Short abstract
Studies comparing gut microbiota profiles of inflammatory bowel disease (IBD) patients have shown several changes in microbiota composition, with marked reduction of local biodiversity relative to that of healthy controls. Modulation of the bacterial community is a promising strategy to reduce the proportion of harmful microorganisms and increase the proportion of beneficial bacteria; this is expected to prevent or treat IBD. The exact mechanism of fecal microbiota transplantation (FMT) remains unknown; however, replacing the host microbiota can reestablish gut microbial composition and function in IBD patients. The present report describes an ulcerative colitis patient who underwent FMT. A 17-year-old male with moderate to severe clinical activity, which was refractory to mesalazine, azathioprine, and infliximab, underwent FMT as alternative therapy. The patient exhibited clinical improvement after the procedure; however, the symptoms returned. A second FMT was performed 8 months after the first procedure, but the patient did not improve. In conclusion, despite the FMT failure observed in this patient, the procedure is a promising therapeutic option for IBD patients, and more in-depth studies of this method are needed.
Keywords: Fecal microbiota transplantation, ulcerative colitis, gastrointestinal microbiome, dysbiosis, inflammatory bowel disease, mesalazine, azathioprine, infliximab
Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) that is observed predominantly in developed countries; it is the result of interactions between genetic and environmental factors that are associated with changes in gut microbiota. These alterations activate immune and non-immune cells, causing chronic inflammation. The goal of therapy for UC is to induce and maintain steroid-free remission, both clinically and endoscopically.1 Maintenance therapy includes aminosalicylates, thiopurines, and anti-tumor necrosis factor therapy or vedolizumab.
Considering the importance of gut microbiota in the development of gastrointestinal tract diseases, there is a growing scientific interest in the use of microbiota modulation for IBD treatment, especially in patients with refractory UC. Studies comparing IBD patients and healthy individuals have shown changes in microbiota composition and a general reduction in local biodiversity.2 The present report describes a patient with refractory UC who underwent fecal microbiota transplantation (FMT) and includes a literature review. The study was approved by the local Research Ethics Committee (protocol CAAE: 66661317.3.0000.5411) and the patient provided written informed consent to participate.
Case report
A 17-year-old male Caucasian patient presented to a Dermatology appointment searching for acne treatment in 2011; at that time, alterations in liver enzymes were detected, as follows: aspartate aminotransferase = 51 U/L (<46 U/L), alanine aminotransferase = 124 U/L (<50 U/L), gamma-glutamyl transpeptidase (GGT) = 73 U/L (<60 U/L), and alkaline phosphatase = 113 U/L (<115 U/L). The patient was referred for specialized evaluation; a discrete hepatomegaly was found, with no other clinical changes. Viral hepatitis markers were negative. Antinuclear antibody titer was 1/80, gamma globulin was 1.7 g/dL (<1.5 g/dL), and anti-mitochondria and anti-smooth muscle antibodies were negative. Liver ultrasonography was normal. A liver biopsy was performed, containing 35 portal spaces. The hepatic parenchyma showed a preserved structure, with discrete fibrous expansion and moderate mononuclear infiltrate. According to the autoimmune hepatitis scoring system, these findings of mild histological activity were compatible with autoimmune hepatitis. The first medical treatment comprised prednisone 40 mg/day, which achieved normalization of liver functional tests after 2 months. However, the patient maintained high GGT levels (88 U/L; reference: < 60 U/L). Despite the persistent alteration in GGT levels, the prednisone was gradually reduced to 15 mg/day, and azathioprine 50 mg/day was started. After 4 months, the patient stopped azathioprine use on his own. In July 2012, 1 year after the initial diagnosis, the patient underwent another liver biopsy containing > 40 portal spaces; this showed a preserved liver structure, with portal septa formation and a moderate portal lymphocytic infiltrate, as well as many plasma cells and ductal epithelium aggression. Partial periductal concentric fibrosis was observed, suggesting a diagnosis of primary sclerosing cholangitis (PSC).
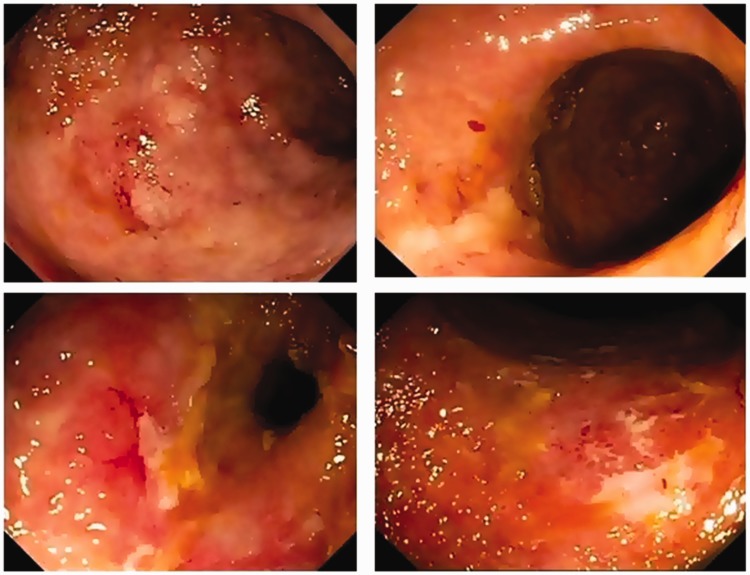
After 6 months of starting medical treatment for autoimmune hepatitis, in December 2011, the patient exhibited bloody diarrhea, with eight bowel movements per day and severe abdominal pain. A colonoscopy showed intense inflammation of the entire colon, characterized by marked erythema, friability, spontaneous bleeding, and ulceration, compatible with UC exhibiting moderate to severe disease activity (Mayo endoscopic score = 2). Histological examination revealed intense colitis and crypt microabscesses. Mesalazine 3 g/day was initiated and prednisone was increased to 60 mg/day; however, despite clinical improvement, the symptoms relapsed after stopping corticosteroid treatment. Azathioprine (2 mg/kg/day) was reintroduced, but no clinical response was achieved; furthermore, the patient became corticosteroid-dependent, maintaining bloody diarrhea, abdominal pain, tenesmus, rectal urgency, fever, asthenia, weakness, and malaise. A second colonoscopy showed moderate endoscopic activity, classified as Mayo endoscopic score of 2 (Figure 1), characteristic of refractory disease.
Figure 1.
Colonoscopy photographs showing erythema, absent vascular pattern, friability, and erosions characteristic of moderate to intense inflammatory activity (Mayo endoscopic activity = 2).
The use of infliximab (IFX) 5 mg/kg, combined with azathioprine, was indicated for induction and maintenance therapy; this produced incomplete clinical and endoscopic responses. The patient continued receiving combined therapy for 2 years, demonstrating clinical recurrence even when receiving corticosteroids, as well as after biological therapy optimization, which consisted of increasing the IFX dose to 10 mg/kg and decreasing the application interval to every 4–6 weeks. A new colonoscopy showed intense endoscopic activity (Mayo score of 3). A colectomy was indicated, but was refused by the patient.
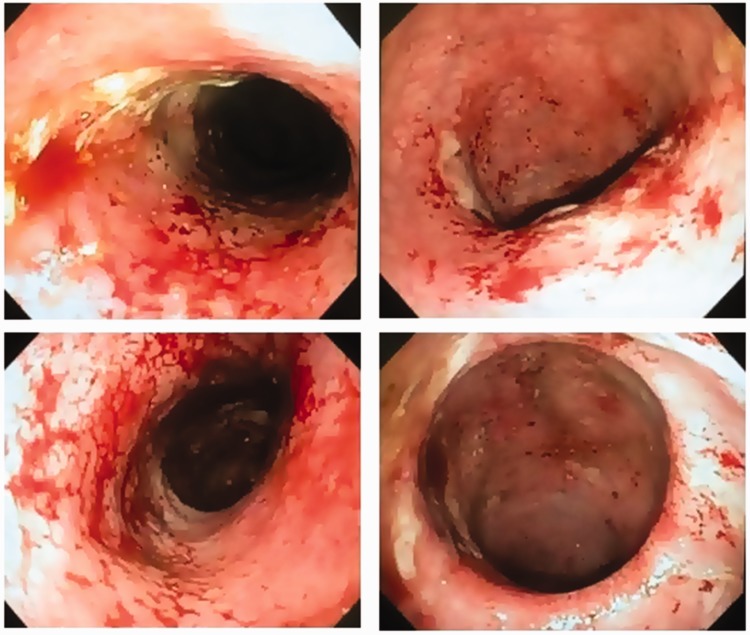
FMT was proposed as an alternative therapy, using fresh feces donated by a close relative (the patient’s father). The donor denied a history of IBD or irritable bowel syndrome; was screened for viral, bacterial, and protozoal infection; and was advised not to use antibiotics for a period of 3 months prior to the procedure. The patient received the same advice regarding antibiotic usage. The amount of stool was approximately 50 grams; this was diluted in saline solution to reach a total volume of 200 mL. The stool was prepared in an aerobic environment and the fecal suspension was infused into the patient's cecum through colonoscopy; this led to clinical improvement that lasted for 1 month, including reduction of the number of bowel movements and cessation of blood and mucus elimination. Subsequently, the symptoms recurred and biological therapy was modified to include the use of adalimumab, with no response. A second FMT was performed 8 months after the first procedure; however, the patient did not show improvement (Figure 2). A colectomy was again indicated, but was refused by the patient. The patient was included in a clinical trial and is receiving anti-integrin therapy; this has achieved partial control of the inflammatory activity, but corticosteroid treatment remains necessary. The clinical treatment and disease activity of the patient are shown in Table 1.
Figure 2.
Colonoscopy photographs showing erythema, friability, spontaneous bleeding, and ulceration characteristic of intense inflammatory activity (Mayo endoscopic activity = 3).
Table 1.
Clinical and endoscopic disease activity, C-reactive protein and medications according to the patient evolution.
| July 2011 | Dec 2011 | 2012–2014 | May 2015 | Jun 2015 | Jan 2016 | Mar 2016 | Aug 2016 – Now | |
|---|---|---|---|---|---|---|---|---|
| Partial Mayo Score (points) | – | 8 | 3–8 | 8 | 8 | 8 | 8 | 8 |
| Mayo Endoscopic Score | – | 2 | 2–3 | 3 | – | 3 | 3 | 3 |
| C-reactive protein (mg/dl) | – | – | 1.3–12.0 | – | 35.40 | 6.0 | – | 13.8 |
| Medications: | ||||||||
| Prednisone (mg/day) | 40 | 60 | 30–60 | 30 | 15–40 | 30 | 20–30 | 30–60 |
| Azathioprine (mg/day) | 50 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
| Mesalazine (g/day) | – | 3 | – | – | – | – | – | – |
| Infliximab (mg/kg) | – | – | 5–10 | – | – | – | – | – |
| Adalimumab (mg) | – | – | – | – | 160, then 80, then 40 | 40 eow | 40 per week | – |
| Anti-integrin (clinical trial) | – | – | – | – | – | – | – | X |
| Fecal microbiota transplantation | X | X | ||||||
| Indication of colectomy | X | X |
eow: every other week
Discussion
FMT consists of the infusion of a fecal suspension from a healthy individual into a recipient gastrointestinal tract in an attempt to treat a specific disease. FMT emerged as an effective treatment for recurrent Clostridium difficile infection, which led to speculation that it could be used in therapy for other bowel diseases, such as IBD.3 The first successful FMT procedure in a UC patient was reported in 1989 by Bennet and Brinkman, who observed prolonged clinical remission after administration of single fecal enema in a UC patient.4 More than a decade later, six UC patients who were non-responsive to conventional therapy were treated with a single daily enema for 5 consecutive days; this approach achieved improvement in clinical symptoms, serum biomarkers, and endoscopic findings throughout a follow-up period of 1 to 13 years.5 The efficacy of FMT for induction of clinical remission has also been demonstrated by recent studies, such as a systematic review published in 20126 and a meta-analysis published in 2014.7 However, we did not obtain sustained clinical and endoscopic improvement after FMT in the present case. In some reports,8,9 clinicians have applied serial FMT infusions, which may have contributed to the establishment of the bacteria in the affected intestine, thereby favoring improved results. Notably, some case reports have shown good results, even when a single infusion is performed.4,10
A pilot study was performed in China11 to evaluate the efficacy and safety of a new methodology, the “step-up” FMT strategy. Patients who did not respond positively to the first round of FMT received a second round of FMT; patients who did not respond positively to the second round of FMT were switched to steroid therapy. In total, 57.1% of patients achieved clinical improvement, suggesting that this methodology might be an effective strategy for steroid-dependent UC patients.11 The intestinal modulation promoted by serial FMT could alter the host immune status and intestinal barrier, improving clinical response to medications.11,12 Although serial FMT was not performed in the present case, our patient received a lower dose of corticosteroid (15–40 mg/day) after both procedures, compared with the previous doses (30–60 mg/day).
The fecal solution can be infused through the upper digestive tract through nasogastric/nasoenteric tubes or through upper digestive endoscopy. However, this route of infusion has been associated with the presence of side effects, such as regurgitation, aspiration of fecal contents, nausea, and vomiting.3 It can also be infused through the lower digestive tract, via enema, sigmoidoscopy, or colonoscopy.3 Although fecal enemas are inexpensive and easily applied at home, the use of colonoscopy in FMT is preferred because it is better accepted by patients and allows infusions throughout the colon. The combination of enemas and sigmoidoscopy may also be an option,9 especially when the patient exhibits severe colitis and colonic distention.3 The stool preservation mode may influence the clinical response to FMT. The use of fresh stool has shown better results than frozen or lyophilized stool, as discussed by Zhang et al.12 in a recent review; those authors also discussed the concepts of selective microbiota transplantation, methods of FMT delivery, and the “step-up” strategy.12
Although the effectiveness of FMT in IBD patients may be related to recipient conditions, it may also be related to the donor stool. As suggested by previous studies, the donor should be healthy, not taking antibiotics in the most recent 3 months, and not exhibit any evidence of viral hepatitis, human immunodeficiency virus, or syphilis.3 However, some questions remain unanswered: Who are the best donors for IBD patients? Should donors be intimate or universal? Is a multi-donor pool better than a single donor? In a systematic review, Andrews et al.13 reported that the use of stool from an intimate donor (family member or spouse/intimate partner) had the highest success rates (87.2% and 90.5%, respectively) in patients infected by C. difficile, compared with the use of unrelated donor stool (84%). However, recent studies using fecal preparations from “universal” donors showed success rates of approximately 90%.14
Two recent randomized, double-blind, placebo-controlled studies9,15 used a pool of stools from 3–7 donors and demonstrated good clinical and endoscopic remission rates in UC patients. Clinical remission was observed in 44% of those who received FMT, compared with 20% in the placebo group (relative risk: 2.2, 95% confidence interval 1.1–4.5; p = 0.021).9 Endoscopic remission was observed in 55% of patients receiving FMT, compared with 17% of patients receiving placebo (p < 0.01).15 The authors of that study reported that a pool of stools has greater microbial diversity than individual stool; they speculated that donor species richness may predict the therapeutic success of FMT for IBD. In the present case, at the time of the procedure, there was insufficient scientific evidence for the use of a multi-donor pool of stool, instead of single donor stool, for UC patients. Other factors related to the divergent results may be associated with different patterns of gut dysbiosis,16 different degrees of disease severity,7 age, and geographical areas.16 Gut microbiota composition varies among geographical regions16 and is closely related to diet and other life habits, such as prior antibiotic use, medications, previous infections, and lifestyle.16
IBD dysbiosis seems to be characterized by a reduced number of species and a reduced alpha diversity, particularly reduced proportions of Lachnospiracea, Clostridia, Bifidobacterium, Lactobacillus, and Faecalibacterium prausnitzii, as well as increased proportions of Bacteroidetes, Fusobacteria, Proteobacteria, and Escherichia coli.17 Microbiome analysis after FMT showed that stools from UC patients who responded to the procedure exhibited significant increases in microbial diversity at week 6, compared with placebo (p = 0.02).8 In the present case, a quantitative analysis of the presence of bacterial DNA in the feces of the recipient was performed prior to the procedure, which showed a reduced quantity of bacterial DNA in the sample. Unfortunately, analysis of the bacterial population was not performed after the stool transplantation procedure; this might have shown the success of the procedure if it had indicated increased bacterial diversity. Prior antibiotic use by the recipient is a controversial topic; although some studies recommend it,18 this practice has not been adopted in other protocols.8
In the present case, the patient presented PSC-UC. Studies have shown that patients with PSC exhibit alterations in intestinal microbiota, independently of comorbidity with IBD, with increased proportions of Enterococcus, Lactobacillus, and Fusobacterium, as well as reduced bacterial diversity.19,20 These prior findings supported the hypothesis that affected patients could benefit from intestinal microbiota modulation through FMT, as seen in patients with IBD.
In conclusion, the exact mechanism by which FMT acts in IBD patients remains unknown; however, microbiota replacement can restore gut composition and function in some of the affected patients. In addition, the role of FMT in IBD treatment needs further study to clarify relevant points, such as the appropriate time to perform the procedure, the best predictors of a positive response, the need for using concomitant medications, the best solution preparation for each section of the intestine, and the periodicity of application, as well as the ideal donor and the ideal recipient preparation prior to the procedure. Despite all of the inherent difficulties and uncertainties, FMT is a promising therapeutic option for IBD patients, and more in-depth and advanced studies on this topic are required.
Acknowledgements
We thank Botucatu Medical School at São Paulo State University (UNESP) for the support for this article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. DOI: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 2.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014; 146: 1489–1499. DOI: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt LJ, Aroniadis OC. An overview of fecal microbiota transplantation: techniques, indications, and outcomes. Gastrointest Endosc 2013; 78: 240–249. DOI: 10.1016/j.gie.2013.03.1329. [DOI] [PubMed] [Google Scholar]
- 4.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989; 1: 164. DOI: 10.1016/S0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 5.Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37: 42–47. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther 2012; 36: 503–516. [DOI] [PubMed] [Google Scholar]
- 7.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8: 1569–1581. DOI: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015; 149: 102–109. DOI: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017; 389: 1218–1228. DOI: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 10.Uygun A, Ozturk K, Demirci H, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine (Baltimore) 2017; 96: e6479. DOI: 10.1097/MD.0000000000006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med 2015; 13: 298. DOI: 10.1186/s12967-015-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Cui B, He X, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell 2018; 9: 462–473. DOI: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews P, Borody TJ, Shortis NP, et al. Bacteriotherapy for chronic constipation–long term follow-up. Gastroenterology 1995; 108: A563. DOI: 10.1016/0016-5085(95)26563-5. [Google Scholar]
- 14.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplantation for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107: 1079–1087. DOI: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 15.Costello SP, Waters O, Bryant RV, et al. Short duration, low intensity, pooled fecal microbiota transplantation induces remission in patients with mild-moderately active ulcerative colitis: a randomized controlled trial. Gastroenterology 2017; 152: S198–S199. DOI: 10.1016/S0016-5085(17)30969-1. [Google Scholar]
- 16.Serban DE. Microbiota in inflammatory bowel disease pathogenesis and therapy: is it all about diet? Nutr Clin Pract 2015; 30: 760–779. DOI: 10.1177/0884533615606898. [DOI] [PubMed] [Google Scholar]
- 17.Mondot S, Kang S, Furet JP, et al. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm Bowel Dis 2011; 17: 185–192. DOI: 10.1002/ibd.21436. [DOI] [PubMed] [Google Scholar]
- 18.Wang ZK, Yang YS, Chen Y, et al. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol 2014; 20: 14805–14820. DOI: 10.3748/wjg.v20.i40.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabino J, Vieira-Silva S, Machiels K, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016; 65: 1681–1689. DOI: 10.1136/gutjnl-2016-312137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodhouse CA, Patel VC, Singanayagam A, et al. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther 2018; 47: 192–202. DOI: 10.1111/apt.14397. [DOI] [PubMed] [Google Scholar]