Short abstract
Objective
To investigate the value of serum galactomannan antigen (GM) testing combined with chest computed tomography (CT) as a noninvasive method for early diagnosis of invasive pulmonary aspergillosis (IPA) in patients with hematological malignancies with febrile neutropenia after antifungal drug treatment.
Methods
We retrospectively analyzed the data of 376 patients with febrile neutropenia from January 2015 to August 2017. All patients were given broad-spectrum antibiotics and divided into the control group (effective antibiotic treatment, no antifungal drugs given) and the observational group (ineffective antibiotic treatment, antifungal drugs given). The serum GM testing, chest CT, and microbiological examination findings were compared between the two groups.
Results
The false-positive rates of GM testing for IPA in the control and observational groups were 4.04% and 8.65%, respectively, and the false-negative rates in the two groups were 1.10% and 9.62%, respectively. Sixty-five patients in the observational group and 11 in the control group had typical features of CT imaging.
Conclusion
Clinical weekly screening of serum GM and chest CT may be an effective combined approach to the early diagnosis of IPA in patients with febrile neutropenia, even if they have undergone antifungal treatment.
Keywords: Galactomannan antigen testing, invasive pulmonary aspergillosis, hematological malignancies, febrile neutropenia, antifungal treatment, computed tomography
Introduction
Patients with hematological malignancies undergoing cytotoxic chemotherapy or hematopoietic stem cell transplantation commonly develop neutropenia, which makes these patients vulnerable to fungal infections. Fungal infections can occur in many locations. The incidence of invasive pulmonary aspergillosis (IPA) has rapidly increased in recent years. IPA is a life-threatening fungal infection that requires prompt diagnosis and treatment because it is associated with significantly high morbidity and mortality rates.1 The traditional approach to diagnosing IPA includes a combination of clinical signs, radiographic evidence, and histopathological examination of specimens obtained via invasive procedures. However, patients with febrile neutropenia often have impaired hemostasis and thrombocytopenia, making diagnostic lung biopsy difficult to perform. Galactomannan antigen (GM) testing offers a potential noninvasive diagnostic method. However, some antifungals or antibiotics may influence the GM testing sensitivity, and false-positive or false-negative results are sometimes obtained. This retrospective study was performed to evaluate the utility of serum GM testing combined with computed tomography (CT) for the monitoring of IPA in patients who have hematological malignancies with febrile neutropenia after antifungal treatments.
Methods
Patients and study design
We retrospectively analyzed the role of serum GM testing combined with CT scans for identifying IPA in patients who had hematological malignancies with febrile neutropenia from January 2015 to August 2017. The inclusion criteria were the presence of hematological malignant disease, neutropenia (<500 neutrophils/mm3), and fever (axillary temperature of >38°C for ≥1 hour) and application of broad-spectrum antibiotics such as carbapenem or piperacillin/tazobactam for the febrile neutropenia. The exclusion criteria were an age of <12 years, no use of broad-spectrum antibiotics for febrile neutropenia, and anticipation that the patient would be unavailable to contact during the 30-day follow-up period. All patients with febrile neutropenia were given broad-spectrum antibiotics. According to their response to the antibiotic treatment, the patients were divided into two groups. In the control group, the broad-spectrum antibiotics were effective and the patients were given no antifungal drugs. In the observational group, the broad-spectrum antibiotics were ineffective or the body temperature increased again during antibiotic treatment, and the patients were given antifungal drugs such as voriconazole, posaconazole, itraconazole, caspofungin, or amphotericin-B. The study was approved by the ethics board of the medical faculty in the First Affiliated Hospital of Xi’an Jiaotong University. All patients provided written informed consent.
GM testing
Serum GM was measured using the Platelia Aspergillus EIA (Bio-Rad Laboratories, Marnes-la-Coquette, France) according to the manufacturer’s instructions. All serum samples were taken from patients in their neutropenic period, and the first sample was collected at the first occurrence of a fever of >38°C for ≥1 hour. The serum GM level was then measured once weekly until neutrophil recovery. Serum samples were considered positive if the GM index was equivalent to a 0.5 GM antigen level.
CT evaluation
Chest CT scans with or without contrast enhancement (usually high-resolution CT) were performed at three time points: after a fever of >38°C had persisted for ≥1 hour in patients with neutropenia, after the body temperature had returned to normal, and at the time of neutrophil recovery. CT scans were performed using a 64-multislice scanner (Philips, Best, the Netherlands). The main CT findings were divided into angio-invasive and airway-invasive. Angio-invasive findings were determined to be present if at least two of the following features were seen: a halo sign, an infarct-shaped consolidation, or an internal low-attenuation, cavity, or air-crescent sign. Airway-invasive findings were determined to be present if at least two of the following features were seen: small airway lesions, peri-bronchial consolidation, or bronchiectasis. Dense nodules were considered a typical sign of Aspergillus. CT evaluation was performed by two experienced thoracic radiologists in a retrospective manner. Where disagreement occurred, the final decision was made by consensus.
Microbiological examination
Before use of broad-spectrum antibiotics, every patient underwent a microbiological examination of their sputum and blood for the presence of fungal microorganisms. Plates were incubated for fungal growth at 30°C for 14 days and inspected regularly.
Definitions of IPA
Patients were diagnosed with IPA according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) 2008 criteria.2 Patients were classified as having proven, probable, possible, or no IPA.
Statistical analysis
Continuous variables were compared using a t test, and categorical variables were compared using the chi-squared test. SPSS for Windows, Version 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and a P value of <0.05 was considered statistically significant.
Results
Patient characteristics
In total, 376 patients were included in the study. The demographic and clinical characteristics of all enrolled patients are presented in Table 1. In total, 814 blood samples were collected from patients with febrile neutropenia. The median number of tests per patient was two. There were no statistically significant differences in the demographic data between the two groups. All patients in the observational group were diagnosed with IPA; 5 cases were proven, 86 were probable, and 13 were possible. Twenty patients in the control group were diagnosed with IPA (Table 2). Among the patients with IPA, 103 had leukemia, 10 had non-Hodgkin’s lymphoma, and 11 had plasma cell disease.
Table 1.
Demographic and clinical characteristics of enrolled patients.
| Characteristic | Observational group (n = 104) | Control group (n = 272) |
|---|---|---|
| Sex | ||
| Male | 56 (53.8) | 131 (48.2) |
| Female | 48 (46.2) | 141 (51.8) |
| Age, years | 49 (16–65) | 45 (15–71) |
| Diagnosis | ||
| AML | 31 29.8) | 104 (38.2) |
| ALL | 30 (28.8) | 93 (34.1) |
| CLL | 6 (5.8) | 2 (0. 7) |
| NHL | 20 (19.2) | 65 (23.8) |
| MM | 15 (14.4) | 7 (2.5) |
| Waldenstrom macroglobulinemia | 1 (1.0) | 0 (0.0) |
| Plasmacytoma | 1 (1.0) | 1 (3.7) |
| Duration of neutropenia, days | ||
| Mean | 14.5 | 14.1 |
| Median | 15 | 13 |
| Range | 7–29 | 5–34 |
| Patients given antifungal drugs | ||
| Fluconazole | 34 (32.8) | 0 (0.0) |
| Voriconazole | 56 (53.8) | 0 (0.0) |
| Itraconazole | 6 (5.8) | 0 (0.0) |
| Caspofungin | 4 (3.8) | 0 (0.0) |
| Amphotericin-B | 4 (3.8) | 0 (0.0) |
Data are presented as n (%) or median (range) unless otherwise indicated
AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; MM, malignant melanoma
Table 2.
Invasive pulmonary aspergillosis in the two groups.
| Invasive pulmonary aspergillosis | Observational group (n = 104) | Control group (n = 272) |
|---|---|---|
| Proven | 5 (4.8) | 0 (0.0) |
| Probable | 86 (82.7) | 20 (7.4) |
| Possible | 13 (12.5) | 0 (0.0) |
Data are presented as n (%)
GM testing
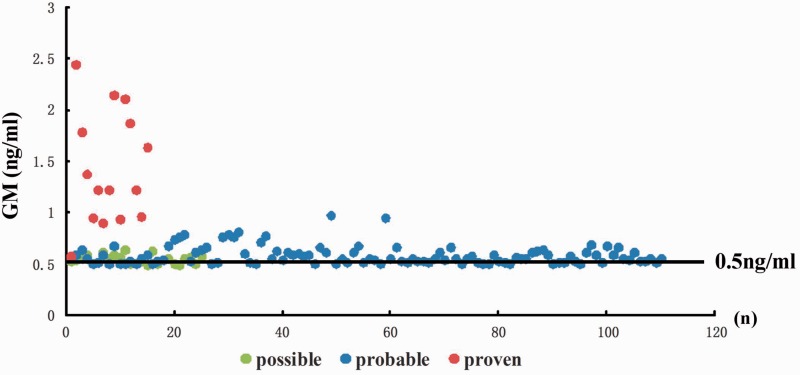
Serum GM testing was positive in 91 patients in the observational group and 20 patients in the control group. The median GM titer was significantly higher in the observational group than in the control group (P < 0.05). An interactive scatter diagram displaying the distribution of GM values in the observational group with a cut-off point of 0.5 ng/mL is presented in Figure 1. The false-positive rates of GM testing for IPA in the control and observational groups were 4.04% and 8.65%, respectively. The false-negative rates of GM testing for IPA in the control and observational groups were 1.10% and 9.62%, respectively.
Figure 1.
Scatter diagram displaying the GM distribution among the observational group with a cut-off point of 0.5 ng/mL. GM, galactomannan antigen
Chest CT scans
Among all patients with IPA, 65 patients in the observational group (6 patients with 3 typical features of CT imaging, 27 patients with 2 typical features of CT imaging, and 32 patients with 1 typical feature of CT imaging) and 11 patients in the control group (9 patients with 2 typical features of CT imaging and 2 patients with 1 typical feature of CT imaging) had typical features of CT imaging. There was a significant difference in the frequency of typical Aspergillus signs between the observational and control groups (P < 0.05) (Table 3).
Table 3.
Results of computed tomography scans in the two groups.
| Observational group (n = 104) | Control group (n = 272) | |
|---|---|---|
| Nodular infiltrate | 22 (21.2)* | 2 (0.7) |
| Halo sign | 14 (13.5)* | 0 (0.0) |
| Wedge-shaped pleura-associated consolidation | 26 (25.0)* | 6 (2.2) |
| Focal ground-glass opacities | 25 (24.0)* | 9 (3.3) |
| Caverns/air-crescent sign | 8 (7.7)* | 2 (0.7) |
| Tree-in-bud pattern/centrilobular nodules | 2 (1.9)* | 0 (0.0) |
| Peribronchial consolidations | 2 (1.9)* | 0 (0.0) |
| Diffuse ground-glass opacities | 5 (4.8)* | 1 (0.4) |
Data are presented as n (%)
*P < 0.05 compared with control group
Microbiological examination
Aspergillus species were detected in cultures of sputum or blood samples (observational group) in four patients (1.06% of all study patients). The isolated species were A. fumigatus (two patients), A. flavus (one patient) and A. niger (one patient). Histological examination findings of specimens obtained from lung biopsy were available in four patients, including one patient with a negative sputum and blood culture and three with a positive sputum or blood culture.
Antimycotic treatment and outcome
Overall, 124 patients received antimycotic treatment for IPA. Voriconazole was the most commonly used antimycotic agent. Sixteen patients died of IPA, giving a mortality rate of 13.4% in the observational group and 0.7% in the control group.
Discussion
The EORTC/MSG revised criteria for defining IPA require a microbiological and or histopathological diagnosis to prove the presence of an infection. A lung tissue biopsy can be obtained by needle aspiration or thoracoscopic biopsy. Bronchial lavage specimens are commonly used in the clinical setting.2,3 However, many patients who have hematological malignancies with thrombocytopenia or impaired coagulation cannot tolerate an invasive operation. Fungal culture is time-consuming; additionally, the fungal culture-positive rate is lower than the bacterial culture-positive rate, and if no fungal species is cultured, the diagnosis of IPA still cannot be excluded.4 Therefore, a noninvasive diagnostic method for IPA is needed. Many reports have described assessment of the value of Aspergillus polymerase chain reaction (PCR) in the clinical setting. The sensitivity and specificity of Aspergillus PCR for diagnosing IPA are 84% and 76%, respectively.5 The sensitivity of samples from bronchoalveolar lavage (BAL) fluid is higher than that from blood samples, but the specificity is lower.6–11 In patients who have hematological malignancies with neutropenia and fever, obtaining a BAL fluid sample is difficult due to myelosuppression and thrombocytopenia. Aspergillus PCR is not recommended for routine use in the clinical setting because few assays have been standardized.
GM testing is a noninvasive diagnostic assay for detection of Aspergillus, and its sensitivity varies in different conditions according to host factors. In patients undergoing allogeneic hematopoietic stem cell transplantation, the sensitivity of GM testing was approximately 70% in serum and approximately 82% in BAL fluid.12 In the present study, the sensitivity of serum GM testing in patients with neutropenia was much higher than the reported sensitivity in patients without neutropenia. This difference may be due to the fact that patients with neutropenia are vulnerable to fungal infection, and the fungal burden is high in these patients; additionally, macrophage deficiency limits the GM clearance from the blood.12–15 False-positive results have been reported in patients given certain antibiotics such as piperacillin-tazobactam and amoxicillin clavulanate, and false-negative results have been reported in patients who undergo antifungal prophylaxis with agents such as echinomycin.16–18 In the present study, the false-positive rates of GM testing for IPA in the control and observational groups were 4.04% and 8.65%, respectively. The false-negative rates of GM testing for IPA in the control and observational groups were 1.10% and 9.62%, respectively, which is in accordance with the literature. These findings show that antibiotic and antifungal treatment in patients with neutropenia does not significantly affect the sensitivity of GM testing.
Chest CT performed at fever onset may help to identify the cause of fever and provide clinical information before GM testing is positive.19–21 In the present study, 65 patients in the observation group and 11 patients in the control group had typical features of CT imaging. There was a significant difference in the frequency of typical Aspergillus signs between the two groups.
Our study has two main limitations. The number of patients with histologically proven IPA was small, and the different sensitivities of GM testing and CT scans among different antifungal treatments was not compared.
In conclusion, clinicians should be aware of the potential antifungal treatment effects on GM testing and with the appropriate GM cut-off optical density index values as well as the proper interpretation and identification of possible false-positive or false-negative results. The GM assay has the potential to become an effective tool for diagnosing IPA. Once-weekly screening of GM in serum and chest CT scans may be an effective combined approach to the early diagnosis of IPA in patients who have hematological malignancies with febrile neutropenia, even if they have accepted antifungal treatment.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Nature Science Fund of China (grant no. 81600179).
References
- 1.Baddley JW, Stephens JM, Ji X, et al. Aspergillosis in Intensive Care Unit (ICU) patients: epidemiology and economic outcomes. BMC Infect Dis 2013; 13: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taccone FS, Van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortensen KL, Johansen HK, Fuursted K, et al. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis 2011; 30: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitis M, Ziakas PD, Zacharioudakis IM, et al. PCR in diagnosis of invasive aspergillosis: a meta-analysis of diagnostic performance. J Clin Microbiol 2014; 52: 3731–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avni T, Levy I, Sprecher H, et al. Diagnostic accuracy of PCR alone compared to galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis: a systematic review. J Clin Microbiol 2012; 50: 3652–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heng SC, Morrissey O, Chen SC, et al. Utility of bronchoalveolar lavage fluid galactomannan alone or in combination with PCR for the diagnosis of invasive aspergillosis in adult hematology patients: a systematic review and meta-analysis. Crit Rev Microbiol 2015; 41: 124–134. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder M, Simon M, Katchanov J, et al. Does galactomannan testing increase diagnostic accuracy for IPA in the ICU?A prospective observational study. Crit Care 2016; 20: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White PL, Wingard JR, Bretagne S, et al. Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis 2015; 61: 1293–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He H, Ding L, Sun B, et al. Role of galactomannan determinations in bronchoalveolar lavage fluid samples from critically ill patients with chronic obstructive pulmonary disease for the diagnosis of invasive pulmonary aspergillosis: a prospective study. Crit Care 2012; 16: R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrissey CO, Chen SC, Sorrell TC, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis 2013; 13: 519–528. [DOI] [PubMed] [Google Scholar]
- 12.Maertens JA, Klont R, Masson C, et al. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin Infect Dis 2007; 44: 1329–1336. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 2006; 42: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 14.Ku NS, Han SH, Choi JY, et al. Diagnostic value of the serum galactomannan assay for invasive aspergillosis: it is less useful in non-haematological patients. Scand J Infect Dis 2012; 44: 600–604. [DOI] [PubMed] [Google Scholar]
- 15.Petraitiene R, Petraitis V, Bacher JD, et al. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1→3)-β-D-glucan in experimental invasive pulmonary aspergillosis. Med Mycol 2015; 53: 558–568. [DOI] [PubMed] [Google Scholar]
- 16.Vergidis P, Razonable RR, Wheat LJ, et al. Reduction in false-positive Aspergillus serum galactomannan enzyme immunoassay results associated with use of piperacillin-tazobactam in the United States. J Clin Microbiol 2014; 52: 2199–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarzinger M, Sagaon-Teyssier L, Cabaret O, et al. Performance of serum biomarkers for the early detection of invasive aspergillosis in febrile, neutropenic patients: a multi-state model. PLoS One 2013; 8: e65776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duarte RF, Sanchez-Ortega I, Cuesta I, et al. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis 2014; 59: 1696–1702. [DOI] [PubMed] [Google Scholar]
- 19.Nucci M, Nouer SA, Grazziutti M, et al. Probable invasive aspergillosis without prespecified radiologic findings: proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clin Infect Dis 2010; 51: 1273–1280. [DOI] [PubMed] [Google Scholar]
- 20.Li XS, Zhu HX, Fan HX, et al. Pulmonary fungal infections after bone marrow transplantation: the value of high-resolution computed tomography in predicting their etiology. Chin Med J (Engl) 2011; 124: 3249–3254. [PubMed] [Google Scholar]
- 21.Georgiadou SP, Sipsas NV, Marom EM, et al. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis 2011; 52: 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]