Short abstract
Objectives
Betatrophin is a widely used diagnostic marker for type 2 diabetes mellitus (DM), but its clinical utility in diagnosing gestational DM (GDM) is unclear. We evaluated the relationship between betatrophin and the risk of GDM as well as the ability of betatrophin to predict postpartum type 2 DM (PDM).
Methods
In total, 386 patients were categorized into those with and without PDM. All underwent the oral glucose tolerance test while pregnant. Betatrophin was assessed to examine the diagnostic characteristics of GDM.
Results
The betatrophin concentration was remarkably higher in patients with than without GDM. The patients were categorized into three groups; those with a betatrophin concentration of 300 to 600 pg/mL and >600 pg/mL had a higher risk of GDM after adjusting for body mass index, age, homeostatic model assessment–insulin resistance (HOMA-IR) concentration, and betatrophin concentration than those with a betatrophin concentration of <300 pg/mL. The HOMA-IR concentration tended to increase as the betatrophin concentration increased, and betatrophin was independently associated with GDM after adjusting for confounders. The betatrophin concentration was higher among pregnant patients with than without PDM.
Conclusions
Betatrophin has high sensitivity but low specificity for diagnosing GDM and may be a promising predictor of PDM.
Keywords: Gestational diabetes mellitus, postpartum diabetes mellitus, betatrophin, biomarker, prognosis, homeostatic model assessment–insulin resistance
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that occurs during pregnancy.1,2 The annual worldwide incidence of GDM ranges from 3% to 14% and has continued to increase. In north urban China, 9.3% of all pregnant mothers develop GDM.3 GDM usually disappears spontaneously after childbirth.4,5 However, it may cause complications such as macrosomia.6,7 Hyperglycemia during pregnancy is related to the development and progression of macrosomia, emergency cesarean section (CS), and vaginal trauma after birth.8,9 Pregnant women with GDM are at higher risk of emergency CS, type 2 diabetes mellitus (T2DM), and pre-eclampsia.8 In general, T2DM is diagnosed based on the World Health Organization criteria, which consider the results of the oral glucose tolerance test (OGTT), random fasting plasma glucose (FPG) concentration, and glycosylated hemoglobin concentration.
The majority of betatrophin is produced in the liver.10 Several studies have demonstrated that betatrophin negatively affects the revival of pancreatic β cells.11,12 Another study showed a positive relationship between the betatrophin concentration and indicators of glycemic regulation in patients with T2DM.13 However, insufficient data are available regarding betatrophin in pregnant women.14,15 We examined the correlation between the betatrophin level and GDM and whether betatrophin can be used as a predictor of postpartum T2DM (PDM). Our research focused on two factors: the applicability of betatrophin in the diagnosis of GDM and in predicting the development and progression of PDM.
Materials and methods
Patients
Pregnant women with GDM and healthy pregnant women (controls) were recruited from the Affiliated Hospital of Jiangsu University. Women with a history of DM or comorbidities and those with a lack of betatrophin measurement within 1 month of the 75-g OGTT were excluded from the study. All participants were nondrinkers and nonsmokers and did not have hyperparathyroidism. The baseline body weight, height, and blood pressure were assessed. The glucose, glycosylated hemoglobin, and lipid levels were evaluated using blood samples obtained after overnight fasting. The experimental design was approved by the local Ethics Committee of the Affiliated Hospital of Jiangsu University. All patients provided consent for the use of their samples in this investigation.
GDM is routinely evaluated during weeks 24 to 28 of gestation. GDM was diagnosed in the present study as follows. A 75-g 2-hour OGTT was performed in all participants. The procedure for the OGTT was discussed in detail with each participant. The pregnant women were categorized into three groups according to their betatrophin concentration: T1 (<300 pg/mL), T2 (300–600 pg/mL), and T3 (>600 pg/mL).
The OGTT was performed in the morning after an overnight fast of at least 8 hours. GDM was diagnosed based on the American Diabetes Association criteria as follows: FPG of ≥5.1 mmol/L, plasma glucose (PG) of ≥10.0 mmol/L at 60 minutes, and/or PG of ≥8.5 mmol/L at 120 minutes.
Methods
The demographic and clinical characteristics of all patients were obtained using questionnaires that assessed the pregnancy period, alcohol and cigarette consumption, and maternal history of malignancy and other comorbidities. An enzyme-linked immunosorbent assay (Wuhan EIAab Science Co., Ltd., Wuhan, China) was used to evaluate betatrophin when GDM was diagnosed. An automated bioanalyzer (7600-020; Hitachi, Tokyo, Japan) was used to evaluate the levels of triglycerides, high-density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, and uric acid. The Glamour 2000 autoanalyzer (Molecular Devices, Sunnyvale, CA, USA) was used to assess the glucose concentration. A chemiluminescent enzyme immunoassay (Roche, Basel, Switzerland) was used to evaluate the insulin concentration. The homeostatic model assessment–insulin resistance (HOMA-IR) concentration was used to investigate insulin resistance (IR) according to the following formula: fasting glucose (mmol/L) × fasting insulin (μU/mL) / 22.5 (threshold value > 2.7).
In terms of prognosis, only patients with GDM who had at least 3 months of follow-up information subsequent to childbirth were chosen. These patients were categorized into two groups: those with T2DM (PDM) and those without T2DM (non-PDM). The betatrophin concentration was evaluated, and laboratory tests for diagnosis of DM were conducted.
Statistical analysis
IBM SPSS Statistics for Windows, Version (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Every variable was assessed for a normal distribution. The results are presented as mean ± standard deviation. Student’s t-test and the Mann–Whitney U test were used to evaluate distinctions between groups. Hierarchical variables manifested themselves as frequency proportion. The chi-square test was also applied to evaluate distinctions between groups. The correlation between betatrophin and HOMA-IR was demonstrated via Spearman’s correlation evaluation. Linear regression analysis was also used to assess the risk of GDM in terms of various betatrophin quartiles. A receiver operating characteristic curve was then generated to determine the cutoff point of betatrophin as an indicator of GDM. A two-sided p-value of <0.05 was considered statistically significant.
Results
Patient characteristics
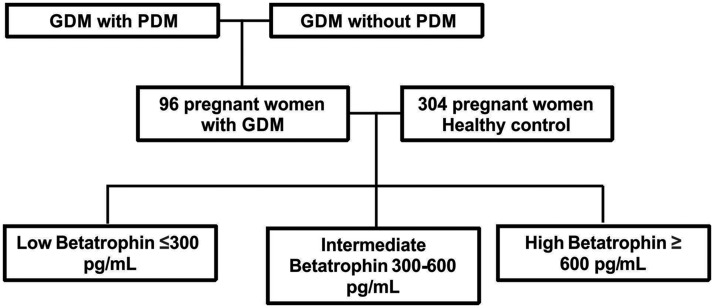
In total, 400 pregnant women (96 with GDM and 304 healthy controls) were recruited from the Affiliated Hospital of Jiangsu University. The mean age of the 96 women with GDM was 32.34 ± 2.25 years, and that of the 304 healthy controls was 28.62 ± 1.47 years. The T1, T2, and T3 groups comprised 102, 176, and 108 patients, respectively. In total, 72 patients with GDM who had at least 3 months of follow-up information subsequent to childbirth were chosen for the prognostic evaluation. Of these patients, 17 had PDM and 55 did not.
Evaluation of clinical differences between patients with GDM and healthy controls
The characteristics of the pregnant women with and without GDM are shown in Table 1. In total, 386 participants with a mean age of 30.36 ± 3.45 years were recruited for this study. Age, FPG level, body mass index (BMI), 60-min PG level, 120-min PG level, uric acid level, triglyceride level, HOMA-IR (p < 0.01), and systolic and diastolic blood pressure (p < 0.05) were significantly different between patients with GDM and healthy controls. No significant difference was found in the blood urea nitrogen, alanine transaminase, aspartate transaminase, low-density lipoprotein cholesterol, or high-density lipoprotein cholesterol concentrations between the two groups. The betatrophin level was higher in patients with GDM than in healthy controls (p < 0.001). These findings indicate that betatrophin can be used as a predictor of GDM (Figure 1).
Table 1.
Clinical characteristics.
| Characteristics | GDM (n = 96) | Non-GDM (n = 270) | p-value |
|---|---|---|---|
| Betatrophin (pg/mL) | 589 ± 132 | 343 ± 87 | <0.001 |
| Age (years) | 32.34 ± 2.25 | 28.62 ± 1.47 | <0.01 |
| BMI (kg/m2) | 24.89 ± 3.67 | 22.67 ± 3.01 | <0.01 |
| SBP (mmHg) | 115.25 ± 15.23 | 113.29 ± 14.97 | <0.05 |
| DBP (mmHg) | 69.92 ± 10.14 | 67.12 ± 9.12 | <0.05 |
| 60-min PG (mmol/L) | 9.78 ± 1.38 | 8.12 ± 1.01 | <0.01 |
| 120-min PG (mmol/L) | 8.54 ± 1.29 | 6.23 ± 0.98 | <0.01 |
| HbA1c (%) | 5.34 (4.92–5.76) | 5.01 (4.78–5.29) | <0.01 |
| FPG (mmol/L) | 4.92 ± 0.65 | 4.28 ± 0.43 | <0.01 |
| UA (mmol/L) | 223.65 ± 63.27 | 205.23 ± 39.87 | <0.01 |
| TG (mmol/L) | 1.78 (1.26–2.56) | 1.44 (1.02–2.11) | <0.01 |
| BUN (mmol/L) | 2.65 ± 0.55 | 2.63 ± 0.51 | 0.432 |
| HOMA-IR | 2.22 (1.52–3.09) | 1.43 (1.02–2.16) | <0.01 |
| ALT (U/L) | 16 (12–20) | 17 (12–21) | 0.321 |
| AST (U/L) | 18 (14–24) | 19 (14–25) | 0.287 |
| LDL-C (mmol/L) | 1.79 ± 0.28 | 1.79 ± 0.32 | 0.639 |
| HDL-C (mmol/L) | 2.65 ± 0.62 | 2.62 ± 0.59 | 0.531 |
Data are presented as mean ± standard deviation or median (range).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment–insulin resistance; LDL-C, low-density lipoprotein cholesterol; PG, plasma glucose; SBP, systolic blood pressure; TG, triglycerides; UA, uric acid.
Figure 1.
Study flow chart. GDM, gestational diabetes mellitus; PDM, postpartum diabetes mellitus.
Prevalence of HOMA-IR and GDM with regard to distinct betatrophin quartiles
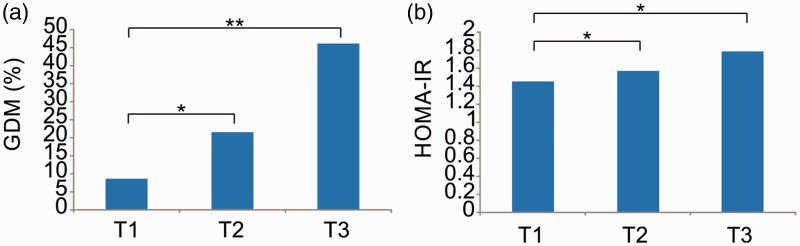
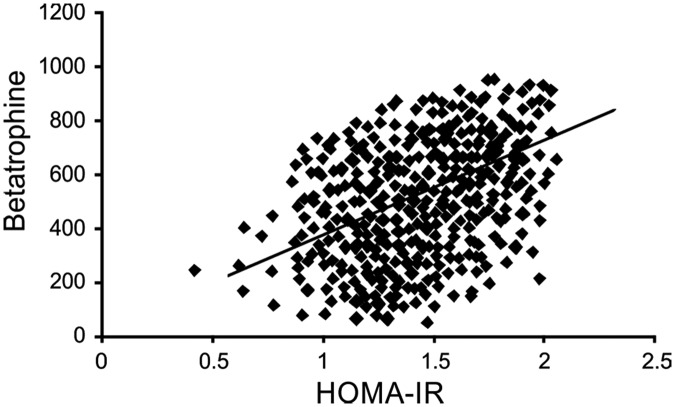
With the T1 group as a reference, the prevalence of GDM was remarkably higher in the T2 group (21.6% vs. 8.72%, respectively; p < 0.05) and T3 group (46.30% vs. 8.72%, respectively; p < 0.01). Moreover, the prevalence of GDM tended to increase in the three groups (p < 0.05) (Figure 2(a)). After adjusting for age, betatrophin level, HOMA-IR, and BMI, the logistic regression analysis demonstrated that compared with patients in the T1 group, those in the T2 group (odds ratio, 1.675; p < 0.05) and T3 group (odds ratio, 3.237; p < 0.01) had a higher risk of GDM (Table 2). The HOMA-IR level tended to increase in the three groups (p < 0.05). Additionally, the HOMA-IR level was higher in the T2 and T3 groups than in the T1 group (p < 0.05) (Figure 2(b)). Finally, the HOMA-IR level was correlated with the betatrophin level (Figure 3).
Figure 2.
(a) Prevalence of GDM in the different betatrophin groups. (b) HOMA-IR level in the different betatrophin groups. GDM, gestational diabetes mellitus; HOMA-IR, homeostatic model assessment–insulin resistance.
Table 2.
Association of betatrophin with risk of gestational diabetes mellitus.
| Level of betatrophin | OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| T1 | 1 | 1 | ||
| T2 | 2.12 (1.07–5.19) | <0.05 | 1.675 (1.02–3.42) | <0.05 |
| T3 | 2.87 (1.43–3.68) | <0.01 | 3.237 (1.79–4.07) | <0.01 |
CI: confidence interval; OR: odds ratio.
T1, betatrophin of <300 pg/mL; T2, betatrophin of 300–600 pg/mL; T3, betatrophin of >600 pg/mL.
Figure 3.
Correlation of HOMA-IR and betatrophin concentration. HOMA-IR, homeostatic model assessment–insulin resistance.
Predictive value of betatrophin for IR and GDM
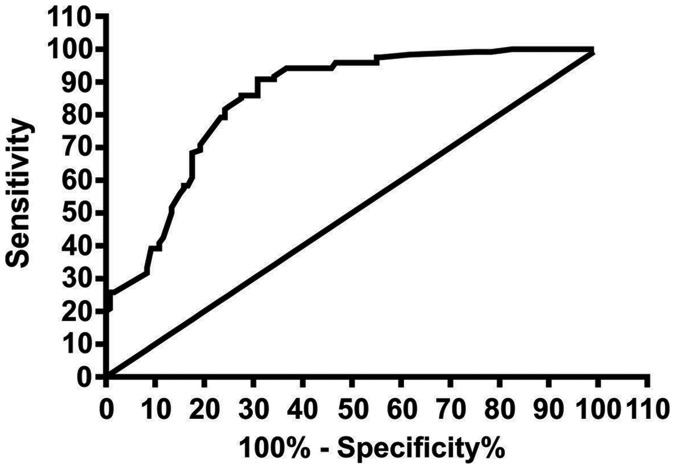
Receiver operating characteristic analysis was used to determine the accuracy of betatrophin as a predictor of GDM. The best cutoff threshold of betatrophin for indicating GDM was 0.95 × 106 pg/mL (area under the curve, 0.812; 95% confidence interval, 0.698–0.879; p < 0.01; Youden’s index, 0.122; sensitivity, 68.8%; specificity, 84.1%) (Figure 4).
Figure 4.
Receiver operating characteristic analysis of betatrophin for diagnosis of gestational diabetes mellitus.
Betatrophin as an indicator of PDM
To assess the predictive capability of betatrophin for PDM, a proportion of the patients with GDM underwent evaluation. The characteristics of these participants are presented in Table 3. The BMI was higher in patients with than without PDM. The betatrophin concentration was also significantly different between patients with and without PDM (Table 3). The mean betatrophin concentration was 505 ± 51 pg/mL in patients without PDM and 738 ± 65 pg/mL in those with PDM (p < 0.001).
Table 3.
Characteristics of GDM with or without PDM.
| Characteristics | non-PDM (n = 55) | PDM (n = 17) | p-value |
|---|---|---|---|
| Age (years) | 31.12 ± 2.19 | 32.62 ± 2.34 | 0.09 |
| Follow-up months | 13.23 ± 10.75 | 14.56 ± 11.31 | 0.11 |
| BMI (kg/m2) | 23.43 ± 3.09 | 26.03 ± 4.15 | 0.013 |
| Betatrophin (pg/mL) | 505 ± 121 | 738 ± 145 | <0.001 |
BMI, body mass index; GDM, gestational diabetes mellitus; PDM, postpartum diabetes mellitus.
Discussion
Several studies have shown that GDM is an IR syndrome and can progress to DM.16,17 GDM increases the risk of preeclampsia and the need for emergency CS.18 Additionally, women with GDM have a high risk of developing T2DM after pregnancy, and the risk of T2DM is high in the offspring, particularly when accompanied by obesity.19,20 Studies have shown that betatrophin promotes IR.21,22 An increased betatrophin concentration is a compensatory reaction to IR because it enhances the secretory capability of the liver and the β-cell mass.12,23,24 A significant independent relationship was noted between betatrophin and the prevalence of GDM without dependence on confounders. The current study also demonstrated a relationship between metabolic disorders and an increased betatrophin concentration leading to GDM. Consequently, the relationship between the betatrophin concentration and GDM can be attributed to the close relationship between IR and betatrophin. Furthermore, previous research has indicated that betatrophin and IR are reliable predictors of T2DM.25 IR is known to contribute to the development and progression of GDM. Consequently, IR is hypothesized to be involved in the relationship between GDM and betatrophin despite the insufficient understanding of its underlying etiology.
In our study, the betatrophin concentration and BMI were significantly different between pregnant women with and without GDM. We also demonstrated that betatrophin was positively associated with BMI and HOMA-IR. The increased prevalence of GDM led to an increased betatrophin concentration. Nevertheless, the specificity and sensitivity of these markers are relatively limited. The BMI and age of the participants with GDM were higher than those of the healthy controls, indicating that the incidence of GDM declines with a good health status and young age.
Apart from evaluating the applicability of betatrophin for diagnosing GDM, we also explored whether betatrophin is a predictive indicator of T2DM among patients with GDM. The American Diabetes Association criteria include betatrophin as a determinant of preliminary DM, and betatrophin serves as indicator of a high risk of T2DM. Patients with GDM are more vulnerable to T2DM. Therefore, the application of betatrophin in evaluating the risk of PDM was examined. The extent of preliminary DM was assessed according to the betatrophin levels in the PDM subgroup. On the basis of the betatrophin concentration during pregnancy, pregnant women at risk for T2DM can be recognized and given more appropriate clinical management. Our study is the first to assess the applicability of betatrophin in predicting GDM among Chinese women.
Our study has some limitations. First, the sample size, particularly that of patients with GDM, was relatively small. Second, several women who underwent the OGTT were excluded from our research because betatrophin was not measured simultaneously. Third, several confounders including diet, a family history of DM, and physical exercise were not eliminated. Fourth, the 3-hour 75-g OGTT rather than the 2-hour 75-g OGTT, which is the recommended test for accurate GDM diagnosis, was used in our research. Finally, the patients were not screened for T2DM at baseline before delivery. Well-designed prospective studies are needed to address these limitations and determine the role of betatrophin in the development of GDM and its progression to T2DM.
In summary, our research showed a significant correlation between IR and the betatrophin level. Betatrophin was proven to be an independent risk factor for GDM progression. We also showed that an increased betatrophin concentration at the time of GDM diagnosis was associated with a higher risk of T2DM after delivery. Betatrophin was an independent predictor of PDM with high specificity. To determine the applicability of betatrophin in diagnosing GDM, betatrophin should be assessed using both laboratory tests and clinical outcomes to more fully determine the applicability of betatrophin in diagnosing GDM.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by Zhenjiang Social Development Project (No. SH2016034 and SH2018071).
References
- 1.Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes 2015; 6: 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mpondo BC, Ernest A, Dee HE. Gestational diabetes mellitus: challenges in diagnosis and management. J Diabetes Metab Disord 2015; 14: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrar D, Simmonds M, Bryant M, et al. Treatments for gestational diabetes: a systematic review and meta-analysis. BMJ Open 2017; 7: e015557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theriault S, Forest JC, Masse J, et al. Validation of early risk-prediction models for gestational diabetes based on clinical characteristics. Diabetes Res Clin Pract 2014; 103: 419–425. [DOI] [PubMed] [Google Scholar]
- 5.Rudge MV, Piculo F, Marini G, et al. [Translational research in gestational diabetes mellitus and mild gestational hyperglycemia: current knowledge and our experience]. Arq Bras Endocrinol Metabol 2013; 57: 497–508[in Portuguese, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 6.Koning SH, Hoogenberg K, Lutgers HL, et al. Gestational diabetes mellitus: current knowledge and unmet needs. J Diabetes 2016; 8: 770–781. [DOI] [PubMed] [Google Scholar]
- 7.Santangelo C, Zicari A, Mandosi E, et al. Could gestational diabetes mellitus be managed through dietary bioactive compounds? Current knowledge and future perspectives. Br J Nutr 2016; 115: 1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carolan OMC. Educational and intervention programmes for gestational diabetes mellitus (GDM) management: an integrative review. Collegian 2016; 23: 103–114. [DOI] [PubMed] [Google Scholar]
- 9.Caissutti C, Berghella V. Scientific evidence for different options for GDM screening and management: controversies and review of the literature. Biomed Res Int 2017; 2017: 2746471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espes D, Martinell M, Liljeback H, et al. Betatrophin in diabetes mellitus: the epidemiological evidence in humans. Curr Diab Rep 2015; 15: 104. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Farha M, Abubaker J, Tuomilehto J. ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes Metab Res Rev 2017; 33: e2919 10.1002/dmrr.2919 [DOI] [PubMed] [Google Scholar]
- 12.Abu-Farha M, Al Madhoun A, Abubaker J. The rise and the fall of betatrophin/ANGPTL8 as an inducer of beta-cell proliferation. J Diabetes Res 2016; 2016: 4860595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Rawashdeh A, Kasabri V, Bulatova N, et al. The correlation between plasma levels of oxytocin and betatrophin in non-diabetic and diabetic metabolic syndrome patients: a cross sectional study from Jordan. Diabetes Metab Syndr 2017; 11: 59–67. [DOI] [PubMed] [Google Scholar]
- 14.Ejarque M, Borlaug M, Vilarrasa N, et al. Angiopoietin-like protein 8/betatrophin as a new determinant of type 2 diabetes remission after bariatric surgery. Transl Res 2017; 184: 35–44. [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Sun W, Yu S, et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 2014; 37: 2718–2722. [DOI] [PubMed] [Google Scholar]
- 16.Barbour LA, McCurdy CE, Hernandez TL, et al. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007; 30: S112–S119. [DOI] [PubMed] [Google Scholar]
- 17.Boerschmann H, Pfluger M, Henneberger L, et al. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 2010; 33: 1845–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka K, Yamada K, Matsushima M, et al. Increased maternal insulin resistance promotes placental growth and decreases placental efficiency in pregnancies with obesity and gestational diabetes mellitus. J Obstet Gynaecol Res 2018; 44: 74–80. [DOI] [PubMed] [Google Scholar]
- 19.Noctor E, Dunne FP. Type 2 diabetes after gestational diabetes: the influence of changing diagnostic criteria. World J Diabetes 2015; 6: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strehlow SL, Mestman JH. Prevention of T2DM in women with a previous history of GDM. Curr Diab Rep 2005; 5: 272–277. [DOI] [PubMed] [Google Scholar]
- 21.Qu Q, Zhao D, Zhang F, et al. Serum betatrophin levels are increased and associated with insulin resistance in patients with polycystic ovary syndrome. J Int Med Res 2017; 45: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Lin Y, Zhou H, et al. The correlation between circulating betatrophin and insulin resistance in general population: a meta-analysis. Horm Metab Res 2017; 49: 760–771. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Lee SG, Lee CJ, et al. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci Rep 2016; 6: 24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Farha M, Abubaker J, Al-Khairi I, et al. Higher plasma betatrophin/ANGPTL8 level in type 2 diabetes subjects does not correlate with blood glucose or insulin resistance. Sci Rep 2015; 5: 10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Farha M, Abubaker J, Noronha F, et al. Lack of associations between betatrophin/ANGPTL8 level and C-peptide in type 2 diabetic subjects. Cardiovasc Diabetol 2015; 14: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]