of cancer-induced bone resorption directly stimulate acid-sensing ion channel receptors of primary afferent nociceptors (Figure 5).20,22,40,69
Figure 5.
Cells in the tumour microenvironment, nerve injury, and activated bone-resorbing osteoclasts, all contribute to the induction and maintenance of metastatic bone pain. Noxious agents secreted by cells in the tumour microenvironment, and microenvironment acidity generated by protons secreted by activated osteoclasts, directly excite primary afferent sensory neurons in the bone. This causes peripheral and central sensitization, resulting in metastatic bone cancer pain.20,67
Acidosis in the metastatic cancer microenvironment can also be generated by ions and acid metabolites released by increased numbers of apoptotic and inflammatory cells,20,51 and together with ischaemia and increased mechanical pressure generated by rapid growth of the tumour or its metastases, with consequent local proteolysis, can cause injury to primary afferent nociceptors with peripheral and central sensitization, resulting in neuropathic pain.20,22 Pain related to malignant metastatic bone disease is usually dull, constant and localized, gradually becoming more intense with growth of the metastases.40,71 The intensity of the pain usually fluctuates.64,68,71
Metastatic spinal cord compression (MSCC) is a severe cancer complication involving compression of the dural sac and its components (spinal cord, cauda equina) by extra- or intradural tumour mass. MSCC occurs in up to 10% of all patients with cancer, and at the time of MSCC diagnosis, approximately 23% of patients may not have been aware that they had cancer.72,73 Lung, breast and prostate cancers account for more than 50% of cases of MSCC.73 The pathogenesis of MSCC is complex, and involves the spread of primary cancer cells to the spine, either haematogenously or by direct invasion from a nearby tumour. Damage to the spinal cord with consequent pain is brought about firstly, by prolonged direct pressure by the tumour mass, causing vascular injury-mediated spinal cord infarction and nerve demyelinization; and secondly, by tumour-induced pathological fracture and collapse of the affected vertebrae.72,73 Back pain of increasing intensity is the most common first symptom of MSCC. Pain may be localized to the immediate area of the spinal cord compression, or may be more peripheral affecting the area innervated by the compressed nerve roots.72,73
Metastasis of cancer to the jaws is uncommon but usually originates from primary cancer of the breast, lung, kidney or prostate.65,74–80 Prevalence of metastases in the jaws is almost equal in females and males, with the mandible more frequently affected than the maxilla.65,74–80 Signs and symptoms of malignant metastatic disease of the jaw include pain, local swelling, paraesthesia and loosening of teeth. The bone lesions are nearly always osteolytic, but occasionally, for reasons related to the nature of the primary tumour and to the specific characteristics of the niche in which the metastatic cells lodge, the metastatic bone lesions may be osteoblastic.65,74 Usually metastatic jaw cancer arises from a known primary cancer, but in approximately 30% of cases, the discovery of metastatic cancer in the jaw, often associated with metastatic disease at other sites, prompts a search for the undiagnosed primary cancer.65,74–80
The jaws are less frequently affected by metastatic bone disease than are the long bones, ribs or vertebrae, where larger sinusoidal spaces occupied by haematopoietically active red bone marrow, with a rich blood supply, favour the lodgement of metastatic cells. The mandible is more frequently affected than the maxilla, probably because the posterior area of the mandible is richer in red bone marrow with its sinusoidal spaces, than is any other part of the jaws.65,75,76,79
When pain is directly related to the cancer itself, surgery, chemotherapy, radiotherapy or a combination of these, should be considered as the first-line of treatment.20,43 Anti-inflammatory agents and opioids are beneficial in the management of early cancer pain induced by algogenic mediators, while opioids together with adjunctive agents such as anticonvulsants, antidepressants and NMDA-receptor inhibitors are beneficial in the later phase of cancer-related neuropathic pain.20,22,43 Relaxation, cognitive-behavioural therapy and psychological and spiritual coaching are additional supportive measures.44,66,81
Non-steroidal anti-inflammatory drugs (NSAIDs) alone, but more particularly in combination with opioids, are usually effective in treating malignant metastatic bone pain. NSAIDs inhibit prostaglandin production, tumour-related inflammatory osteolysis and the associated oedema, thus reducing intraosseous pressure and stretching of the periosteum.40,64 Bisphosphonates can inhibit osteoclastic bone resorption and the acidity brought about by osteoclasis, thus relieving the pain of bone metastases.22,67
In general, the clinician’s judgement and decision-making regarding the most appropriate patient-specific pain treatment is guided by personal experience and the expert opinion of colleagues. There are unavoidable variations in detail between practitioners regarding clinical judgement and decision-making, thus well-design algorithms and guidelines incorporating principles of statistics and evidence-based data, obtained from randomized trails and meta-analytical studies, can be useful adjuncts.82,83 Not all evidence-based information is of unquestionable scientific value, however, due to biases and inconsistencies caused by variabilities in study design and methodology, sample size, methods of statistical analysis, criteria for exclusion of patients, follow-up periods and drop-out rates. Although evidence-based practice is likely to improve clinical care and treatment outcomes,84 there will be non-pharmacological treatments and pharmacological agents that, according to evidence-based research, are not completely effective in the randomized average patient, but nevertheless may be beneficial to certain patients.85–87
The clinician has to consider the unpredictable variability in side effects of different drug preparations, and patient-specific differences in drug absorption, metabolism and functional activity of relevant receptors, which may result in insufficient relief with a specific agent. In such cases, switching to a different drug is advisable, though without any assurance of greater benefits. Furthermore, as pain is a subjective experience, different patients with comparable pain, using the same drug, will require different doses and possibly different routes of administration to achieve the same degree of pain relief.88–90
The pain mechanism, aetiopathogenesis, severity and clinical course, the potency and pharmacokinetics of the drug to be used, the required duration of analgesic effect, the known side effects of the particular agent, and previous experience of the patient with the chosen agent, are all factors to be considered when prescribing an analgesic.89,90
Conclusion
The persistent barrage of neural signals delivered by primary nociceptors induces structural and functional changes in the dorsal horn of the spinal cord, and this neural plasticity facilitates the development of persistent central sensitization, and consequently promotes postsurgery pain and neuropathic cancer pain. The neural plasticity is characterized by expansion of receptive fields, alterations in the expression of genes encoding neuropeptides, and suppression of pain inhibitory mechanisms. Inherited predisposition and genetic polymorphism play important roles in developing central sensitization.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Price DD, Mao J, Mayer DJ. Central consequences of persistent pain states In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z. (eds) Proceedings of the 8th world congress on pain: Progress in pain research and management. Seattle: IASP Press, 1997, pp.155–184. [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. [DOI] [PubMed] [Google Scholar]
- 3.Dubner R. Neural basis of persistent pain: sensory specialization, sensory modulation, and neuronal plasticity In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z. (eds) Proceedings of the 8th world congress on pain: Progress in pain research and management. Seattle: IASP Press, 1997, pp.243–257. [Google Scholar]
- 4.National Research Council. Mechanisms of pain. In: National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals (eds) Recognition and alleviation of pain in laboratory animals Washington DC: The National Academies Press, 2009, pp.33–46. [Google Scholar]
- 5.Costigan M, Befort K, Karchewski L, et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 2002; 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008; 138: 22–28. [DOI] [PubMed] [Google Scholar]
- 9.Bannister K, Kucharczyk M, Dickenson AH. Hopes for the future of pain control. Pain Ther 2017; 6: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg 2004; 99: 482–495. [DOI] [PubMed] [Google Scholar]
- 11.Borsook D, Kussman BD, George E, et al. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg 2013; 257: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth 2008; 101: 8–16. [DOI] [PubMed] [Google Scholar]
- 13.Waddell G. Low back pain: a twentieth-century health care enigma In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z. (eds) Proceedings of the 8th world congress on pain: Progress in pain research and management. Seattle: IASP Press, 1997, pp.101–112. [Google Scholar]
- 14.Bannister K, Patel R, Goncalves L, et al. Diffuse noxious inhibitory controls and nerve injury: restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 2015; 156: 1803–1811. [DOI] [PubMed] [Google Scholar]
- 15.De Felice M, Sanoja R, Wang R, et al. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain 2011; 152: 2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feller L, Fourie J, Bouckaert M, et al. Burning mouth syndrome: aetiopathogenesis and principles of management. Pain Res Manag 2017; 2017: 1926269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baert IAC, Lluch E, Van Glabbeek F, et al. Short stem total hip arthroplasty: potential explanations for persistent post-surgical thigh pain. Med Hypotheses 2017; 107: 45–50. [DOI] [PubMed] [Google Scholar]
- 18.Nijs J, Torres-Cueco R, van Wilgen CP, et al. Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician 2014; 17: 447–457. [PubMed] [Google Scholar]
- 19.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008; 101: 77–86. [DOI] [PubMed] [Google Scholar]
- 20.Mantyh PW, Clohisy DR, Koltzenburg M, et al. Molecular mechanisms of cancer pain. Nat Rev Cancer 2002; 2: 201–209. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 2010; 46: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benoliel R, Epstein J, Eliav E, et al. Orofacial pain in cancer: part I–mechanisms. J Dent Res 2007; 86: 491–505. [DOI] [PubMed] [Google Scholar]
- 23.Di Franco R, Falivene S, Ravo V, et al. Impact of procedural pain in radiotherapy treatment. WCRJ 2017; 4: e884–e890. [Google Scholar]
- 24.Ripamonti CI, Bossi P, Santini D, et al. Pain related to cancer treatments and diagnostic procedures: a no man's land? Ann Oncol 2014; 25: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 25.Katz J. Perioperative predictors of long-term pain following surgery In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z. (eds) Proceedings of the 8th world congress on pain: Progress in pain research and management. Seattle: IASP Press, 1997, pp.231–242. [Google Scholar]
- 26.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 27.Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth 2014; 113: 1–4. [DOI] [PubMed] [Google Scholar]
- 28.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000; 93: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 29.Brandsborg B, Dueholm M, Nikolajsen L, et al. A prospective study of risk factors for pain persisting 4 months after hysterectomy. Clin J Pain 2009; 25: 263–268. [DOI] [PubMed] [Google Scholar]
- 30.Stubhaug A, Breivik H, Eide PK, et al. Ketamine reduces postoperative hyperalgesia In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z. (eds) Proceedings of the 8th world congress on pain: Progress in pain research and management. Seattle: IASP Press, 1997, pp.333–342. [Google Scholar]
- 31.Wolf PS, Park JO, Bao F, et al. Preoperative chemotherapy and the risk of hepatotoxicity and morbidity after liver resection for metastatic colorectal cancer: a single institution experience. J Am Coll Surg 2013; 216: 41–49. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Pottabathini R, Bhatnagar A, et al. Pharmacological management of neuropathic pain: current trends and possible approaches. Arch Neurosci 2017; 4: e28998. [Google Scholar]
- 33.Wibbenmeyer L, Eid A, Kluesner K, et al. An evaluation of factors related to postoperative pain control in burn patients. J Burn Care Res 2015; 36: 580–586. [DOI] [PubMed] [Google Scholar]
- 34.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001; 91: 165–175. [DOI] [PubMed] [Google Scholar]
- 36.Macrae WA. Chronic pain after surgery. Br J Anaesth 2001; 87: 88–98. [DOI] [PubMed] [Google Scholar]
- 37.Baert IA, Lluch E, Mulder T, et al. Does pre-surgical central modulation of pain influence outcome after total knee replacement? A systematic review. Osteoarthritis Cartilage 2016; 24: 213–223. [DOI] [PubMed] [Google Scholar]
- 38.Renton T. Persistent pain after dental surgery. Rev Pain 2011; 5: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer 2011; 117: 1994–2003. [DOI] [PubMed] [Google Scholar]
- 40.Luger NM, Mach DB, Sevcik MA, et al. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage 2005; 29: S32–S46. [DOI] [PubMed] [Google Scholar]
- 41.Ripamonti C, Dickerson ED. Strategies for the treatment of cancer pain in the new millennium. Drugs 2001; 61: 955–977. [DOI] [PubMed] [Google Scholar]
- 42.Longo DL. Neoplastic disorders In: Kasper DL, Fauci AS, Hauser SL, et al. (eds) Harrison's principles of internal medicine. 19th ed New York: McGraw Hill, 2015, pp.467–475. [Google Scholar]
- 43.Martin LA, Hagen NA. Neuropathic pain in cancer patients: mechanisms, syndromes, and clinical controversies. J Pain Symptom Manage 1997; 14: 99–117. [DOI] [PubMed] [Google Scholar]
- 44.Chen SC, Liao CT, Chang JT. Orofacial pain and predictors in oral squamous cell carcinoma patients receiving treatment. Oral Oncol 2011; 47: 131–135. [DOI] [PubMed] [Google Scholar]
- 45.Feller L, Essop R, Wood NH, et al. Chemotherapy- and radiotherapy-induced oral mucositis: pathobiology, epidemiology and management. SADJ 2010; 65: 372–374. [PubMed] [Google Scholar]
- 46.Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med 2001; 2: 8–14. [DOI] [PubMed] [Google Scholar]
- 47.Wang XM, Lehky TJ, Brell JM, et al. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine 2012; 59: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavaletti G, Alberti P, Frigeni B, et al. Chemotherapy-induced neuropathy. Curr Treat Options Neurol 2011; 13: 180–190. [DOI] [PubMed] [Google Scholar]
- 49.Paice JA. Chronic treatment-related pain in cancer survivors. Pain 2011; 152: S84–S89. [DOI] [PubMed] [Google Scholar]
- 50.Dios PD, Leston JS. Oral cancer pain. Oral Oncol 2010; 46: 448–451. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt BL, Hamamoto DT, Simone DA, et al. Mechanism of cancer pain. Mol Interv 2010; 10: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett MI. Cancer pain terminology: time to develop a taxonomy that promotes good clinical practice and allows research to progress. Pain 2010; 149: 426–427. [DOI] [PubMed] [Google Scholar]
- 53.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain 1999; 81: 129–134. [DOI] [PubMed] [Google Scholar]
- 54.Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev 1998; 24: 425–432. [DOI] [PubMed] [Google Scholar]
- 55.Mystakidou K, Tsilika E, Parpa E, et al. Exploring the relationships between depression, hopelessness, cognitive status, pain, and spirituality in patients with advanced cancer. Arch Psychiatr Nurs 2007; 21: 150–161. [DOI] [PubMed] [Google Scholar]
- 56.Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014; 32: 1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997; 20: 78–84. [DOI] [PubMed] [Google Scholar]
- 58.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol 2010; 29: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vierck CJ., Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain 2006; 124: 242–263. [DOI] [PubMed] [Google Scholar]
- 60.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol 2010; 6: 1863–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol 2013; 49: 887–892. [DOI] [PubMed] [Google Scholar]
- 62.Thornton LM, Andersen BL, Schuler TA, et al. A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med 2009; 71: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janssen SA, Arntz A, Bouts S. Anxiety and pain: epinephrine-induced hyperalgesia and attentional influences. Pain 1998; 76: 309–316. [DOI] [PubMed] [Google Scholar]
- 64.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997; 69: 1–18. [DOI] [PubMed] [Google Scholar]
- 65.Feller L, Kramer B, Lemmer J. A short account of metastatic bone disease. Cancer Cell Int 2011; 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chwistek M. Recent advances in understanding and managing cancer pain. F1000Res 2017; 6: 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pacharinsak C, Beitz A. Animal models of cancer pain. Comp Med 2008; 58: 220–233. [PMC free article] [PubMed] [Google Scholar]
- 68.Honore P, Rogers SD, Schwei MJ, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000; 98: 585–598. [DOI] [PubMed] [Google Scholar]
- 69.Schwei MJ, Honore P, Rogers SD, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 1999; 19: 10886–10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathway. Pain 2010; 149: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeppetella G. Impact and management of breakthrough pain in cancer. Curr Opin Support Palliat Care 2009; 3: 1–6. [DOI] [PubMed] [Google Scholar]
- 72.Al-Qurainy R, Collis E. Metastatic spinal cord compression: diagnosis and management. BMJ 2016; 353: i2539. [DOI] [PubMed] [Google Scholar]
- 73.Nair C, Panikkar S, Ray A. How not to miss metastatic spinal cord compression. Br J Gen Pract 2014; 64: e596–e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pruckmayer M, Glaser C, Marosi C, et al. Mandibular pain as the leading clinical symptom for metastatic disease: nine cases and review of the literature. Ann Oncol 1998; 9: 559–564. [DOI] [PubMed] [Google Scholar]
- 75.Hirshberg A, Leibovich P, Buchner A. Metastatic tumors to the jawbones: analysis of 390 cases. J Oral Pathol Med 1994; 23: 337–341. [DOI] [PubMed] [Google Scholar]
- 76.Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, et al. Metastatic tumours to the oral cavity - pathogenesis and analysis of 673 cases. Oral Oncol 2008; 44: 743–752. [DOI] [PubMed] [Google Scholar]
- 77.van der Waal RI, Buter J, van der Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg 2003; 41: 3–6. [DOI] [PubMed] [Google Scholar]
- 78.Katsnelson A, Tartakovsky JV, Miloro M. Review of the literature for mandibular metastasis illustrated by a case of lung metastasis to the temporomandibular joint in an HIV-positive patient. J Oral Maxillofac Surg 2010; 68: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 79.Aksoy S, Orhan K, Kursun S, et al. Metastasis of prostate carcinoma in the mandible manifesting as numb chin syndrome. World J Surg Oncol 2014; 12: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashab Yamin MR, Kalantarhormozi A, Hamdamjo F, et al. Breast ductal carcinoma metastasis to jaw bones: a case report. Novel Biomed 2014; 2: 31–35. [Google Scholar]
- 81.Kane CM, Mulvey MR, Wright S, et al. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med 2018; 32: 276–286. [DOI] [PubMed] [Google Scholar]
- 82.Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine”. Am J Med 1997; 103: 529–535. [DOI] [PubMed] [Google Scholar]
- 83.Djulbegovic B, Elqayam S, Dale W. Rational decision making in medicine: implications for overuse and underuse. J Eval Clin Pract 2018; 24: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992; 268: 2420–2425. [DOI] [PubMed] [Google Scholar]
- 85.Frieden TR. Evidence for health decision making - beyond randomized, controlled trials. N Engl J Med 2017; 377: 465–475. [DOI] [PubMed] [Google Scholar]
- 86.Carlson CL. Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review. J Pain Res 2016; 9: 515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khammissa RAG, Ballyram R, Jadwat Y, et al. Vitamin D deficiency as it relates to oral immunity and chronic periodontitis. Int J Dent 2018; 2018: 7315797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engstrom JW, Deyo RA. Back and neck pain In: Kasper DL, Fauci AS, Hauser SL, et al. (eds) Harrison's principles of internal medicine. 19th ed New York: McGraw Hill, 2015, pp.111–113. [Google Scholar]
- 89.Breitbart W. Pain in AIDS In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z. (eds) Proceedings of the 8th world congress on pain: Progress in pain research and management. Seattle: IASP Press, 1997, pp.63–100. [Google Scholar]
- 90.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10: 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]