ABSTRACT
Toxoplasmosis is an infection caused by Toxoplasma gondii, a widespread zoonotic protozoan which poses a great threat to human health and economic well-being worldwide. It is usually acquired by ingestion of water contaminated with oocysts from the feces of infected cats or by the ingestion of raw or undercooked foodstuff containing tissue cysts. The oocyst can contaminate irrigation water and fresh edible produce. It is estimated that approximately one-third of the human population worldwide harbor this parasite. Infection with T. gondii is an important cause of diseases of the central nervous system and the eye in immunocompromised and immunocompetent individuals. The purpose of this study was to evaluate the efficacy and applicability of thermal (heating, cooking, freezing and low temperature), non-thermal (high pressure processing, ionizing irradiation and curing) and chemical and biochemical (disinfection, essential oils and biochemical methods such as enzymes, nanoparticles, antibiotics and immune response) treatments for the inactivation, inhabitation or to kill T. gondii in foodstuff intended for public consumption and under experimental conditions.
KEYWORDS: Toxoplasma gondii, foodborne toxoplasmosis, inactivation treatment, thermal, non-thermal, chemical and biochemical
1. Introduction
Foodborne diseases are caused by a number of agents that can affect or compromise the life of the consumer and in most cases are of biological origin, such as bacteria, viruses and parasites. Food and waterborne infections have received considerable attention for many years. Parasites are organisms that acquire nourishment and protection from other living organisms such as humans and animals that are known as hosts. They can cause illness in humans when present in food or water. Many parasites can be transmitted by food and food handlers, including protozoa and helminths.
In many countries, the most common foodborne parasites are protozoa such as Cryptosporidium spp., Entamoeba histolytica, Cyclospora cayetanensis, Giardia intestinalis, Sarcocystis (hominis and suihominis), Toxoplasma gondii, roundworms such as Anisakis spp. and Trichinella spp., flatworms such as Fasciola hepatica, Fasciolopsis buski and Paragonimus sppand tapeworms such as Diphyllobothrium spp., Taenia spp. and Echinococcus spp. One common zoonotic parasitic disease worldwide is toxoplasmosis, an infection caused by T. gondii [1].
T. gondii cannot grow outside of a suitable host, in all food types or in other environments; however, findings have shown that T. gondii infection can be transmitted by the ingestion of oocysts (from contamination of the environment through cat feces) and can contaminate drinking or surface water, soil (an oocyst can survive in soil for up to two years) [2] and fruits and vegetables or by viable tissue cysts found in raw or undercooked meat of intermediate hosts (all warm-blooded animals, including most livestock and humans) [3,4]. The oocysts are highly infectious to herbivores, as are the bradyzoites to cats. There are three infectious stages of T. gondii: in groups or clones as a tachyzoites, in tissue cysts as a bradyzoites and in oocysts as sporozoites. Biological life cycle of T. gondii is classified in the sexual and asexual stages. The sexual cycle is restricted to the feline intestine and lead to shedding of oocysts in cat feces. The activated oocysts (excretion by cat) become extremely infectious and can survive in the environment for long time (several months) and possibly years. The asexual cycle is initiated when any other warm-blooded animal ingests these infectious oocysts [5].
Food handlers such as farmers, sellers, butchers or housewives that directly or indirectly deal with the production, preparation, processing and distribution of foods between communities are identified in most cases as the most likely sources of toxoplasmosis infection in humans. One of the best ways to prevent contamination is compliance with good hygiene practices during food production and processing [6].
In response to natural infection, most farm animals are seropositive for T. gondii. Serological studies have shown infectious parasites of T. gondii in meat production of farm animals [7]. Food animals such as pigs (extreme seroprevalence in many countries) [8,9], poultry (seroprevalence up to 65% in free-range chickens and 81% seropositive in birds) [10] and sheep and goats (seroprevalence of 75 to 92% in many areas of the world) [11] become infected by the same routes, resulting in meat products containing tissue cysts which can then infect consumers [12]. Scientists have reported that at least a third of the global population is infected with the parasite, making it one of the most successful parasitic infections [13].
Researchers have indicated that although infection with T. gondii in healthy humans may be asymptomatic, it can be cause substantial risks and be fatal to immunocompromised individuals such as young children, the elderly, HIV/AIDS and cancer patients and organ transplant recipients [14–18]. In addition to consumption of contaminated meat or infection with feline feces, it can cause trans-placental infection in pregnant women when the infection is acquired during pregnancy [19]. Studies have shown a 30 to 90% toxoplasmosis infection rate in Central and South America and continental Europe [20–22]. The estimated disease burden (incidence, mortality and sequelae) of congenital toxoplasmosis in the Netherlands was 620 (range of 220–1900) disability-adjusted life years (DALY) [23], which is similar to that reported in the US [24]. High burdens have been reported in South America and some Middle Eastern and low-income countries as well [25]. Hoffmann et al. reported that the annual cost of illness and quality-adjusted life year loss (QALY) in the US is due to foodborne pathogens. They estimate that the cost of T. gondii is $3 billion annually (11,000 QALY) [24].
2. Impact of T. gondii on health and reproduction of farm animals
The development of the food chain supply, extension of international travel, and increase in the populations of vulnerable groups, changes in dietary habits and improved diagnostic methods and communication are factors that have given rise to the investigation of foodborne parasitic diseases globally [26]. In two advanced studies of death and disability attributable to pathogens of foodborne diseases, toxoplasmosis was classified as very high [27] and many researchers have presented similar findings in different parts of the world [24,28,29]. Economically, it causes tremendous loss of valuable livestock (cattle, pigs, sheep, goats and poultry).
Infection of dairy goats with T. gondii is widespread and constitutes a public health concern [30], resulting in significant reproductive loss [31,32]. Jacobs and Hartley reported that infection within 30 days after mating led to fetal death and resorption in 42% of ewes. When infected 90 days after mating, 16% of ewes aborted, and 16% gave birth to dead lambs and 52% to live lambs [33].
These results and risk assessment suggest that T. gondii infected animals are a food safety concern. In terms of food safety, the US Food and Drug Administration reports that about 85% of pregnant women in the US are at risk of being infected with toxoplasmosis. About 50% of toxoplasmosis infections in the US each year are acquired from food [34]. The consumption of lamb, beef and meat products such as salami are more common in northern and central European countries than in Italy. Cook et al. found that the proportion of toxoplasmosis infections attributed to eating salami was 14, 11, 4, 3, 10 and 5% in Naples, Milan, Copenhagen, Oslo, Brussels and Lausanne, respectively [35].
3. Food contamination
Contamination of food and the environment by T. gondii can directly or indirectly cause infections in humans consuming high risk food products such as contaminated meat (sheep, goats, pigs, cattle and birds), unpasteurized milk (unpasteurized or inadequately processed milk or fresh cheese), fresh or raw fruits, vegetables and plant products, as well as contaminated water [36]. Infection may also occur by consumption of raw or undercooked meat containing the cysts or exposure to cross-contamination through water and food contaminated with feline feces [19]. Cook et al. showed that 30 to 63% of infections in European countries could be attributed to raw and undercooked meat consumption [35]. Different types of foodstuff, such as pork [37], sheep [38], poultry [21] and cattle [39], the unpasteurized milk of goats [4], fresh plants and plant products [40], water [3] and raw or undercooked seafood [41] are food vehicles in the transmission of toxoplasmosis to humans (Figure 1).
Figure 1.
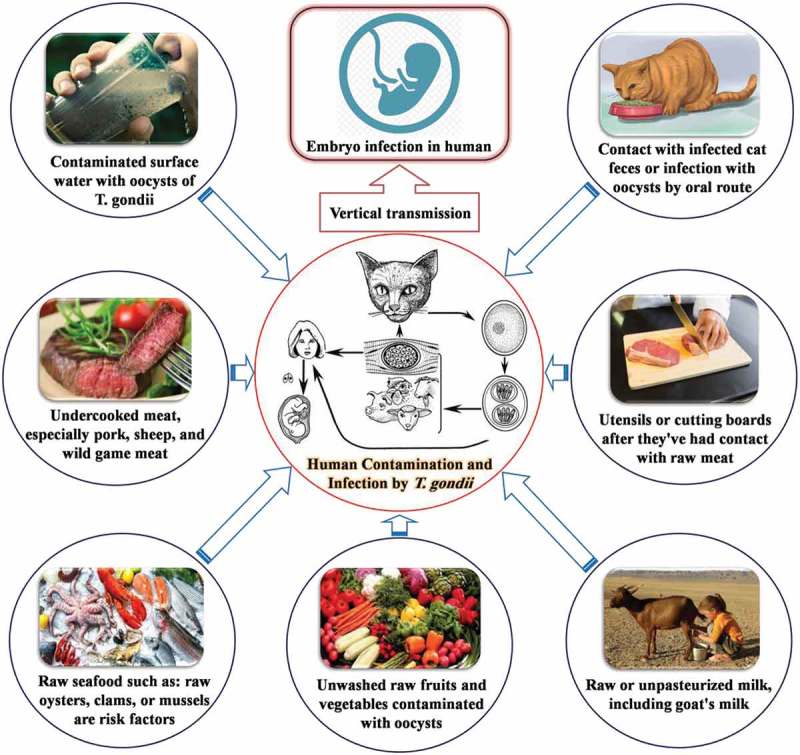
Main sources of T. gondii infection in food or by oral entry route in humans.in humans.
As noted, toxoplasmosis is acquired by ingesting food and water contaminated with oocysts from the feces of infected cats. However, it can also occur by the ingestion of infected meat containing tissue cysts of an infected intermediary host [3]. In the US, assessment of 698 retail outlets showed that the prevalence of viable T. gondii tissue cysts in commercially available fresh pork products was 0.38% [42]. Investigation of 71 meat samples obtained from UK retail outlets showed the presence of this parasite in 27 of the meat samples [43]. Another study reported that T. gondii was detected in ready-to-eat cured meat samples [44]. Evaluation of the prevalence of T. gondii in meat and meat products (100 samples consisting of tongue, heart and muscle taken from 50 lamb and 50 beef samples) in Ahvaz, Iran showed that 7 lamb samples (14%) and 2 beef samples (4%) were found positive for T. gondii cysts [45].
Although outbreaks of toxoplasmosis infection in humans are recognized infrequently, outbreaks associated with water and food are shown in Table 1. The world’s largest outbreak of waterborne toxoplasmosis occurred in British Columbia in Canada, for which drinking water was the source [46].
Table 1.
Reported toxoplasmosis outbreaks associated with water and foods.
| Years | Country | Type of food | Ref. |
|---|---|---|---|
| 1992 | France | Undercooked or cured meat products | [47] |
| 1994 | South Korea | Uncooked pork | [48] |
| 1994–1995 | Denmark-Belgium-Italy-Switzerland-Norway | Undercooked or cured meat products | [35] |
| 2001 | India | Contaminated water | [49] |
| 2001–2002 | Brazil | Contaminated water and/or foods | [50] |
| 2001–2002 | Brazil | Ice cream prepared with contaminated water | [50] |
| 2004 | India | Contaminated water | [51] |
| 1999–2004 | United States | Contaminated water | [52] |
| 2005 | Suriname | Contaminated water | [53] |
| 2006–2008 | Poland | Fruits and vegetables | [40] |
| 2009 | Brazil | Vegetable | [54] |
| 2009–2010 | United States | Contaminated water | [52] |
| 2014 | Tunisia | Unpasteurized goat milk | [55] |
4. Inactivation treatments for T. gondii
Tachyzoites, bradyzoites and sporozoites are the three infectious stages of T. gondii in humans and other warm-blooded animals. Humans and animals become infected mainly by ingesting bradyzoites or oocysts [56]. T. gondii oocysts are highly resistant to environmental influence and are rapidly inactivated following exposure to temperature extremes, irradiation, chemical agents and other physical methods.
It has been found that the oocyst wall is bilayered, with the outer layer being thinner than the inner layer [57]. Experimental results have shown that layers are not tightly bound together and the outer layer can be stripped off easily using chemical agents [58]. Furthermore, the oocyst wall is composed of more than 90% cysteine- and tyrosine-rich protein [59] and thermal methods such as high heating, cooking or physical methods can denature the proteins and cause inactivation or killing of T. gondii. The use of drugs or freezing can inactivate or prevent T. gondii infection by preventing the parasite from attaching and entering cells [60] or by disrupting metabolism and the formation of ice crystals [61,62], respectively. Under experimental and industrial conditions, physical methods (thermal and non-thermal) or other applied technologies (chemical and biochemical methods) can be used to control, remove or inactivate T. gondii. The prevention of cross-contamination in raw and processed food products is significant in this regard (Figure 2).
Figure 2.
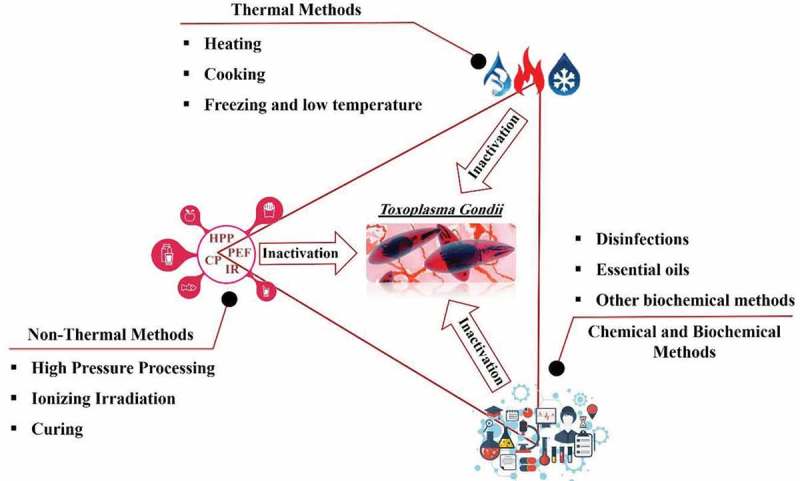
Schematic of applicable methods for inactivation of T. gondii in food.
5. Thermal methods
The killing or inactivation of the parasitic cysts in animal tissue and oocysts in the environment is essential to preventing T. gondii infection in man and animals. Studies have reported beneficial effects from heating, freezing and cooking for inactivation of T. gondii [63].
5.1. Heating
Heat processing is an important method for extending the storage life of foodstuff. The basic purpose of heat treatment of food is to reduce or destroy all microbes or spoilage caused by bacteria and bacterial spores, viruses and parasites. Heat processing of food products for sterilization must be intense enough to inactivate or kill most heat-resistant microorganisms [64]. T. gondii is susceptible to heat inactivation and studies have shown that heating can destroy T. gondii oocysts of both unsporulated and sporulated strains [65].
Dubey et al. reported on the destructive effect of heating on tissue cysts [66]. Another study showed that heating at 58°C was sufficient to inactivate all oocysts [67]. Wainwright et al. indicated that T. gondii oocysts may not always be inactivated when exposed to a minimum of 60°C for 1 min and that the water heating time, cooling time and volume of water treated must be considered when evaluating radiofrequency (RF) or thermal heating for oocyst inactivation [68]. Table 2 shows that heating methods are effective for causing T. gondii to become inviable. Shorter times and lower temperatures are needed to kill tachyzoites found in the heart (a blood-filled organ) that recently have been acquired from the animal compared to bradyzoites, which could be found in muscle tissue and most other tissues. Long times/temperatures/treatments are needed for oocysts found in contaminated vegetables or in the gut tissue of felines.
Table 2.
Summary of effects of thermal methods to inactivation of T. gondii and its infectious forms.
| Type of methods | Temperature(s) (°C) | Time (min/h/day) | Main finding | Ref. |
|---|---|---|---|---|
| Heating | 50 | 2.5 min | Sporulation of Toxoplasma oocysts inhibited. | [65] |
| 50 | 30 min | Infectivity of sporulated oocysts disappears. | [65] | |
| 55 | 30 min | Tissue cysts are destroyed. | [66] | |
| 58 | 30 min | No evidence of parasites in infected murine brains. | [67] | |
| 58 | 15 min | Sufficient to inactivate all oocysts. | [67] | |
| 61 | 3.6 min | Tissue cysts were generally rendered nonviable. | [75] | |
| 60 or 100 | 1 min | No viable T. gondii infective stages isolated from meat samples. | [76] | |
| 63 | 30 min | T. gondii tachyzoites RH strain die in pasteurized milk. | [77] | |
| 75 | 1 h | Heat treatment like boiling water can inactivate T. gondii oocysts. | [78] | |
| Cooking | 63, 71, 82 | – | Beef, lamb and veal roasts and steaks should be cooked to at least 63°C. Pork, ground meat and wild game should be cooked to 71°C before eating. Whole poultry should be cooked to 82°C in the thigh to ensure doneness. | [27] |
| 67 | – | Tissue cysts in meat are killed by heating meat throughout. | [61] | |
| 50 | 1 h | Heating inactivates tissue cysts. | [73] | |
| 67 | 0.01–96 min | Kills tissue cysts in meat. | [79] | |
| 66 | - | Heating meat throughout to reach a temperature is sufficient to kill cysts in meat. | [80] | |
| Freezing and low temperature | −12.37 | 11.2 day | Nonviable T. gondii tissue cysts in pork upon freezing. | [61] |
| −20 | 1–2 day | Tissue cysts stored at −20°C could infection after 24 and 48 h of storage. | [81] | |
| −21 | 1 1/2 h | After 1 1/2 h of exposure to −21°C, many cysts seem to lose their infectivity. | [62] | |
| −10 or −20 | 3 day | Freezing of meat at −10°C for 3 days or at −20°C for 2 days killed parasite and cysts could not recover. | [76] | |
| −7 | 4 day | Inactivation of T gondii tissue cysts was achieved by freezing at −7°C for 4 days. | [82] | |
| −20 | 21 day | Sporulated oocysts were inactivated by freezing. | [82] | |
| −20 | 2 day | Freezing for 2 days at −20°C was sufficient to inactivate parasite. | [72] | |
| −25 | 6–35 day | Experiments with meat from pigs fed with T. gondii infected mice showed that all meat samples were rendered non-infectious. | [83] | |
| −7−12 | 4 day | Parasites in meat from experimentally infected pigs did not survive. | [82] | |
| −20 | 3 day | Temperature and time required to inactivate isolated tissue cysts. | [81] |
5.2. Cooking
The primary control factor for prevention of T. gondii infection in meat consumption is adequate cooking and prevention of cross-contamination by separation of cooked meat and raw or undercooked meat [69]. Hill et al. showed that T. gondii is killed in 5.6 min at 49°C, in 44 sec at 55°C and in 6 sec at 61°C if the temperature is evenly distributed and maintained throughout the meat [70]. Other studies have reported similar findings (Table 2). Overall, meat should be cooked to 67°C before consumption and tasting meat while cooking or while seasoning should be avoided [71]. Briefly, cooking meat and its products to reach the desired temperature throughout (60–70°C) is sufficient to kill T. gondii cysts.
5.3. Freezing and low temperature
Freezing of food by consumers is common in many countries, but an important factor in consumer attitudes towards freezing is the perceived loss of sensory qualities [7]. Studies have shown that freezing can inactivate the T. gondii tissue cysts. The effect of freezing on T. gondii cyst viability was first described in 1965 by Sommer et al., who stated that freezing for two days at −20°C was sufficient to inactivate the parasite [72]. Other studies have shown that to inactivate the isolated tissue cysts, at least three days at −20°C is required and these results are in accordance with earlier reports by other researchers [73,74]. Table 2 shows similar studies on the effect of low temperature and freezing on the killing or inactivation of T. gondii and its infectious forms. It has been recommended that meat be stored for at least three days at −20°C to reduce the T. gondii load in contaminated meat.
6. Non-thermal methods
In the last decade, non-thermal technologies have become of interest to food scientists, manufacturers and handlers because they have minimal impact on the sensory properties and nutritional properties of food. They also can extend the shelf life by inactivation or killing of foodborne pathogens [84,85]. Novel non-thermal technologies such as pulsed electric fields (PEF), pulsed light treatment (PLT), high pressure processing (HPP), cold plasma (CP) and extremely low frequency (ELF) and ionizing radiation (IR) have the ability to inactivate a range of bacteria, viruses and parasites [86,87]. This section summarizes some non-thermal processing technologies that are currently available or in development for the inactivation of T. gondii.
6.1. High pressure processing
HPP technology is a relatively new, non-thermal method that subjects food products (liquid or solid) to pressures of up to 1000 MPa to inactivate or kill many of the microorganisms which are found in foods with rapid processing times, even at low temperatures, without losing minerals, vitamins, flavor and color molecules in the process [84,88,89]. HPP is also known as high hydrostatic pressure (HHP) or ultra high pressure processing. It has been shown to be effective for the elimination of microbial spores from a variety of food products [90]. Studies on the effectiveness of HPP in the killing or inactivation of foodborne parasites show the sensitivity of the parasites and achieves their destruction at relatively low pressures [7].
Lindsay et al. have shown that HPP using 340–550 MPa can inactivate T. gondii tissue cysts under laboratory conditions [91]. However, the effects of high pressure treatment on the color and texture of food have limited consumer acceptance [10]. Similar studies on HPP treatment of T. gondii are presented in Table 3. The results suggests that 340–400 MPa for 1 min is applicable to control or inactivate T. gondii in food, but further studies are needed to determine the amount of time and HPP needed for each T. gondii life cycle.
Table 3.
Summary of effects of non-thermal methods to inactivation of T. gondii and its infectious forms.
| Type of methods | Experimental Conditions | Main finding | Ref. |
|---|---|---|---|
| High Pressure Processing | 400 or 300 MPa to 30, 60, or 90 sec | No mice inoculated with tissue cysts exposed to 400 or 300 MPa became infected. | [104] |
| 340 MPa to 1 min | Use of HPP at 340 MPa for 60 sec required to render oocysts spot inoculated on raspberries non-infectious for mice. | [104] | |
| 550 MPa, 480 MPa, 400 MPa, or 340 MPa to 1 min | T. gondii oocysts in HBSS (without calcium or magnesium) or distilled water treated with HPP at 550, 480, 400 or 340 MPa for 60 sec were rendered noninfectious for mice. | [105] | |
| Ionizing Irradiation |
Gamma (70 krad) | Use of 70 krad gamma radiation was minimum effective dose for fresh pork. | [93] |
| Gamma (40 krad) | T. gondii in tissue cysts killed by exposure to 40 krad of gamma irradiation. | [93] | |
| Gamma (50 krad or more) | Tissue cysts irradiated with 40 krad were infectious when inoculated in mice, but when irradiated with 50 krad or more, tissue cysts were not detected. | [107] | |
| Gamma (70 krad or 100 krad) | Tissue cysts in murine brains and edible pig flesh irradiated with 30 and 50 krad doses were not effective, whereas irradiation with 70 or 100 krad did not infect cats or mice in bioassay. | [108] | |
| Gamma (20 krad) | Irradiation treatments at doses as low as 20 krad effectively inactivated T. gondii oocysts on blueberry surfaces with minimal impact on texture, color, or anthocyanin content of treated berries. | [109] | |
| Gamma (60 krad and 45 krad) | The minimal effective dose for Chinese NT strain and the American ME-49 and TS-2 strains of T. gondii cysts in mouse and pig tissues was 60 krad. The infectivity for mice of NT strain bradyzoites irradiated at 45 krad decreased 10,000-fold. | [95] | |
| Ultraviolet (>20 mJ/cm2) | A 4-log inactivation of the oocyst/sporozoite infectivity was obtained for UV fluence. | [110] | |
| Ultraviolet (4 mJ/cm2 and 10 mJ/cm2) | The results from the animal bioassay show that 1- and 3-log10 inactivation was achieved with 4 mJ/cm2 UV and 10 mJ/cm2 low-pressure UV, respectively. | [94] | |
| Ultraviolet (40 mJ/cm2) | A 2-log10 reduction of T. gondii oocyst infectivity was achieved at 40 mJ/cm2. | [111,112] | |
| Ultraviolet (>500 mJ/cm2) | Inactivation of T. gondii oocysts occurred with exposure to pulsed and continuous UV radiation, as evidenced by mouse bioassay. Even at >500 mJ/cm2, some oocysts retained their viability. | [113] | |
| Ultraviolet (1 min UV exposure) | Using 1 min UV light at 3689.04 µJ/cm2/sec powers for a total energy exposure, tachyzoites were unable to replicate in vitro or produce parasite cysts in vivo. | [96] | |
| Curing | 3.9% NaCl, 25 mg/kg nitrate, and 3 mg/kg nitrite; 14 months | The last curing salt concentration of 3.9% NaCl, 25 mg/kg nitrate and 3 mg/kg nitrite for a duration of curing of 14 months inactivated T. gondii. | [114] |
| 2.5% of sodium nitrite; 14 days | About 2.5% of initial amount of sodium nitrite was effective for killing T. gondii cysts in 14 days. | [115] | |
| 7% nitrates, 4% nitrites, sodium ascorbate, and sodium chloride; 9–12 months | The viability of T. gondii was higher in hams cured for 9 months compared to those cured for 12 months. | [116] | |
| 2.0% NaCl or 1.4% or higher lactate-based salt solutions; 8 h | The injection of 2.0% NaCl or 1.4% or higher lactate-based salt solutions into pork loins containing infective tissue cysts within 8 h prevented transmission of T. gondii. | [103] | |
| salt and sugar for 64 h at 4°C; smoking at 50°C to 24–28 h | Curing of lamb meat with salt and sugar for 64 h at 4°C or smoking salt-injected meat at temperatures not exceeding 50°C for 24 to 28 h was effective for killing T. gondii. | [110] | |
| 6% NaCl; 4–20°C; 3–56 days | In various time intervals and all temperatures examined, tissue cysts were killed in 6% NaCl solution. | [101] | |
| 2.0 and 2.5% of salt; 48 hours | Pig sausage experimentally inoculated with T. gondii showed that salt in concentrations of 2.0 and 2.5% inactivated the parasite within 48 h of onset of curing. | [117] | |
| 3% table salt; 3–7 days | About 3% table salt after 3–7 days killed T. gondii tissue cysts. | [118] | |
| 2.5 and 3.0%, NaCl and 0.5% nitrite; 1–8 day | The cysts lost their infectivity in concentrations of 2.5 and 3.0% NaCl after 1 day. NaCl plus 0.5% nitrite had a stronger effect on T. gondii cysts than common table salt. | [119] |
6.2. Ionizing irradiation
IR is a non-thermal food pasteurization process that reduces, inactivates or eliminates spoilage of pathogenic microorganisms, insects, fungi and other pests and retards the spoilage of food. IR can use gamma rays from radioisotopic sources such as cobalt-60 or cesium-137, electrons, x-rays from beta rays, electron beam accelerators or ultraviolet rays (from an electromagnetic spectrum UV region) [85]. Irradiation has been evaluated for inactivation or killing of T. gondii tissue cysts in meat and has demonstrated its potential as an effective intervention [92,93,94].
Studies have reported that T. gondii is rendered unviable by irradiation at doses of 0.4–1 kGy [79,82,93,95]. UV irradiation has been shown to inactivate T. gondii tachyzoites [96], but recently published protocols are based on prolonged exposure (i.e. up to 60 min) to UV irradiation [97,98]. Overall, the differences between gamma and UV electromagnetic radiations include the origin of production. Gamma rays are produced from the nucleus of energetic atoms whereas UV rays are produced from atomic orbitals with lower energy levels; thus, gamma rays can cause ionization in food media and penetrate deeper than UV irradiation, making it more effective for inactivation of T. gondii under similar conditions. Research on the effects of extremely low frequency electromagnetic fields (ELF-EMF) on T. gondii by Ozlem-Caliskan et al. showed that pulsed and continuous EMF exposure (75 Hz) reduced the number of T. gondii tachyzoites in comparison with the controls [99]. Table 3 shows the positive findings of some studies about the use of IR on T. gondii.
6.3. Curing
Curing treatments are used to preserve meat by the addition of a combination of salt, nitrates, nitrite or sucrose and low temperature smoking [100,101]. Studies indicate that tissue cysts of T. gondii are killed during salt curing, although the inactivation of theses cysts depends on the maturation time, temperature of storage and salt concentration in the curing process [10,101–103]. Hill et al. reported that injection within 8 h of 2.0% NaCl or 1.4% or higher of lactate-based salt solution into pork loins containing infective tissue cysts prevented transmission of T. gondii [103]. Lunden and Uggla also showed that the curing of lamb meat with salt and sugar for 64 h at 4°C or smoking salt-injected meat at temperatures not exceeding 50°C for 24–28 h was effective for killing T. gondii [100]. Similar results have been reported by researchers as presented in Table 3. Nonetheless, additional studies are required to evaluate the safety of meat products cured under different curing conditions with regard to time and salt and nitrite concentration.
7. Chemical and biochemical methods
The control of T. gondii in human foodstuff is important principally to protect the human food chain from contamination by T. gondii derived from infected animals. Several complementary strategies have been used to control feed and food contamination which include a range of chemical and biochemical treatments. The principal agents used are organic acids and their salts, solvents and other oxidant compounds, formaldehyde, disinfectants, alcohols and other chemicals, enzymes, food additives and plant essential oils (EO) [120–122]. Many products use blends of agents from the same or different chemical groups to achieve synergistic or combined effects. The present review describes the various modes of action and efficacies of different chemical and biochemical agents delivered in food against T. gondii occurring in food or the environment.
7.1. Disinfection
At present, two chemicals (chlorine and ozone) are most commonly used to treat food, especially drinking water, because their ability to inactivate T. gondii oocysts [123]. Both chlorine and ozone are strong oxidizing agents that can cause cell death through inhibition of enzymatic activity, damage to cells by modifying cellular components, alterations in cell permeability or damage to DNA and RNA [124]. A major advantage for the use of these chemical agents is that they are easier to handle than gaseous chlorine or calcium hypochlorite and require shorter contact times and dosages than chlorine for sodium hypochlorite and ozone, respectively. On the other hand, disinfectants are hazardous waste because they contain halogenated compounds, making it essential to use caution in their use in food to prevent cross-contamination. Principally, disinfectants or sanitizers can only function adequately for preventing cross-contamination of raw fruits and vegetables when the required disinfectant residual is controlled during washing by automated monitoring and dosing of the disinfectant [125].
Wainwright et al. studied the chemical inactivation of T. gondii oocysts in water by chlorine and ozone. They exposed T. gondii oocysts to 100 mg/L of chlorine for 30 min or 2, 4, 8, 16 and 24 h or 6 mg/L of ozone for 1, 2, 4, 8 and 12 min. The results indicated that neither sodium hypochlorite nor ozone effectively inactivated T. gondii oocysts, even when used at high concentrations [126]. Dubey et al. exposed cat feces containing T. gondii oocysts to 5.25% aqueous sodium hypochlorite for 24 h, but it did not kill the oocysts [66]. Another study showed that oocysts were not inactivated by ozone after exposure to 9.4 mg min l−1 in water at 20°C [110]. The results of some studies have also indicated that oocyst exposure to chlorine is generally limited to 15–30 min [126].
Finch et al. showed that the oocysts of a related chlorine-resistant parasitic protozoan were inactivated (≥99%) following exposure to 3 or 4 mg × 1 min/L of ozone [127]. Frenkel et al. found that a strong concentration of ammonia (28%) killed all oocysts of T. gondii within 10 min and a strong tincture of iodine did so within 30 min [78]. Another study found that 5% and 10% concentrations of ammonia for 30 and 10 min, respectively, inactivated oocysts [66]. Another study found that the use of iodine (7% I2 + 5% KI) for 30 min or 1% to 10% formaldehyde for 24 h eliminated all oocysts [128]. Ito et al. showed that sensitization with 1% Neo Kurehasol (coccidiocidal disinfectant) for 120 min, with 1% Lomasept (coccidiocidal disinfectant) for 10 or 15 min or with 5% Lomasept for 5 min completely inhibited sporulation of unsporulated oocysts. In addition, sensitization with Neo Kurehasol or peracetic acid for 48 h, with ethanol for 24 h or with methanol for 12 h killed the oocysts [121]. A summary of the effect of different disinfectants on T. gondii oocysts is listed in Table 4 and is based on the findings of Dubey [31].
Table 4.
Effect of some disinfectants on T. gondii Oocysts.
| Disinfections | Concentration | Treatment time (min/h/day) | Effective | Ref. |
|---|---|---|---|---|
| Formalin | 10% | 48 hr | No | [121] |
| Sulfuric acid +dichromate | 63/7% | 30 min | No | [66] |
| Ethanol +acetic acid | 95/5% 95/5% |
1 hr 24 hr |
No Yes |
[66] |
| Ammonium hydroxide | 5.0% 5.0% |
10 min 30 min |
No Yes |
[66] |
| Sodium hyporchlorite (Purex) | 6.0% | 24 hr | No | [66] |
| Sodium lauryl sulfate | 0.1% | 24 hr | No | [66] |
| Cetyl trimethyl ammonium | 0.1% | 24 hr | No | [66] |
| Tween 80 | 0.1% | 24 hr | No | [66] |
| Ammonia, liquid | 5.5% 5.5% |
1 hr 3 hr |
No Yes |
[78] |
| Tincture of iodine | 2.0% 2.0% 7.0% |
10 min 3 hr 10 min |
No Yes Yes |
[78] |
| Aldesol (contains benzalchoniumchloride, glutaraldehyde, and gloxal) | 33% | 24 hr | No | [129] |
| Tincture of hibisept (contains chlorhexidine gluconate in ethanol) | - | 24 hr | No | [129] |
| Izosan-G (contains sodium dichloroizicyanurate-dihydrate in granulate) | .02% | 24 hr | No | [129] |
| Lomasept | 1% | 1 hr 3 hr |
No Yes |
[121] |
| Neo Kurehasol | 5% | 24 hr | No | [121] |
| Paracetic acid | 5% | 48 hr | Yes | [121] |
| Sodium chloride +potassium or sodium lactate | 2% and ≥1.4% respectivily | 14 days at 4°C | yes | [103] |
| Chlorination of water | 100 mg/L | 24 hr | No | [126] |
| Ozone treatment of water | 6 mg/L | 12 min | No | [126] |
| Ozone treatment of water | 9.4 mg/L | 20 min | No | [110] |
7.2. Essential oils
Plant extracts and their essential oils (EO) are widely used as alternative treatments against foodborne parasites, especially T. gondii. These extracts may have the potential to decrease the side effects of the toxoplasmosis treatment drugs such as the combination of sulfadiazine and pyrimethamine [130]. Dahbi et al. reported the total absence of intra-cerebral cysts in mice who received thyme EO (20 μg), signifying that they blocked the appearance of the cysts. No abnormalities were observed in the control mice who received the EO of thyme [131]. Other studies on mice have shown that extracts of Nigella sativa oil in combination with pyrimethamine had a synergistic effect in the treatment of toxoplasmosis. They reported an increased survival rate and decreases in the parasite density and pathological insult to both the liver and spleen, but Nigella sativa oil alone had no direct anti-toxoplasmosis effect [132]. Leesombun et al. reported that Thai piperaceae plant extract had the potential to act as a treatment for toxoplasmosis by inhibiting parasitic growth in human foreskin fibroblast cells [130]. Table 5 lists the efficacy of some plant species and their EO against T. gondii.
Table 5.
In vitro and in vivo studies on anti-toxoplasmosis effects of herbal medicine.
| Plants and essential oils | Concentration | Result | Ref. |
|---|---|---|---|
| Satureja khuzestanica essential oil | 0.2 and 0.3ml/kg | Mortality rate of infected mice was 8 days after oral administration of EO at 0.2 and 0.3 ml/kg. | [133] |
| Bunium persicum (Boiss) Essential Oil | 0.05 and 0.1 mL/kg | Potential of Boiss EO for production of new preventive agent against toxoplasmosis. | [134] |
| Zingiber officinale (Ginger) extract | 500 μg/ml | GE/F1 (fraction 1 obtained from GE) induced anti-T. gondii effects inactivating apoptotic proteins in infected host cells through the direct inhibition of T. gondii and has antiparasitic properties which inhibited inflammatory cytokine secretion in vivo. | [135] |
| Myristica Fragrans Houtt. Essential Oil | 24.45 μg/mL | In vitro anti-T. gondii assay, oil extract caused significant inhibition with EC50 of 24.45 μg/mL. | [136] |
| Thymus broussonetii Boiss essential oil | 20 μg/animal orally | Total absence of intracerebral cysts in mice who received EO of thyme, which appear to block appearance of cysts. No abnormality observed in control mice who received the EO of thyme. | [131] |
| Psidium guajava L. essential oil | 3.94 ± 0.39 µg/mL | In vitro anti-T. gondii assay showed that guava leaf EO showed promising EC of 3.94 ± 0.39 µg/mL, as compared to the standard drug clindamycin (EC50 = 6.24 ± 0.53 µg/mL). | [137] |
| Curcuma longa water extracts | 100 and 200 mg/kg/day | Most effective extract was Curcuma longa ethanol extract which showed a 98.6 and 99.2% inhibition of growth of T. gondii tachyzoites in 100 and 200 doses, respectively, compared to control. | [138] |
| Curcumin from the plant Curcuma longa | 12.9 ± 0.5 μM and 38.3 ± 0.9 μM | Curcumin at the tested doses inhibited the enzymatic activity of recombinant TgGlo1 amplified from T. gondii cDNA and parasitic propagation of in vitro cultured T. gondii. Ki and IC50 were 12.9 ± 0.5 μM and 38.3 ± 0.9 μM, respectively. | [139] |
| N. sativa oil (NSO) +Pyrimethamine (PYR) | PYR (12.5mg/kg) and NSO (5 ml/kg) body weight/day | NSO+PYR combination markedly improved the antioxidant capacity of T. gondii infected mice compared to infected untreated controls. In total, combination of NSO and PYR had synergistic effect in treatment of toxoplasmosis. | [132] |
7.3. Other biochemical methods
Studies have evaluated the effect of other biochemical treatments against T gondii. Pfefferkorn et al. showed that when intracellular T. gondii were treated with a concentration of emimycin (originally isolated from the culture filtrate of a Streptomyces species), it partially inhibited parasite RNA synthesis and much less emimycin was incorporated into the RNA than would be predicted by the amount of intracellular emimycin riboside triphosphate [140]. Sonda et al. showed that although aureobasidin A (a cyclic depsipeptide antibiotic isolated from the filamentous fungus Aureobasidium pullulans R106) treatment did not induce tachyzoite to bradyzoite stage of conversion in T. gondii, it did result in a loss of intracellular structure and vacuolization within the parasite. Moreover, aureobasidin A inhibited sphingolipid synthesis in T. gondii [141]. Palencia et al. found that mice infected with T. gondii and treated orally with benzoxaborole AN3661 (a boron‐containing compound) did not show an apparent increase in disease, while untreated control groups had a significant number of lethal infections.
TgCPSF3 (Toxoplasma CPSF3: TGGT1-285200) is a promising new purpose of T. gondii that provides the opportunity for the extension of anti-parasitic medicine [142]. A previous study reported that benzoxaborole AN6426 inhibits the growth in human cells of T. gondii and provided evidence that the target AN6426 in this organism is the LeuRS editing site [143]. Borges et al. proved that the BnSP-7 PLA2 (BnSP-7 toxin, a Lys49 phospholipase A2 (PLA2) homologue of Bothrops pauloensis snake venom) exerts an anti-toxoplasmosis effect at a lower dose than that needed to induce cytotoxicity in HeLa cells and also modulates the immune response of host cells [144].
Research on reduction of T. gondii development through inhibition of parasitic antioxidant enzymes by dinuclear iron (III) have shown that, in the presence of this compound, the redox environment becomes an oxidant in the LLC-MK2 cells. A reduction in catalase and superoxide dismutase activity in the treated parasites and the presence of reactive oxygen species in the parasitophorous vacuoles was observed, indicating an impaired protozoan response against these radicals. These results suggest that this compound disorders the redox balance of T. gondii, inducing cystogenesis and parasitic death [145].
Suzuki et al. reported that CD8(+) T cells can remove T. gondii cysts by their perforin-mediated cytotoxic activity. They provided a novel mechanism of the immune system against chronic infection with T. gondii [146]. Ochiai et al. found that CD8(+) T cells are capable of removing T. gondii cysts by recognizing epitopes commonly expressed in type II and III strains of T. gondii or cross-reactions between these two genotypes [147].
A review article by Assolini et al. on nano-medicine advances in toxoplasmosis shows that nano-materials smaller than 1000 nm particle in size are currently being investigated as an alternative for the treatment of T. gondii infection [148]. Similarly, Adeyemi et al. reported that inorganic gold, silver and platinum nanoparticles (NPs: 0.01–1,000 μg/mL) caused 90% inhibition of T. gondii growth with EC50 values of ≤7, ≤1 and ≤100 μg/mL for gold, silver, and platinum NPs, respectively [149]. In an evaluation of the effect of enzymes on T. gondii, de Carvalho et al. demonstrated that incubation of macrophages with trypsin significantly inhibited the uptake of T. gondii and confirmed that treatment of macrophages with cytochalasin D under such conditions blocks the typical phagocytic process and partially inhibits T. gondii infection in the cells [150].
8. Conclusion
The results of research clearly indicates that food contaminated with all structural forms of T. gondii (tachyzoites, bradyzoites, oocysts, sporocysts, sporozoites and enteroepithelials) pose a risk to public health if consumed in raw or undercooked meat, unpasteurized milk, raw vegetables and water contaminated with T. gondii oocysts from cat feces. Food preservation technologies are based on the prevention if the growth, the inactivation or killing of the microorganism. This review describes all appropriate and applicable methods and their parameters for effective inactivation, prevention or killing of T. gondii in different types of food and tissues. There are other ways to inactivate T. gondii that have not been the focus of research thus far. We therefore suggest that further research should evaluate the treatment of food samples with novel thermal or non-thermal technologies and chemical and biochemical processing methods for the inactivation of T. gondii. These include PEF, PLT, CP, pulsed UV, tumbling and injection, enzymes, active packaging materials and Hurdle technology. Many technological, economic and regulatory barriers must to be overcome before the food supply can benefit from these methods.
Funding Statement
This work was supported by the Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Acknowledgments
This study is related to the project NO. 1396/54047 from Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Lundén A, Uggla A.. Infectivity of Toxoplasma gondii in mutton following curing, smoking, freezing or microwave cooking. Int J Food Microbiol. 1992;15(3–4):357–363. [DOI] [PubMed] [Google Scholar]
- [2].Nesbakken T. Food safety in a global market—do we need to worry? Small Ruminant Res. 2009;86(1):63–66. [Google Scholar]
- [3].Pereira KS, Franco RMB, Leal DAG. Chapter 1 - transmission of toxoplasmosis (Toxoplasma gondii) by foods In: Taylor SL, editor. Advances in food and nutrition research. Vol. 60 Oxford, UK: Academic Press; 2010. p. 1–19. [DOI] [PubMed] [Google Scholar]
- [4].Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12):1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64(3):607–623. PubMed PMID: PMC99006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jones JL, Ogunmodede F, Scheftel J, et al. Toxoplasmosis-related knowledge and practices among pregnant women in the United States. Infect Dis Obstet Gynecol. 2003;11(3):139–145. PubMed PMID: PMC1852280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bayarri S, Gracia MJ, Lázaro R, et al. Toxoplasma gondii in meat and food safety implications-a review. Zoonosis: InTech; 2012. [Google Scholar]
- [8].Herrero L, Gracia MJ, Pérez-Arquillué C, et al. Toxoplasma gondii pig seroprevalence, associated risk factors and viability in fresh pork meat. Vet Parasitol. 2016;224:52–59. [DOI] [PubMed] [Google Scholar]
- [9].Dubey J. Toxoplasmosis in pigs—the last 20 years. Vet Parasitol. 2009;164(2):89–103. [DOI] [PubMed] [Google Scholar]
- [10].Kijlstra A, Jongert E. Control of the risk of human toxoplasmosis transmitted by meat. Int J Parasitol. 2008;38(12):1359–1370. [DOI] [PubMed] [Google Scholar]
- [11].European Food Safety A Surveillance and monitoring of Toxoplasma in humans, food and animals - Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2007;5(12):583–n/a. [Google Scholar]
- [12].Hill DE, Dubey JP. Toxoplasma gondii prevalence in farm animals in the United States. Int J Parasitol. 2013;43(2):107–113. [DOI] [PubMed] [Google Scholar]
- [13].Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012;44(11):805–814. [DOI] [PubMed] [Google Scholar]
- [14].Da Cunha S, Ferreira E, Ramos I, et al. Cerebral toxoplasmosis after renal transplantation. Case report and review. Acta Med Port. 1994;7:61–66. [PubMed] [Google Scholar]
- [15].Pott H Jr, Castelo A. Isolated cerebellar toxoplasmosis as a complication of HIV infection. Int J STD AIDS. 2013;24(1):70–72. [DOI] [PubMed] [Google Scholar]
- [16].Agrawal SR, Singh V, Ingale S, et al. Toxoplasmosis of spinal cord in acquired immunodeficiency syndrome patient presenting as paraparesis: a rare entity. J Glob Infect Dis. 2014;6(4):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lu N, Liu C, Wang J, et al. Toxoplasmosis complicating lung cancer: a case report. Int Med Case Rep J. 2015;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Z-D, Liu -H-H, Ma Z-X, et al. Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front Microbiol. 2017;8:389 PubMed PMID: PMC5343064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].WHO. Foodborne Disease Outbreaks: guidelines for investigation and control. Geneva: World Health Organization; 2008. [Google Scholar]
- [20].Dubey J, Jones J. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–1278. [DOI] [PubMed] [Google Scholar]
- [21].Dubey JP. Toxoplasmosis of animals and humans. 2nd ed. Boca Raton, FL, USA: CRC press; 2010. [Google Scholar]
- [22].Minbaeva G, Schweiger A, Bodosheva A, et al. Toxoplasma gondii infection in Kyrgyzstan: seroprevalence, risk factor analysis, and estimate of congenital and AIDS-related toxoplasmosis. PLoS Negl Trop Dis. 2013;7(2):e2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Havelaar A, Kemmeren J, Kortbeek L. Disease burden of congenital toxoplasmosis. Clin Infect Dis. 2007;44(11):1467–1474. [DOI] [PubMed] [Google Scholar]
- [24].Hoffmann S, Batz MB, Morris JJG. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot. 2012;75(7):1292–1302. [DOI] [PubMed] [Google Scholar]
- [25].Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ. 2013;91(7):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dorny P, Praet N, Deckers N, et al. Emerging food-borne parasites. Vet Parasitol. 2009August7;163(3):196–206. PubMed PMID: WOS:000268648000003; English. [DOI] [PubMed] [Google Scholar]
- [27].Hussain MA, Stitt V, Szabo EA, et al. Toxoplasma gondii in the food supply. Pathogens. 2017;6(2):21.PubMed PMID: PMC5488655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mostafavi S, Jalali Monfared L. Toxoplasmosis epidemiology in Iran: a systematic review. J Isfahan Med School. 2012;176(30):1–15. [Google Scholar]
- [29].Havelaar AH, Haagsma JA, Mangen M-J-J, et al. Disease burden of foodborne pathogens in the Netherlands, 2009. Int J Food Microbiol. 2012;156(3):231–238. [DOI] [PubMed] [Google Scholar]
- [30].Zhao G-H, Zhang M-T, Lei L-H, et al. Seroprevalence of Toxoplasma gondii infection in dairy goats in Shaanxi Province, Northwestern China. Parasites Vectors. 2011;4(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dubey JP. Toxoplasmosis of animals and humans. Second ed. Boca Raton: CRC Press; 2009. [Google Scholar]
- [32].Walsh C, Hammond S, Zajac A, et al. Survival of Toxoplasma gondii tachyzoites in goat milk: potential source of human toxoplasmosis. J Eukaryot Microbiol. 1999;46(5):73S–74S. [PubMed] [Google Scholar]
- [33].Jacobs L, Hartley WJ. Ovine toxoplasmosis: studies on parasitaemia, tissue infection, and congenital transmission in ewes infected by various routes. Br Vet Jl. 1964;120(8):347–364. [Google Scholar]
- [34].FDA. Food Safety for Moms-To-Be: while you’re pregnant - Toxoplasma: U.S. Food and Drug Administration; 2018. Available from: https://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083327.htm
- [35].Cook A, Holliman R, Gilbert R, et al. Sources of Toxoplasma infection in pregnant women: european multicentre case-control studyCommentary: congenital toxoplasmosis—further thought for food. Bmj. 2000;321(7254):142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hill DE, Dubey JP. Toxoplasma gondii as a parasite in food: analysis and control. Microbiol Spectr. 2016;4(4). doi: 10.1128/microbiolspec.PFS-0011-2015 [DOI] [PubMed] [Google Scholar]
- [37].Batz MB, Hoffmann S, Morris JJG. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot. 2012;75(7):1278–1291. [DOI] [PubMed] [Google Scholar]
- [38].Rossi G, Cabral D, Ribeiro D, et al. Evaluation of Toxoplasma gondii and Neospora caninum infections in sheep from Uberlândia, Minas Gerais State, Brazil, by different serological methods. Vet Parasitol. 2011;175(3):252–259. [DOI] [PubMed] [Google Scholar]
- [39].Jones JL, Dubey J. Foodborne toxoplasmosis. Clin Infect Dis. 2012;55(6):845–851. [DOI] [PubMed] [Google Scholar]
- [40].Lass A, Pietkiewicz H, Szostakowska B, et al. The first detection of Toxoplasma gondii DNA in environmental fruits and vegetables samples. Eur J Clinl Microbiol Infect Dis. 2012;31(6):1101–1108.PubMed PMID: PMC3346938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jones JL, Dargelas V, Roberts J, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009;49(6):878–884. [DOI] [PubMed] [Google Scholar]
- [42].Dubey J, Hill D, Jones J, et al. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol. 2005;91(5):1082–1093. [DOI] [PubMed] [Google Scholar]
- [43].Aspinall TV, Marlee D, Hyde JE, et al. Prevalence of Toxoplasma gondii in commercial meat products as monitored by polymerase chain reaction–food for thought? Int J Parasitol. 2002;32(9):1193–1199. [DOI] [PubMed] [Google Scholar]
- [44].Warnekulasuriya MR, Johnson JD, Holliman RE. Detection of Toxoplasma gondii in cured meats. Int J Food Microbiol. 1998;45(3):211–215. [DOI] [PubMed] [Google Scholar]
- [45].Rahdar M, Samarbaf-Zadeh A, Arab L. Evaluating the prevalence of Toxoplasma gondii in meat and meat products in Ahvaz by PCR method. Jundishapur J Microbiol. 2012;5(4):570–573. [Google Scholar]
- [46].Isaac-Renton J, Bowie WR, King A, et al. Detection of Toxoplasma gondii oocysts in drinking water. Appl Environ Microbiol. 1998;64(6):2278–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Laurence Baril TAVGPTVT-FBC. Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand J Infect Dis. 1999;31(3):305–309. [DOI] [PubMed] [Google Scholar]
- [48].Choi W-Y, Nam H-W, Kwak N-H, et al. Foodborne outbreaks of human toxoplasmosis. J Infect Dis. 1997;175(5):1280–1282. [DOI] [PubMed] [Google Scholar]
- [49].Palanisamy M, Madhavan B, Balasundaram MB, et al. Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian J Ophthalmol. 2006;54(2):129. [DOI] [PubMed] [Google Scholar]
- [50].De Moura L, Lmg B-O, Wada MY, et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg Infect Dis. 2006;12(2):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Balasundaram MB, Andavar R, Palaniswamy M, et al. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol. 2010;128(1):28–32. [DOI] [PubMed] [Google Scholar]
- [52].Krueger WS, Hilborn ED, Converse RR, et al. Drinking water source and human Toxoplasma gondii infection in the United States: a cross-sectional analysis of NHANES data. BMC Public Health. 2014;14:711.PubMed PMID: PMC4105121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Demar M, Ajzenberg D, Maubon D, et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007;45(7):e88–e95. [DOI] [PubMed] [Google Scholar]
- [54].Ekman CCJ, MFdV C, Meireles LR, et al. Case-control study of an outbreak of acute toxoplasmosis in an industrial plant in the state of São Paulo, Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 2012;54(5):239–244. [DOI] [PubMed] [Google Scholar]
- [55].Amairia S, Rouatbi M, Rjeibi MR, et al. Molecular prevalence of Toxoplasma gondii DNA in goats’ milk and seroprevalence in Northwest Tunisia. Vet Med Sci. 2016;2(3):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28(7):1019–1024. [DOI] [PubMed] [Google Scholar]
- [57].Speer C, Clark S, Dubey J. Ultrastructure of the oocysts, sporocysts, and sporozoites of Toxoplasma gondii. J Parasitol. 1998;84(3):505–512. [PubMed] [Google Scholar]
- [58].Dumètre A, Dubey JP, Ferguson DJP, et al. Mechanics of the Toxoplasma gondii oocyst wall. Proc Natl Acad Sci U S A. 2013;110(28):11535–11540. PubMed PMID: PMC3710823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Possenti A, Cherchi S, Bertuccini L, et al. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int J Parasitol. 2010;40(14):1639–1649. [DOI] [PubMed] [Google Scholar]
- [60].Ryning FW, Remington JS. Effect of cytochalasin D on Toxoplasma gondii cell entry. Infect Immun. 1978;20(3):739–743. PubMed PMID: PMC421921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kotula A, Dubey J, Sharar A, et al. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J Food Prot. 1991;54(9):687–690. [DOI] [PubMed] [Google Scholar]
- [62].Hellesnes I, Mohn S. Effects of freezing on the infectivity of Toxoplasma gondii cysts for white mice. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene Erste Abteilung Originale Reihe A: Medizinische Mikrobiologie und Parasitologie. 1977;238(1):143–148. [PubMed] [Google Scholar]
- [63].Gross U. Toxoplasma gondii. current topics in microbiology and immunology. CT microbiology. Vol. 219 Berlin Heidelberg: Springer; 1996. [Google Scholar]
- [64].Safefood 360 Inc Thermal processing of food 2014. Available from: http://safefood360.com/resources/Thermal-Processing-of-Food.pdf
- [65].Ito S, Tsunoda K, Taki T, et al. Destructive effect of heating against Toxoplasma oocysts. Natl Inst Anim Health Q (Tokyo). 1975;15(3):128–130. [PubMed] [Google Scholar]
- [66].Dubey J, Miller NL, Frenkel J. Characterization of the new fecal form of Toxoplasma gondii. J Parasitol. 1970;56(3):447–56. [PubMed] [Google Scholar]
- [67].Kuticic V, Wikerhauser T. Effects of some chemical and physical factors on the viability of Toxoplasma gondii. Veterinarski Arhiv. 1994;64:89–93. [Google Scholar]
- [68].Wainwright K, Lagunas‐Solar M, Miller M, et al. Radiofrequency‐induced thermal inactivation of Toxoplasma gondii oocysts in water. Zoonoses Public Health. 2010;57(1):74–81. [DOI] [PubMed] [Google Scholar]
- [69].McCurdy SM, Takeuchi MT, Edwards ZM, et al. Food safety education initiative to increase consumer use of food thermometers in the United States. Br Food J. 2006;108(9):775–794. [Google Scholar]
- [70].Gamble HR, Nas Hill DE. Toxoplasma - pork safety fact sheet: National Pork Board, Des Moines, IA USA; 2013. Available from: https://www.porkcdn.com/sites/porkorg/library/2010/04/Toxoplasma.pdf
- [71].Saridewi R, Lukman DW, Sudarwanto M, et al. Survival of Toxoplasma gondii in goat milk after pasteurization with low temperature and long time. 2013;11(6):789–793. [Google Scholar]
- [72].Sommer R, Rommel M, Levetzow R. Die Uberlebensdauer von Toxoplasmazysten in fleish und fleishzubereitungen. Fleischwirtschaft. 1965;5(454):e457. [Google Scholar]
- [73].Jacobs L, Remington JS, Melton ML. The resistance of the encysted form of Toxoplasma gondii. J Parasitol. 1960;46(1):11–21. [PubMed] [Google Scholar]
- [74].Dubey J. Effect of freezing on the infectivity of Toxoplasma cysts to cats. J Am Vet Med Assoc. 1974;165:534–536. [PubMed] [Google Scholar]
- [75].Dubey J, Kotula A, Sharar A, et al. Effect of high temperature on infectivity of Toxoplasma gondii tissue cysts in pork. J Parasitol. 1990; 76(2);201–204. [PubMed] [Google Scholar]
- [76].El-Nawawi FA, Tawfik MA, Shaapan RM. Methods for inactivation of Toxoplasma gondii cysts in meat and tissues of experimentally infected sheep. Foodborne Pathog Dis. 2008;5(5):687–690. [DOI] [PubMed] [Google Scholar]
- [77].Hill D, Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634–640. [DOI] [PubMed] [Google Scholar]
- [78].Frenkel J, Dubey J. Toxoplasmosis and its prevention in cats and man. J Infect Dis. 1972;126(6):664–673. [DOI] [PubMed] [Google Scholar]
- [79].Dubey J. The scientific basis for prevention of Toxoplasma gondii infection: studies on tissue cyst survival, risk factors and hygiene measures In: Ambroise-Thomas P., & Petersen P.E. editor, Congenital toxoplasmosis. Paris: Springer; 2000. p. 271–275. [Google Scholar]
- [80].Goldsmid J, Speare R, Bettiol S. The parasitology of food In: Hocking AD, editor. Foodborne microorganisms of public health significance. 6th ed. Waterloo, Australia: Australian Institute of Food Science and Technology Inc., NSW Branch, Food Microbiology Group; 2003. p. 705–721. [Google Scholar]
- [81].Djurković-Djaković O, Milenković V. Effect of refrigeration and freezing on survival of Toxoplasma gondii tissue cysts. Acta Veterinaria (Beograd). 2000;50(5/6):375–380. [Google Scholar]
- [82].Kuticic V, Wikerhauser T. Studies of the effect of various treatments on the viability of Toxoplasma gondii tissue cysts and oocysts In: Gross U, editor, Toxoplasma gondii. Berlin, Heidelberg: Springer Berlin Heidelberg; 1996. p. 261–265. [DOI] [PubMed] [Google Scholar]
- [83].Grossklaus D, Baumgarten H. Die überlebensdauer von Toxoplasma-cysten in schweinefleisch I. Mitteilung: ergebnisse von lagerungsversuchen bei verschiedenen temperaturen. Fleischwirtschaft. 1968;48:930–932. [Google Scholar]
- [84].Rashida Rajuva TA, Divya B, Joy PP. [Google Scholar]
- [85].Morris C, Brody AL, Wicker L. Non‐thermal food processing/preservation technologies: a review with packaging implications. Packaging Technol Sci. 2007;20(4):275–286. [Google Scholar]
- [86].Pivarnik LF, Worobo R. Non‐thermal or alternative food processing methods to enhance microbial safety and quality-frequently asked questions: USDA-NIFA; 2014. Available from: http://ucfoodsafety.ucdavis.edu/files/227891.pdf
- [87].Afshari R, Hosseini H. Non-thermal plasma as a new food preservation method, Its present and future prospect. J Paramedical Sci. 2013;5(1):116–120. [Google Scholar]
- [88].Considine KM, Kelly AL, Fitzgerald GF, et al. High‐pressure processing–effects on microbial food safety and food quality. FEMS Microbiol Lett. 2008;281(1):1–9. [DOI] [PubMed] [Google Scholar]
- [89].Tewari G, Jayas D, Holley R. High pressure processing of foods: an overview. Sci Aliments. 1999;19(6):619–661. [Google Scholar]
- [90].Black EP, Setlow P, Hocking AD, et al. Response of spores to high‐pressure processing. Compr Rev Food Sci Food Saf. 2007;6(4):103–119. [Google Scholar]
- [91].Lindsay DS, Collins MV, Holliman D, et al. Effects of high-pressure processing on Toxoplasma gondii tissue cysts in ground pork. J Parasitol. 2006;92(1):195–196. [DOI] [PubMed] [Google Scholar]
- [92].Dubey JP. Toxoplasma gondii In: Samuel B, editor. Medical microbiology. Galveston, Texas: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- [93].Dubey J, Thayer D. Killing of different strains of Toxoplasma gondii tissue cysts by irradiation under defined conditions. J Parasitol. 1994;80(5):764–767. [PubMed] [Google Scholar]
- [94].Ware MW, Augustine SA, Erisman DO, et al. Determining UV inactivation of Toxoplasma gondii oocysts by using cell culture and a mouse bioassay. Appl Environ Microbiol. 2010;76(15):5140–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chang-Cun S, Xing-Zheng Y, Li-Ying S, et al. The effect of cobalt-60 irradiation on the infectivity of Toxoplasma gondii. Int J Parasitol. 1993;23(1):89–93. [DOI] [PubMed] [Google Scholar]
- [96].Kannan G, Prandovszky E, Steinfeldt CB, et al. One minute ultraviolet exposure inhibits Toxoplasma gondii tachyzoite replication and cyst conversion without diminishing host humoral-mediated immune response. Exp Parasitol. 2014;145:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lu F, Zheng H, Guo H, et al. Cellular immune response in mice vaccinated with uv-attenuated Toxoplasma gondii ZS1 strain trophozoites. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi= Chin J Parasitol Parasitic Dis. 1999;17(6):384–386. [PubMed] [Google Scholar]
- [98].Zhao Y, Huang B, Huang S, et al. Evaluation of the adjuvant effect of pidotimod on the immune protection induced by UV-attenuated Toxoplasma gondii in mouse models. Parasitol Res. 2013;112(9):3151–3160. [DOI] [PubMed] [Google Scholar]
- [99].Ozlem-Caliskan S, Ertabaklar H, Bilgin MD, et al. Assessment of the effects of extremely low frequency electromagnetic fields on Toxoplasma gondii. Iran J Parasitol. 2016;11(2):159. [PMC free article] [PubMed] [Google Scholar]
- [100].Bayarri S, Gracia MJ, Perez-Arquillue C, et al. Toxoplasma gondii in commercially available pork meat and cured ham: a contribution to risk assessment for consumers. Journal of Food Protection. 2012;75(3):597–600. [DOI] [PubMed] [Google Scholar]
- [101].Dubey J. Survival of Toxoplasma gondii tissue cysts in 0.85–6% NaCl solutions at 4–20 C. J Parasitol. 1997;83(5):946–949. [PubMed] [Google Scholar]
- [102].Hill D, Benedetto S, Coss C, et al. Effects of time and temperature on the viability of Toxoplasma gondii tissue cysts in enhanced pork loin. J Food Prot. 2006;69(8):1961–1965. [DOI] [PubMed] [Google Scholar]
- [103].Hill D, Sreekumar C, Gamble H, et al. Effect of commonly used enhancement solutions on the viability of Toxoplasma gondii tissue cysts in pork loin. J Food Prot. 2004;67(10):2230–2233. [DOI] [PubMed] [Google Scholar]
- [104].Lindsay DS, Holliman D, Flick GJ, et al. Effects of high pressure processing on Toxoplasma gondii oocysts on raspberries. J Parasitol. 2008;94(3):757–758. [DOI] [PubMed] [Google Scholar]
- [105].Lindsay DS, Collins MV, Jordan CN, et al. Effects of high pressure processing on infectivity of Toxoplasma gondii oocysts for mice. J Parasitol. 2005;91(3):699–701. [DOI] [PubMed] [Google Scholar]
- [106].Wilkinson VM, Gould G. Food irradiation: a reference guide. Cambridge, England: Woodhead Publishing; 1996. 180 p. [Google Scholar]
- [107].Dubey J, Brake R, Murrell K, et al. Effect of irradiation on the viability of Toxoplasma gondii cysts in tissues of mice and pigs. Am J Vet Res. 1986;47(3):518–522. [PubMed] [Google Scholar]
- [108].Wikerhauser T, Kuticic V, Orsanic L. Effect of xirradiation or gamma rays on infectivity of Toxoplasma gondii cysts in the flesh of pigs. Veterinarski-Arhiv. 1989;59:113–116. [Google Scholar]
- [109].Lacombe A, Breard A, Hwang C-A, et al. Inactivation of Toxoplasma gondii on blueberries using low dose irradiation without affecting quality. Food Control. 2017;73:981–985. [Google Scholar]
- [110].Dumetre A, Le Bras C, Baffet M, et al. Effects of ozone and ultraviolet radiation treatments on the infectivity of Toxoplasma gondii oocysts. Vet Parasitol. 2008;153(3–4):209–213. [DOI] [PubMed] [Google Scholar]
- [111].Shin G, Linden K, Dubey J, et al. Inactivation of Toxoplasma gondii oocysts by UV irradiation. The International Ultraviolet Association Second International Congress on UV Technologies; Vienna, Austria: International Ultraviolet Association; 2003. [Google Scholar]
- [112].Simmons OD, Linden KG, Sobsey MD, et al. UV inactivation of Toxoplasma gondii oocysts. The Water Quality Technology Conference; Denver, CO: American Water Works Association; 2006. [Google Scholar]
- [113].Wainwright KE, Lagunas-Solar M, Miller MA, et al. Physical inactivation of Toxoplasma gondii oocysts in water. Appl Environ Microbiol. 2007;73(17):5663–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bayarri S, Gracia MJ, Lázaro R, et al. Determination of the viability of Toxoplasma gondii in cured ham using bioassay: influence of technological processing and food safety implications. J Food Prot. 2010;73(12):2239–2243. [DOI] [PubMed] [Google Scholar]
- [115].Neumayerová H, Juránková J, Saláková A, et al. Survival of experimentally induced Toxoplasma gondii tissue cysts in vacuum packed goat meat and dry fermented goat meat sausages. Food Microbiol. 2014;39:47–52. [DOI] [PubMed] [Google Scholar]
- [116].Herrero L, Gracia MJ, Pérez-Arquillué C, et al. Toxoplasma gondii in raw and dry-cured ham: the influence of the curing process. Food Microbiol. 2017;65:213–220. [DOI] [PubMed] [Google Scholar]
- [117].Navarro IT, Vidotto O, Giraldi N, et al. Resistência do Toxoplasma gondii ao cloreto de sódio e aos condimentos em linguica de suínos. Bol Of Sanit Panam. 1992;112(2):138–143. [PubMed] [Google Scholar]
- [118].Jamra LMF, Martins MC, Vieira MDPL. Acao do sal de cozinha sobre o Toxoplasma gondii. Revista do Instituto de Medicina Tropical de São Paulo. 1991. [PubMed] [Google Scholar]
- [119].Pott S, Koethe M, Bangoura B, et al. Effects of pH, sodium chloride, and curing salt on the infectivity of Toxoplasma gondii tissue cysts. J Food Prot. 2013;76(6):1056–1061. [DOI] [PubMed] [Google Scholar]
- [120].Wales AD, Allen VM, Davies RH. Chemical treatment of animal feed and water for the control of Salmonella. Foodborne Pathog Dis. 2010;7(1):3–15. [DOI] [PubMed] [Google Scholar]
- [121].Ito S, Tsunoda K, Shimada K, et al. Disinfectant effects of several chemicals against Toxoplasma oocysts. Nihon Juigaku Zasshi Japanese Journal Veterinary Science. 1975;37(3):229–234. [DOI] [PubMed] [Google Scholar]
- [122].Sharif M, Sarvi S, Pagheh AS, et al. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: a systematic review. Can J Physiol Pharmacol. 2016;94(12):1237–1248. [DOI] [PubMed] [Google Scholar]
- [123].Betancourt WQ, Rose JB. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet Parasitol. 2004;126(1–2):219–234. [DOI] [PubMed] [Google Scholar]
- [124].U.S. Environmental Protection Agency Combined sewer overflow technology fact sheet: chlorine disinfection. Washington, D.C.: Office of Water; EPA-832-F-99-034 1999. [Google Scholar]
- [125].Banach JL, Sampers I, Van Haute S, et al. Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int J Environ Res Public Health. 2015;12(8):8658–8677. PubMed PMID: PMC4555240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wainwright KE, Miller MA, Barr BC, et al. Chemical inactivation of Toxoplasma gondii oocysts in water. J Parasitol. 2007;93(4):925–931. [DOI] [PubMed] [Google Scholar]
- [127].Finch G, Black E, Gyürék L, et al. Ozone inactivation of cryptosporidium parvum in demand-free phosphate buffer determined by in vitro excystation and animal infectivity. Appl Environ Microbiol. 1993;59(12):4203–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].New Zealand Ministry of Health (MoH) Toxoplasma gondii New Zealand: institute of Environmental Science and Research Ltd (ESR); 2001. Available from: http://www.okyanusbilgiambari.com/bilgiambari/GG/Hazards/MO/Toxoplasma-gondii.pdf
- [129].Kuticic V, Wikerhauser T. Effects of three disinfectants on the viability of Toxoplasma gondii oocysts. Period Biol. 1993;95:345. [Google Scholar]
- [130].Leesombun A, Boonmasawai S, Shimoda N, et al. Effects of extracts from Thai piperaceae plants against infection with Toxoplasma gondii. PloS one. 2016;11(5):e0156116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Dahbi A, Bellete B, Flori P, et al. The effect of essential oils from thymus broussonetii boiss on transmission of Toxoplasma gondii cysts in mice. Parasitol Res. 2010;107(1):55–58. [DOI] [PubMed] [Google Scholar]
- [132].Mady RF, El-Hadidy W, Elachy S. Effect of Nigella sativa oil on experimental toxoplasmosis. Parasitol Res. 2016;115(1):379–390. [DOI] [PubMed] [Google Scholar]
- [133].Mahmoudvand H, Beyranvand M, Nayebzadeh H, et al. Chemical composition and prophylactic effects of Saturja Khuzestanica essential oil on acute toxoplasmosis in Mice. Afr J Traditional Complement Altern Medicines (AJTCAM). 2017;14(5):49–55. [Google Scholar]
- [134].Kareshk AT, Keyhani A, Mahmoudvand H, et al. Efficacy of the Bunium persicum (Boiss) essential oil against acute toxoplasmosis in mice model. Iran J Parasitol. 2015;10(4):625. [PMC free article] [PubMed] [Google Scholar]
- [135].Choi W, Jiang M, Chu J. Antiparasitic effects of zingiber officinale (Ginger) extract against Toxoplasma gondii. J Appl Biomed. 2013;11(1):15–26. [Google Scholar]
- [136].Pillai S, Mahmud R, Lee WC, et al. Anti-parasitic activity of myristica fragrans houtt. Essential oil against Toxoplasma gondii parasite. APCBEE Procedia. 2012;2:92–96. [Google Scholar]
- [137].Lee WC, Mahmud R, Noordin R, et al. Free radicals scavenging activity, cytotoxicity and anti-parasitic activity of essential oil of Psidium guajava L. leaves against Toxoplasma gondii. J Essent Oil Bear Plants. 2013;16(1):32–38. [Google Scholar]
- [138].Al-Zanbagi NA. In vivo effect of some home spices extracts on the Toxoplasma gondii tachyzoites. J Family Community Med. 2009May-Aug;16(2):59–65. PubMed PMID: PMC3377031. [PMC free article] [PubMed] [Google Scholar]
- [139].Goo Y-K, Yamagishi J, Ueno A, et al. Characterization of Toxoplasma gondii glyoxalase 1 and evaluation of inhibitory effects of curcumin on the enzyme and parasite cultures. [Journal Article]. Parasites Vectors. 2015December23;8(1):654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Pfefferkorn E, Eckel ME, McAdams E. Toxoplasma gondii: the biochemical basis of resistance to emimycin. Exp Parasitol. 1989;69(1):129–139. [DOI] [PubMed] [Google Scholar]
- [141].Sonda S, Sala G, Ghidoni R, et al. Inhibitory effect of aureobasidin A on Toxoplasma gondii. Antimicrob Agents Chemother. 2005;49(5):1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Palencia A, Bougdour A, Brenier‐Pinchart MP, et al. Targeting Toxoplasma gondii CPSF3 as a new approach to control toxoplasmosis. EMBO Mol Med. 2017;9(3):3s85–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Palencia A, Liu R-J, Lukarska M, et al. Cryptosporidium and Toxoplasma parasites are inhibited by a benzoxaborole targeting leucyl-tRNA synthetase. Antimicrob Agents Chemother. 2016;60(10):5817–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Borges IP, Castanheira LE, Barbosa BF, et al. Anti-parasitic effect on Toxoplasma gondii induced by BnSP-7, a Lys49-phospholipase A2 homologue from bothrops pauloensis venom. Toxicon. 2016;119:84–91. [DOI] [PubMed] [Google Scholar]
- [145].Portes J, Souza T, Dos Santos T, et al. Reduction of Toxoplasma gondii development due to inhibition of parasite antioxidant enzymes by a dinuclear iron (III) compound. Antimicrob Agents Chemother. 2015;59(12):7374–7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Suzuki Y, Wang X, Jortner BS, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol. 2010;176(4):1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ochiai E, Sa Q, Perkins S, et al. CD8+ T cells remove cysts of Toxoplasma gondii from the brain mostly by recognizing epitopes commonly expressed by or cross-reactive between type II and type III strains of the parasite. Microbes Infect. 2016;18(7–8):517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Assolini JP, Concato VM, Gonçalves MD, et al. Nanomedicine advances in toxoplasmosis: diagnostic, treatment, and vaccine applications. Parasitol Res. 2017;116(6):1603–1615. [DOI] [PubMed] [Google Scholar]
- [149].Adeyemi OS, Murata Y, Sugi T, et al. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int J Nanomedicine. 2017;12:1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].De Carvalho L, Yan CYI, De Souza W. Effect of various digestive enzymes on the interaction of Toxoplasma gondii with macrophages. Parasitol Res. 1993;79(2):114–118. [DOI] [PubMed] [Google Scholar]


