Short abstract
Objective
This study was performed to assess the impact of risk factors on the presence and progression of coronary calcification in patients with type 2 diabetes.
Methods
We prospectively enrolled 45 patients without cardiovascular or kidney disease. Coronary calcification was measured with multidetector computed tomography at baseline and 18 months. We also measured blood pressure; body mass index; serum levels of calcium, phosphate, and 25-hydroxyvitamin D; mineral bone density; and levels of alkaline phosphatase, parathormone, fetuin-A, high-sensitivity C-reactive protein, fibrinogen, albumin, homocysteine, lipids, HbA1c, and average preprandial and postprandial blood glucose at 18 months. Information about severe hypoglycemia and smoking was recorded. Spearman’s correlation coefficients were calculated. Multiple linear regression was used for the multivariate analysis.
Results
The median baseline calcium score was 63, and that at 18 months was 100. In the univariate analysis, albumin was significantly correlated with the baseline calcium score. Fetuin-A and postprandial glycemia were correlated with calcium score progression. In the multivariate model, postprandial glycemia and fetuin-A were independently associated with calcium score progression.
Conclusions
Fetuin-A and postprandial glycemia influence coronary calcification progression in patients with type 2 diabetes. The absence of some correlations could be due to pharmacological treatments for cardiovascular risk reduction.
Keywords: Diabetes mellitus, type 2, (non-traditional/new) risk factors, coronary calcification, fetuin-A, hyperglycemia, postprandial
Introduction
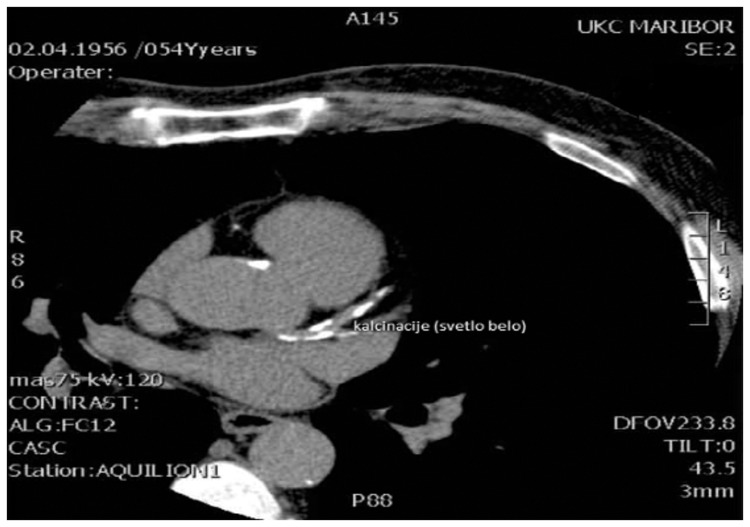
Cardiovascular disease (CVD), coronary artery disease in particular, is the leading cause of morbidity and mortality in patients with type 2 diabetes (T2D).1 The higher prevalence and rapid progression of CVD in these patients can only partly be explained by traditional risk factors such as arterial hypertension, dyslipidemia, and smoking. The search for other, less well-known risk factors is therefore warranted.2 In patients with T2D, coronary calcification appears in the arterial intima as part of the atherosclerotic process, but it also develops in the media as Monckeberg’s sclerosis of large and medium arteries. Coronary calcification is a tightly controlled and active process, similar to the formation of bone.3 Coronary calcification can easily be located and quantified by noncontrast cardiac computed tomography (CT), and multidetector spiral CT has mainly been used for this purpose in recent years.4,5 Figure 1 shows coronary calcification as seen on CT.
Figure 1.
Coronary calcification as seen on computed tomography.
The most extensively studied and most commonly used method of coronary calcification quantification was developed by Agatston. In this method, coronary calcification is quantified as the calcium score (CS).6,7 CS reflects the lifetime exposure to CVD risk factors.6 Many studies have revealed a connection between a higher CS and higher cardiovascular morbidity and mortality as well as a connection between a higher CS and higher all-cause mortality in the general population.3,8 These connections have also been proven in patients with T2D.9,10 In these patients, the CS seems to predict the CVD risk better than the Framingham or UK Prospective Diabetes Study risk assessment tools and can therefore improve CVD risk stratification.11–13 Measurement of CS progression offers better insight into the current disease activity and overall risk factor exposure during the studied period than does individual determination. No uniform definition of significant progression has been established. With newer protocols, the expected variability between two measurements taken 1 year apart is considered to be less than the expected CS progression in the same period.14 Some past studies have revealed a link between traditional CVD risk factors (such as blood pressure, age, and sex) and the CS in patients with T2D11 as well as an association between CS progression and traditional risk factors.15,16
In recent years, there has been a growing interest in potential new CVD risk factors in the general population and in patients with T2D. We included some of these risk factors in the present study. Among them is 25-hydroxyvitamin D (25-OHD), which is also a marker of vitamin D sufficiency: insufficiency of 25-OHD is more common in patients with than without T2D and seems to impact insulin sensitivity and beta-cell function.17,18 Lower vitamin D levels could be connected to all-cause and cardiovascular mortality.19 Homocysteine could also be a CVD risk factor in patients with T2D and perhaps an even more important risk factor than in the general population.20,21 Low-grade inflammation is a well-known process in patients with T2D. High-sensitivity C-reactive protein (hsCRP) and fibrinogen are markers of low-grade inflammation that have been linked to CVD risk and mortality in some studies of patients with T2D.22–24 T2D is characterized by chronic hyperglycemia and glucose fluctuations. Glucose fluctuations, especially postprandial hyperglycemia, have been shown to be harmful by deteriorating endothelial function, inflammation, and oxidative stress. In many studies, postprandial hyperglycemia (but not preprandial hyperglycemia) has been found to increase cardiovascular risk and mortality.25–27 Severe hypoglycemia (i.e., that requiring another person’s help) has also been independently linked to cardiovascular events.28–30
The bone-vascular axis could prove to be important for CVD risk. The bone mineral density (BMD) is higher in patients with than without T2D, but the fracture risk is paradoxically increased in T2D. In the general population, low BMD seems to be correlated with overall mortality.31–33 High-normal levels of serum phosphate could also be linked to increased cardiovascular mortality independently of kidney function.34 Serum calcium appears to be higher and parathormone appears to be lower in patients with than without T2D. The potential association of these factors with CVD risk is unknown.35,36
Fetuin-A is a systemic inhibitor of extraskeletal calcification. In patients with T2D, fetuin-A also increases insulin resistance through its action on insulin receptors. Lower fetuin-A levels could even be protective in some subpopulations of patients with T2D, such as those of advanced age.37,38 The association of serum alkaline phosphatase with CVD risk and mortality has also been reported in the general population.39 Serum albumin is an established risk predictor in many diseases and is being used in nutritional state assessment.40 In patients with T2D complications, especially diabetic nephropathy, a lower serum albumin level is associated with higher CVD risk.41
Both the presence and progression of coronary calcification appear to be clinically relevant in patients with T2D. Therefore, in the present study, we assessed the impact of various potential new risk factors on the presence and progression of coronary calcification in these patients.
Patients and methods
Patients
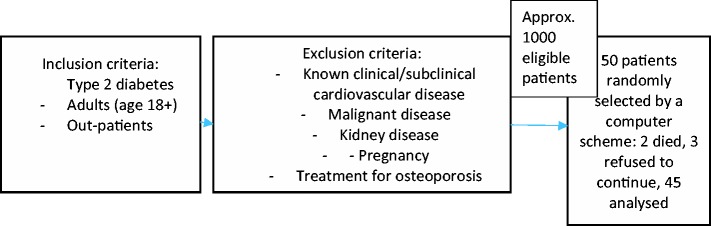
Randomly selected adult patients with T2D were enrolled in this prospective clinical study from 2008 to 2010, as shown in Figure 2. We excluded all patients with known clinical or subclinical CVD. Kidney disease was present when any one of the following three criteria was met: the estimated glomerular filtration rate was <60 ml/min/1.73 m2; one or more parameters of kidney injury were present, such as proteinuria, albuminuria, abnormal sediment, electrolyte and other disorders due to tubule injury, or histological or morphological abnormalities; or the patient had undergone kidney transplantation.
Figure 2.
Study flow chart with inclusion and exclusion criteria.
Ethics
All patients were diabetology outpatients of our institution (Maribor University Medical Centre) and were managed according to the newest professional recommendations. The Medical Ethics Commission of the Republic of Slovenia approved the study (N. 58/09/06, issued on 18 October 2006 and N.112/12/08, issued on 28 December 2008). All patients provided written informed consent after they had been comprehensively informed about the study’s aims, methods, and possible risks and had all their questions answered. The provisions of the Declaration of Helsinki were strictly followed.
Measurements
All measurements (including the patients’ scans) were performed twice at enrollment in the study and 18 months later; the exceptions were BMD and intact parathormone, which were measured only once at the beginning of the study. We measured coronary calcification according to Agatston (i.e., as the CS) with a 64-multidetector CT system (Aquilion; Toshiba, Tokyo, Japan). The process was prospectively gated at 75% of the R-R interval, the tube voltage was 120 kVp, and the current depended on the size and form of the patient (approximately 200–400 mA). We imaged the area between the trachea and cardiac apex during a single breath-hold. The average effective radiation dose in our patients was 1.7 mSv. Analysis was performed by a single radiologist (M.R.) with dedicated software (VScore; Vital Images, Minnetonka, MN, USA). Agatston units were used for reporting, which was done using Agatston’s method as previously described.
The morning after an overnight fast, blood samples were withdrawn for analysis into Vacutainer tubes (BD Biosciences, Plymouth, UK), according to the manufacturer’s requirements. The samples were then processed without delay in our institution’s central laboratory. Using standard methods, we measured the serum concentrations of calcium, phosphate, intact parathormone, cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, hsCRP, fibrinogen, alkaline phosphatase, 25-OHD, fetuin-A, homocysteine, and albumin. If an infectious or other obvious cause of hsCRP elevation was found, we resolved this cause and remeasured the hsCRP level. The patients performed self-measurement of their capillary blood glucose level with a glucometer available in Slovenia, usually the Accu-Chek (Roche, Basel, Switzerland) or the Contour (Bayer, Leverkusen, Germany). At minimum, three preprandial and three postprandial measurements were required each month. The patients were asked to measure the postprandial values 120 minutes after starting a meal. Self-measurements were otherwise performed according to the recommendations given at the regular outpatient clinic follow-ups. Blood glucose data were collected at the follow-up visits, and all available values were collected after 18 months and individually analyzed. The patients were instructed to write down all measured glucose values in a notebook and to bring the notebook, together with their glucometer, to a diabetologist, who they would typically see one to four times a year. The glucose measurements and technique with which they were obtained were discussed with the diabetologist and a diabetic nurse. The notebooks and glucometers were also compared for correctness of recording the values. The patients self-reported severe hypoglycemia and smoking (in pack-years). Severe hypoglycemia was reported at the time of the event.
We measured femoral neck BMD (in g/cm2 using dual X-ray absorbtiometry (Explorer; Hologic, Marlborough, MA, USA). Blood pressure was measured according to European guidelines.42
Statistical analysis
We performed the statistical analysis with SPSS for Windows, version 21 (IBM Corp., Armonk, NY, USA). Spearman’s correlation coefficient was used to assess the correlation between the CS, its absolute and relative progression, and the studied parameters (univariate analysis). Due to the non-normal positively skewed distribution of the CS, we ln-transformed it for multiple linear regression (CS ln (CS+1)). After the transformation, basic assumptions of multiple linear regression were satisfied. A p value of <0.05 was considered statistically significant. Spearman’s correlation coefficients for associations between the baseline parameters and baseline CS and between the baseline parameters and absolute and relative progression of the CS were calculated.
Results
In total, 45 patients with T2D were included in this study. The patients’ demographic and clinical characteristics are shown in Table 1.
Table 1.
Patients’ demographic and clinical characteristics.
| N | 45 |
| Sex | |
| Male | 23 (51) |
| Female | 22 (49) |
| Caucasian ethnicity | 45 (100) |
| Age, yrs | 59 ± 8 |
| Male | 60 ± 7 |
| Female | 58 ± 8 |
| Diabetes duration, yrs | 10 ± 8 |
| Male | 11 ± 9 |
| Female | 10 ± 8 |
| Smokers, past + present | 16 (36) |
| Male | 10 |
| Female | 6 |
| Baseline therapy | |
| Metformin | 35 (78) |
| Insulin | 20 (44) |
| Insulin + metformin | 11 (24) |
| Statin | 37 (82) |
| ACEI/sartan | 34 (76) |
| HbA1c, % | 7.2 ± 0.9 |
| Male | 7.3 ± 0.8 |
| Female | 7.1 ± 1.0 |
| Body mass index, kg/m2 | 31 ± 5 |
| Male | 32 ± 5 |
| Female | 30 ± 4 |
| Metabolic syndrome | 39 (87) |
| Male | 20 |
| Female | 19 |
Data are presented as average ± standard deviation, n (%), or n.
ACEI, angiotensin-converting enzyme inhibitor.
Comparison by sex showed no important differences in characteristics between men and women (p > 0.05) except for smoking, which was more common in men.
Measurements of the studied parameters (at baseline and 18 months) are presented in Table 2.
Table 2.
Parameters measured at baseline and 18 months.
| Parameter | Baseline (n = 45) | 18 months (n = 45) |
|---|---|---|
| Systolic blood pressure, mmHg | 152 ± 18 | 151 ± 16 |
| Diastolic blood pressure, mmHg | 86 ± 10 | 88 ± 11 |
| Calcium, mmol/L | 2.38 ± 0.11 | 2.34 ± 0.09 |
| Phosphate, mmol/L | 1.15 ± 0.17 | 1.11 ± 0.16 |
| 25-OHD, nmol/L | 58.0 ± 35.1 | 40.6 ± 18.9 |
| Femoral neck BMD, g/cm2 | 0.82 ± 0.16 | – |
| Alkaline phosphatase, μkat/L | 1.21 ± 0.30 | 1.21 ± 0.27 |
| iPTH, pg/ml | 36.2 ± 17.3 | – |
| Fetuin-A, ng/ml | 26.2 ± 5.5 | 28.0 ± 7.0 |
| Male | 25.9 ± 5.3 | 28.3 ± 7.3 |
| Female | 26.3 ± 5.6 | 27.7 ± 6.8 |
| hsCRP, mg/L | 6.9 ± 8.0 | 2.8 ± 2.6 |
| Fibrinogen, g/L | 4,04 ± 0,96 | 3.96 ± 0.82 |
| Albumin, g/L | 41.0 ± 3.7 | 39.7 ± 4.3 |
| Homocysteine, μmol/L | 10.8 ± 3.2 | 13.0 ± 5.2 |
| Triglycerides, mmol/L | 1.60 ± 1.14 | 1.84 ± 0.95 |
| Cholesterol, mmol/L | 4.80 ± 0.81 | 4.30 ± 1.01 |
| LDL cholesterol, mmol/L | 2.83 ± 0.71 | 2.47 ± 0.68 |
| HDL cholesterol, mmol/L | 1.31 ± 0.41 | 1.28 ± 0.45 |
| Preprandial glucose, mmol/L | – | 7.84 ± 0.84 |
| Postprandial glucose, mmol/L | – | 9.50 ± 1.62 |
| Male | 9.60 ± 1.68 | |
| Female | 9.40 ± 1 |
Data are presented as average ± standard deviation.
25-OHD, 25-hydroxyvitamin D; BMD, bone mineral density; iPTH, intact parathormone; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Comparison by sex showed no important difference in any of the listed characteristics between men and women (p > 0.05).
As shown in the tables, most patients did not reach the recommended targets (as suggested in the guidelines of professional societies such as the American Diabetes Association Standards of Medical Care in Diabetes) for blood pressure and LDL cholesterol, although most of them took appropriate medications. Most patients were overweight or obese and had metabolic syndrome. The average HbA1c and preprandial glucose levels should be interpreted in the context of individualization of glycemic goals.43
Measurements of the CS are presented in Table 3.
Table 3.
Analysis of CS.
| Baseline | 18 months | |
|---|---|---|
| Median CS | 63 | 100 |
| Range | 0–3213 | 0–3078 |
|
Interquartile range |
6–384 |
13–532 |
|
Category distribution* |
Patients, n (%) |
|
| CS (0) | 10 (22) | 8 (18) |
| CS (1–100) | 16 (36) | 15 (33) |
| CS (101–400) | 6 (13) | 11 (24) |
| CS (401–1000) | 7 (16) | 3 (7) |
| CS (>1000) | 6 (13) | 8 (18) |
*Categories correspond to increasing levels of cardiovascular risk.
CS, calcium score.
During the study, the patients performed 54 to 1652 preprandial glucose self-measurements and 54 to 609 postprandial glucose self-measurements. Two events consistent with the definition of severe hypoglycemia occurred in two different insulin-treated patients.
Table 4 shows the statistically significant Spearman’s correlation coefficients between the baseline parameters and baseline CS and between the baseline parameters and absolute and relative progression of the CS. We found a significant correlation between fetuin-A and the relative change in the CS (p = 0.02) and between albumin and the baseline CS (p = 0.03). Postprandial glucose was significantly correlated with both absolute and relative changes in the CS (p = 0.049 and 0.01, respectively). All other correlations between the baseline CS, its absolute or relative progression in 18 months, and the studied parameters were statistically insignificant. The studied parameters that we examined for correlations with the baseline CS and the absolute and relative change in the CS are listed in Table 2. We also examined the diabetes duration, smoking in pack-years, body mass index, and HbA1c for correlations with the baseline CS and the absolute and relative change in the CS; however, no significant correlations were found. We included the significant parameters in the multivariate analysis.
Table 4.
Spearman’s correlation coefficients.
| Parameter | Coefficient for baseline CS | Coefficient for absolute change in CS | Coefficient for relative change in CS |
|---|---|---|---|
| Fetuin-A | −0.133 | 0.041 | −0.345* (0.02) |
| Albumin | 0.329 (0.03)* | −0.012 | −0.076 |
| Postprandial glucose | 0.136 | 0.295 (0.049)* | 0.376 (0.01)* |
*Denotes statistical significance; p-values are shown in parentheses.
CS, calcium score.
The initial multiple linear regression model, which was based on simultaneous entry of postprandial glucose and fetuin-A as independent variables and the difference in ln-transformed CS as a dependent variable, was statistically significant (R = 0.497; p = 0.006; p for postprandial glucose = 0.038; p for fetuin-A = 0.044; standardized beta coefficient for postprandial glucose = 0.308; standardized beta coefficient for fetuin-A = −0.298). To yield the most appropriate regression equation, we also built several other models by a forward selection and backward elimination process. Other potentially important predictors based on published data (such as LDL cholesterol, HbA1c, age, sex, systolic blood pressure, and preprandial glucose) were added or deleted if not significant (threshold p = 0.05). These other models were not found to be statistically significant. None of the multivariate models that included the baseline CS as a dependent variable was significant.
Discussion
Past studies of the possible connections between coronary calcification and newer (non-traditional or emerging) CVD risk factors in patients with T2D have sometimes been conflicting, and some of our parameters have not yet been studied in this context.
In the present study, the concentration of 25-OHD was not correlated with the patients’ outcomes. The 25-OHD concentration was <75 nmol/L in 73% of patients at baseline and 96% of patients at 18 months. The 25-OHD concentration was <30 nmol/L in 18% of patients at baseline and 29% of patients at 18 months. According to the National Institutes of Health guidelines, a 25-OHD level of ≥50 nmol/L is sufficient in the vast majority of people.44 The differences in these percentages are influenced by the seasonal variation of ultraviolent light exposure, which is known to impact the findings of studies involving vitamin D, and which we did not take into account.45 Joergensen et al.46 found that very low 25-OHD levels (<12.5 nmol/L) were independently associated with a higher CS, but only in patients with albuminuria; such patients were excluded from our study. Among patients with diabetes in the MESA study, a low 25-OHD level was correlated with the appearance of coronary calcification during the study period.47 Our findings regarding the absence of a correlation between homocysteine/hsCRP and coronary calcification are consistent with those of the PREDICT study.48 Some authors have considered that hsCRP reflects the dynamic processes of arterial plaque remodeling (rupture, thrombosis, and instability) more accurately than the presence of atherosclerosis and calcification.49,50 Contrary to our data, which did not confirm the link between fibrinogen and the outcomes in patients with T2D, some data have shown that fibrinogen is independently associated with coronary calcification progression in patients with type 1 diabetes.51 In our patients, who performed self-measurements of glucose for 18 months, the average postprandial glycemia (but not preprandial glycemia or HbA1c) seemed to be an important factor affecting the progression of coronary calcification; this was also true in the multivariate model that included postprandial glycemia and fetuin-A. Postprandial hyperglycemia is known to worsen endothelial dysfunction (including impaired vasodilation, the synthesis and availability of nitric oxide, and the formation of advanced glycation end products), inflammation, and oxidative stress (characterized by expression of adverse adhesion molecules and cytokines, activation of lymphocytes and monocytes, and formation of free oxygen species). These factors contribute to vascular smooth muscle cell proliferation, plaque formation, and calcification of the arterial intima and media.27 However, the role of postprandial glucose in cardiovascular outcomes is not universally established. In the HEART2D study, which involved the treatment of diabetic survivors of acute myocardial infarction with prandial versus basal strategies, the data showed differences in fasting blood glucose levels, smaller-than-expected differences in postprandial blood glucose levels, similar levels of HbA1C, and no difference in the risk of future cardiovascular events.52 In the NAVIGATOR trial, the most powerful independent predictors of cardiovascular events in patients with impaired glucose tolerance included both established risk factors and other variables, excluding measures of glycemia such as 2-hour postprandial glucose.53 In Chinese patients with coronary heart disease and impaired glucose tolerance, acarbose, which primarily impacts postprandial glucose, did not reduce the risk of major adverse cardiovascular events but did reduce the incidence of diabetes.54 However, these studies differed in their design and populations. The resolution of unanswered questions requires the inclusion of postprandial glycemia in future research of CVD. The incidence of severe hypoglycemia was too low in our study (two events in two patients) to reach valid conclusions about its effect on the outcomes. A study by Saremi et al.55 indicated that in patients undergoing standard therapy for T2D, serious hypoglycemia was associated with increased coronary calcification. Femoral neck BMD did not impact the outcomes of our study; in contrast, Bandeira et al.56 found that femoral neck and lumbar spine BMD were correlated with coronary calcification in men of advanced age with diabetes. More data on this topic are expected from the Diabetes Heart Study and its bone substudy.57 The serum calcium, phosphate, and parathormone levels did not influence coronary calcification or its progression in patients with T2D, which has not been previously studied. Some data indicate negative cardiovascular effects of high-normal serum phosphate34 and high serum calcium35,58 in patients with T2D; these effects are in the opposite direction from those of the above-mentioned parameters. Fetuin-A seemed to inhibit the progression of coronary calcification in our patients. The effect of fetuin-A on CVD is known to be greatly influenced by the presence of diabetes mellitus.59 Emoto et al.60 reported a link between fetuin-A and carotid and femoral plaques in patients with T2D without kidney disease, which also illustrates the protective effect of fetuin-A against arterial calcification. The present data suggest that the inclusion of fetuin-A in CVD studies is reasonable, preferably also with insulin resistance parameters.
Alkaline phosphatase was not correlated with patient outcomes in our study. In the univariate analysis, we found that serum albumin was correlated with the baseline CS. All of our patients had normal albumin levels, and most were overweight and obese; none of them had malnutrition. We consider this finding coincidental because it was not reproduced when the albumin level at 18 months and the average albumin level (at baseline and 18 months) were analyzed for the same correlation with the outcomes. Additionally, the p value of this correlation was relatively high (0.03). No published data indicate a link between serum albumin and coronary calcification in patients with T2D. There were no correlations of the CS or its progression with traditional CVD risk factors (blood pressure, body mass index, cholesterol, LDL cholesterol, HDL cholesterol, and smoking in pack-years). The absence of such correlations is most likely due to the fact that most patients used medications aimed at multifactorial cardiovascular risk reduction (antihyperglycemics, antihypertensives, and hypolipemics). Because the vast majority of our patients were treated with various angiotensin-converting enzyme inhibitors or sartans (76%) and statins (82%) during the study, and most of the remaining patients had been treated with such medications in the past, we did not estimate the effects of these drugs on the results. For most patients with T2D with very high cardiovascular risk, the LDL cholesterol and blood pressure values were above the recommended targets.43
In our study, a clear minority of patients had no detectable atherosclerosis; i.e., they had undetectable coronary calcification (CS of 0; 22% at baseline and 18% at 18 months). Such patients are thought to have lower mortality rates comparable with those of people without diabetes.11 In two patients with a baseline CS of 0, coronary calcification appeared after 18 months.
Most of the parameters in our study were measured and not reported by the patients. Comprehensive medical documentation was available for all of the patients. Our patients were enrolled from everyday clinical practice and were heterogeneous in their therapies, age, and duration of diabetes. They were managed according to the newest professional recommendations. CS measurements were performed by a single radiologist who was unaware of the patients’ characteristics.
This study has several limitations. Our study sample was small due to limited funding and staff, which influenced the probability of accurate determination of the results’ significance, especially for weaker correlations. The CS is a marker of coronary artery disease and not a hard point. Power calculations for multiple regression nevertheless allowed four predictors to be tested with our sample size. The study duration (18 months) could have been too short for some parameters to influence the CS. Only Caucasians were included in our study. The inter-rater variability for the presence or absence of calcification and the total CS was not checked. Glucose self-measurements were performed and reported by the patients themselves and therefore might have sometimes been unreliable or false, although we tried to prevent this as described in the Methods. The generalizability of our results to all patients with T2D is limited because of the exclusion criteria. Other factors that might have contributed to the results include the chosen linear regression models, omission bias, and accuracy of self-reported smoking. There is possibility of type 1 error for the significance of postprandial glycemia and fetuin-A. The lack of statistical significance of the associations of the other investigated predictors could have been type 2 error due to omission bias and/or model selection. Additionally, the results for fetuin-A are not necessarily robust because the absolute difference in the CS was not significant. Evaluation of relative differences in the CS carries the risk of heavier weighting of patients with a low CS.
In conclusion, postprandial glycemia and fetuin-A seem to be associated with the progression of coronary artery calcification in patients with T2D, but larger studies are needed to replicate these findings. Serum calcium, phosphate, parathormone, alkaline phosphatase, fibrinogen, and traditional risk factors did not influence coronary calcification or its progression in our study. Again, larger studies are necessary to replicate these findings.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Sattar N. Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia 2013; 56: 686–695. [DOI] [PubMed] [Google Scholar]
- 2.Zittermann A, Koerfer R. Vitamin D in the prevention and treatment of coronary heart disease. Curr Opin Clin Nutr Metab Care 2008; 11: 752–757. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol 2009; 6: 681–688. [DOI] [PubMed] [Google Scholar]
- 4.Budoff MJ, Gul KM. Expert review on coronary calcium. Vasc Health Risk Manag 2008; 4: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006; 114: 1761–1791. [DOI] [PubMed] [Google Scholar]
- 6.Erbel R, Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: subclinical atherosclerosis: the memory of lifetime risk exposure. Eur Heart J 2012; 33: 1201–1213. [DOI] [PubMed] [Google Scholar]
- 7.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. J Am Coll Cardiol Img 2009; 2: 675–688. [DOI] [PubMed] [Google Scholar]
- 8.Oudkerk M, Stillman AE, Halliburton SS, et al. Coronary artery calcium screening: current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Eur Radiol 2008; 18: 2785–2807. [DOI] [PubMed] [Google Scholar]
- 9.Kramer CK, Zinman B, Gross JL, et al. Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ 2013; 346: f1654. doi: 10.1136/bmj.f1654. [DOI] [PubMed] [Google Scholar]
- 10.Kiramijyan S, Ahmadi N, Ismaeel H, et al. Impact of coronary artery calcium progression and statin therapy on clinical outcome in subjects with and without diabetes mellitus. Am J Cardiol 2013; 111: 356–361. [DOI] [PubMed] [Google Scholar]
- 11.Elkeles RS. Coronary calcium and cardiovascular risk in diabetes. Atherosclerosis 2010; 210: 331–336. [DOI] [PubMed] [Google Scholar]
- 12.Becker A, Leber AW, Becker C, et al. Predictive value of coronary calcifications for future cardiac events in asymptomatic patients with diabetes mellitus: a prospective study in 716 patients over 8 years. BMC Cardiovasc Disord 2008; 8: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand DV, Lim E, Lahiri A, et al. The role of non-invasive imaging in the risk stratification of asymptomatic diabetic subjects. Eur Heart J 2006; 27: 905–912. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy JW, Blaha MJ, DeFilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? J Am Coll Cardiol 2010; 56: 1613–1622. [DOI] [PubMed] [Google Scholar]
- 15.Elkeles RS, Godsland IF, Rubens MB, et al. The progress of coronary heart disease in type 2 diabetes as measured by coronary calcium score from electron beam computed tomography (EBCT): the PREDICT Study. Atherosclerosis 2008; 197: 777–783. [DOI] [PubMed] [Google Scholar]
- 16.Anand DV, Lim E, Darko D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes. J Am Coll Cardiol 2007; 50: 2218–2225. [DOI] [PubMed] [Google Scholar]
- 17.Stivelman E, Retnarakan R. Role of vitamin D in the pathophysiology and treatment of type 2 diabetes. Curr Diabetes Rev 2012; 8: 42–47. [DOI] [PubMed] [Google Scholar]
- 18.Kositsawat J, Freeman VL, Geber BS, et al. Association of A1c levels with vitamin D status in U.S. adults: data from the National Health and Nutrition Examination Survey. Diabetes Care 2010; 33: 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joergensen C, Gall MA, Schmedes A, et al. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care 2010; 33: 2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogoveen EK, Kostense PJ, Jakobs K, et al. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes; 5-year follow-up of the Hoorn study. Circulation 2000; 1010: 1506–1511. [DOI] [PubMed] [Google Scholar]
- 21.Hoogoveen EK, Kostense PJ, Beks PJ, et al. Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin dependent diabetes mellitus: a population-based study. Arteriolscler Thromb Vasc Biol 1998; 18: 133–138. [DOI] [PubMed] [Google Scholar]
- 22.Kengne AP, Batty GD, Hamer M, et al. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status. Diabetes Care 2012; 35: 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulze MB, Rimm EB, Li T, et al. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care 2004; 27: 889–894. [DOI] [PubMed] [Google Scholar]
- 24.Soedamah-Muthu SS, Chaturvedi N, Pickup JC, et al. Relationship between plasma sialic acid and fibrinogen concentration and incident micro- and macrovascular complications in type 1 diabetes. Diabetologia 2008; 51: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2011; 10: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Node K, Inoue T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol 2009; 8: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006; 91: 813–819. [DOI] [PubMed] [Google Scholar]
- 28.Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347: f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 29.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 30.Duckworth WC, Abraira C, Moritz TE, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011; 25: 355–361. [DOI] [PubMed] [Google Scholar]
- 31.Mussolino ME, Gillum RF. Low bone mineral density and mortality in men and women: the Third National Health and Nutrition Examination Survey linked mortality file. Ann Epidemiol 2008; 18: 847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001; 86: 32–38. [DOI] [PubMed] [Google Scholar]
- 33.Strotmeyer ES, Cauley JA, Schwarty AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 2005; 165: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 34.Chonchol M, Dale R, Schrier RW, et al. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122: 380–386. [DOI] [PubMed] [Google Scholar]
- 35.Haglin L, Backman L, Tornkvist B. A structural equation model for assessment of links between changes in serum triglycerides, urate and glucose and changes in serum calcium, magnesium and phosphate in type 2 diabetes and non-diabetes metabolism. Cardiovasc Diabetol 2011; 10: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto M, Yamaguchi T, Nawata T, et al. Decreased PTH levels accompanied by low bone formation are associated with vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2012; 97: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin GA, Cummins KM, Wassel CL, et al. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. J Am Coll Cardiol 2012; 59: 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen MK, Bartz TM, Mukamal KJ, et al. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: the cardiovascular health study. Diabetes Care 2013; 36: 1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Shaper AG, Whincup PH, et al. Alkaline phosphatase, serum phosphate, and incident cardiovascular disease and total mortality in older men. Atherioscler Thromb Vasc Biol 2013; 33: 1070–1076. [DOI] [PubMed] [Google Scholar]
- 40.Fuhrman MP. The albumin-nutrition connection: separating myth from fact. Nutrition 2002; 18: 199–200. [DOI] [PubMed] [Google Scholar]
- 41.Anavekar NS, Gans DJ, Berl T, et al. Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: a case for albuminuria. Kidney Int Suppl 2004; 92: S50–S55. [DOI] [PubMed] [Google Scholar]
- 42.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014; 23: 3–16. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Standards of Medical Care in Diabetes. http://care.diabetesjournals.org/content/41/Supplement_1/S1 (accessed 5 September 2018).
- 44.National Institutes of Health, Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed 10 August 2018).
- 45.Rosecrans R, Dohnal JC. Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem 2014; 47: 670–672. [DOI] [PubMed] [Google Scholar]
- 46.Joergensen C, Reinhard H, Schmedes A, et al. Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care 2012; 35: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeBoer IH, Kestenbaum B, Schoben AB, et al. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 2009; 20: 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godsland IF, Elkeles RS, Feher MD, et al. Coronary calcification, homocysteine, C-reactive protein and the metabolic syndome in type 2 diabetes: the Prospective Evaluation of Diabetic Ischaemic Heart Disease by Coronary Tomography (PREDICT) Study. Diabet Med 2006; 23: 1192–1200. [DOI] [PubMed] [Google Scholar]
- 49.Swords JN, Brown ER, Detrano R, et al. Associations of inflammatory markers with coronary artery calcification: results from the multi-ethnic study of atherosclerosis. Atherosclerosis 2010; 209: 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis 2008; 201: 1–7. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues TC, Snell-Bergeon JK, Maahs DM, et al. Higher fibrinogen levels predict progression of coronary artery calcification in adults with type 1 diabetes. Atherosclerosis 2010; 210: 671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009; 32: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preiss D, Thomas LE, Sun JL, et al. Predictors of cardiovascular events in contemporary population with impaired glucose tolerance: an observational analysis of the nateglinide and valsartan in impaired glucose tolerance outcomes research (NAVIGATOR) trial. BMJ Open 2012; 2: e001925. doi: 10.1136/bmjopen-2012-001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holman RR, Coleman RL, Chan JCN, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017; 5: 877–886. [DOI] [PubMed] [Google Scholar]
- 55.Saremi A, Bahn GD, Reaven PD. A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bandeira E, Neves AP, Costa C, et al. Association between vascular calcification and osteoporosis in men with type 2 diabetes. J Clin Densitom 2012; 15: 55–60. [DOI] [PubMed] [Google Scholar]
- 57.Bowden DW, Cox AJ, Freedman BI, et al. Review of the diabetes heart study (DHS) family of studies: a comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev Diabet Stud 2010; 7: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendrick J, Targher G, Smits G, et al. 25-Hydoxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis 2009; 205: 255–260. [DOI] [PubMed] [Google Scholar]
- 59.Ix JH, Barrett-Connor E, Wassel CL, et al. The associations of fetuin-A with subclinical cardiovascular disease in community-dwelling persons: the Rancho Bernardo study. J Am Coll Cardiol 2011; 58: 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emoto M, Mori K, Lee E, et al. Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism 2010; 59: 873–878. [DOI] [PubMed] [Google Scholar]