ABSTRACT
Waterborne protozoa (WBP) are important cause of several outbreaks all over the world. The report system of WBP in Africa is weak. More than one third of African countries (21/54) reported WBP with absent reports in the remaining countries (33/54). The top reported WBP were Cryptosporidium, Giardia, FLA and Entamoeba contaminating different African water resources. Other protozoa were less documented even though it is abundant and robust. More than one protozoa were detected in contaminated African water including drinking sources, a prediction index to popular epidemics and real presence of undocumented WBP outbreaks. Risk factors in Africa were observed to be abundant and multi-factorial ‘socioeconomic, governmental, pathogen in water and climate change. Climate change is an important factor impacting Africa. Increasing droughts in Africa with other extreme weather events will lead to water crises. Incidence and transmission of WBP will change, with new manifested strains/species. Recognizing future consequences of water crises in Africa are important. Governments and population unity will be needed to protect against expected raise and spread of WBP diseases and water shortages.
KEYWORDS: Water-borne protozoa, transmission, water, risk factors, climate change, review, Africa
Introduction
Africa, the second-largest continent in the world after Asia, covers 20.4% of the earth’s total land area with 54 recognized countries. In 2017, it accounted for approximately 16% of the world’s human population being a home for 1.250 billion citizens [1].
This continent has about 9% of the world’s fresh water resources [2]. However, the variability of the African climate and water resources characteristics, made some regions have sufficient water, while others like Sub-Saharan Africa (SSA) face numerous challenges concerning water issues that constrict economic growth and limit the livelihoods of its people [3].
Waterborne infectious diseases are the main cause of morbidity and mortality worldwide, causing around 801,000 children to die annually, due to diarrheal diseases [4–7] compounded by malnutrition. Diarrheal diseases were classified as the fourth cause of disability in children after iron-deficiency anaemia, skin diseases and protein-energy malnutrition [8].
In Africa, drinking water might not provide the ideal microbiological quality that guarantee safe water for African communities [9] adding more load of waterborne infectious diseases which already cause death of 2.2 million per annum and far more cases of illness every day [10]. In SSA, World Health Organization (WHO) grouped diarrheal diseases due to viral, bacterial and parasitic causes in group 1 category to cause human death [7].
Waterborne protozoa (WBP) are a group of parasites that cause diarrheal diseases. Diversity of WBP can be found in water. Cryptosporidium spp. and Giardia duodenalis (intestinalis) took the lead among other protozoan parasites to account the majority of waterborne outbreaks (524, 344 outbreaks respectively) [10–12]. Whereas Acanthamoeba spp., Balantidium coli, Blastocystis spp., Cyclospora cayetanensis, Cystoisospora belli, Microsporidium spp., Naegleria spp., Sarcocystis spp., and Toxoplasma gondii are less reported parasites [10–12].
Out of them, Cryptosporidium is reported to be the second just after rotavirus to cause moderate to severe diarrhea during the first two years of life. In seven regions of Asia and SSA, Cryptosporidium is considered the fourth major contributors to diarrhea [5].
In SSA and Asia (developing settings), the common water enteric pathogenic protozoa include G. duodenalis (intestinalis), Entamoeba spp., Cryptosporidium spp., Cyclospora cayetanensis, and Microsporidia. Whereas other species such as Blastocystis spp. and Dientamoeba fragilis are usually isolated from developed countries [13,14].
The transmission path of the previous protozoa is feacal-oral route causing infection to humans and/or animals. WBP utilizes the path to contaminate water through passages of feces that contains shed oo (cysts) from infected animals and/or humans into the water system [11,15,16]. Infections usually are present where there is no or inadequate access to clean water or good sanitation [17].
The worldwide distribution of WBP outbreaks was published in previous reviews [10–12]. Such reviews covered the occurrence rate of WBP outbreaks in the period of 1954–2014. About 905 WBP outbreaks were recorded to cause human diseases. Association between enteric protozoa and waterborne transmission increased the concern of scientists and two reviews highlighted the currently available information about Cryptosporidium and Giardia epidemiology, genetic diversity, and distribution in the African continent. Absence of outbreaks in Africa was critically addressed [3,18]. In Latin America as well, WBP as a cause of outbreaks were also highlighted [19].
Even in developed countries, the operational surveillance systems include only a few or no parasitic protozoa, because the main focus is only on infections caused by viruses and bacteria. Frequently enteric protozoa are ignored and overlooked as a cause of diarrhea because these regions have better hygienic conditions [13].
In SSA, there is inadequate water supply, unclean water, underdevelopment, poverty, population density, malnutrition, poor sanitation and water shortage. There is critical absence of governmental systems to document diseases, particularly protozoal infections or waterborne outbreaks. Such factors are negative factors predisposing disease spread among African populations [20].
During the last few decades, the increasing world population steadily strained demands of fresh water creating a real threat of fresh water scarcity to human society [3,12,18,19,21]. It is expected that Africa will have the largest population growth from now to 2050, which will add more population than any world region (1.3 billion). Almost all of this growth will occur in the poorest regions of SSA [22]. This scenario will raise the demand for water sources, a situation require critical response.
Africa is a very sensitive continent to climate change, particularly nations of SSA, the waterborne enteric protozoa are susceptible to climate change especially temperature and precipitation. Extreme meteorological changes in Africa with high expectation of drought will lead to spread of diarrheal diseases. Variable precipitation and drought in some regions of Africa influences the availability of fresh water [23]. Death from infection, dehydration and/or malnutrition (due to nutritional effects of drought as reduced dietary range/low affordability) will be an expected outcome.
Our objective is to find out the size of burden of WBP with associated risk factors in Africa, taking into consideration the recent crises of climate change and its impact on abundance and transmission of WBP across this continent.
Literature review
This study is conducted to analyze situation of waterborne protozoa parasites in the African water resources. The search and selection of literature was done using databases as Scopus, PubMed, Google Scholar, Web of Knowledge, African Journals online, Library Genesis Scientific Articles and Egyptian Knowledge Bank. The literature review starts from 1982 to the first trimester of 2018. The literature review was defined using Booleans descriptors and combination of keywords: ‘Balantidium coli’, ‘Blastocystis hominis’, ‘Cryptosporidium’, ‘Cyclospora’, ‘Dientamoeba fragilis’, ‘Entamoeba’, ‘free living amoebae, (FLA)’, ‘Giardia’, ‘Isospora belli’, ‘Microsporidia’, ‘Sarcocystis spp.’ ‘Toxoplasma’ and ‘name of each one of countries from Africa’, and also ‘water’, ‘climate’, ‘crisis’, ‘outbreak’, ‘transmission’, ‘incidence/prevalence’ to collect relevant articles.
Results and discussion
A total of relevant 148 reports were found, including full text and abstracts, in which 120 were selected for analysis of addressed topics and the WBP in Africa to be included in the review. We excluded 28 reports from articles, chapters and reports which contained ambiguous information and poor quality citation ‘absence of abstract, full text, no publisher data, and no volume or issue data’.
A total of 21 countries reported presence of WBP in different water bodies (wells, lakes, rivers, taps, and ground water). The countries with higher numbers of publications were Egypt [36] >South Africa [13] >Nigeria [11] and Tunisia [11]. To our knowledge, 33 countries have no documented reports on WBP in their territories.
Data concerning reports distribution among geographical divisions of Africa and incidental rate of each parasite among total number of reports were mentioned in Figure1. The data on the distribution of WBP reports by African countries is presented in Figure 2. Dominant information on WBP in relation to risk factors is summarized in Table 1. Mechanisms of climate changes and their effect on occurrence and distribution of WBP outbreaks are presented in Figure 3. All of the previous figures and table will be discussed later in the following sections.
Figure 1.
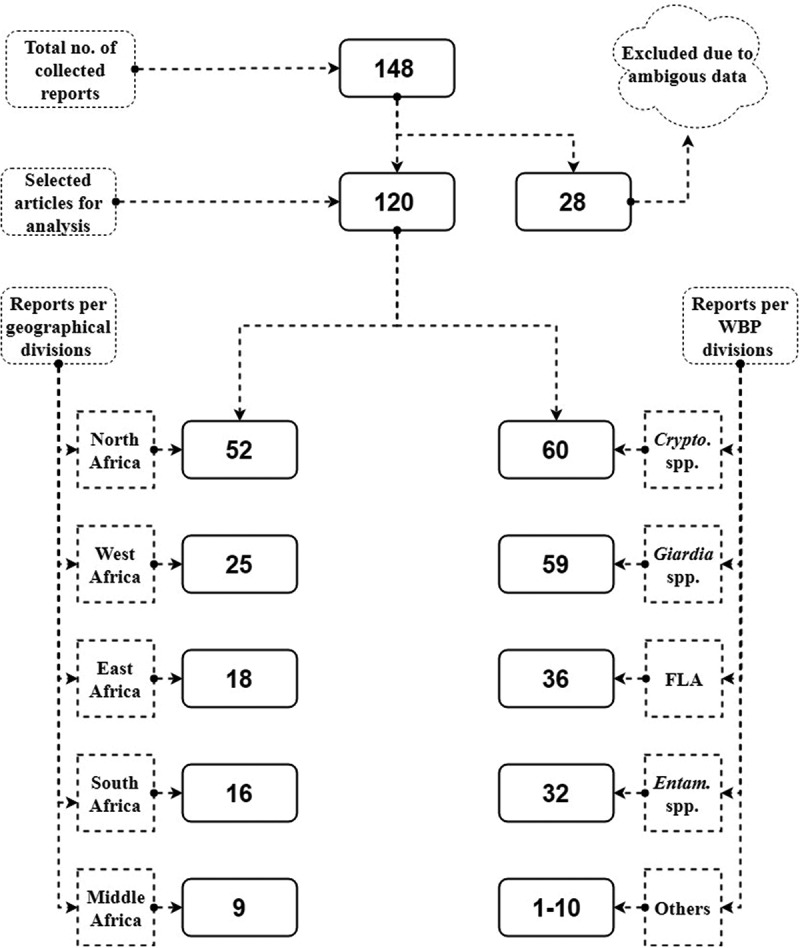
Analytical results of WBP reports in Africa and its numerical distribution according to geographic and parasitic divisions Sub-title: Crypto: Cryptosporidium, FLA: Free living amoebae; Entam: Entamoeba spp.; Other: Balantidium coli, Blastocystis hominis, Cyclospora cayetanensis, Dientamoeba fragilis, Isospora belli, Microsporidia, Sarcocystis spp. and Toxoplasma gondii.
Figure 2.
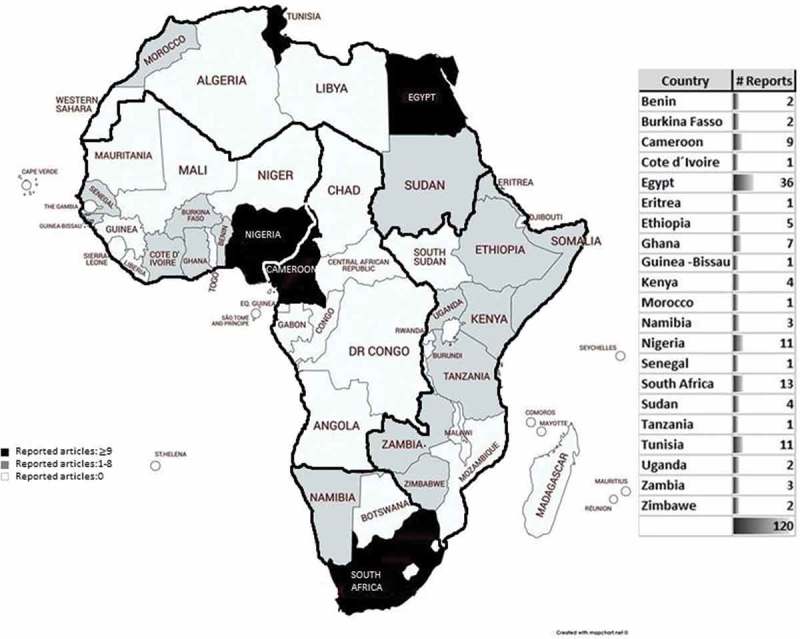
Allocation of WBP reports in different countries on the African map.
Table 1.
Factors present in Africa that jeopardize the infection with WBP.
| Country/Region | Risk factor (s) | Population studied | WBP | Reference |
|---|---|---|---|---|
| Western-Kenya | Contact with Animals (feces): Goats, sheep, chicken, ducks, donkeys, dog, cats | Children <5 years and adults | Giardia and Cryptosporidium | [79] |
| Dagoretti, Nairobi, Kenya | Social and gender determinants: gender, age and role in the household. | Children <5 years and 50–60 years | Cryptosporidium | [81] |
| Chencha town, Southern Ethiopia | Absence of washing facility, home cleanness condition and type of latrine | Children <5 years | E. histolytica/dispar, and G. lamblia | [78] |
| Kumasi Metropolis-Ghana | Irrigation water into the food chain, wastewater | Farmers | Cryptosporidium spp. | [49] |
| Settat, Morocco | Irrigation with raw wastewater in agriculture | Children between 3–9 years | Giardia intestinalis | [51] |
| Fulanis in Kuraje rural settlement of Zamfara state, Nigeria | Poor housing and sanitary conditions (open air defecation), lack of potable water and illiteracy | Children <5 years and adults | Giardia lamblia, and E. histolytica | [52] |
| Nigeria | Co-infection with malaria, stunting, younger age <2, low levels of maternal education and socioeconomic status. | Children <5 years | Cryptosporidium: C. parvum and C. hominis | [53] |
| Ibadan South East Local Government Area, Nigeria | Type of toilet facility used, source of drinking water, and knowledge of parasite transmission patterns | Children <5 years and adults | Isospora, and Cryptosporidium | [54] |
| Archetypal African urban slum in Nigeria | Unsafe drinking water, education, bad environmental hygiene | School aged children | E. histolytica/dispar, G. duodenalis, Entamoeba coli, and Blastocystis hominis | [55] |
| Eastern Cape Province of South Africa | Farm animals contact | >18 years old and HIV infected | Cryptosporidium spp. | [56] |
| Rural western Kenya | Clinical, environmental and behavioural conditions | Children from 0 to 5 years old | Cryptosporidium spp. | [57] |
Figure 3.
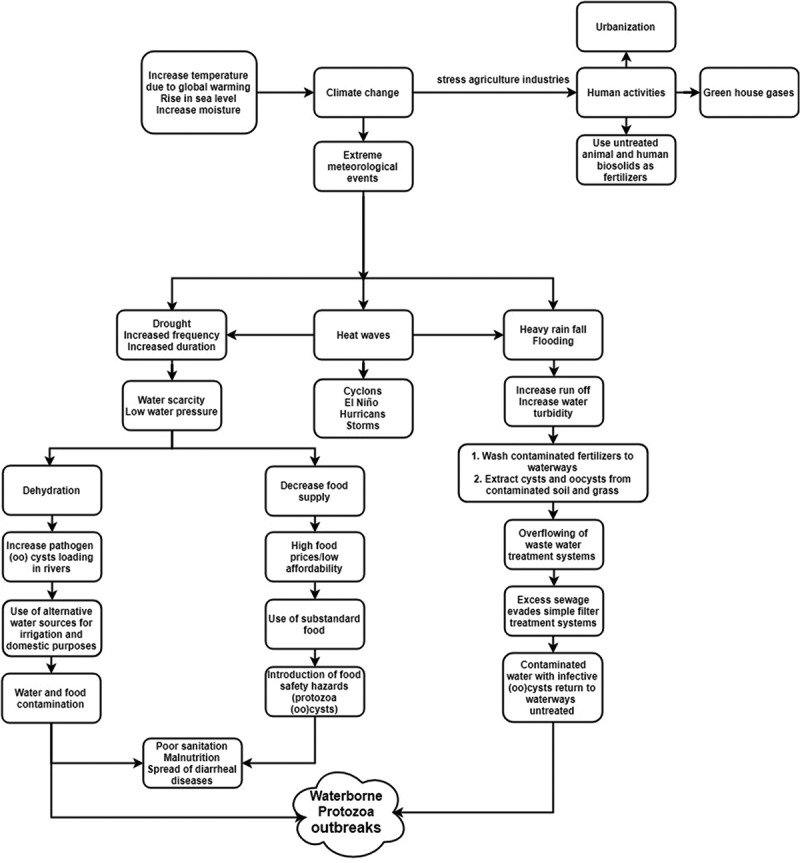
Mechanisms of extreme meteorological events that impact WBP status.
Burden of waterborne protozoa in the African continent
In developing countries lack of access to drinking water supplies, lack of adequate sanitation and deficient waste disposal made scientists expect high prevalence rates of waterborne infectious diseases [11].
The present review reports show that North Africa leads other African geographical divisions (West, East, South and Middle) in the reporting of WBP with significant statistical difference (P value ≤0.05) (Figure 1). According to country classification, Egypt took the lead to document nearly 1/3 (36/120) of WBP reports in Africa. South Africa, Nigeria and Tunisia were the subsequent top countries in reporting WBP forming another 1/3 (35/120). It is noted that 33 African countries have no documented reports about WBP (Figure 2).
On the African continent, a large number of countries still face huge challenges attempting to achieve the United Nations water/sanitation-related Millennium Development Goals (MDGs). Although Africa is a major region trailing improvement in accomplishing the MDGs on sanitation by 2015, there is a noticeable gap between North Africa (90%) and SSA (30%) coverage (http://www.un.org/waterforlifedecade/africa.shtml).
Based on the Human Development Index (HDI), most of the top reporting countries are the most developed in Africa. However, HDI is an economic tool represents a small part of human development [24].
SSA is identified to be the poorest and the least developed region in the world. Living sustenance of half of its population is less than one dollar/day. The deep and widespread poverty severely restricts many countries of SSA to provide proper water and sanitation services (http://www.un.org/waterforlifedecade/africa.shtml). Clear picture about institutional capacity of health system research, financial resources and political security will provide adequate relevant research findings for decision makers and explain the shortage in documentation present in more than half of the African countries [25]. In such countries usage of contaminated water justified the infection of the population and contamination of their food. For example in Eritrea, agriculture reuse of untreated wastewater was a major cause for the increase of giardiasis among farmers of this area [26].
Concerning the WBP in African reports, Cryptosporidium spp. and G. duodenalis were reported in 12 and 16 African countries with high incident rate. Half of African reports of different water sources documented both parasites (Figure 1). Cryptosporidium and Giardia are known to be the most prevalent WBP in developing countries [11]. The multiple routs of exposure of both parasites might explain their abundance in African water. Besides, African populations suffer multiple risk factors (explained later) which result in cycling of these two parasites and increase their burden in water ecosystems. Moreover, small size of oo (cysts)/spores (1–17 µm) and their resistance against common water disinfectants, increase their penetration into multi-barrier water treatment systems [27,28]. Cryptosporidium and Giardia oo (cysts) are able to maintain viability in water up to 6–12 months (Cryptosporidium oocysts even longer) with high stability. Characteristics possibly cause epidemics even after consumption of purified drinking water [29].
FLA (Acanthamoeba and Naegleria spp.) ranked the 3rd leading cause of waterborne protozoal contamination forming more than 1/3 of the documented reports in ten African countries (Uganda, Tunisia, Sudan, South Africa, Namibia, Guinea Bissau, Ethiopia, Egypt, Central African Republic, and Benin).
FLAs are common protozoa present in fresh water bodies. While encysted FLA become resistant to conditions of extreme temperature, osmolarity and pH [30,31]. FLA protozoa have been observed in different water bodies (dental water lines, haemodialysis water, and tap water) in African reports [32–34]. Pathogenic forms of FLA are opportunistic and can cause fatal encephalitis and sight threatening infection (keratitis) [35]; however, in the previous African reports species were not identified. Noted cases in different African countries displayed infection with FLA. Such cases were reported as Acanthamoeba keratitis in Tunisia [36], Ghana [37], and South Africa [38] and as Acanthamoeba meningoencephalitis in Senegal [39]. Raised reports might explain the obvious interest of researchers to stress work on FLA and hence increase its reporting data.
Entamoeba spp., were presented in ten African countries (Zimbabwe, Tunisia, Sudan, Nigeria, Morocco, Ethiopia, Egypt, Cote d´Ivoire, Cameroon, Burkina Fasso). Diarrhea and dysentery are the most common symptoms after infection with this parasite. Extra-intestinal complications are less frequent to occur; however, high mortality can be associated. In recent cross sectional study in South Africa, E. histolytica was highly loaded in the diarrhea samples [40]. In Ethiopian patients, Entamoeba spp. were the highest predominant protozoa in the diarrhea samples from adults [41] and one of the most identified parasites in children under five years [42]. In Cameroon, Egypt and Sudan, Entamoeba spp., were detected in drinking water [42–44]. Direct wet mount/iodine is a simple, cost effective popular method used in African reports to identify cysts of Entamoeba spp. in water samples which probably raises its reporting numbers.
There are scarce reports in Africa concerning other WBP (Balantidium coli ‘7’, Blastocystis hominis ‘4’, Cyclospora cayetanensis ‘10’, Dientamoeba fragilis ‘1’, Isospora belli ‘4’, Microsporidia ‘3’, Sarcocystis spp. ‘2’ and Toxoplasma gondii ‘4’ ranging from (1–10 reports). These parasites are abundant and robust in the environment augmenting the potential for waterborne transmission. The (oo) cysts/spores of these WBP are similar to, or larger than those of Giardia and Cryptosporidium [12]. Even though these less frequent WBP have been the aetiology of waterborne outbreaks [10,11], information about its detection and prevalence are not available in sufficient way. African researchers are less attentive to these parasites – except E. histolytica – giving much more focus to Cryptosporidium and Giardia. The lack of sensitive methods to recover and detect their exogenous stages in water possibly is the cause of their under-reporting [45].
All examined water bodies in the 21 African countries were contaminated with one or mixed waterborne protozoa. A clear fact emphasized well in previous reviews [3,18]. Most important is drinking water which was co-contaminated with more than one protozoa and have been published in several African reports [33,43,46]. In this situation, co-infections will be highly expected in people, who use these water sources for drinking. There is danger of an epidemic, particularly if popular sources are used (ex. sachet water).
Finally, even though WBP caused a significant number of outbreaks (905) documented worldwide until 2014 [10–12] and although the high reporting of protozoa incidence in African waters that are explained in this review, there are still no reported outbreaks in the African continent.
Monitoring disease outbreaks is dependent on having efficient and reliable surveillance and notification system. Despite an efficient surveillance system, a degree of under estimation should be considered [47].
We can surmise that no functional surveillance system to report waterborne disease outbreaks in Africa exist, which is well established in some European countries (e.g. Germany, UK, Sweden), however, indirect prevalence data, reporting incidence, together with the level of contamination may be used as risk predictors [18].
Predisposing factors in the favor of waterborne protozoa distribution
Africa leads the world in terms of diarrheal diseases. Infections by WBP are highly prevalent among rural communities in warm and humid regions mainly due to water, inadequate hygienic and sanitation facilities. The absence of environmental sanitation facilitates the increase of rubbish, other wastes, sewage and wastewater which not properly treated [6,9,13,33,48–50].
Addressing previous threats to surface water is a major challenge in Africa and it depends significantly on recognizing the following predisposing factors and taking action to overcome it. Africa similar to other developing regions worldwide, hold an important route of transmission of WBP, summarized in the following points:
- Water problems
- i.i. Insufficient water resources controlled by climate change
- i.ii. Poor quality of potable water controlled by inadequate water industries and bad human practices around water supplies
Social and behavioral aspects of people
Geographic locations, which can cause isolation of people
Governmental inequality in distributing financial resources
Recent publications about risk factors in Africa have been illustrated in Table 1. Equally other developing regions suffered similar risk factors [16,19].
For better comprehension, the frequent reported risk factors have been grouped in categories: Behavioral, Governmental, Socioeconomic, Sanitation-Water/Pathogen factors.
Behavioral factors (hazards habits of African native population)
Behavior contributes significantly in elevating (oo) cysts level in water sources. Hazardous habits were reported in numerous articles that lead to contamination of water systems with human fecal waste resulting in protozoal threats.
Uncontrolled sewage discharge is a major risk factor that leads to increase prevalence of protozoa in water sources. WHO/UNICEF reported that practicing open defecation is linked to a higher risk of stunting or chronic malnutrition, leaving 161 million children worldwide with irreversible physical and cognitive damage (www.WHO.intl).
Direct defecation on the ground, sewage spills and usage of pit latrines; dominate human to human transmission of (oo) cysts [58]. Half of SSA population still use pit latrines as a mean of excreta disposal, particularly among low income areas [59].
In Uganda, drainage of sewage directly to water bodies from hotels and fishing communities, using shallow latrines and run off excreta of wild and domestic animals from land to water explained contamination of natural bodies of water (river, lake, and channel) and tap water resources with free living amoebae, Cryptosporidium spp. and G. duodenalis (intestinalis) [33]. The distribution of Cryptosporidium and Giardia (oo) cysts in the Mfoundi mainstream/Cameroon returns to the anthropogenic activities carried out by waterside inhabitants who open their toilets directly into the streams and throw their waste into the waterways [60].
Transmission of specific species dynamics and host specificity play a role in the movement of protozoan pathogens between hosts. Humans, animals and wildlife can contribute to environmental transmission of protozoa [58].
In Egypt, pasturing infected animals, watering infected livestock, contact with their manure and its usage as fertilizers contribute to increase the prevalence of E. histolytica and G. duodenalis in drinking ground water [61].
The absence of garbage treatment mechanisms put communities at risk of severe gastroenteritis [62]. Swimming in lakes and ponds, cleaning of utensils, throwing dead animals and garbage and dumping sewage in canals, ponds, wells and rivers are reported behaviors in the Egyptian communities [9,61], Botswana [58], Uganda [33,59], Nigeria [63], Cameroon [64,65] raising the exposure to different WBP.
Using unprotected water sources (spring, dam, wells) for drinking water increased prevalence of G. duodenalis and C. parvum in Ethiopian population [66]. In Zimbabwe, protected and unprotected deep wells, bore holes pose a risk to acquire G. duodenalis, Entamoeba spp., Cryptosporidium spp. and C. cayetanensis [67].
The selection of consumed water in developing countries depends on its aesthetic quality ‘No color appearance of water and no lees’, neglecting its microbiological and/or chemical quality. In Cameroon, 63% of different drinking water sources (wells, boreholes, springs, tap) were contaminated with enteric pathogenic protozoa, even though clean and/or clear its water appearance [43]. Using spring water for drinking without prior treatment is also a habit of the Cameroon population [65].
Land use is an important predictor of water contamination with Cryptosporidium oocysts [68]. As human activity increases, water supply will be inadequate with insufficient sanitary resources. Rapid urbanization caused municipal lake pollution with high accumulation of Cryptosporidium and Giardia spp. in Yaunde, Cameroon [62]. The study of water pollution of the Mingoa River, (springs and wells) further confirms the influence of rampant urbanization and usage of traditional pit latrines on the quality of water and its linkage to the 40% prevalence of diarrhea and parasite infestation [69].
Governmental factors
Governments of African countries have a critical role to play in exacerbating water infectious diseases. African governments are unable to address the growing water scarcity in their countries because of weak political will, pauper institutions and improper governance [70]. The governments in Africa lack consensus to face challenges for providing adequate sanitation infrastructure and hygiene [18]. There is a serious shortage in skilled health care workers and laboratory facilities. African populations moreover suffer lack of transportation and communication infrastructures [3]. Such deficit causes many diseased individuals to face difficulty in seeking medical advice and have no other option but to use home remedies to overcome diarrheal conditions. True obstacles will definitely hamper accurate diagnosis and underestimate reporting of infectious diseases in African countries. Government corruption and civil wars are actions provoking long term damage to public health and spread of infectious diseases. Such interventions lead to massive loss of life and health, particularly in children and women [71]. The rampant war and conflict present in SSA, harsh dictatorship and unresolved political agendas disrupted health care and education and increased prevalence of gastrointestinal diseases and malnutrition. Under corruption and war conditions, there is scarcity of agricultural amenities resulting in rationing of foods and water. Thereby, chronic hunger and starvation will be aggravated leading to increased morbidity and mortality [20]. Similar scenarios are present among the populations from other developing countries, such as Colombia [72]. These problems exceed the capacity of science or people, because decision makers need to develop human polices and be more aware of their decisions in turn to justify and ensure equal distribution of financial resources.
Socio-economic factors
African populations suffer poverty and illiteracy. Widespread problems have made populations unaware of the danger of using untreated water for household drinking and usage. Certain practices will increase the transmission of diarrheal diseases [73,74]. The concept of large family size is popular among African population. Greater than five persons/household were observed in Africa and Middle East [75]. Hence population has increased, a fact that decrease affordability per person and increase malnutrition among family members particularly children. Shortage in health education programs and lack of personal hygiene help increase emission of protozoa infection and spread of diarrheal diseases [3,76].
Sanitation and water/pathogen factors
Treated water must reach healthy standards before used for drinking. Since 1999, it is a criminal offence if concentration of Cryptosporidium and Giardia oo (cysts) is greater than 1 (oo) cysts/10 liters [77]. African countries have their own risk factors for transmission of WBP. In Ethiopia for instance school children aged 5–15 years suffer the highest infection rate and worm burden that attributes to poor sanitation and hygiene. The illiteracy of adults and the use of open defecations systems with the lack of practices such as hand washing using soap put the children at higher risk. Only 19.5% of Ethiopian homes have washing facilities while 80.5% of homes do not have hygienic practices. As a result, people who were poor remain deprived from health, contributing to economic instability and social marginalization [78].
In Kenya, there is high vulnerability to acquire and transmit infectious diseases in different ages/sex. Children under five years, have recognized the exposure to domestic animals mainly faeces of chicken, cats and dogs [79]. Such exposure is a significant risk factor to get diarrheal diseases caused by WBP ‘Giardia and Cryptosporidium’ [80]. In addition to children, Kenyan farm workers and people aged 50 to 65 years had much contact with cattle. Women as well had greater contact with raw milk with higher risk to exposure to Cryptosporidium spp. via involvement in milking activities, feeding and watering of cattle [81].
Poor countries reported difficulty in detecting parasites such as Giardia, Cryptosporidium, T. gondii or C. cayetanensis in water [19]. One of the reasons is the use of less effective methods for concentration of (oo) cysts due to inability to follow standardized methods [28,83]. A similar situation is present in Africa. In particular, Cryptosporidium is not only difficult to detect, but also to treat and its control is limited due to the lack of specific drugs and/or vaccinations. The profile of distribution of all these parasites is high, and will be higher, if management and control strategies are not implemented or direct political and financial decisions are unable to establish efficient and effective surveillance systems.
The clear challenge is water supply and its quality. The water supplies have been impacted routinely by anthroponotic, agricultural and industrial practices, resulting in the decreased availability of surface and ground water supplies. The treatment of water for human consumption, has been difficult in removing parasites by practical water treatment and mainly in the disinfection process, the use of chlorine has been seriously questioned since 1986 [84]. To efficiently eliminate WBP, high dosages of disinfectant are required, which is impractical and represents hazardous scenario of high organic water content [19,85]. High dosages of such organics in water imply high risk of toxicity per potential disinfection by products (DBPs) formation [86].
The science and new technological possibilities have provided tools to detect, identify and genotype WBP; however, poor countries can’t use them despite the fact that they are the most affected with high prevalence of parasitic diseases. At the end, the WBP will continue to take advantage of adapting to the hosts and environment by diversifying genetically, while humans and animals continue to fall ill.
Waterborne protozoa across Africa in terms of climate change
Globally, the temperature will increase by 2–3 ºC and relative humidity will rise [87,88], result in provoking marked changes in the surrounding climate. The alteration of climate pattern in return, causes extreme meteorological events ‘heat waves, drought, and heavy rain fall’ in a way that leads to the occurrence of waterborne and foodborne outbreaks.
By 2030 and 2050, extreme climate is anticipated to cause approximately 250,000 additional deaths per year from malnutrition, diarrheal diseases, heat stress and malaria [89].
The human activities play a significant role in accelerating climate change throughout demographic urbanization, elevating green house gasses emission, and the use of animal sewage and human biosolids as fertilizers to retain crop productivity [12,88,90,91].
Africa is the most sensitive continent to climate change, particularly nations of SSA. Its countries suffer notable critical change in climate with extreme water related weather events (El Niño, cyclones, and hurricanes) [92–94]. As a developing continent, most of its area suffers weak health infrastructure, poverty, armed conflicts and inability of institutional capacity to adapt rapid environmental changes and deal with additional health challenges [95–98].
The extreme water related weather in return will affect precipitation in some regions of Africa and influences the availability of fresh water. Additional water stress will be created in addition to what the SSA already suffers. Currently, over half of SSA populations have no access to safe water and 10% of population still use surface water as drinking source [23].
The climate of the five different geographic regions of Africa is variable; however, climate recently tends to be warmer and drier in all of them with expected high vulnerability to drought. North Africa Mediterranean part (NAMP) are going through a gradual, persistent decrease in rainfall [99]. The climate models of the Fifth Assessment Report of the Intergovernmental Panel on Climate Change approved that there is a sign of forced Mediterranean drying trend with great ambiguity of its rate [100,101]. The situation has forced the population of NAMP to use ground water [102]. Central Africa is known to be the wettest part of Africa. It is predicted to get warmer by 3 oC [103] with rainfall deficits in a large portion of its region [104]. Moreover, central Africa is vulnerable to intense thunderstorms and strong lightning flashes which will influence climate and weather across Africa and the globe [105]. In West Africa, Sahelian storms increase precipitation over the Sahel with consequent warming weather and dryness [105]. Drought will occur as a result, which will critically impact the population of this region exposing them to poor sanitation and limited access to clean water [106,107]. East Africa has been recently affected with a series of devastating droughts following long rain season [108]. At the horn of Africa, El Niño events induce drought crises. The number of drought affected people has been doubled with critical and emergent food insecurity levels, leaving East Africa’s future uncertain [92,108]. South region of Africa as well suffers drought and water scarcity, its governments have declared national drought emergencies. In particular, Madagascar suffers severe drought with critical water scarcity affecting more than 850,000 people [93,94]. During 2017, a dangerous category four tropical cyclone Enawo affected 760,000 people in Madagascar. It was described as the strongest cyclone in the past 13 years. Flooding and water stagnation from such climate crises will end up with outbreaks of life-threatening waterborne diseases [94].
Indeed all previous extreme meteorological events in Africa will significantly alter the seasonal pattern and therefore change the incidence of excreta related diseases [15,108], which by turn will increase the water-borne diarrheal diseases [109] (Figure 3). An impact will clearly appear on the occurrence, transmission and distribution of WBP parasites. Water is often connected to disease spread due to its role in the life cycle of parasites or its direct effect on the health of people [97]. Temperature changes are known to be linked to development rates of the parasite, while relative humidity has been stated to continue parasitic pressure [48,110,111]. In water, (oo) cysts need optimum temperature and moisture conditions to maintain the viability of these infective stages waiting for host to continue the next step of their life cycle [48]. Such linkage influences the richness of parasitic species and intensity of infection [48,90,91,108,112]. The rainfalls and temperatures as well have an impact on the survival, viability and dissemination of parasitic protozoa [113]. In warm and wet locations, precipitation can serve as liable predictor for incidence of WBP [15,91].
Either rainfall or drought in Africa will result in WBP outbreaks (Figure 3) in the form of polluted drinking and recreational water. Hence affected water quality and availability will pose a threat to millions of people [13,114]. African people then either will change their hygienic practices ending with water and food outbreaks [88,91,97,114] or massive migration patterns of populations will occur as a response to climate change. Therefore, it is predicted that many diseases will travel with migrants establishing both, new infectious species or strains, and emergent demand of water resources.
Amebiasis, cryptosporidiosis, giardiasis and toxoplasmosis can all be transmitted through contaminated drinking water. Very low doses of Cryptosporidium spp. and G. duodenalis (oo) cysts are needed to cause infection to healthy individual [115–117]. In Africa, the distribution of these parasites in the environment varies significantly depending on climatic season as in other regions of the world. High frequency was reported in summer and spring, while high intensity with viability of the dormant transmissive stages was documented in summer [9]. Other authors documented different seasons: summer and autumn [44], or winter [118,119]. Such variation proves that the occurrence of Cryptosporidium and Giardia protozoa tends to have a multi-modal pattern in Africa.
Conclusions
The load of WBP is indeed underestimated and underreported in Africa, 56% of African countries have no WBP reports even though Africa leads the world in terms of death due to diarrheal diseases and malnutrition. Cryptosporidium, Giardia, FLA and Entamoeba were the most reported protozoa in African water resources although, other protozoa were less reported due to deficient effective diagnosis. North African countries reported nearly half of the total reported numbers in the continent. High reporting countries were known to be the most developed which might explain the raised record of reporting WBP. The rest of African countries still need government recognition of the future burden of waterborne protozoa diseases and react rapidly to detect and diagnose WBP in an efficient way.
Africa suffers poverty, weak institutional infrastructure, political conflict and its populations are illiterate and practice poor hygiene. Moreover the warm climate and future climate changes to drought in Africa will provoke flare up of diseases. All previous risk factors actively affect the quality and the quantity of drinking water despite the fact that Africa has 9% of the world’s water resources. Such abundant risk factors in Africa are predisposing factors for WBP outbreaks, however, not a single outbreak has been reported yet.
Water crises in Africa will occur, while African governments will not have the capacity and infrastructure to deal with them. African populations therefore either will use other water sources without adequate treatment or migrate to other places. Diseases and malnutrition will rise and waterborne protozoal diseases in particular will impact the incidence, transmission and strains or species manifestation.
African countries should unite and put the welfare of their populations as a priority in their political agendas. Financial resources should be distributed equally to assist and protect low income areas. Only then the devastating effects of water-borne diseases and their future consequences could be mitigated in environments of climate change and other risk factors. African population should receive health education to recognize the impact and react immediately to change their habits and work with the government to avert expected water crises.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].PRB World population data sheet. Washington, DC 20009 USA: Population Reference Bureau; 2017. [Google Scholar]
- [2].Donkor S, Shawel K.. The United Nations world water development report 2016: water and jobs. UNESCO, editor United Nations: UNESCO;201666. [Google Scholar]
- [3].Squire SA, Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasit Vectors. 2017;10(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].CDC Diarrhea: common illness, global killer. US: Center for Disease Control and Prevention; 2015. [Google Scholar]
- [5].Checkley W, White AC, Jaganath D, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis. 2015;15(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kotloff KL. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am. Elsevier Inc; 2017;64(4): 799–814. [DOI] [PubMed] [Google Scholar]
- [7].WHO FACTSHEET: the leading causes of death in Africa - Africa Check. World Health Organization; 2017. [Google Scholar]
- [8].Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].El-Kowrany SI, El- Zamarany EA, El-Nouby KA, et al. Water pollution in the Middle Nile Delta, Egypt: an environmental study. J Adv Res. 2016;7(5):781–794. [Google Scholar]
- [10].Efstratiou A, Ongerth JE, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - An update 2011–2016. Water Res. 2017;114:14–22. [DOI] [PubMed] [Google Scholar]
- [11].Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - An update 2004-2010. Water Res. 2011;45(20):6603–6614. [DOI] [PubMed] [Google Scholar]
- [12].Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5(1):1–38. [DOI] [PubMed] [Google Scholar]
- [13].Fletcher SM, Stark D, Harkness J, et al. Enteric protozoa in the developed world : a public health perspective. Clin Microbiol Rev. 2012;25(3):420–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kwakye-Nuako G, Borketey P, Mensah-Attipoe I, et al. Sachet drinking water in accra: the potential threats of transmission of enteric pathogenic protozoan organisms. Ghana Med J. 2007;41(2):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jagai JS, Castronovo DA, Monchak J, et al. Seasonality of cryptosporidiosis: A meta-analysis approach. Environ Res. 2009;109(4):465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mahmoudi MR, Ongerth JE, Karanis P. Cryptosporidium and cryptosporidiosis: the Asian perspective. Int J Hyg Environ Health. 2017;220(7):1098–1109. [DOI] [PubMed] [Google Scholar]
- [17].Speich B, Croll D, Fürst T, et al. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(1):87–99. [DOI] [PubMed] [Google Scholar]
- [18].Aldeyarbi HM, Abu El-Ezz NMT, Karanis P. Cryptosporidium and cryptosporidiosis: the African perspective. Environ Sci Pollut Res. 2016;23(14):13811–13821. [DOI] [PubMed] [Google Scholar]
- [19].Rosado-García FM, Guerrero-Flórez M, Karanis G, et al. Water-borne protozoa parasites: the Latin American perspective. Int J Hyg Environ Health. 2017;220(5):783–798. [DOI] [PubMed] [Google Scholar]
- [20].Uchendu FN. Hunger influenced life expectancy in war-torn Sub-Saharan African countries. J Health Popul Nutr. 2018;37(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mekonnen M, Hoekstra YA. Four billion people experience water scarcity. Sci Adv. 2016;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haub C, Kaneda T. World population data sheet. Population Reference Bureau; 2013. [Google Scholar]
- [23].WHO/UNICEF Progress on drinking water, sanitation and hygiene. World Health Organization/ United Nations Children's Fund; 2017. [Google Scholar]
- [24].Ann T. Most developed countries in Africa - Comprehensive ranking for 2018 - Afrikanza; 2018. [Google Scholar]
- [25].Simba D, Mukose A, Bazeyo W. Institutional capacity for health systems research in East and Central African schools of public health: strengthening human and financial resources. Heal Res Policy Syst. 2014;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Srikanth R, Naik D. Prevalence of giardiasis due to wastewater reuse for agriculture in the suburbs of Asmara City, Eritrea. Int J Environ Health Res. 2004;14(1):43–52. [DOI] [PubMed] [Google Scholar]
- [27].Karanis P. The truth about in vitro culture of Cryptosporidium species. Parasitol. 2017;16:1–10. [DOI] [PubMed] [Google Scholar]
- [28].Efstratiou A, Ongerth J, Karanis P. Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res. 2017;123:96–112. [DOI] [PubMed] [Google Scholar]
- [29].Omarova A, Tussupova K, Berndtsson R, et al. Protozoan parasites in drinking water: A system approach for improved water, sanitation and hygiene in developing countries. Int J Environ Res Public Health. 2018;15(3):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mahmoudi MR, Rahmati B, Seyedpour SH, et al. Occurrence and molecular characterization of free-living amoeba species (Acanthamoeba, Hartmannella, and Saccamoeba limax) in various surface water resources of Iran. Parasitol Res. 2015;114(12):4669–4674. [DOI] [PubMed] [Google Scholar]
- [31].Mahmoudi MR, Taghipour N, Eftekhar M, et al. Isolation of Acanthamoeba species in surface waters of Gilan province-north of Iran. Parasitol Res. 2012;110(1):473–477. [DOI] [PubMed] [Google Scholar]
- [32].Trabelsi H, Sellami A, Dendena F, et al. Free-living Amoebae (FLA): morphological and molecular identification of Acanthamoeba in dental unit water. Parasite. 2010;17(1):67–70. [DOI] [PubMed] [Google Scholar]
- [33].Sente C, Erume J, Naigaga I, et al. Prevalence of pathogenic free-living amoeba and other protozoa in natural and communal piped tap water from Queen Elizabeth protected area, Uganda. Infect Dis Poverty. 2016;5(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dendana F, Sellami H, Jarraya F, et al. Free-living amoebae (FLA): detection, morphological and molecular identification of Acanthamoeba genus in the hydraulic system of an haemodialysis unit in Tunisia. Parasite. 2008;15(2):137–142. [DOI] [PubMed] [Google Scholar]
- [35].Lass A, Guerrero M, Li X, et al. Detection of Acanthamoeba spp. in water samples collected from natural water reservoirs, sewages, and pharmaceutical factory drains using LAMP and PCR in China. Sci Total Environ. 2017;584–585:489–494. [DOI] [PubMed] [Google Scholar]
- [36].Dendana F, Sellami H, Trabelsi H, et al. Acanthamoeba T4 genotype associated with keratitis infections in Tunisia. Parasitol Res. 2013;112(1):401–405. [DOI] [PubMed] [Google Scholar]
- [37].Leck AK, Matheson MM, Hagan M, et al. Acanthamoeba keratitis in Ghana. Br J Ophthalmol. 2002;86(10):1187–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dini LA, Cockinos C, Frean JA, et al. Unusual case of Acanthamoeba polyphaga and Pseudomonas aeruginosa keratitis in a contact lens wearer from Gauteng, South Africa. J Clin Microbiol. 2000;38(2):826–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ndiaye M, Diop AG, Dieng Y, et al. A case of meningoencephalitis caused by Acanthamoeba sp. in Dakar. Med Trop (Mars). 2005;65(1):67–68. [PubMed] [Google Scholar]
- [40].Ngobeni R, Samie A, Moonah S, et al. Entamoeba species in South Africa: correlations with the host microbiome, parasite burdens, and first description of Entamoeba bangladeshi outside of Asia. J Infect Dis. 2017;216(12):1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Berhe B, Bugssa G, Bayisa S, et al. Foodborne intestinal protozoan infection and associated factors among patients with watery diarrhea in Northern Ethiopia; a cross-sectional study. J Heal Popul Nutr. 2018;37(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zemene T, Shiferaw MB. Prevalence of intestinal parasitic infections in children under the age of 5 years attending the Debre Birhan referral hospital, North Shoa, Ethiopia. BMC Res Notes. 2018;11(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shanan S, Abd H, Bayoumi M, et al. Prevalence of protozoa species in drinking and environmental water sources in Sudan. Biomed Res Int. 2015;2015:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nsoh FA, Wung BA, Atashili J, et al. Prevalence, characteristics and correlates of enteric pathogenic protozoa in drinking water sources in Molyko and Bomaka, Cameroon: a cross-sectional study. BMC Microbiol. 2016;16(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Elshazly AM, Elsheikha HM, Soltan DM, et al. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2007;37(1):51–64. [PubMed] [Google Scholar]
- [46].Plutzer J, Karanis P. Neglected waterborne parasitic protozoa and their detection in water. Water Res. 2016;101:318–332. [DOI] [PubMed] [Google Scholar]
- [47].Ekwunife C, Okafor S, Ukaga C, et al. Parasites associated with sachet drinking water (Pure Water) in Awka, South-Eastern, Nigeria. Sierra Leone J Biomed Res. 2010;2(1):23–27. [Google Scholar]
- [48].Gibbons CL, Mangen M-J-J, Plass D, et al. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Alum A, Absar IM, Asaad H, et al. Impact of environmental conditions on the survival of Cryptosporidium and Giardia on environmental surfaces. Interdiscip Perspect Infect Dis. 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sampson A, Owusu-Ansah ED-GJ, Mills-Robertson FC, et al. Probabilistic quantitative microbial risk assessment model of farmer exposure to Cryptosporidium spp. in irrigation water within Kumasi Metropolis-Ghana. Microb Risk Anal. 2017;6:1–8. [Google Scholar]
- [51].Bengis RG, Leighton FA, Fischer JR, et al. The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech. 2004;23(2):497–511. [PubMed] [Google Scholar]
- [52].El Kettani S, Azzouzi E-M, Maata A. Prevalence of Giardia intestinalis in a farming population using sewage water in agriculture, Settat, Morocco. Med Mal Infect. 2006;36(6):322–328. [DOI] [PubMed] [Google Scholar]
- [53].Jombo GT, Damen JG, Safiyanu H, et al. Human intestinal parasitism, potable water availability and methods of sewage disposal among nomadic Fulanis in Kuraje rural settlement of Zamfara state. Asian Pac J Trop Med. 2010;3(6):491–493. [Google Scholar]
- [54].Molloy SF, Tanner CJ, Kirwan P, et al. Sporadic Cryptosporidium infection in Nigerian children: risk factors with species identification. Epidemiol Infect. 2011;139(6):946–954. [DOI] [PubMed] [Google Scholar]
- [55].Adekeye TA, Thompson E, Awobode HO. Environmental contamination and public health risk of soil parasites in Ibadan South East Local Government Area, Nigeria. Zool Ecol. Taylor & Francis; 2016;26(2): 150–157. [Google Scholar]
- [56].Gyang VP, Chuang T-W, Liao C-W, et al. Intestinal parasitic infections: current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. J Microbiol Immunol Infect. 2017;pii:S1684–1182(17)30072–5. [DOI] [PubMed] [Google Scholar]
- [57].Omoruyi B, Matongo F, Nkwetshana NT, et al. Environmental and demographic risk factors associated with the prevalence of Cryptosporidium infection in the Alice rural settlements of the Eastern Cape Province of South Africa: a pilot study. Rev Environ Health. 2011;26(2):127–133. [DOI] [PubMed] [Google Scholar]
- [58].Delahoy MJ, Omore R, Ayers TL, et al. Clinical, environmental, and behavioral characteristics associated with Cryptosporidium infection among children with moderate-to-severe diarrhea in rural western Kenya, 2008-2012: the Global Enteric Multicenter Study (GEMS). Kang G, editor PLoS Negl Trop Dis. 2018;12(7):e0006640 J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Alexander K, Herbein J, Zajac A. The occurrence of Cryptosporidium and Giardia infections among patients reporting diarrheal disease in Chobe District, Botswana. Adv Infect Dis. 2012;2(4):143–147. [Google Scholar]
- [60].Nakagiri A, Niwagaba CB, Nyenje PM, et al. Are pit latrines in urban areas of Sub-Saharan Africa performing? A review of usage, filling, insects and odour nuisances. BMC Public Health. 2016;16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ajeagah G, Njine T, Bilong Bilong C, et al. Seasonal distribution of enteric opportunistic Cryptosporidium spp. oocysts and Giardia spp. cysts in a tropical water basin, Cameroon. Water. 2010;2:44–57. [Google Scholar]
- [62].Elfadaly HA, Hassanain NA, Hassanain MA, et al. Evaluation of primitive ground water supplies as a risk factor for the development of major waterborne zoonosis in Egyptian children living in rural areas. J Infect Public Health. 2017;2:203-208. [DOI] [PubMed] [Google Scholar]
- [63].Ajeagah G, Njine T, Foto S, et al. Enumeration of Cryptosporidium sp. and Giardia sp. (oo)cysts in a tropical eutrophic lake. Int J Env Sci Tech. 2007;4(2):223–332. [Google Scholar]
- [64].Chollom SC, Iduh MU, Gyang BJ, et al. Parasitological evaluation of domestic water sources in a rural community in Nigeria. Br Microbiol Res J. 2013;3(3):1–21. [Google Scholar]
- [65].Chia PN, N PCU, Yongabi PKA, et al. Baseline study on the occurrence of Cryptosporidium spp from streams water, after torrential rains in Bamenda, Cameroon. J Biol Agric Health. 2015;4(3):62–69. [Google Scholar]
- [66].Ajeagah GA. Occurrence of bacteria, protozoans and metazoans in waters from two semi-urbanized areas of Cameroon. Ecohydrol Hydrobiol. 2013;13(3):218–225. [Google Scholar]
- [67].Tigabu E, Petros B, Endeshaw T. Prevalence of giardiasis and cryptosporidiosis among children in relation to water sources in selected village of Pawi Special District in Benishangul-Gumuz Region, northwestern Ethiopia. Ethiop J Heal Dev. 2010;24(3):205–213. [Google Scholar]
- [68].Mtapuri-Zinyowera S, Ruhanya V, Midzi N, et al. Human parasitic protozoa in drinking water sources in rural Zimbabwe and their link to HIV infection. Germs. 2014;4(4):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Phillip DAT, Rawlins SC, Baboolal S, et al. Relative importance of the various environmental sources of oocysts in three watersheds. J Water Health. 2008;6(1):23–24. [DOI] [PubMed] [Google Scholar]
- [70].Youmbi JGT, Feumba R, Njitat VT, et al. Pollution de l’eau souterraine et risques sanitaires à Yaoundé au Cameroun. Comptes Rendus - Biol. 2013;336(5–6):310–316. [DOI] [PubMed] [Google Scholar]
- [71].Dos Santos S, Adams EA, Neville G, et al. Urban growth and water access in sub-Saharan Africa: progress, challenges, and emerging research directions. Sci Total Environ. 2017;607–608:497–508. [DOI] [PubMed] [Google Scholar]
- [72].Ghobarah HA, Huth P, Russett B. The post-war public health effects of civil conflict. Soc Sci Med. 2004;59(4):869–884. [DOI] [PubMed] [Google Scholar]
- [73].Webster PC. Health in Colombia: a system in crisis. Cmaj. 2012;184(6):E289–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].CDC Global water, sanitation, and hygiene epidemiology team. Atlanta: Center for Disease Control and Prevention; 2014. [Google Scholar]
- [75].WHO Combating waterborne disease at the household level: the international network to promote household water treatment and safe storage. World Health Organization; 2007. p. 34. [Google Scholar]
- [76].UN Population facts. United Nations; 2017. [Google Scholar]
- [77].Hofstra N, Vermeulen LC. Impacts of population growth, urbanisation and sanitation changes on global human Cryptosporidium emissions to surface water. Int J Hyg Environ Health. 2016;219(7):599–605. [DOI] [PubMed] [Google Scholar]
- [78].Standard Operating Protocol Standard operating protocol for the monitoring of Cryptosporidium oocysts in treated water supplies. Standard Operation Protocol; 1999. [Google Scholar]
- [79].Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014;14(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Conan A, O’Reilly CE, Ogola E, et al. Animal-related factors associated with moderate-to-severe diarrhea in children younger than five years in western Kenya: A matched case-control study. Ryan ET, editor PLoS Negl Trop Dis. 2017;11(8):e0005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kotloff KL, Blackwelder WC, Nasrin D, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kimani VN, Mitoko G, McDermott B, et al. Social and gender determinants of risk of cryptosporidiosis, an emerging zoonosis, in Dagoretti, Nairobi, Kenya. Trop Anim Health Prod. 2012;44(Suppl 1 (S1)):S17–23. [DOI] [PubMed] [Google Scholar]
- [83].Method EPA. 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. United states Environmental Protection Agency; 2005. [Google Scholar]
- [84].Hoff JC, Akin EW. Microbial resistance to disinfectants: mechanisms and significance. Environ Health Perspect. 1986;69:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Karanis P, Schoenen D, Seitz HM. Distribution and removal of Giardia and Cryptosporidium in water supplies in Germany. Water Sci Technol. 1998;37(2):9–18. [Google Scholar]
- [86].Collivignarelli M, Abbà A, Benigna I, et al. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability. 2017;10(2):86. [Google Scholar]
- [87].Patz JA, Githeko AK, McCarty JP, et al. Climate change and infectious diseases. Infect Dis. 2008;9(6):103–132. [Google Scholar]
- [88].Fuhrimann S, Winkler MS, Kabatereine NB, et al. Risk of intestinal parasitic infections in people with different exposures to wastewater and fecal sludge in Kampala, Uganda: A cross-sectional study. Bethony JM, editor PLoS Negl Trop Dis. 2016;10(3):e0004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].WHO Climate change and health. World Health Organization; 2017. [Google Scholar]
- [90].Short E, Caminade C, Thomas B. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect Dis Res Treat. 2017;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yan C, Liang L-J, Zheng K-Y, et al. Impact of environmental factors on the emergence, transmission and distribution of Toxoplasma gondii. Parasit Vectors. 2016;9(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].OCHA El Niño in East Africa | OCHA. United Nations Office for the Coordination of Humanitarian Affairs; 2016a. [Google Scholar]
- [93].OCHA El Niño in Southern Africa | OCHA. United Nations Office for the Coordination of Humanitarian Affairs; 2016b. [Google Scholar]
- [94].OCHA Madagascar: tropical cyclone Enawo likely to affect 760,000 people. United Nations Office for the Coordination of Humanitarian Affairs; 2017. [Google Scholar]
- [95].Bain LE, Awah PK, Geraldine N, et al. Malnutrition in Sub-Saharan Africa: burden, causes and prospects. Pan Afr Med J. 2013;15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Heffernan O. Adapting to a warmer world: no going back. Nature. 2012;491(7426):659–661. [DOI] [PubMed] [Google Scholar]
- [97].Owens OC, Okereke C, Webb J, et al. Climate change and Health Across Africa: issues and options. United Nations Economic Commission for Africa (UNECA); 2011. p. 48. [Google Scholar]
- [98].Schmidhuber J, Tubiello FN. Global food security under climate change. Proc Natl Acad Sci. 2007;104(50):19703–19708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kushnir Y. Mediterranean climate future: an insightful look into the Basin’s precipitation response to greenhouse gas forcing. Environ Res Lett. 2015;10:111001. [Google Scholar]
- [100].IPCC Climate change 2013: the physical science basis In: Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change; 2013; p. 33. [Google Scholar]
- [101].Zappa G, Hoskins BJ, Shepherd TG. The dependence of winter time Mediterranean precipitation on the atmospheric circulation response to climate change. Environ Res Lett. 2015;10(10):104012. [Google Scholar]
- [102].Kuper M, Amichi H, Mayaux P-L. Groundwater use in North Africa as a cautionary tale for climate change adaptation. Water Int. 2017;42(6):725–740. [Google Scholar]
- [103].IPCC Climate Change 2007: impacts, adaptation and vulnerability In: Parry M, Canziani O, Palutikof J, et al, editors. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge (UK): Cambridge University Press; 2007. p. 976. [Google Scholar]
- [104].The WMO. Climate in Africa: 2013. World Meteorological Organization; 2015. p. 29. [Google Scholar]
- [105].Creese A, Pokam W. Central Africa’s climate system In: Africa’s climate helping decision‑makers make sense of climate information. Future climate for Africa; 2016. p. 4–10. [Google Scholar]
- [106].Taylor CM, Belušić D, Guichard F, et al. Frequency of extreme Sahelian storms tripled since 1982 in satellite observations. Nature. 2017;544(7651):475–478. [DOI] [PubMed] [Google Scholar]
- [107].Skinner CB, Diffenbaugh NS. Projected changes in African easterly wave intensity and track in response to greenhouse forcing. Proc Natl Acad Sci. 2014;111(19):6882–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Araujo J, Marsham J, Rowell D, et al. East Africa’s climate: planning for an uncertain future In: Joubert L, editor. Africa’s climate: Helping decision-makers make sense of climate information. South Africa: Climate & Development Knowledge Network; 2016. p. 11-17. [Google Scholar]
- [109].Rosenthal J. Climate change and the geographic distribution of infectious diseases. Ecohealth. 2009;6(4):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hudson PJ, Cattadori IM, Boag B, et al. Climate disruption and parasite-host dynamics: patterns and processes associated with warming and the frequency of extreme climatic events. J Helminthol. 2006;80(2):175–182. [DOI] [PubMed] [Google Scholar]
- [111].Kutz SJ, Hoberg EP, Polley L, et al. Global warming is changing the dynamics of Arctic host-parasite systems. Proceedings Biol Sci. 2005;272(1581):2571–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mas-Coma S, Valero MA, Bargues MD. Effects of climate change on animal and zoonotic helminthiases. Rev Sci Tech. 2008;27(2):443–457. [PubMed] [Google Scholar]
- [113].Schijven J, Bouwknegt M, de Roda Husman AM, et al. A decision support tool to compare waterborne and foodborne infection and/or illness risks associated with climate change. Risk Anal. 2013;33(12):2154–2167. [DOI] [PubMed] [Google Scholar]
- [114].Patz JA, Graczyk TK, Geller N, et al. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30(12–13):1395–1405. [DOI] [PubMed] [Google Scholar]
- [115].Ankarklev J, Jerlström-Hultqvist J, Ringqvist E, et al. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat Rev Microbiol. 2010;8(6):413–422. [DOI] [PubMed] [Google Scholar]
- [116].Carmena D. Waterborne transmission of Cryptosporidium and Giardia: detection, surveillance and implications for public health In: Mendez-Vilas A, editor. Current research, technology and education topicas in applied microbiology and microbial biotechnology formatex microbiology series. Formatex Research Center; 2010. p. 3–14. [Google Scholar]
- [117].Castro-Hermida JA, García-Presedo I, González-Warleta M, et al. Cryptosporidium and Giardia detection in water bodies of Galicia, Spain. Water Res. 2010;44(20):5887–5896. [DOI] [PubMed] [Google Scholar]
- [118].Khairy AE, El Sebaie O, Abdel Gawad A, et al. The sanitary condition of rural drinking water in a Nile Delta village. I. Parasitological assessment of “zir” stored and direct tap water. J Hyg (Lond). 1982;88(1):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sayed FG, Hamza AI, Galal LA, et al. Detection of Cryptosporidium parvum oocysts contaminating hospitals drinking water supply using different techniques during winter/summer season. Glob Adv Res J Microbiol. 2016;5(6):68–79. [Google Scholar]


