Short abstract
Objectives
The purpose of this study was to investigate the effect of remotely delivered telemedicine dietary advice on monitoring of blood glucose levels and weight gain of women with gestational diabetes mellitus (GDM).
Methods
Women with GDM were recruited and randomly allocated into two groups: a Tele-GDM group that received a telemonitoring device, and a control group that was followed-up traditionally. A telemonitoring service calculated the ratio of reaching or exceeding the pregnancy weight gain target (according to pre-pregnancy weight), following Institute of Medicine guidelines for healthy pregnancy weight gain.
Results
The sample comprised 27 women in the Tele-GDM group and 30 in the control group. At the end of pregnancy, the Tele-GDM group showed significantly lower 2-hour postprandial glucose levels than the control group. Most women in the Tele-GDM group reached their recommended range of weight gain at the end of pregnancy. Additionally, the Tele-GDM group showed significantly lower weight gain than the control group.
Conclusions
Telemonitoring can facilitate close monitoring of women with GDM and motivate patients to adopt a healthy lifestyle.
Keywords: Gestational diabetes mellitus, hyperglycaemia, gestational weight gain, telediabetes, telemedicine, telemonitoring
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance of varying levels that is initially diagnosed during pregnancy and typically disappears postpartum.1 It is widespread, affecting all populations worldwide.2 It is estimated that GDM affects 5% of all pregnancies3 depending on the diagnostic criteria used and the population studied,3 and there is an exponential increase in incidence proportional to body mass index (BMI).4
The pathophysiology of GDM is unclear. However, it seems that insulin resistance develops under the influence of pregnancy hormones as pregnancy progresses, and a failure to adjust physiologically to the decline in insulin sensitivity results in glucose intolerance and GDM.5,6 GDM is associated with multiple complications, such as abnormal labour, increased risk of caesarean section, macrosomia, and stillbirth.3,7 Women with a history of GDM have an increased risk of developing type 2 diabetes mellitus (DM),8 and foetuses exposed to GDM have a greater risk of obesity and type 2 DM later in life.9,10
Multiple factors increase the risk of GDM in pregnant women, such as a history of GDM, a previous infant >4.5 kg, polycystic ovary syndrome, parity, and maternal obesity.11–13
Automated smart health monitoring systems can be used to monitor weight.14 These are smart devices that facilitate remote, real-time collection and analysis of biomedical informatics data to manage chronic illnesses.14 Telemedicine is a clinically and financially effective approach to the treatment of chronic illnesses, such as heart failure, hypertensive diseases, and type 2 DM.15,16 Many teletechnology studies of diabetic patients have demonstrated improvement in glycaemic control and glycosylated haemoglobin (HbA1c).17,18 However, there have been no investigations of the use of telemedicine to manage GDM.
The purpose of this study was to investigate the effect of remotely delivered telemedicine dietary advice on monitoring of blood glucose levels and weight gain in diabetic women throughout pregnancy. The aim was to determine whether telemedicine is an effective method for managing hyperglycaemia in pregnant women.
Materials and methods
Ethical approval
Ethical approval was obtained from the Unit of Biomedical Ethics Research Committee at King Abdulaziz University Hospital (KAUH) (reference number 336-16), and the study complied with the requirements of the National Committee on Bio and Medical Ethics (registration number HA-02-J-008). All participants provided written informed consent before their enrolment in the study.
Participants
Women with established GDM diagnosed using the criteria of the International Association of Diabetes and Pregnancy Study Group (IADPSG) (75 g oral glucose tolerance test)19 were recruited from the GDM unit at KAUH and enrolled in a randomised open label controlled study. All women diagnosed with GDM at a gestational age of 24–28 weeks were invited to participate in the study. Women diagnosed with type 1 or type 2 DM, and those receiving cortisone or experiencing any other illness, were excluded from the study. The study aim and procedure, including consent procedures, were explained to all participants. Then participants were categorised into two groups according to the randomisation protocol (Figure 1).
Figure 1.
Random allocation of women with gestational diabetes mellitus. GDM: gestational diabetes mellitus; DM: diabetes mellitus; BMI: body mass index.
Both groups maintained routine visits to GDM and antenatal clinics and all participants were referred to a dietician for appropriate dietary recommendations. All pregnant women attending our unit were encouraged to eat a low carbohydrate and high protein diet. For consistency, all participants received structured dietary advice, an assessment of dietary structure, and standard national healthy eating information for pregnancy.
Telemonitoring
After a face-to-face explanation of the study, an information sheet was given to volunteers prior to obtaining written consent. The participant was supplied with a telemonitoring device (a Smartphone-Glucometer and a Glucomail application installed in their phones, obtained from ePoint Healthcare (Limburg, Belgium) with a contract for 12 months of open service20) and given full instructions and training on using the system. Telemonitoring began from the day of diagnosis with GDM (24–28 weeks of gestation) and continued until 6 weeks post-delivery.
The telemonitoring device was used to monitor blood sugar and weight gain. Participants were asked to download their daily blood sugar readings and weekly weight measurements and fill out the questionnaire every week. This information was reviewed weekly by the diabetic care team at the GDM clinic to evaluate whether participants needed further interventions, such as lifestyle monitoring or insulin/medication adjustments.
Glucose monitoring plan
Based on the IADPSG guidelines, the study targeted a mean fasting plasma glucose (FPG) and 2-hour postprandial plasma glucose (PPG) less than 5.1 and 8.5 mmol/L, respectively. Patients were advised to measure their blood sugar four times daily: once early in the morning before breakfast (FPG), ensuring at least 6 hours of fasting, and three readings 2 hours after meals (breakfast, lunch, and dinner). The Glucomail application was adjusted to signal an emergency alert for hyperglycaemia or hypoglycaemia. For example, when three thresholds of high or low blood glucose levels were met, the doctor was immediately informed by SMS or email (Figure 2a) and further actions taken.
Figure 2.
Compliance of patients with GDM in telemonitoring. Proactive communication graphs showing an alert system for the healthcare provider using an email (a), a questionnaire link (b), and a coaching message (c) to indicate the health status of the patient. Average number of monitoring episodes per week for participants in the telemedicine group for glucose (GLU) readings, weight (WT) and questionnaire (QUS) response. GDM: gestational diabetes mellitus.
Weight management plan
Maternal weight gain during pregnancy was the second major outcome of this study. Body weight was recorded every week using the Glucomail application. This application was developed to observe weight gain during pregnancy and to ensure patients remain within the normal ranges of recommended weight gain. The gestational weight gain target was estimated by the Glucomail application using an online calculator (https://www.calculator.net/pregnancy-weight-gain-calculator.html) to calculate the weight gain per week. The calculations used weight and height measurements before pregnancy and followed guidelines from the Institute of Medicine for healthy weight gain during pregnancy.21
Conversation map
A feedback questionnaire and conversation map were used to enable proactive communication between healthcare professionals and patients. Patients were asked to regularly complete an online questionnaire about their health condition (Figure 2b). The questionnaire assesses weekly factors that may affect GDM, including healthy food intake, glucose intake, medications, exercise, toileting, stress, and pain. Most items use yes/no responses.
An automated message from the application to the patient was generated weekly. The coaching system was adjusted for each pregnant woman according to her due date (Figure 2c). The message included some useful facts about body changes, baby size and growth stage, healthy food, and what to avoid during pregnancy (Figure 2c). Additionally, appropriate dietary advice was delivered to each patient in the Tele-GDM group by text message after hyperglycaemia or hypoglycaemia alerts were received (Figure 2a).
The average glucose values, weight, and participant responses recorded by the Glucomail application were recorded per week for all cases (Figure 2d).
Laboratory tests
At the end of pregnancy (38–40 weeks gestation), blood tests, including the two-point glucose test (FPG and 2-hour PPG) and HbA1c, were performed for all studied cases and controls.
Sample size calculation
The estimated GDM prevalence, based on local figures, ranged from 32.4% to 51% in the most recent epidemiological studies in the Kingdom of Saudi Arabia, published in 2014 and 2015, respectively.22 We chose the primary study outcome, a reduction in hyperglycaemia, to calculate the appropriate sample size. Our estimates of the standard deviation in the study and control groups were obtained from pilot studies and analysed by Power and Sample Size from NCSS software (NCSS, Kaysville, UT, USA). A sample size of 24 in each group has 95% power to detect a difference between means of 1.56 mmol/L with a significance level (alpha) of 0.05 (two-tailed). Allowing for 20% additional patients to account for dropout and protocol violation, a total of 60 patients were required (30 in each group).
Statistical analysis
GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA) was used to calculate descriptive statistics for the telemonitoring service data, including patient compliance, daily blood glucose levels, and BMI. Additionally, unpaired t-tests were used to compare the Tele-GDM group and the control group on glucose levels, HbA1c levels, and weight gain. A chi-square test was used to compare the number of women that reached/exceeded the recommended range of weight gain in the Tele-GDM group between the end-of-pregnancy and post-delivery periods.
Results
Patients were enrolled in the study. The Tele-GDM group (n = 30) was monitored with the telemonitoring system. Only 27 pregnant women continued with telemonitoring for 18–20 weeks throughout pregnancy and 6 weeks post-delivery. Three pregnant women joined the study for less than 2 weeks but were excluded from the study. Cases were closely monitored at the GDM clinic (weekly visit); the results of the control group (n = 30) were compared with the Tele-GDM group. Information on the anthropometric and sociodemographic characteristics of all participants was obtained via closed-question questionnaire interviews (Table 1).
Table 1.
Sociodemographic characteristics of respondents.
| Variablesa | GDM(n = 30) | Tele-GDM(n = 27) | P-value |
|---|---|---|---|
| Age (years) | 32.4 ± 5.3 | 32.5 ± 5.8 | NS |
| Gestational age at GDM diagnosis (weeks) | 27.6 ± 3.2 | 26.5 ± 4.4 | NS |
| Gravidity | 4.6 ± 2.9 | 4 ± 2.6 | NS |
| Parity | 2.4 ± 1.8 | 1.5 ± 1.2 | NS |
| Abortion or IUFD | 1.4 ± 1.5 | 1.8 ± 2.1 | NS |
| BMI | 30.2 ± 5.5 | 31 ± 5.7 | NS |
| Waist circumference (inches) | 42.6 ± 4.2 | 42.7 ± 5.3 | NS |
| Activity >30 minutes per dayb | |||
| Yes | 17 | 17 | |
| No | 13 | 10 | |
| Educational levelc | |||
| High | 15 | 15 | |
| Intermediate | 10 | 8 | |
| Low | 5 | 4 | |
aData show frequencies or means ± standard deviations.
bActivity >30 minutes per day was defined as exercise such as walking.
cA high level of education was defined as a bachelor’s degree or higher. Intermediate education was defined as completed intermediate or high school. Low education was defined as no formal education or completed elementary school
Abbreviations: BMI: body mass index; GDM: gestational diabetes mellitus; IUFD: intrauterine foetal death; NS: not significant.
Well-monitored postprandial glucose
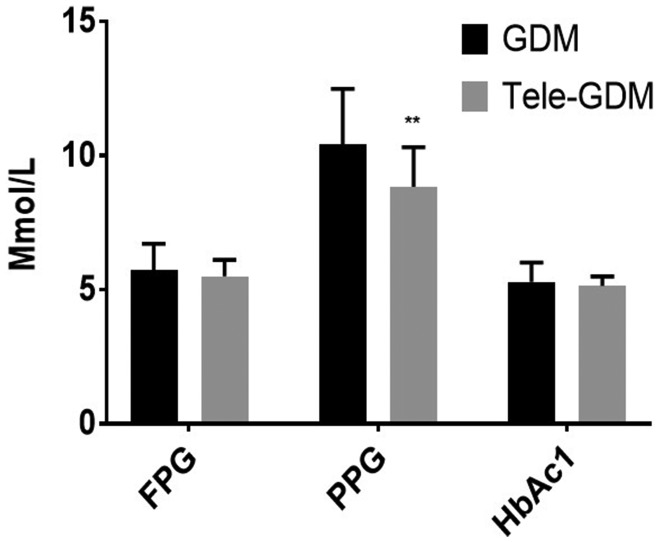
We examined the mean blood glucose levels with 95% confidence intervals for women with GDM using the Glucomail application; data were obtained from the service for the Tele-GDM group only. Glucose level readings were obtained at the end of pregnancy, either by telemonitoring or by traditional measures, after the completion of the follow-up study. The mean FPG was still high (6.5 ± 0.8 mmol/L; 95% confidence interval, 6.0–6.9 mmol/L). The 2-hour PPG levels were well-monitored (mean, 6.9 ± 0.6 mmol/L; 95% confidence interval, 6.6–7.2).
This result was consistent with laboratory tests (Figure 3) showing that the 2-hour PPG in the Tele-GDM group was significantly less than in the GDM control (P-value = 0.002). However, there were no significant between-group differences in FPG and HbA1c.
Figure 3.
Results of laboratory investigations, including FPG, 2-hour PPG and glycated haemoglobin (HbA1c) are compared between the Tele-GDM and control groups. Values are expressed as mean ± standard error of the mean. Statistical significance was determined by unpaired t-tests (n =57); **P < 0.001.
Management of weight during pregnancy
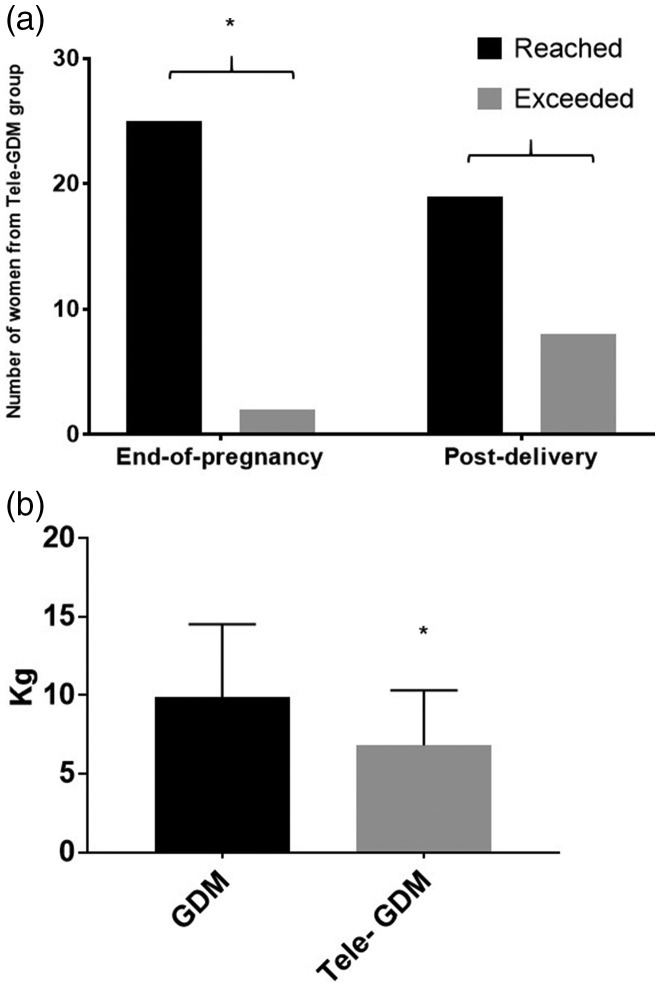
In the Tele-GDM group, more women reached the recommended range of weight gain at the end of pregnancy than exceeded it. Equivalent results were obtained for the post-delivery time. However, the weight management was more effective at the end of pregnancy than in the post-delivery period (P-value = 0.01; Figure 4a).
Figure 4.
Improvement in weight gain during pregnancy in the Tele-GDM group. (a) The number of women from the Tele-GDM group categorised as reaching or exceeding the recommended weight either at the end-of-pregnancy or post-delivery period. (b) The average weight gain (in kilograms) during pregnancy in both groups is expressed as mean ± standard error of the mean. Statistical significance was determined using a chi-square test or an unpaired t-test (n = 57); *P < 0.05.
Additionally, the weight gain in the Tele-GDM group was significantly lower than in the control group (P-value = 0.03; Figure 4b).
Discussion
Our results demonstrate that data from close monitoring of PPG levels during pregnancy were reflected in the laboratory results at the end of pregnancy. Weight management in pregnant women is a critical issue that demands the targeting of blood glucose levels in women with GDM. Intensive monitoring of GDM patients via telemonitoring with an appropriate notification system for hyperglycaemia or hypoglycaemia might help patients to control their blood glucose levels by adjusting their diet or physical activities.
In this study, we demonstrated that telemedicine is an efficient new technique to control 2-hour PPG. Several studies indicate that PPG levels are an important contributor to glycaemic control and can guide the management of diabetes.23–25 Furthermore, PPG has been identified as an independent risk factor for cardiovascular disease,25 with some reports showing that patients with a high 1-hour PPG had a worse cardiovascular risk profile (hypertension, inflammatory markers, and high uric acid levels).26,27 Another study demonstrated an association between carotid intima-media thickness and 1- and 2-hour PPG.28 Although there is strong evidence that monitoring PPG with FPG is crucial to achieving better glycaemic control, there is controversial evidence showing that increased PPG levels might be an independent risk factor for cardiovascular complications.29,30
In pregnant women with GDM, PPG is the best predictor of neonatal macrosomia.31 Further studies conducted among women with GDM suggest that preprandial and postprandial hyperglycaemia are important predictors of foetal weight.32,33 Although the use of FPG and PPG values is useful in monitoring blood glucose levels in women with GDM,34 it is unclear whether 2-hour PPG values are superior to 1-hour PPG values in monitoring these patients. Thus, given the lack of definitive evidence, clinicians typically recommend that patients measure their capillary glucose levels four times per day (fasting glucose before breakfast and PPG level 1 to 2 hours after meals). We used a similar approach for regular glucose self-monitoring among our patients.
Telemedicine is a clinically and financially effective approach to the treatment of chronic illnesses, such as heart failure, hypertensive diseases, and type 2 diabetes.15,16 Many teletechnology studies of diabetic patients have demonstrated improvements in glycaemic control and HbA1c.17,18 Two studies that investigated the impact of telemedicine on weight management, eating habits, and exercise showed that an electronic behavioural intervention was effective in promoting a healthy lifestyle35 and preventing excessive weight gain during pregnancy.36 In another large-scale study conducted on GDM patients assigned to a telephone nurse management program at 12 health centres in the USA, investigators found that the risk of macrosomia was lower among women obtaining care at centres that had frequent referrals to the telephone nurse management service.37
A group from the UK explored the feasibility of telemedicine for patients with GDM living in rural areas.38 The results showed that patients expressed satisfaction with not having to commute long distances for clinic visits. However, healthcare practitioners have expressed concerns that telemedicine should not be used as a substitute for face-to-face contact with patients,39,40 but should instead be considered a tool to facilitate face-to-face contact.40 Some researchers have suggested that telemonitoring can allow physicians to allocate more time to patients who need extra care.38 The focus of this study was glucose monitoring and weight control. In patients with GDM, insulin resistance leads to hyperglycaemia; FPG level is an early sign of hyposensitivity that can affect healthy lifestyle during the rest of the day. Our data suggest that the Glucomail application motivates patients to reduce high carbohydrate diet or to increase exercise to avoid high glucose levels detected in the early morning. This might explain why 2-hour PPG levels were well-controlled compared with FPG levels.
This study has several limitations. First, the number of times that women in the intervention group had to control their blood glucose might have caused some participants not to monitor their glucose levels as required. Second, factors such as educational level and language barriers might have led to some withdrawals. Telemonitoring technology should be developed to be user-friendly to appeal to a wide range of people. Finally, our sample size does not permit us to generalise the findings to patients in other countries; more extensive prospective studies on different populations are needed.
Conclusions
Overall, our results suggest that telemonitoring is a useful tool to facilitate close monitoring of pregnant women with GDM. Furthermore, it can be used to motivate patients to adopt a healthy lifestyle and prevent weight gain during pregnancy.
Acknowledgements
The authors wish to thank Mr. Ronny Broekx, manager, and Hamzeh Awad and biotech employees from ePoint, the electronic point of care, for supplying the web-based application. We are very grateful to all the participants, including the patients, doctors, midwives, and nutritionist at the KAUH Gestational Diabetes Mellitus Unit. We especially thank Mrs. Halima M Dabroom, the head midwife, and Mrs. Fadia Abozaid for their assistance in patient recruitment and data collection. We also thank Dr. Princila Mukoko for editorial assistance.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah (grant no. G-697-248-37). The authors acknowledge with thanks the DSR for technical and financial support.
References
- 1.Reece EA, Moore T. The diagnostic criteria for gestational diabetes: to change or not to change? Am J Obstet Gynecol 2013; 208: 255–259. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a world health organization guideline. Diabetes Res Clin Pract 2014; 103: 341–363. [DOI] [PubMed] [Google Scholar]
- 3.Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes 2015; 6: 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SY, England L, Sappenfield W, et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004-2007. Prev Chronic Dis 2012; 9: E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 2007; 50: 938–948. [DOI] [PubMed] [Google Scholar]
- 6.Poulakos P, Mintziori G, Tsirou E, et al. Comments on gestational diabetes mellitus: from pathophysiology to clinical practice. Horm Athens Greece 2015; 14: 335–344. [DOI] [PubMed] [Google Scholar]
- 7.Swierzewska P, Kosiński M, Wójcik M, et al. , Family, anthropometric and biochemical factors affecting birth weight of infants born to GDM women. Ginekol Pol 2015; 86: 499–503. [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002; 25: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012; 8: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman BL, Rizzo TA, Cho NH, et al. Long-term effects of the intrauterine environment. The Northwestern University diabetes in pregnancy center. Diabetes Care 1998; 21(Suppl 2): B142–B149. [PubMed] [Google Scholar]
- 11.Hedderson MM, Ferrara A, Williams MA, et al. Androgenicity of progestins in hormonal contraceptives and the risk of gestational diabetes mellitus. Diabetes Care 2007; 30: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 12.Aktun LH, Yorgunlar B, Karaca N, et al. Predictive risk factors in the treatment of gestational diabetes mellitus. Clin Med Insights Women's Health 2015; 8: 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aktun HL, Yorgunlar B, Acet M, et al. The effects of polycystic ovary syndrome on gestational diabetes mellitus. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol 2016; 32: 139–142. [DOI] [PubMed] [Google Scholar]
- 14.Baig MM, Gholamhosseini H. Smart health monitoring systems: an overview of design and modeling. J Med Syst 2013; 37: 9898. [DOI] [PubMed] [Google Scholar]
- 15.Board on Health Care Services, Institute of Medicine. The role of telehealth in an evolving health care environment: workshop summary [Internet]. Washington (DC): National Academies Press (US), 2012. [cited 2018 Mar 9]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK207145/ [PubMed] [Google Scholar]
- 16.Zhai Y, Zhu W, Cai Y, et al. Clinical and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltimore) 2014; 93: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pressman AR, Kinoshita L, Kirk S, et al. A novel telemonitoring device for improving diabetes control: protocol and results from a randomized clinical trial. Telemed J E-Health 2014; 20: 109–114. [DOI] [PubMed] [Google Scholar]
- 18.Lieber BA, Taylor B, Appelboom G, et al. Meta-analysis of telemonitoring to improve HbA1c levels: promise for stroke survivors. J Clin Neurosci 2015; 22: 807–811. [DOI] [PubMed] [Google Scholar]
- 19.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al . International association of diabetes and pregnancy study group recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holthe H, Serrano JA. ePoint.telemed–An open web-based platform for home monitoring of patients with chronic heart failure. Stud Health Technol Inform 2015; 216: 74–78. [PubMed] [Google Scholar]
- 21.Institute of Medicine. Weight gain during pregnancy: re-examining the guidelines. Washington (DC): National Academies Press (US), 2009. [Google Scholar]
- 22.Alzzaqani AH, Alzemily MA, Alshahrani HS. A status on gestational diabetes mellitus in Saudi Arabia: a systematic review. Central African Journal of Public Health 2016; 2: 83–88. [Google Scholar]
- 23.Monnier L, Colette C. Target for glycaemic control: concentrating on glucose. Diabetes Care 2009; 32(Suppl 2): S199–S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossetti P, Quirós C, Moscardó V, et al. Closed-loop control of postprandial glycemia using an insulin-on-board limitation through continuous action on glucose target. Diabetes Technol Ther 2017; 19: 355–362. [DOI] [PubMed] [Google Scholar]
- 25.Ceriello A. Targeting one-hour postmeal glucose: is it time for a paradigm switch in diabetes management? Diabetes Technol Ther 2017; 19: 493–497. [DOI] [PubMed] [Google Scholar]
- 26.Perticone F, Sciacqua A, Perticone M, et al. Serum uric acid and 1-h postload glucose in essential hypertension. Diabetes Care 2012; 35: 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Chen H, Wang Y, et al. The relationship between coronary risk factors and elevated 1-h postload plasma glucose levels in patients with established coronary heart disease. Clin Endocrinol (Oxf) 2013; 78: 67–72. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med 1999; 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 29.Ceriello A, Davidson J, Hanefeld M, et al. Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis 2006; 16: 453–456. [DOI] [PubMed] [Google Scholar]
- 30.Standl E, Schnell O, Ceriello A. Postprandial hyperglycaemia and glycaemic variability: should we care? Diabetes Care 2011; 34(Suppl 2): S120–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jovanovic L. Continuous glucose monitoring during pregnancy complicated by gestational diabetes mellitus. Curr Diab Rep 2001; 1: 82–85. [DOI] [PubMed] [Google Scholar]
- 32.Herranz L, Pallardo LF, Hillman N, et al. Maternal third trimester hyperglycaemic excursions predict large-for-gestational-age infants in type 1 diabetic pregnancy. Diabetes Res Clin Pract 2007; 75: 42–46. [DOI] [PubMed] [Google Scholar]
- 33.Aschwald CL, Catanzaro RB, Weiss EP, et al. Large-for-gestational-age infants of type 1 diabetic mothers: an effect of preprandial hyperglycaemia? Gynecol Endocrinol 2009; 25: 653–660. [DOI] [PubMed] [Google Scholar]
- 34.Gabbe SG, Gregory RP, Power ML, et al. Management of diabetes mellitus by obstetrician-gynecologists. Obstet Gynecol 2004; 103: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 35.Kelders SM, Van Gemert-Pijnen JE, Werkman A, et al. Effectiveness of a Web-based intervention aimed at healthy dietary and physical activity behavior: a randomized controlled trial about users and usage. J Med Internet Res 2011; 13: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez ID, Groth SW, Reschke JE, et al. eMoms: electronically-mediated weight interventions for pregnant and postpartum women. Study design and baseline characteristics. Contemp Clin Trials. 2015; 43: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara A, Hedderson MM, Ching J, et al. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol 2012; 206: 491.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Given JE, Bunting BP, O’Kane MJ, et al. Tele-Mum: a feasibility study for a randomized controlled trial exploring the potential for telemedicine in the diabetes care of those with gestational diabetes. Diabetes Technol Ther 2015; 17: 880–888. [DOI] [PubMed] [Google Scholar]
- 39.Hopp FP, Hogan M. Community-based tele-health systems for persons with diabetes: development of an outcomes model. Soc Work Health Care 2009; 48: 134–153. [DOI] [PubMed] [Google Scholar]
- 40.Pols J. The heart of the matter. About good nursing and telecare. Health Care Anal HCA J Health Philos Policy 2010; 18: 374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]