Short abstract
Objective
Acute lung injury is responsible for mortality in seriously ill patients. Previous studies have shown that systemic inflammation is attenuated by remote ischemic preconditioning (RIPC) via reducing nuclear factor-kappa B (NF-κB). Therefore, we investigated whether lipopolysaccharide (LPS)-induced indirect acute lung injury (ALI) can be protected by RIPC.
Methods
RIPC was accomplished by 10 minutes of occlusion using a tourniquet on the right hind limb of mice, followed by 10 minutes of reperfusion. This process was repeated three times. Intraperitoneal LPS (20 mg/kg) was administered to induce indirect ALI. Inflammatory cytokines in bronchoalveolar lavage fluid were analyzed using an enzyme-linked immunosorbent assay. Pulmonary tissue was excised for histological examination, and for examining NF-κB activity and phosphorylation of inhibitor of κBα (IκBα).
Results
NF-κB activation and LPS-induced histopathological changes in the lungs were significantly alleviated in the RIPC group. RIPC reduced phosphorylation of IκBα in lung tissue of ALI mice.
Conclusions
RIPC attenuates endotoxin-induced indirect ALI. This attenuation might occur through modification of NF-κB mediation of cytokines by modulating phosphorylation of IκBα.
Keywords: Acute lung injury, ischemic preconditioning, cytokine, inflammation, mice, survival rate
Introduction
Many cases of acute lung injury (ALI) are accompanied by sepsis, with approximately one fourth of acute respiratory distress syndrome (ARDS) cases stemming from severe sepsis.1 Mortality is higher and recovery from lung injury is poorer in sepsis-related ARDS than in nonsepsis-related ARDS.1,2 ALI is common even for sepsis with a suspected nonpulmonary source.3 In the setting of systemic inflammation, ALI is featured by diffuse parenchymal pulmonary inflammation, alveolar-capillary destruction, and edema. Mortality rates from ALI have decreased in recent decades, although marked efforts and multiple therapeutic strategies for ALI remain insufficient.4,5
Remote ischemic preconditioning (RIPC) is a powerful protective treatment against subsequent and more severe ischemic organ damage caused by transient events of ischemia on a distant tissue or organ.6–11 Intraperitoneal (IP) injection of lipopolysaccharide (LPS) is a common experimental model in mice for LPS-induced ALI from a non-pulmonary origin. This model features major pathological components of ALI, such as profound neutrophilic infiltration, vascular leakage/lung edema, and alveolar hemorrhage, all of which are observed in patients with ALI.12–14 Our previous study15 showed that RIPC attenuated systemic inflammation induced by IP administration of LPS and increased survival rates in a septic mouse model. However, little is known regarding the anti-inflammatory effects of RIPC on ALI.
Nuclear factor-kappa B (NF-κB) is a critical regulator of immediate transcriptional responses in inflammatory diseases. Additionally, sustained NF-κB activation in the airway epithelium results in neutrophilic lung inflammation and severe lung injury.16 Therefore, controlling the NF-κB signaling pathway may be important in treatment of ALI. The inhibitor of kappa B-alpha (IκBα) phosphorylation pathway is an important controlling pathway of NF-κB activity. In this study, we examined the IκBα phosphorylation pathway associated with NF-κB for inflammatory responses in lung tissue. We also aimed to evaluate whether RIPC can reduce LPS-induced ALI in mice and to determine the defensive mechanism associated with NF-κB.
Methods
Animals
This study obtained approval from the Ethical Committee on Animal Research of the Korea University College of Medicine (KUIACUC-2014-164). Animals were cared for in accordance with the institutional guidelines for experimental animals. Six-week-old male BALB/c mice (20–25 g; Hanlim Co., Hwasung, South Korea) were maintained in specific pathogen-free facilities on a 12-hour light/12-hour dark schedule for at least 7 days before the start of the study.
ALI mouse
LPS-induced ALI was induced by an IP injection of 20 mg/kg LPS (Escherichia coli, O127:B8) with 0.4 mL saline. RIPC was achieved in the mice by occluding the right hind limb using a tourniquet for 10 minutes, followed by a 10-minute reperfusion period. This procedure was repeated three times. Ischemia was judged to have occurred if the affected limb appeared pale.
Thirty-two mice were evenly divided into four groups as follows: (1) saline-only group, which received an IP injection of 0.4 mL saline; (2) RIPC/saline group, which underwent RIPC and immediately received an IP injection of 0.4 mL saline; (3) RIPC/LPS group, which underwent RIPC before receiving an IP injection of LPS; and (4) LPS-only group, which received an IP injection of LPS.
Survival rate
The survival rate was measured in the LPS-only group (n = 10) and RIPC/LPS group (n = 10), every 8 hours for 120 hours. After 5 days, all living mice were euthanatized with an IP injection of thiopental sodium (50 mg/kg).
Histological examination
Fourteen hours after saline or LPS injection, the mice were sacrificed with IP administration (50 mg/kg) of Zoletil (zolazepam and tiletamine, Virbac; Fort Worth, TX, USA). The right lower lung, which was stained with hematoxylin and eosin, was examined in a blinded manner by microscopists for pathological changes under light microscopy. The remaining right lung tissues were frozen to be examined for myeloperoxidase (MPO) activity. We measured the lung injury score using four criteria used by Schingnitz et al. as follows17: alveolar congestion, hemorrhage, infiltration or aggregation of neutrophils, and thickness of the alveolar wall/hyaline membrane formation. For each criteria, a five-point scale was applied from 0 (i.e., minimal damage) to 4 (i.e., maximal damage). The total lung injury score was the sum of the points of the four criteria.17
Wet/dry weight ratio
For assessment of lung tissue edema, we calculated the lung wet/dry (W/D) weight ratio. The left lower lobe was measured to obtain the wet weight and was then incubated at 80°C for 72 hours to obtain the dry weight. The W/D weight ratio was calculated by dividing the wet weight by the dry weight.
Bronchoalveolar lavage fluid and cytokine measurement
Bronchoalveolar lavage fluid (BALF) was collected by cannulating the upper part of the trachea and lavaging three times with 1.0 mL phosphate-buffered saline. The lavage fluid recovery rate was greater than 90%. For cytokine assays, BALF supernatant was acquired by centrifugation (4 minutes at 4000 rpm) and stored at −80°C. Concentrations of cytokines, which included tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), were measured by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) using the optical density of 450 nm.
MPO, NF-κB, and heme oxygenase-1 enzyme activity in lung tissue
To measure MPO activity, lung tissues were homogenized in a KPO4 buffer solution and centrifuged. The supernatant was mixed in a reaction solution according to the manufacturer’s guidelines. MPO activity was checked spectrophotometrically by measuring changes in absorption at 460 nm.18 Measurements are represented as units of MPO activity per milligram of tissue (mU/mg).
Fourteen hours after LPS (or saline) injection, NF-κB activity was measured in the lung tissue homogenate. A total of 5 µg of nuclear protein of tissue was evaluated to identify NF-κB activation by the NF-κB p65 assay kit (TransAM p65; Active Motif, Carlsbad, CA, USA) according to the manufacturer’s guidelines.
Fourteen hours after LPS (or saline) injection, heme oxygenase-1 (HO-1) was evaluated in the supernatant of homogenized lung tissue using the mouse HO-1 ELISA kit (Cusabio Bio-Tech, Wuhan, China). The homogenates were centrifuged for 5 minutes at 5000 × g at 4°C. The supernatant was diluted 1500-fold for the HO-1 assay.
Assessment of phosphorylation of IκBα by western blotting
Fourteen hours after LPS (or saline) injection, phosphorylation of IκBα was measured in lung tissue homogenate. Cells were homogenized in 1× sodium dodecyl sulfate (SDS) lysis buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% w/v SDS, 10% v/v glycerol, and 0.002% w/v bromophenol blue. Protein content was measured using the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Equal amounts of protein were separated by 8% SDS-polyacrylamide gel electrophoresis and transferred to membranes. Membranes were blocked with 5% w/v nonfat dry milk in phosphate-buffered saline containing 0.1% v/v Tween-20 at 4°C overnight. The membranes were incubated with primary antibody for 3 hours, followed by incubation with horseradish peroxidase-conjugated secondary antibody for 1 hour. Phospho-IκBα (Ser32) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), anti-p38 was purchased from Santa Cruz (Santa Cruz, CA, USA), and monoclonal anti-β-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). Immunoreactive proteins were visualized by enhanced chemiluminescence solutions (Amersham, Arlington Heights, IL, USA). For relative quantification, the integrated band density was determined by independently repeated samples using the Gel-Doc EQ program (Bio-Rad, Hercules, CA, USA). Each density of target proteins was normalized with beta-actin levels as an internal control. The density of the immunoblots was measured with ImageJ 1.39u software (NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was conducted using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). All numerical values are expressed as mean ± standard error of the mean. Survival data were analyzed using the Kaplan–Meier log-rank test. To detect significant differences between groups, the Kruskal–Wallis test was used. When significant differences were evident, post-hoc analysis was performed by the median test. Differences were considered to be significant when P values were less than 0.05.
Results
RIPC improves the survival rate
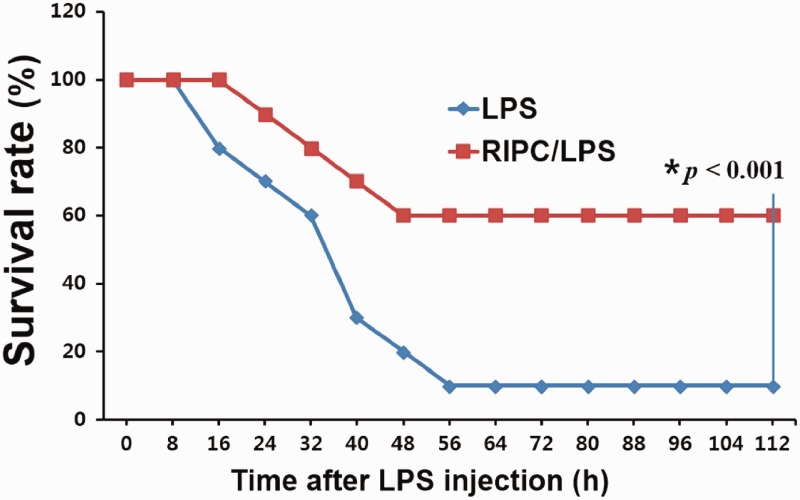
The survival rates were evaluated for 120 hours. The RIPC/LPS group showed a significantly improved survival rate compared with the LPS-only group (60% versus 10%, P < 0.001) (Figure 1).
Figure 1.
Kaplan–Meier survival curves. At 5 days post-LPS or saline injection, the survival rate of the mice was evaluated in the LPS-only group (n = 10) and the RIPC/LPS group (n = 10). LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
RIPC improves lung histopathology and pulmonary edema
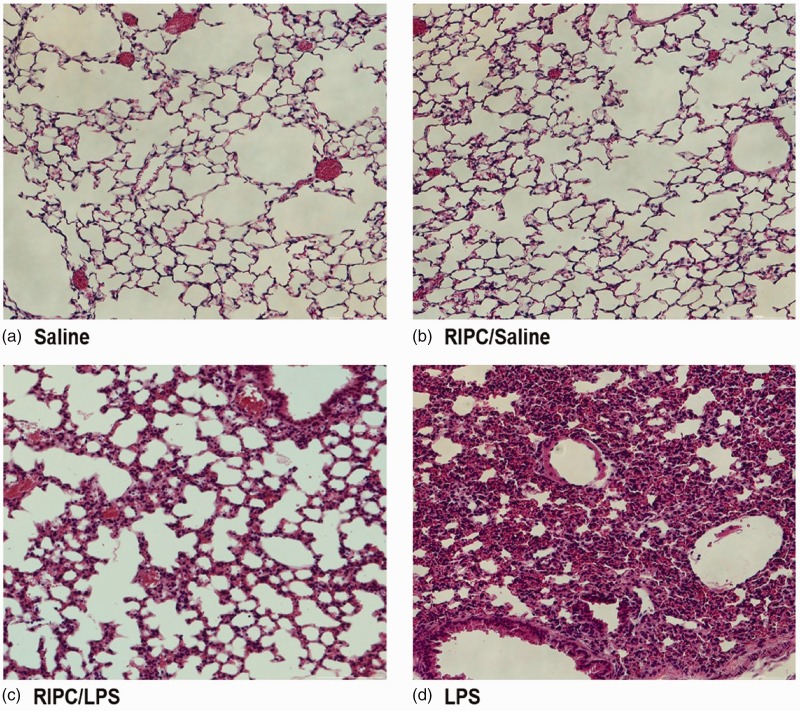
After LPS injection, morphological examination of lung tissue showed inflammatory cell infiltration, alveolar congestion, and hemorrhage. This finding indicated successful induction of ALI in our study. The saline-only group showed no histological changes. Histopathological changes in the pulmonary tissue of mice that received RIPC before LPS injection appeared to be reduced (Figure 2).
Figure 2.
Histopathological changes in murine pulmonary tissue. Fourteen hours after instillation of LPS or saline, lung tissues were collected and histologically examined. Representative histological changes in hematoxylin and eosin-stained lung sections from the four experimental groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group (hematoxylin and eosin staining; magnification, 200×). LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
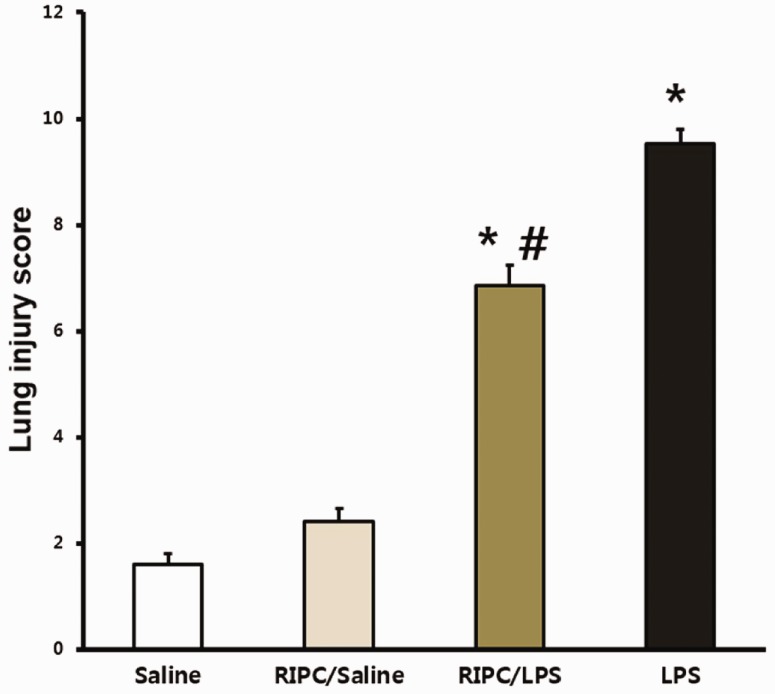
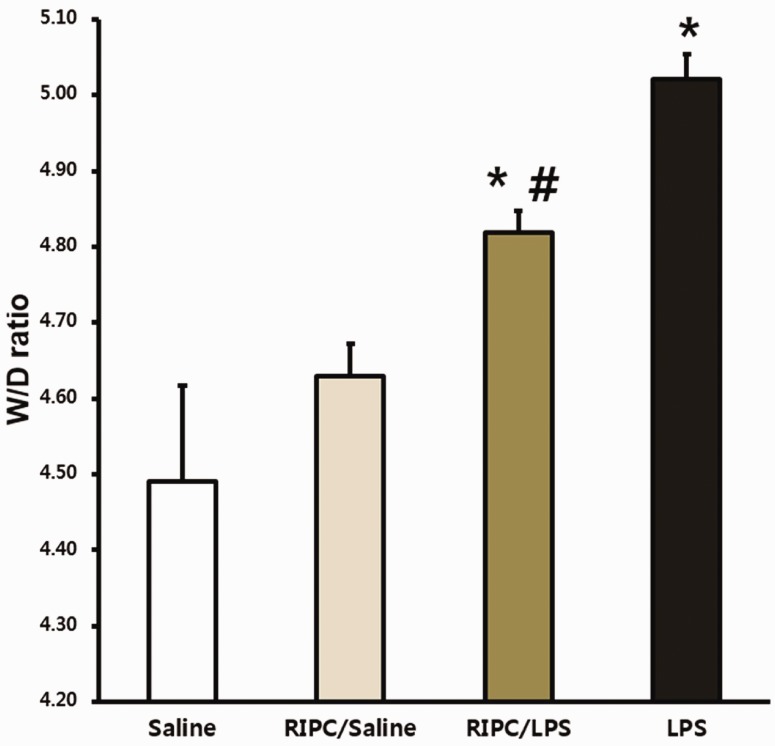
Lung injury scores from the LPS-only and RIPC/LPS groups were significantly greater than those in the saline-only group (both P < 0.001) (Figure 3). However, the mean lung injury score was lower in the RIPC/LPS group than in the LPS-only group (P = 0.023) (Figure 3). The W/D weight ratio was used to evaluate levels of pulmonary edema. The W/D weight ratio was significantly higher in the LPS-only group than in the saline-only and RIPC/saline groups (both P < 0.05) (Figure 4). However, the W/D weight ratio in the RIPC/LPS group was significantly lower than that of the LPS-only group (P = 0.038) (Figure 4).
Figure 3.
Effect of RIPC on the lung injury score. Fourteen hours after instillation of LPS or saline, lung injury scores were analyzed in pulmonary tissue from four experimental groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. All data are presented as mean ± standard error of the mean from the four experimental groups. *P < 0.001 versus the saline-only and RIPC/saline groups; #P < 0.05 versus the LPS-only group. LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
Figure 4.
Effect of RIPC on the W/D weight ratio. Fourteen hours after an LPS or saline challenge, the W/D weight ratios were measured in pulmonary tissue from the four experimental groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. All data are presented as mean ± standard error of the mean from the four experimental groups. *P < 0.05 versus the saline-only and RIPC/saline groups; #P < 0.05 versus the LPS-only group. W/D, wet/dry; LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
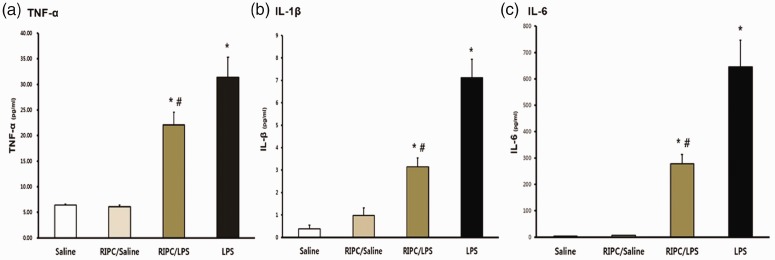
RIPC reduces production of cytokines in BALF
TNF-α, IL-1β, and IL-6 levels in the two LPS groups were significantly higher compared with those in the saline-only group (all P < 0.001) (Figure 5a, b, and c). However, TNF-α, IL-1β, and IL-6 levels were significantly lower in the RIPC/LPS group than in the LPS-only group (P = 0.045, P < 0.001, and P < 0.001, respectively) (Figure 5a, 5, and c).
Figure 5.
Cytokine concentrations in bronchoalveolar lavage fluid. (a) TNF-α, (b) IL-1β, and (c) IL-6 levels in bronchoalveolar lavage fluid were measured by using enzyme-linked immunosorbent assays. Bronchoalveolar lavage fluid was collected 14 hours after injection of LPS or saline in the four groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. All data are presented as mean ± standard error of the mean from the four experimental groups. *P < 0.001 versus the saline-only and RIPC/saline groups; #P < 0.05 versus the LPS-only group. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
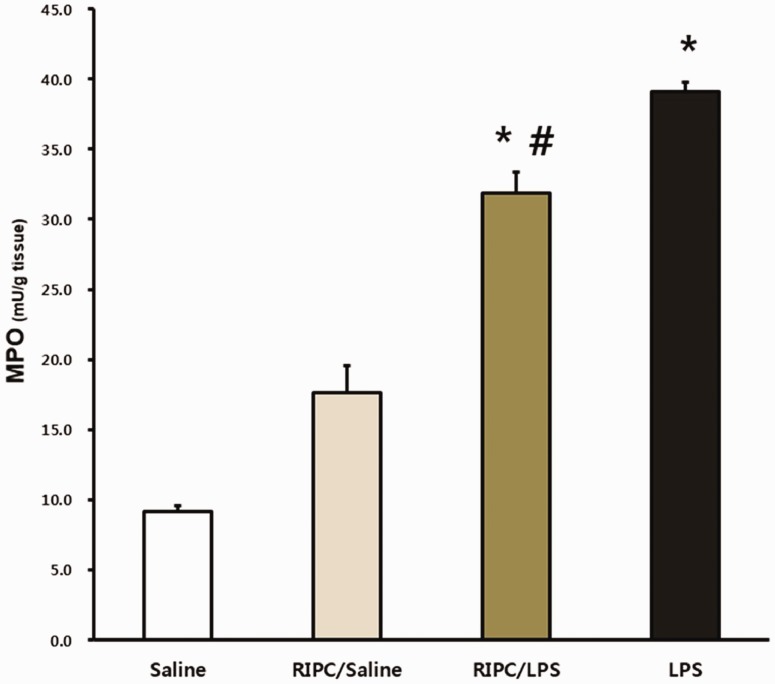
RIPC downregulates MPO Activity
MPO activity was significantly higher in the LPS-only and RIPC/LPS groups than in the saline-only group (both P < 0.001) (Figure 6). However, MPO activity was significantly attenuated by performing RIPC before LPS administration (P = 0.005) (Figure 6).
Figure 6.
MPO activity levels. Fourteen hours after an LPS or saline challenge, pulmonary tissue was collected and MPO activity was detected in pulmonary tissues from the four experimental groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. All data are presented as mean ± standard error of the mean from the four experimental groups. *P < 0.001 versus the saline-only and RIPC/saline groups; #P < 0.05 versus the LPS-only group. MPO, myeloperoxidase; LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
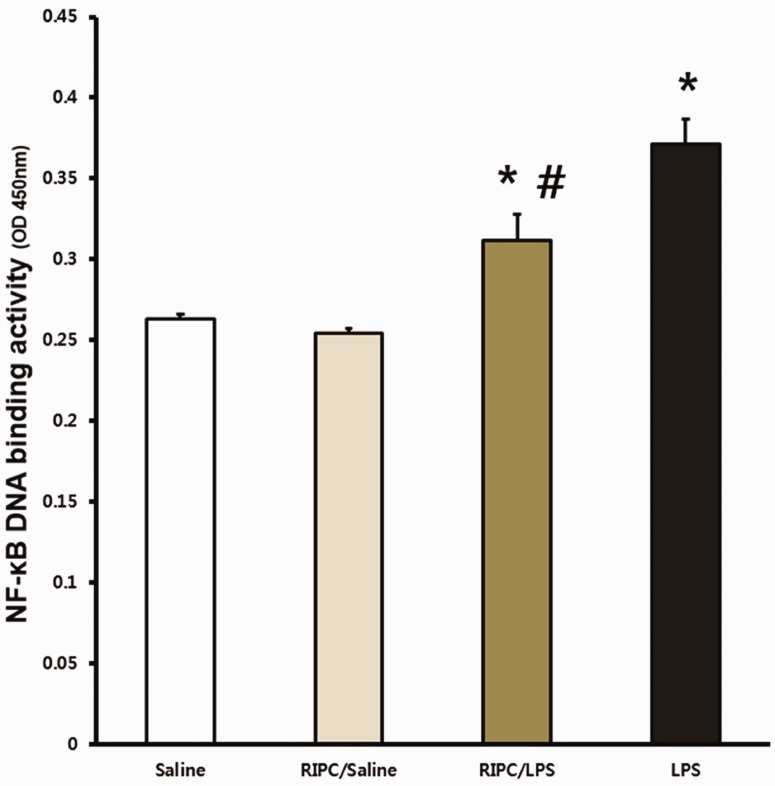
RIPC attenuates NF-κB DNA binding activity
The activity of NF-κB in lung tissue was significantly higher in the RIPC/LPS and LPS-only groups compared with the saline-only and RIPC/saline groups (both P < 0.05) (Figure 7). However, NF-κB activity in the RIPC/LPS group was significantly lower than that in the LPS-only group in lung tissue (P = 0.006) (Figure 7).
Figure 7.
Effect of RIPC on tissue NF-κB activity. Fourteen hours after an LPS or saline challenge, NF-κB activity was measured in pulmonary tissue of four experimental groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. All data are presented as mean ± standard error of the mean from the four experimental groups. *P < 0.05 versus the saline-only and RIPC/saline groups; #P < 0.01 versus the LPS-only group. NF-κB, nuclear factor kappa B; LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
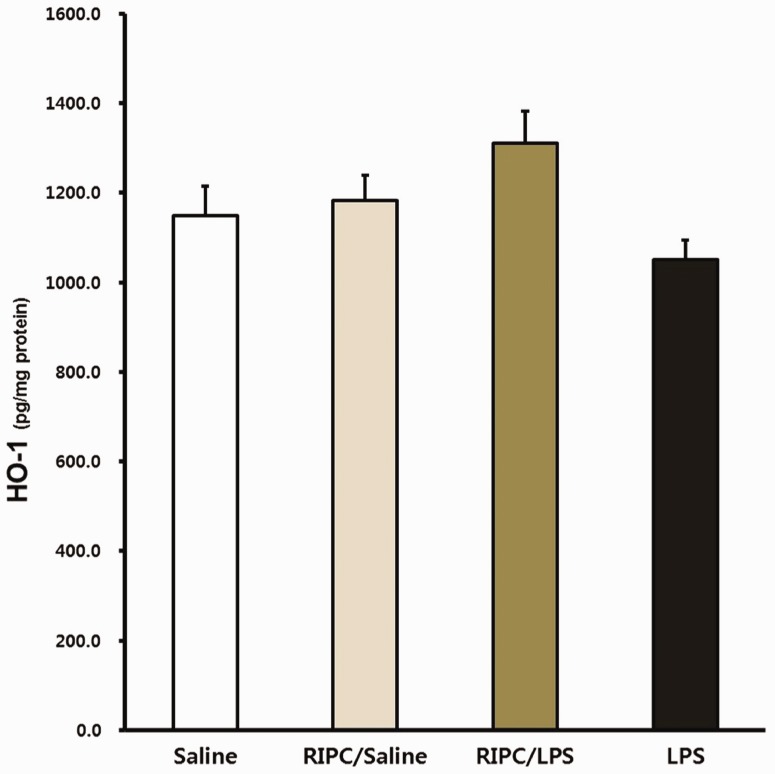
Effects of RIPC on HO-1 expression
No significant difference in HO-1 expression in lung tissue was found among the groups at 14 hours. HO-1 expression levels in the RIPC/LPS group appeared to be higher than those in the LPS-only group, but this was not significant (Figure 8).
Figure 8.
Effect of RIPC on HO-1 expression. Fourteen hours after an LPS or saline challenge, HO-1 expression was measured in pulmonary tissue of the four experimental groups (n = 8 per group). (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. All data are presented as mean ± standard error of the mean from the four experimental groups. HO-1, heme oxygenase-1; LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
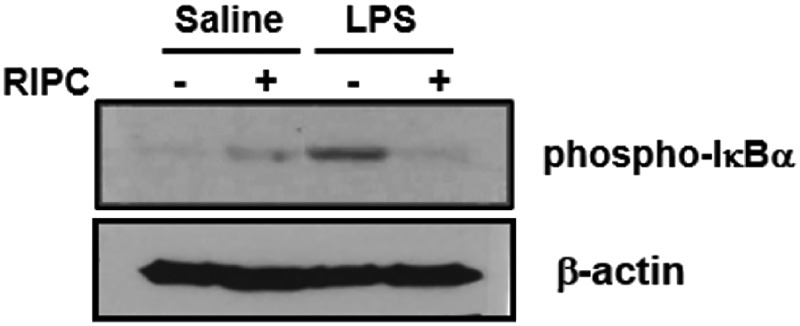
Effect of phosphorylation of IκBα
At 3, 6, 14, and 18 hours after endotoxin injection, IκBα levels were evaluated in lung tissue. RIPC decreased phosphorylation of IκBα in lung tissue at 14 hours after LPS-induced inflammation (Figure 9).
Figure 9.
Effect of RIPC on the level of phosphorylation of IκBα. Fourteen hours after an LPS or saline challenge in the four experimental groups (n = 8 per group), phosphorylation of IκBα in lung tissue was detected using western blotting with phospho-IκBα (Ser32) antibodies. (a) Saline-only group, (b) RIPC/saline group, (c) RIPC/LPS group, (d) LPS-only group. IκBα, inhibitor of κBα; LPS, lipopolysaccharide; RIPC, remote ischemic preconditioning
Discussion
The purpose of this study was to evaluate defensive effects on the lungs and related mechanisms associated with RIPC in an indirect ALI model. LPS-induced ALI was characterized by upregulated expression of NF-κB, production of pro-inflammatory cytokines, and destructive histopathologic changes. Our results indicated that RIPC alleviated LPS-induced inflammatory responses and increased survival rates by modifying the NF-κB-mediated signaling pathway through modulating phosphorylation of IκBα in the lungs.
LPS-injected mice began to die within 16 hours. Therefore, to establish the best time point to measure NF-κB and evaluate tissue, preliminary studies were performed at 1, 6, and 14 hours after LPS administration. Our preliminary study (data not shown) showed that NF-kB levels began to increase 1 hour after LPS injection and were then maintained at high NF-κB levels until 14 hours after LPS injection. Therefore, we performed histological examinations and measured inflammatory cytokines 14 hours after LPS injection in this study.
Disruption of lung epithelial barriers, increased permeability of the alveolar-capillary barrier after an infection, and the resultant pulmonary edema with a proteinaceous alveolar exudate are characteristics of ALI. In our study, analysis of lung pathology using optical microscopy showed that, when RIPC was performed, pathological changes in lung tissue, alveolar bleeding, and infiltration of neutrophils caused by LPS significantly improved. This led to a significant difference in the lung injury score, which quantified these improvements. Moreover, the lung W/D weight ratio, which is widely used to evaluate lung tissue edema, was significantly lower in the RIPC/LPS group than in the LPS-only group.
Lung inflammation can occur as a result of successive inflammatory responses initiated by peritoneal injection of LPS. TNF-α, IL-1ß, and IL-6 are important cytokines related to the inflammatory response process of ALI.19,20 We evaluated the amount of pro-inflammatory cytokines in BALF 14 hours after an LPS challenge. TNF-α, IL-1β, and IL-6 levels remarkably increased in LPS groups. However, RIPC markedly reduced production of cytokines in the pulmonary tissue of LPS-injected mice.
Neutrophils have a major role in ALI.21 Activated neutrophils are able to extravasate and migrate into the alveolar space. In this study, we observed MPO activity, which guides neutrophilic infiltration.22 RIPC significantly reduced MPO activity, which indicated that this procedure may have a critical role in reducing neutrophilic infiltration. Additionally, activation of neutrophils is highly associated with pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6.23 NF-κB, which is related to worse clinical outcomes, is activated by LPS and regulates many pro-inflammatory cytokines that are important in development of ALI.24–26 We found that RIPC reduced activation of NF-κB. Therefore, the protective effects of RIPC in LPS-induced ALI may be due to disruption of the NF-κB signaling pathway, which is a critical step in initiating neutrophilic inflammation.
Inhibiting NF-κB expression reduces pulmonary infiltration by leukocytes and reduces lung edema.16 In our study, the RIPC/LPS group showed significantly lower NF-kB expression and IκBα phosphorylation compared with the LPS-only group. IκB phosphorylation leads to NF-κB nuclear translocation and plays a major role in controlling NF-κB expression. Our results suggest that the lung protective effect of RIPC decreases NF-κB expression through inhibiting IκBα phosphorylation. This action is similar to inhibition of NF-κB expression caused by drugs, such as aspirin and salicylate, which inhibit phosphorylation of IκBα.27 Przyklenk et al.9 first attempted RIPC and demonstrated that it had a protective effect on the heart when they applied non-lethal ischemia–reperfusion to organs far from the heart. This protective effect occurred in the heart, kidney, liver, and lungs.15,28,29 Based on previous findings, the mechanism of signal transmission of remote ischemic conditioning that protects distal organs is highly complex and obscure. However, with activation of peripheral sensory nerves, remote ischemic conditioning is associated with transmission of protective signals from the stimulated region to the target organ by a neuronal pathway and humoral factors, transmission through a systemic response, and with potential intracellular signaling mediators within a target organ.30 Based on these mechanisms, the systemic protective response controls gene expression in intracellular inflammatory pathways by modulating immune cells at the post-translational level or through transcriptional regulation.31 An association between the protective effect of remote ischemic conditioning and NF-κB (especially IκBα phosphorylation), which is the main mechanism of systemic protective responses, indicates a protective effect of RIPC on contrast-induced nephropathy.32 This finding also corresponds to our finding that performing RIPC reduced ALI (caused by LPS) and affected IκBα phosphorylation.
To determine the defensive mechanism of RIPC against LPS-induced lung injury, production of HO-1 was also examined in our study. A previous report showed that RIPC increased HO-1 expression in the kidney, heart, and skeletal muscles, but not in the lungs or skin.33,34 Our results also showed higher HO-1 activation in the RIPC/LPS group compared with the other groups, but this difference was not significant.
Sepsis is the major cause of ALI, and can develop through the following two pathogenetic pathways: direct pulmonary injury or indirect pulmonary injury.21 There is no difference in the degree of neutrophilic infiltration in the indirect lung injury model compared with the direct pulmonary injury model.35 Previous studies assessed damage to the pulmonary epithelium caused by IP exposure to LPS in the indirect pulmonary injury model.36 We only showed that RIPC inhibited infiltration of neutrophils into the alveolar space in an IP LPS injection model. Therefore, further research on the effect of RIPC in a direct pulmonary injury model is required.
Early regulation of neutrophilic activation, which is an important factor in lung injury, is associated with improved outcomes in ALI. Therefore, early use of RIPC could prove beneficial in the treatment of ALI.37 RIPC data from animal models appear promising, but these data merely provide a bridge between the laboratory bench and human patients.38,39 Nonetheless, the current study has implications for more specific additional mechanisms, such as NF-κB and IκBα phosphorylation, in the lungs. The results of these experimental studies can be the basis for several clinical applications. Similar to intermittent pneumatic compression, which is routinely applied to intensive care unit patients, alternative or variant methods of RIPC may be applied in the clinical setting. Further studies are required to establish the potential role of RIPC in the treatment of ALI in humans.
Conclusions
RIPC increases the survival rate and decreases several parameters of LPS-induced ALI, such as production of pro-inflammatory cytokines, neutrophilic infiltration, and destructive pulmonary histological changes. In indirect ALI, the most likely anti-inflammatory mechanism of RIPC is modification of NF-κB-mediated cytokine expression via a direct protective effect through IκBα phosphorylation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (No. NRF-2015R1A2A2A01006734).
References
- 1.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004; 30: 51–61. [DOI] [PubMed] [Google Scholar]
- 2.Sheu CC, Gong MN, Zhai R, et al. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest 2010; 138: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–1349. [DOI] [PubMed] [Google Scholar]
- 5.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 2014; 20: 3–9. [DOI] [PubMed] [Google Scholar]
- 6.Ren C, Gao X, Steinberg GK, et al. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 2008; 151: 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapuria N, Kumar Y, Habib MM, et al. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury–a review. J Surg Res 2008; 150: 304–330. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Birch SE, He R, et al. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg 2010; 251: 292–299. [DOI] [PubMed] [Google Scholar]
- 9.Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic ‘preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993; 87: 893–899. [DOI] [PubMed] [Google Scholar]
- 10.Bagcik E, Ozkardesler S, Boztas N, et al. [Effects of dexmedetomidine in conjunction with remote ischemic preconditioning on renal ischemia-reperfusion injury in rats]. Rev Bras Anestesiol 2014; 64: 382–390. [DOI] [PubMed] [Google Scholar]
- 11.Olguner CG, Koca U, Altekin E, et al. Ischemic preconditioning attenuates lipid peroxidation and apoptosis in the cecal ligation and puncture model of sepsis. Exp Ther Med 2013; 5: 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong J, Wu ZY, Qi H, et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br J Pharmacol 2014; 171: 3539–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filgueiras LR, Jr., Martins JO, Serezani CH, et al. Sepsis-induced acute lung injury (ALI) is milder in diabetic rats and correlates with impaired NFkB activation. PLoS One 2012; 7: e44987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Song Y, Zhao W, et al. Small interfering RNA targeting NF-kappaB attenuates lipopolysaccharide-induced acute lung injury in rats. BMC Physiol 2016; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Yoon DW, Kim JH, et al. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J Inflamm (Lond) 2014; 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DS, Han W, Chen SM, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 2007; 178: 6504–6513. [DOI] [PubMed] [Google Scholar]
- 17.Schingnitz U, Hartmann K, Macmanus CF, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 2010; 184: 5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speyer CL, Gao H, Rancilio NJ, et al. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am J Pathol 2004; 165: 2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 2004; 202: 145–156. [DOI] [PubMed] [Google Scholar]
- 20.Goodman RB, Pugin J, Lee JS, et al. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 2003; 14: 523–535. [DOI] [PubMed] [Google Scholar]
- 21.Perl M, Lomas-Neira J, Venet F, et al. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med 2011; 5: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Q, Dong N, Yao X, et al. Bufexamac ameliorates LPS-induced acute lung injury in mice by targeting LTA4H. Sci Rep 2016; 6: 25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima T, Suarez CJ, Lin KW, et al. T cell pathways involving CTLA4 contribute to a model of acute lung injury. J Immunol 2010; 184: 5835–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2001; 281: L1037–L1050. [DOI] [PubMed] [Google Scholar]
- 25.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 2006; 290: L622–L645. [DOI] [PubMed] [Google Scholar]
- 26.Uwe S. Anti-inflammatory interventions of NF-kappaB signaling: potential applications and risks. Biochem Pharmacol 2008; 75: 1567–1579. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Zhang L, Joo D, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2017; 2: e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Diao Y, Chen G, et al. Remote ischemic conditioning for kidney protection: a meta-analysis. J Crit Care 2016; 33: 224–232. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Xu M, Wu Y, et al. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology 2014; 121: 249–259. [DOI] [PubMed] [Google Scholar]
- 30.Lim SY, Hausenloy DJ. Remote ischemic conditioning: from bench to bedside. Front Physiol 2012; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena P, Newman MA, Shehatha JS, et al. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg 2010; 25: 127–134. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Yin J, Lu Z, et al. Limb ischemic preconditioning protects against contrast-induced nephropathy via renalase. EBioMedicine 2016; 9: 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamion F, Richard V, Renet S, et al. Intestinal preconditioning prevents inflammatory response by modulating heme oxygenase-1 expression in endotoxic shock model. Am J Physiol Gastrointest Liver Physiol 2007; 293: G1308–G1314. [DOI] [PubMed] [Google Scholar]
- 34.Cremers NA, Wever KE, Wong RJ, et al. Effects of remote ischemic preconditioning on heme oxygenase-1 expression and cutaneous wound repair. Int J Mol Sci 2017; 18: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menezes SL, Bozza PT, Neto HC, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol (1985) 2005; 98: 1777–1783. [DOI] [PubMed] [Google Scholar]
- 36.Rittirsch D, Flierl MA, Day DE, et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol 2008; 180: 7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 2003; 167: 1567–1574. [DOI] [PubMed] [Google Scholar]
- 38.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 295: L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004; 172: 2731–2738. [DOI] [PubMed] [Google Scholar]