Short abstract
Objective
This study aimed to assess the value of measuring the tubule diameter during microdissection testicular sperm extraction (micro-TESE) in predicting outcomes in patients with Sertoli cell-only syndrome (SCOS).
Methods
Fifty-six consecutive patients with SCOS were included. Patients were classified into two groups on the basis of the diameter of seminiferous tubules measured against 5/0 surgical suture (≥100 µm or <100 µm).
Results
The sperm retrieval rate (SRR) in men with a tubule diameter ≥100 µm was significantly lower than that in those with <100 µm (3.1% vs. 25.0%). The SRR from the contralateral testis in men with a tubule diameter ≥100 µm was lower than that in those with <100 µm (0% vs. 14.3%). Men with a tubule diameter ≥100 µm had a significantly larger testis and lower follicle-stimulating hormone levels than did men with <100 µm (8.1 ± 2.4 vs. 5.3±1.8 mL, 19.9 ± 9.7 vs. 25.9 ± 7.1 mIU/mL, respectively).
Conclusions
The diameter of tubules is a useful predictor for a successful SRR in men with SCOS. Intraoperative assessment of homogeneous large tubules allows some men to perform a limited (superficial) contralateral micro-TESE after no spermatozoa are initially identified.
Keywords: Nonobstructive azoospermia, microdissection, seminiferous tubule diameter, Sertoli cell-only syndrome, sperm retrieval, microdissection testicular sperm extraction
Introduction
Nonobstructive azoospermia (NOA) due to testicular failure affects approximately 1% of all men and 10% to 15% of infertile men.1 Microdissection testicular sperm extraction (micro-TESE) combined with intracytoplasmic sperm injection (ICSI) can provide an opportunity for reproduction in men with NOA.2,3 Micro-TESE, which is the most effective sperm retrieval technology, can increase the sperm retrieval rate (SRR) and minimize postoperative complications.3,4 When the seminiferous tubule diameter is ≥300 mm, a single tubule biopsy is usually sufficient to harvest enough testicular spermatozoa for ICSI.5
A lot of research has focused on predictors of successful micro-TESE. Most reports have shown that there is no definite factor that can accurately predict the outcome of TESE, including the levels of follicle-stimulating hormone (FSH) and testosterone, testicular volume, and paternal age.6–9 Alfano et al.10 found that levels of anti-Müllerian hormone and the ratio of anti-Müllerian hormone-to-total testosterone achieved independent predictor status for sperm retrieval in men with idiopathic NOA. Histological findings also appear to be a useful predictor for successful micro-TESE.11,12 Additionally, intraoperative evaluation can also provide useful information on operative planning. Previous studies showed that the SRR was higher when identifying larger and more opaque seminiferous tubules.3,5 Intraoperative identification of ≥five motile and/or non-motile spermatozoa at the time of unilateral micro-TESE allowed us to limit the surgical procedure correctly in a previous study.13
Because predicting successful sperm retrieval preoperatively is difficult, predicting no sperm retrieval intraoperatively might be an effective method of reducing any problems. Men with a Sertoli cell-only syndrome (SCOS) pattern are thought to have a lower SRR compared with patients with hypospermatogenesis and maturation arrest.11,14 SCOS is classified into two histological patterns of the pure (primitive) form and the secondary (acquired) form.15 The pure form is a congenital disorder that is characterized by a normal tubule diameter. Therefore, finding spermatozoa by micro-TESE is impossible. In the secondary or acquired form, the seminiferous tubule diameters range from almost normal to small.15 Therefore, this study aimed to assess the value of identifying tubules during micro-TESE for predicting the SRR outcome in patients with SCOS.
Materials and methods
This comparative study received institutional review board approval from the Medical Ethics Committee of First Hospital of Jilin University (No. 2016-389). Written informed consent was obtained from all patients.
Patients
We analyzed 109 consecutive infertile men with NOA who underwent micro-TESE by a single surgeon from 2016 to 2017 in the Centre for Reproductive Medicine and Prenatal Diagnosis of the First Hospital of Jilin University. All of the patients were confirmed to have azoospermia by analysis of at least two centrifuged semen specimens according to World Health Organization criteria. All of the patients underwent karyotype and Y chromosomal microdeletion analyses; men with complete AZFa and AZFb microdeletions were excluded.
Preoperatively identifiable factors included FSH, testosterone, age, a history of an undescended testis, and a history of testicular cancer. Physical examinations were performed to measure testis volume using the Prader orchidometer (Pro-Health, Guangzhou, China) by the same urologist and to detect varicocele. The average volume of the two testicles was used for analysis. Patients who had undergone a previous diagnostic biopsy or TESE elsewhere were not included in this analysis. Testicular histology was used in this study on the basis of results of a testicular tissue examination by a pathologist after micro-TESE. Patients with proven obstructive azoospermia were excluded. All of the procedures were performed by the same surgeon.
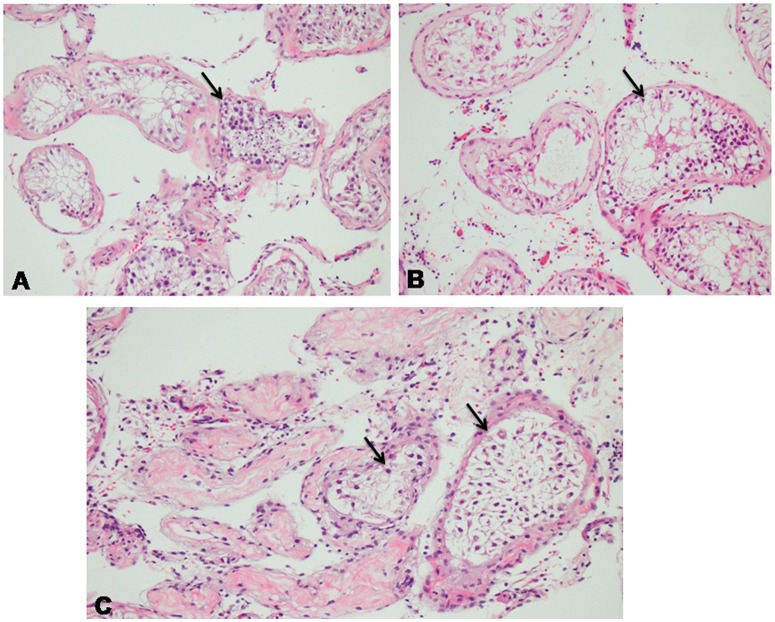
On the basis of the most predominant histopathological pattern, testicular histology was classified as follows: normal spermatogenesis, hypospermatogenesis (Figure 1A), maturation arrest (Figure 1B), and SCOS (Figure 1C). Patients were considered to have SCOS if all intraoperative testis biopsies were consistent with SCOS by a pathologist. Patients with a mixed histopathological pattern were excluded.
Figure 1.
Histopathology of (A) hypospermatogenesis, (B) maturation arrest, and (C) Sertoli cell-only syndrome.
Micro-TESE
The procedure of micro-TESE has been described previously in detail.3 Briefly, under a general anesthetic, a transverse, mid-scrotal incision was made over the left or right testis. The tunica vaginalis was opened to visualize the tunica albuginea. An equatorial incision was widely opened over the tunica albuginea under an operative microscope, taking care to avoid vasculature injury. Homogeneous tubules were measured against 5/0 surgical suture (Polysorb; Covidien, Boulder, CO, USA), which had a diameter of 100 µm. If the tubules were heterogeneous (the difference in diameter between the largest and smallest tubules was ≥50 µm16), we measured the diameters of the most similar tubules. Microdissection was then performed to identify seminiferous tubules at between 12× and 18× magnification under an operating microscope. The superficial tubules were first examined for larger and more opaque tubules and one to three samples were taken to an embryologist in the operating room. If no spermatozoa were found, deeper testicular tissue was microdissected along the seminiferous tubule lobules under optical magnification. If tubules had an identical morphological appearance, at least six specimens were obtained from upper and lower poles of the testicle. If no spermatozoa were observed during micro-TESE, the testicular tissue was thoroughly examined for the presence of spermatozoa in the embryology laboratory 12 to 24 hours later. Tissue specimens were fixed in Bouin’s solution and sent for histopathological analysis.
Diameter of seminiferous tubules
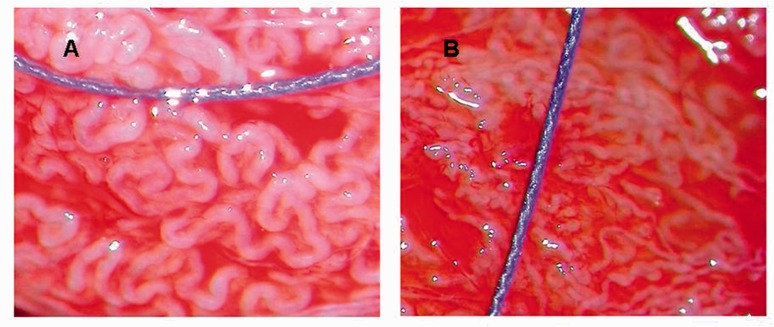
Amer et al.10 reported that the best cutoff level of the seminiferous tubule diameter for harvesting testicular spermatozoa was 110 µm. Tubules with a diameter of ≥100 µm were more likely to contain mature spermatozoa. Therefore, men were divided into two groups on the basis of the diameter of seminiferous tubules: ≥100 µm (larger than the 5/0 surgical suture) and <100 µm (smaller than the 5/0 surgical suture) (Figure 2). The mean SRR values were compared between these two groups.
Figure 2.
(A) Photograph showing seminiferous tubules that are larger in diameter than 5/0 surgical sutures (100 µm). (B) Photograph showing seminiferous tubules that are smaller than 5/0 surgical sutures.
Statistical analysis
All statistical data were analyzed with SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA). For descriptive statistics such as age, testis size, FSH levels, and testosterone levels, the independent-sample t test was used to compare the two groups. Qualitative variables, such as the spermatozoa retrieval rate, were evaluated by Fisher’s exact test. p < 0.05 was considered statistically significant.
Results
A total of 56 patients who suffered from NOA with SCOS pathology were identified in the present study. The mean age of the patients was 30.6 ± 3.8 years (±standard deviation). The median testicular volume undergoing micro-TESE was 6.9 ± 2.6 mL. The overall SRR was 12.5% (7/56).
Of the 56 men, 32 (57%) were classified as having a seminiferous tubule diameter of ≥100 µm, and the remaining 24 (43%) men were classified as having a diameter of <100 µm. The characteristic differences between these groups are shown in Table 1. Testosterone levels were similar in the two groups. Men in the ≥100 µm group had larger testes than did men in the <100 µm group (p < 0.001). Men in the ≥100 µm group also showed lower FSH levels than did men in the <100 µm group (p = 0.014). The SRR in men in the ≥100 µm group was lower than that in men in the <100 µm group (p = 0.035). Of the 52 men who underwent bilateral testicular microdissection, sperm were found on the contralateral side in only three (5.8%) patients after no sperm were identified on the initial side. No sperm were obtained from a tubule diameter of ≥100 µm. All three instances of sperm recovery from the contralateral testis were from tubule diameters of <100 µm (Table 2).
Table 1.
Comparison of a tubule diameter ≥100 µm and that <100 µm.
| Diameter of tubules (µm) |
|||
|---|---|---|---|
| ≥100 | <100 | p value | |
| n | 32 | 24 | |
| Mean (±SD) male age (years) | 30 ± 4 | 31.4 ± 3.5 | 0.174 |
| Mean (±SD) volume of testis (mL) | 8.1 ± 2.4 | 5.3 ± 1.8 | <0.001 |
| Mean (±SD) FSH (mIU/mL) | 19.9 ± 9.7 | 25.9 ± 7.1 | 0.014 |
| Mean (±SD) testosterone (nmol/L) | 13.9 ± 9 | 11.4 ± 4.9 | 0.069 |
| Sperm retrieval rate (%) | 3.1 | 25 | 0.035 |
SD: standard deviation; FSH: follicle-stimulating hormone.
Table 2.
Sperm retrieval of the contralateral side of the testis.
| Diameter of tubules (µm) |
||
|---|---|---|
| ≥100 | <100 | |
| Number | 31 | 21 |
| Sperm retrievalrate, n (%) | 0 | 3/21 (14.3) |
Discussion
Under optical magnification, tubules that are identifiable as larger and more opaque or whiter tubules are thought to contain sperm.3 Tubule atrophy appears to lead to a poor chance of sperm retrieval with micro-TESE. In the present study, in men with SCOS, spermatozoa were successfully retrieved in 25% of those with tubule diameters <100 µm, but they were only retrieved in 3.1% of men with tubule diameters ≥100 µm. Our findings appear to conflict with previous reports. Kamal et al.17 reported that the SRR in biopsies where all tubules were dilated was 14/33 (42%), whereas the SRR was 12/53 (23%) when all of the tubules were collapsed as observed under a stereoscope. In a study of 264 men, Amer et al.5 noted that the SRR was different among men with different tubule diameters, including 31%, 44%, and 84% for ≤200 µm, >200 to <300 µm, and ≥300 µm, respectively. The possible reason for this difference between studies is that most reports had no further identification in histology. A few previous studies18 mentioned histological identification, but no comprehensive research had been performed. In a study of 58 men with NOA, Tsujimura et al.18 observed that seven patients with thick homogeneous tubules had sperm and all of these men were classified as having hypospermatogenesis; 23 patients with homogeneously thin tubules were found to have no sperm and 22 of them were classified as SCOS. These studies provide evidence that a larger tubule diameter is associated with an increased SRR for all unclassified NOA. However, the SRR outcome remains unclear for each specific histological classification. In our study, men with SCOS who had larger tubules had a lower SRR. Therefore, the SRR is not only predicted by the diameter of the tubule, but also by the histopathological classification.
Men with larger tubules had a worse SRR compared with those who had smaller tubules in our series. This finding might not be surprising. Anniballo et al.15 showed that two histological patterns of SCOS can be distinguished as the pure (primitive) form and secondary (acquired) form. In the pure form, tubules are a normal diameter; no histological changes can be seen because of the congenital nature of this disorder, and it is impossible to find spermatozoa by micro-TESE in such patients. Unlike the pure (primitive) form, in men with the secondary (acquired) form, the diameters of tubules range from almost normal to tiny.15 Success of micro-TESE in men with NOA depends on finding heterogeneous areas. Tubules in secondary SCOS are thinner than those in pure SCOS and tubules with sperm are thick. Therefore, differentiation between empty tubules and tubules with sperm is easier in secondary SCOS. If all of the tubules in pure SCOS are a homogeneous pattern and have almost a normal diameter, spermatozoa are unlikely to be identified and require more biopsy.
In our study, the SRR of the contralateral side of the testis was lower in men with SCOS who had larger tubules compared with those who had smaller tubules. Ramasamy et al.19 reported that 40 of 506 (8%) men had sperm on the contralateral side when no sperm were identified on the initial side. A similar result was found in our study. Of the 52 patients who underwent bilateral micro-TESE, three (5.8%) patients had successful sperm retrieval from the contralateral side of the testis. However, no sperm were found in men with larger tubules. Therefore, the diameter of tubules appears to be a useful predictor in guiding intraoperative planning in men with SCOS. If homogeneous large tubules are shown under optical magnification, performing a limited (superficial) contralateral micro-TESE might be a good choice to reduce testicular damage.
We also found that men with larger tubules had a larger testicular size and lower FSH levels than did those with smaller tubules. A possible reason for this finding is that primordial germ cells had not reached their final destination in the primitive testicle in men with congenital SCOS, and the tubules were composed of normal Sertoli and Leydig cells, which tend not to atrophy. Further histopathological studies should be performed to confirm these findings. Testicular atrophy in men with acquired SCOS is more susceptible to occur with decreased testicular size and elevated FSH levels. Therefore, men with SCOS with a larger testicular size and lower FSH levels appear to have a lower SRR.
Conclusion
The diameter of seminiferous tubules is a useful predictor for successful sperm retrieval in men with SCOS. Men with larger tubules have an extremely poor SRR. Relatively low FSH levels and a large testicular volume appear to reflect large tubules in men with SCOS. Under optical magnification, intraoperative assessment of homogeneous large tubules allows some men to perform a limited (superficial) contralateral micro-TESE after no spermatozoa are initially identified.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Nature Science Fund under grant No. 81471515 from China.
References
- 1.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol 1989; 142: 62–65. [DOI] [PubMed] [Google Scholar]
- 2.Devroey P, Liu J, Nagy Z, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod 1995; 10: 1457–1460. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999; 14: 131–135. [DOI] [PubMed] [Google Scholar]
- 4.Amer M, Ateyah A, Hany R, et al. Prospective comparative study between microsurgical and conventional testicular sperm extraction in obstructive non-azoospermia: follow-up by serial ultrasound examinations. Hum Reprod 2000; 15: 653–656. [DOI] [PubMed] [Google Scholar]
- 5.Amer M, Zohdy M, Abd El Naser T, et al. Single tubule biopsy: a new objective microsurgical advancement for testicular sperm retrieval in patients with nonobstructive azoospermia. Fertil Steril 2008; 89: 592. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimura A. Microdissection testicular sperm extraction: prediction, outcome, and complications. Int J Urol 2007; 14: 883–889. [DOI] [PubMed] [Google Scholar]
- 7.Ramasamy R, Lin K, Gosden LV, et al. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril 2009; 92: 590–593. [DOI] [PubMed] [Google Scholar]
- 8.Bryson CF, Ramasamy R, Sheehan M, et al. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol 2014; 191: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada H, Goda K, Yamamoto Y, et al. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter’s syndrome. Fertil Steril 2005; 84: 1662–1664. [DOI] [PubMed] [Google Scholar]
- 10.Alfano M, Ventimiglia E, Locatelli I, et al. Anti-mullerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. Sci Rep 2017; 7: e796–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su LM, Palermo GD, Goldstein M, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol 1999; 161: 112–116. [PubMed] [Google Scholar]
- 12.Abdel Raheem A, Garaffa G, Rushwan N, et al. Testicular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermia. BJU Int 2013; 111: 492–499. [DOI] [PubMed] [Google Scholar]
- 13.Alrabeeah K, Doucet R, Boulet E, et al. Can the rapid identification of mature spermatozoa during microdissection testicular sperm extraction guide operative planning? Androlugy 2015; 3: 467–472. [DOI] [PubMed] [Google Scholar]
- 14.Hussein A. Evaluation of diagnostic testis biopsy and there petition of testicular sperm extraction surgeries in infertility patients. Fertil Steril 2013; 100: 88–93. [DOI] [PubMed] [Google Scholar]
- 15.Anniballo R, Ubaldi F, Cobellis L, et al. Criteria predicting the absence of spermatozoa in the Sertoli cell-only syndrome can be used to improve success rates of sperm retrieval. Hum Reprod 2000; 15: 2269–2277. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Xi Q, Wang R, et al. Heterogenicity of testicular histopathology and tubules as a predictor of successful microdissection testicular sperm extraction in men with nonobstructive azoospermia. Medicine 2018; 97: e10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal A, Fahmy I, Mensour RT, et al. Selection of individual testicular tubules from biopsied testicular tissue with a stereomicroscope improves sperm retrieval rate. J Androl 2004; 25: 123–127. [DOI] [PubMed] [Google Scholar]
- 18.Tsujimura A, Matsumiya K, Miyagawa Y, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod 2002; 17: 2924–2929. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Reifsnyder JE, Husseini J, et al. Localization of sperm during microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol 2013; 189: 643–646. [DOI] [PubMed] [Google Scholar]