ABSTRACT
Dengue disease is the most prevalent mosquito-borne viral infection in humans. At least one half of the global population is estimated at risk of infection and an estimated 390 million people are infected each year. Over the past few years, dengue burden continued to increase, mainly impacting developing countries. Alarming changes in dengue epidemiology were observed highlighting a spread from tropical to subtropical regions as well as urban to rural areas. An increase in the co-infections with the four serotypes has also been noticed, involving a shift in the targeted population from pediatric to adult. Facing these global changes, authorities will have to reinforce preventive actions and adapt healthcare management. New prophylactic strategies are urgently needed to prevent severe forms of dengue disease. The lack of specific antiviral therapies available turns vaccine development into a socio-economic challenge. In this review, we propose an update on the dengue global trends and different vaccine strategies in development. A particular attention will be paid to up-to-date information on dengue transmission and the protective efficacy of newly commercialized tetravalent dengue vaccine Dengvaxia®, as well as the most advanced candidate vaccines in clinical development.
KEYWORDS: dengue, epidemiology, clinical disease, antiviral immunity, prophylaxis, vaccine strategies, live-attenuated viruses, biological markers of dengue disease
Introduction
Dengue virus circulates in many parts of the world, impacting most tropical and subtropical countries. Millions of people are affected each year and global dengue incidence has dramatically increased in recent decades. Dengue fever is a flu-like illness that usually heals after three to seven days. However, dengue disease sometimes causes life-threatening complications. Although dengue disease has been twice classified by the World Health Organization (WHO) in 1997 and in 2009, severe disease prediction and monitoring still remain unsatisfactory. In addition, the burden of dengue disease represents a real threat to affected countries, some of which are facing economic difficulties. An efficient prophylactic vaccine strategy is urgently needed to tackle dengue infections worldwide. We hope that this work, by reviewing the global trends of dengue virus epidemiology, biology, and clinical disease, will help to better understand current vaccination strategies.
Dengue disease
Epidemiology of dengue
Since its discovery in 1779, dengue disease rapidly evolved into a major public health problem which still remains present today. Dengue is now the most medically-important arthropod-borne viral infection with nearly one-half of the world population at risk. Dengue virus (DENV) is predominantly transmitted by Aedes (Ae.) aegypti, and to a lesser extent, by Ae. albopictus mosquitoes [1]. Over the past 50 years, the global dengue incidence dramatically increased to almost 30 fold, reinforcing an already high economic burden causing both human suffering and massive socioeconomic losses [2]. Such figures underscore the failure of implemented procedures to control virus transmission and accentuate the need of adapted vaccine development.
According to the WHO, the actual numbers of dengue cases are underreported and 3.9 billion people, representing half of the global population, are estimated at risk of infection [3]. One recent estimate indicates 390 million dengue infections per year (95% credible interval 284–528 million) instead of the 50 to 100 million cases usually quoted, of which 96 million (67–136 million) manifest clinically, independently of the severity of disease [4]. The geographic range of DENV widely spread from tropical to most subtropical regions where endemicity was facilitated by the abundance of vectors and high population density. Most hyperendemic regions are located in Asia and Latin America, with a particular focus towards Thailand, Philippines, India and Brazil which experienced the highest number of severe outbreaks in the last decades [2]. In recent years, outbreak reports and meta-analysis raised up changes in epidemiological trends of the disease (Figure 1).
Figure 1.
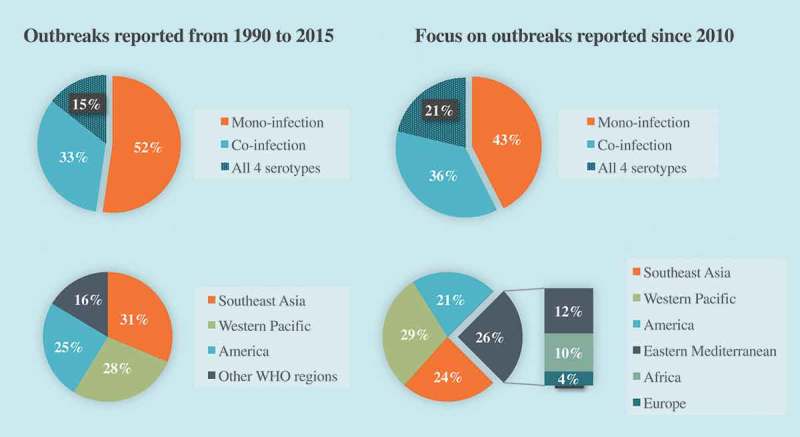
Evolution of epidemiological trends of outbreaks from 1990 to 2015.
Dengue serotypes distribution: data based on 174 outbreaks including 80 from 2010 (46%) outbreaks distribution among WHO regions: data based on 262 outbreaks including 112 from 2010 (43%) (data from GUO et al, 2017).
Although DENV is still persistent and mostly transmitted in urban areas, outbreaks in rural areas are becoming more and more frequent suggesting that dengue is not confined to urban areas anymore. The expansion to rural areas has been especially spotted in Southeast Asia and India where the prevalence of dengue was rising so fast that it has converged towards the high rates detected in urban populations [5]. As villages develop without proper water supplies, inhabitants use water storage vessels that provide breeding sites for the mosquitoes causing dengue fever. Moreover, urbanization prompts persons from rural areas into frequent travel between large cities and relatives in their villages. This way, growing interactions attenuate boundaries from both areas and enhance the risk of transmission. This finding underscores the urgent need to create awareness among the rural population and to conduct education programs providing good habits and behavior that help vector control.
As there are not yet specific medications to treat severe dengue, prevention remains the most important action to reduce the risk of dengue infection (WHO guidelines 2009). Two major ways to prevent dengue infection are efficiently experienced: mosquito control by either larval or adult control, and reducing mosquito bites especially during daylight hours. People experiencing fever from DENV infection should not be in an environment where they may be bitten by mosquitoes. If this is not possible, they should stay at home until they have no fever and are therefore no longer infectious, usually 3 to 5 days after the onset of clinical manifestations of disease.
Replication of dengue virus
Mosquito-borne DENV is a single-stranded positive RNA virus which belongs to Flavivirus genus from Flaviviridae family (reviewed in Screaton et al., 2015 [6]). There are four antigenically different serotypes DENV sharing 60–80% homology: DENV-1, DENV-2, DEN-3 and DENV-4. DENV is a 50-nm virus particle surrounded with a lipid membrane. The genomic RNA of about 11.000 nucleotides of length contains a single open reading frame which encodes a large polyprotein that is co- and post-translationally processed into three structural proteins, capsid (C), precursor membrane (prM) and envelope (E) and seven nonstructural proteins, NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5. The E glycoprotein is the major component of the virion surface and the main target of DENV neutralizing antibodies. The ectodomain of E comprises a centrally located domain I (EDI), a domain II (EDII) containing the fusion loop and a domain III (EDIII) which brings most of the serotype-specific antibody epitopes.
Once the genomic RNA is released into the cytoplasm upon the fusion of viral membrane with endosomal membranes, viral RNA translation can start at the close vicinity of the endoplasmic reticulum (ER) membranes. After the viral replication complex is synthesized, translation switches off, and viral RNA synthesis begins. During DENV replication, the viral genome is degraded by exonuclease XRN1 to generate small species of subgenomic flavivirus RNA (sfRNAs) which correspond to a large part of the non-translated genomic (NTR) region at the 3ʹ end of genomic RNA [7]. Accumulation of sfRNAs contributes to viral replication but also alters the host-cell antiviral immune responses to the benefit of virus replication [8]. De novo synthesized viral RNA molecules are packaged into nucleocapsids or engaged in massive production of viral proteins. Virus assembly occurs at the ER surface, resulting in non-infectious, immature viral particles. Immature viral particles are transported through the Golgi complex and then prM is processed to M by furin-like proteases until the fully mature infectious virus particle is released into the extracellular environment.
Symptomatic features of dengue disease
The four serotypes of mosquito-transmitted DENV can cause a wide spectrum of clinical manifestations which range from asymptomatic or paucisymptomatic to severe infections. After an incubation period of 3 to 7 days, dengue disease is characterized by three phases (reviewed in details in Simmons et al 2012 [9]):
Febrile: typically characterized by high temperature (> 38,5°c) accompanied by headache, vomiting, myalgia, and joint pain, sometimes with a transient macular rash;
Critical: In a small proportion of patients, typically in children and young adults, a systemic vascular leak syndrome becomes apparent around the time of defervescence;
Recovery: reverting spontaneously to a normal level. A second rash may appear during the recovery phase. Adults may have profound fatigue for several weeks after recovery.
It is now well recognized that the majority of people infected with dengue have no obvious clinical signs (asymptomatic) or are paucisymptomatic. However, some individuals can experience clinical symptoms of disease. Patients with dengue fever who improve after defervescence are diagnosed with a non-severe form of dengue, while others are considered to have severe symptoms. Around the time of defervescence, when the temperature drops to 37.5 to 38°C or less and remains below this level, usually on days 3–7 of illness, an increase in capillary permeability may occur. This phenomenon marks the beginning of the critical phase. The period of clinically significant plasma leakage usually lasts 24–48 hours and the degree of plasma leakage varies among patients. When a critical volume of plasma is lost through leakage, acute peripheral circulatory failure occurs leading to insufficient inflow of oxygen-rich blood to body cells. But this condition of low blood perfusion to tissues resulting in cellular injury and inadequate tissue function called ‘shock’ is often preceded by warning signs. For example, changes in the full blood count should be used to guide the onset of the critical phase and plasma leakage, and this way prevents shock. With prolonged shock, the consequent organ hypoperfusion results in progressive organ impairment, metabolic acidosis and disseminated intravascular coagulation. This in turn leads to severe hemorrhage causing the hematocrit to decrease in severe shock which considerably impairs patient vital prognosis if not cared. As a matter of fact, the case fatality rate in untreated dengue patients was reported to reach up to 20% and reduced to less than 1% under expert clinical management.
Today, no specific antiviral therapies are available for dengue virus infection and clinical management of the more severe forms of disease is mainly based on supportive therapy and intravascular volume replacement [10,11].
WHO classification guidelines of dengue disease
Classification history
For greater clarity on distinctions between classic dengue fever and dengue hemorrhagic fever or severe dengue, a World Health Organization (WHO) committee developed case classification guidelines in 1974 (WHO 1975), based on studies of disease patterns in children in Thailand in the 1960s, which were subsequently modified and published a number of times. The 1997 guidelines (2nd edition) classified dengue into Dengue Fever (DF), Dengue Hemorrhagic Fever (DHF Grades 1 and 2) and Dengue Severe Syndrome (DHF Grades 3 and 4) (WHO 1997). The case diagnosis for DF emphasized the need for laboratory confirmation. The experience of this classification system has highlighted a number of limitations. This classification is based in particular on clinical data collected from Thai children, which may not be universally representative of dengue fever after its expansion into other tropical regions and older age groups. A range of clinical trials requiring repetition is also needed, which may be difficult for countries with limited resources to implement. The tourniquet test, a measure of capillary fragility and thrombocytopenia for the diagnosis of DHF, is an integral part of the 1997 case definitions. However, the test does not have sufficient sensitivity and specificity to effectively differentiate cases of DF and DHF, and dengue fever from other febrile diseases. For all these reasons, a new classification was established in 2009 by WHO.
Current classification
The 2009 classification into severity levels is considered to be more sensitive in capturing severe disease than the 1997 guidelines (WHO 2009). The 2009 WHO criteria classify dengue according to levels of severity: dengue without warning signs; dengue with warning signs (abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, liver enlargement, increasing hematocrit with decreasing platelets); and severe dengue (dengue with severe plasma leakage, severe bleeding, or organ failure). Patients who recover from defervescence are considered to have mild dengue fever, but those whose condition does not ameliorate after this period tend to manifest warning signs. These people are likely to recover with intravenous rehydration. However, further deterioration is classified as severe dengue, though recovery is possible if appropriate and timely treatment is given.
Human susceptibility to dengue virus infection
Asymptomatic and symptomatic cases of dengue
It is now well admitted that a majority of dengue infected people are clinically asymptomatic [12]. Unexpectedly, it has been observed that these inapparent infections are potentially capable of infecting Aedes aegypti [13] . Recently, ten Bosch et al. argued that the rapid spread of DENV in endemic areas, as well as enhanced transmission of disease by the invertebrate vectors, could be largely attributable to asymptomatic or paucisymptomatic cases of dengue [14]. Given that the number of dengue cases is probably underestimated, it is a high priority to survey the actual level of dengue endemicity in the regions affected by the disease. Such information would be essential for the introduction of a dengue vaccine as WHO has recommended with the recently commercialized Dengvaxia® vaccine by Sanofi-Pasteur.
People with clinically apparent DENV infection may develop severe dengue requiring hospitalization. The exact causes of severe dengue still remain elusive. The etiology of severe dengue is undoubtedly multifactorial with host factors and immune status having important roles [15–17]. The question of the existence of biological markers of severe dengue as prognostic signatures of the risk of evolution of disease was addressed [18,19]. Nhi et al. identified plasma proteins exhibiting different relative concentrations in patients diagnosed for a severe dengue [20]. Nikolayeva et al. worked on a small number of gene expression markers to detect severe cases of dengue fever from blood samples from young patients with secondary infection [21]. Once the biological prognostic markers for severe dengue fever are identified, their validation will require large and independent cohorts of dengue patients who differ in ages and immune status. Regarding the pathogenic properties of DENV, it has been widely reported that DENV-2 infection causes a greater number of severe cases than other serotypes [22].
Antibody response to dengue virus infection
We now know that humoral immunity plays an important role in eliminating DENV infection and protecting against disease. The effective response of anti-dengue antibodies, stimulated in response to primary DENV infection, lasts about 2 years, after which the individual is susceptible to re-infection with another serotype.
Neutralization of virus infectivity by antibodies is assumed to be the key mechanism by which protection against DENV is achieved [18,23]. The major target of DENV neutralizing antibody is the E protein, even though anti-M and anti-NS1 antibodies have also been shown to be protective [24,25]. The free viral particles are bound by a diverse set of neutralizing and non-neutralizing antibodies directed against multiple different antigenic sites, and to the epitopes of these sites, which act together to effect neutralization. Infection can be prevented by antibodies through various mechanisms including: blocking of binding, inhibition of fusion of the viral membrane with the endocytic vacuole membrane which inhibits the release of viral RNA into the cytoplasm, and lysis of the antibody-coated virus by the complement.
However, specific antibodies against DENV could be associated with the development of severe dengue. Indeed, it has been proposed that infected people with a low neutralizing antibody titer are at a higher risk of severe dengue compared to those with titers above 100 [12]. Severe dengue is associated with secondary infections, suggesting that pre-existing immunity might play a key role in dengue pathogenesis [26]. A hallmark of secondary dengue fever is a more rapid and higher antibody response than the primary response. This is caused by the stimulation of memory B-cells from the primary infection. In fact, in this case, the antibodies produced as a result of the secondary infection remain more fitted to neutralize the serotype responsible for the primary infection than the current infecting virus. This phenomenon has been called ‘Antibody-Dependent Enhancement’ (A.D.E) or ‘Original Antigenic Sin’ as a dominance of B-cell response to primary DENV infection [27]. As such, A.D.E. could enhanced the pathogenicity of DENV infection or additional inflammatory responses, and could also significantly alter vaccine efficacy (to be discussed later) [28]. In secondary infection, the neutralizing antibody response extends over time. A key feature of secondary dengue fever is a durable response that neutralizes or sub-neutralizes multiple serotypes, including those that have not yet infected the individual. Cases of tertiary or quaternary DENV infections have rarely been documented, which supports the notion that secondary infections may stimulate a long-term cross-neutralizing antibody response that may be effective.
Dengue vaccine development
The current challenges for dengue vaccine development
The global spread of dengue disease is mainly linked to urban population growth in endemic areas, climate change, globalization of economic exchanges and intensification of intercontinental travels [11]. Such factors largely facilitated the hyperendemicity of dengue in Latin America and South-East Asia, and contributed to intensive circulation of different dengue serotypes through the tropical regions too [29]. The majority of dengue infections are clinically asymptomatic and inapparent infections may significantly contribute to the burden of disease in endemic areas [14].
An efficient dengue vaccine is expected to afford a rapid and high protection level against all circulating DENV serotypes regardless of individual immune status and age of vaccination. Furthermore, such a vaccine should be efficient in producing long-term, type- or cross-specific neutralizing antibodies against each of the four DENV serotypes. Lastly, neutralization titers needed for protection should be higher than those usually accepted for other flavivirus vaccines such as yellow fever and Japanese encephalitis. Serotype-specific and cross-reactive CD8 T cells could contribute to the long-term immune protection against DENV infection [30]. Although induction of neutralizing antibodies may play a pivotal role in dengue vaccination, recent data argue in favor of the development of dengue vaccine candidates that also induce protective CD8+ T cell response [31–33].
Initially, the development of dengue vaccines was hampered by a series of major obstacles. First, the evolution of virus strains within the four dengue serotypes and their genotypes is often rapid and unpredictable, intensifying the inherent difficulties associated with developing a dengue vaccine candidate. Secondly, the development of effective vaccines against dengue fever remains hindered in the absence of an accurate animal model mimicking the pathogenesis of dengue virus infection in humans, combined with a clear lack of knowledge concerning the major risk factors of severe outcome [11,34,35]. Advances have been recently made on development of appropriate animal models – immunocompromised mice deficient in type-I and -II interferon receptors and the non-human primates (NHP) – suitable for assessing the protective activity of dengue vaccines [36–40]. However, the use of animals and, in first instances, NHPs for biomedical research represents a serious ethical dilemma especially in the European Union. Their progressive substitution by in vitro biological tests is a topical issue.
Considering the important genetic variations that exist between the different serotypes, as well as viral strain diversity within each serotype, a dengue vaccine candidate is expected to induce a balanced and efficient immune response against the different genotypes from the four serotypes [10,34,41]. Such a challenge highlights a first major constraint in the formulation of dengue vaccine. A second important issue relates to the adverse effects associated to A.D.E. phenomenon occurring on secondary infection [10,41–44]. Briefly, the enhanced severity of dengue disease in individuals experiencing a heterologous secondary infection could be due to A.D.E caused by non-protective heterotypic antibodies arising from the primary infection [10,11,34,41–44]. Although in vivo evidence of the phenomenon is still lacking, one of the chief concerns is that a dengue vaccine candidate must be able to stimulate simultaneously a sterilizing immunity against all serotypes. A possible A.D.E between members of flavivirus genus such as Zika virus (ZIKV) and DENV has been suggested due to the antigenic cross-reactivity of dengue antibodies with contemporary epidemic strains of ZIKV [45]. Whether dengue vaccination can have beneficial or detrimental impacts on ZIKV infection due to strong antigenic cross-reactivity between ZIKV and DENV is an important issue to be taken into account in the development of dengue vaccines [42,46,47].
Which remarkable advances have been recently made toward the development of effective dengue vaccines? Several dengue vaccine candidates are in the pipeline. There are live-attenuated and inactivated viruses, DNA constructs, vector-based expression, virus-like particles and recombinant subunit antigens [48–50]. In 2016, the first commercial dengue vaccine called Dengvaxia® has been registered in several endemic dengue countries including Mexico, Brazil, and Philippines [44,51]. Dengvaxia®, as well as the most advanced candidate vaccines are discussed below.
Current status of dengue vaccine development
The development of effective tetravalent dengue vaccines is a high priority especially in regions infested by the mosquito vectors of the disease [10,29,52,53]. The first tetravalent dengue vaccine CYD-TDV (Dengvaxia® developed by the manufacturer Sanofi-Pasteur) has been recently licensed in Asia and Latin America. The efficacy of CYD-TDV was evaluated on the basis of large phase 3 clinical trials where young children had been included. Two additional live-attenuated dengue vaccines are in phase 3 development: the TDV developed by Takeda and the LAV Delta 30 developed by NIH/Butantan Institute. Today, the two live-attenuated vaccines (LAV) for dengue, TDV and LAV Delta 30, are the two most advanced candidates in clinical studies.
Dengue vaccine Dengvaxia®
It is widely accepted that dengue vaccination should induce a balanced protective immunity against all four serotypes [34,41]. Based on this premise, the chimeric tetravalent dengue vaccine CYD-TDV has been generated using a flavivirus vaccine – the live-attenuated 17D strain of yellow fever virus (YF-17D) – as a backbone (Figure 2). CYD-TDV contains four chimeric YF-17D viruses which express the prM and E proteins from each of the four dengue serotypes [43,44]. Started in 2011, the vaccine candidate combined multi-center pivotal Phase 3 clinical trials with tens of thousands of individuals in different regions of Asia and Latin America following the same protocol [51,54]. Neutralization antibody titers higher than 1:10 have been accepted as a correlate of protection against dengue disease. In 2018, the manufacturer Sanofi-Pasteur has already licensed CYD-TDV vaccine (Dengvaxia®) for dengue prevention in twenty endemic countries worldwide. Three doses of the vaccine are administered to recipients at 0, 6 and 12-month intervals.
Figure 2.
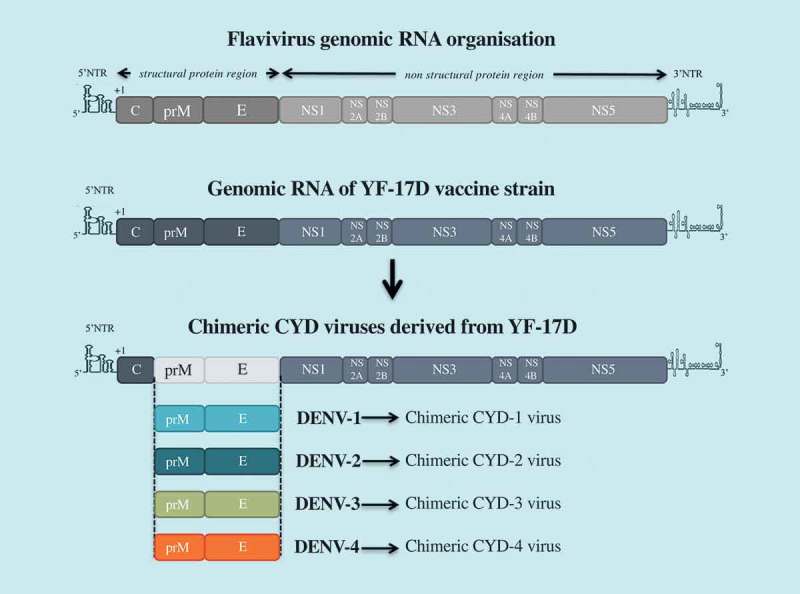
CYD-TDV (Dengvaxia®) vaccine.
The tetravalent dengue vaccine CYD-TDV has been constructed by substituting prM and E genes from DENV-1 to DENV-4 into the backbone of the molecular clone of yellow fever vaccine YF-17D. there are four chimeric CYD-1 to CYD-4 which compose the CYD-TDV.
Clinical studies showed that Dengvaxia® efficacy is less than 70% among recipients. The vaccine has been shown to be poorly effective against DENV-2, which is however the main serotype in terms of medical problems. Moreover, the efficacy of Dengvaxia® significantly varied according to the age and immune status of an individual [55,56]. During the four years of the phase 3 clinical trial, an unexpected number of hospitalizations for clinical dengue infection was significantly observed among vaccinated participants not older than 9-years-old [51]. The risk of hospitalization for severe dengue was mostly observed after inoculation of the first dose of Dengvaxia® [56]. The mechanism explaining this increased risk of dengue disease in the younger recipients of Dengvaxia® is not yet understood. Clinical studies have shown that higher vaccine efficacy has been reported among individuals with pre-existing immunity to flaviviruses, suggesting that natural infection may contribute to establishing long-term adaptive immunity against dengue fever [54]. A contrario, vaccine recipients who had not been previously exposed to dengue or having a limited anti-dengue immunity manifested a higher risk of severe dengue upon vaccination with Dengvaxia® [10,57]. The manifestation of A.D.E. due to a too low antibody titer and/or differences in vaccine efficiency according to the four infecting serotypes could explain the increased risk of severe dengue fever in vaccinated subjects without prior experience of DENV infection [57]. Despite all these restrictions, Sanofi-Pasteur considers Dengvaxia® to be a beneficial vaccine given the lower incidence of severe dengue and the number of people hospitalized in the various endemic countries where it has been introduced [57–60].
In April 2018, WHO published the latest recommendations of the Strategic Advisory Group of Experts on Immunization (SAGE) on the use of Dengvaxia® vaccine in dengue endemic areas (WHO Weekly Epidemiological Record, Friday 8 June 2018). To date, SAGE recommends the deployment of Dengvaxia® vaccine commercialized by Sanofi-Pasteur only in endemic regions where dengue seroprevalence is higher than 70% among the target population, excluding individuals under 9 years of age. The age limit for vaccination would depend on the level of dengue transmission in the area concerned, but is generally 45 years. To prevent adverse events related to the immunological status of vaccinated individuals, strong recommendations have been made to implement a ‘pre-vaccination screening’ strategy in countries where it is necessary to assess population exposure to dengue fever or where the expected transmission rate is low. To this purpose, Sridhar et al. recently developed a new diagnostic test based on DENV NS1 that was performed on blood samples taken from individuals one year after receiving a single dose of Dengvaxia®[57].
Dengue vaccine candidate TDV
Takeda’s tetravalent dengue vaccine (TDV) candidate is based on mutant TDV-2 derived from the original vaccine strain DENV-2-PDK-53, over-attenuated by the introduction of an additional mutation in NS3 [61–63],(Figure 3). Three chimeric viruses TDV-1, TDV-3, and TDV-4 have been constructed by substituting prM and E gene from DENV-1, DENV-3 or DENV-4 into the TDV-2 backbone [64,65]. In clinical studies conducted during phases 1 and 2, TDV was reported as a safe and well-tolerated vaccine candidate that induces neutralizing antibody and CD8+ T cell responses against all four serotypes [66–68]. Another phase 1b study was performed to investigate the immunogenicity of early low-doses of TDV (LD-TDV) following intradermal administration in healthy dengue-naive adults [69]. The administration of two doses of DL-TDV, 3 months apart, induces immune responses against the four dengue serotypes, the highest being DENV-2 and the lowest DENV-4. TDV vaccine recipients had neutralizing antibodies against DENV directed to the EDIII antigenic domain of DENV-2 or EDI of DENV-1 [70]. A two-dose regimen, administered 3 months apart, was selected for the ongoing phase 3 efficacy trial, enrolling about 20,000 individuals aged 4 to 16 years in different countries in Latin America and Asia. Phase 3 is expected to be completed by the end of 2021.
Figure 3.
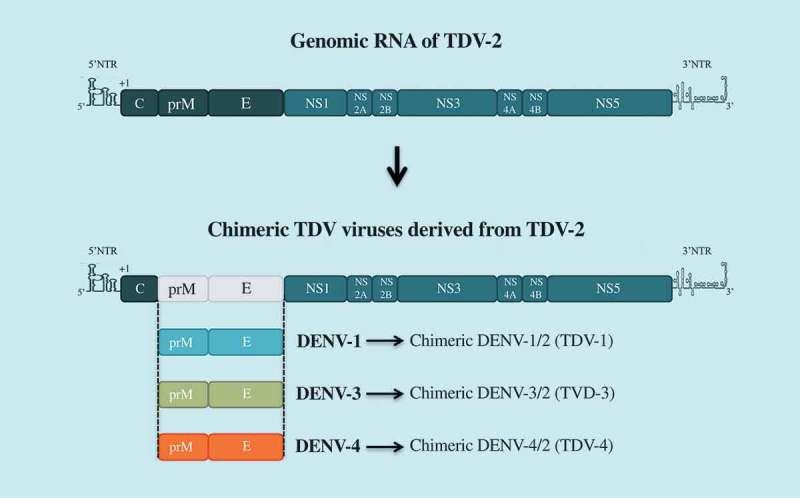
TDV vaccine candidate.
The Takeda tetravalent vaccine TDV has been generated by substituting prM and E genes from DENV-1, DEN-3, and DENV-4 into TDV-2 backbone. TDV2 is an over-attenuated mutant of DENV-2 vaccine candidate DENV-2-PDK53.
Dengue vaccine candidate LAV Delta 30
National Institute of Health (NIH) has developed a tetravalent dengue candidate vaccine, LAV Delta 30, which contains a deletion of 30 contiguous nucleotides (∆30) into the domain II from the 3ʹ end NTR of the genomic RNA. The ∆30 deletion has been used to attenuate DENV-1, −3, and −4 viruses (Figure 4)[71]. A chimeric DENV-2 virus containing the prM and E of DENV-2 in the context of mutant DENV-4 virus ∆30 was also generated. The marked susceptibility of LAV Delta 30 to the antiviral action of type-I interferon may explain the attenuated properties of the LAV due to reduced accumulation of sfRNAs in infected cells [72]. LAV Delta 30 was licensed to Butantan Institute to produce a tetravalent vaccine known as ‘TV003’. This vaccine shares an equal dose of each recombinant DENV of the four serotypes. Studies conducted in phase 1 and 2 demonstrated the safety and immunogenicity of TV003, with seroconversion rates ranging from 50% (DENV-2) to 100% (DENV-1, 3, and −4) after a single dose. However, viremia was still detectable in individuals vaccinated with TV003 [73]. Up to 90% of vaccine receivers developed neutralizing antibodies after a single dose. TV003 vaccination provided protection against a challenge with DENV-2 one year later [74]. A boost of vaccine recipients with a second dose of TV003 significantly increased the seroconversion rate against DENV-2 [74]. Vaccine candidate TV003 is currently in Phase 3 clinical trial which would be completed in 2022.
Figure 4.
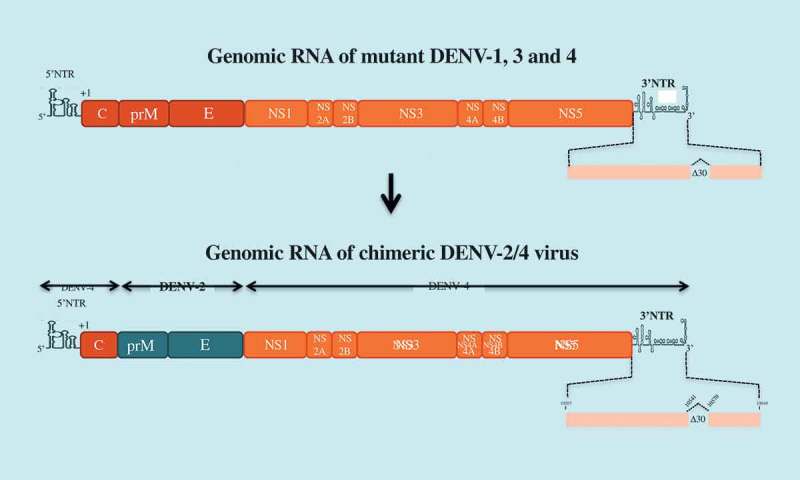
LAV Delta 30 vaccine candidate.
The tetravalent dengue vaccine LAV Delta 30 (or TV003) is composed of four mutants DENV-1 to DEN-4 which contain a deletion of 30 nucleotides (∆30) in the domain II of the 3ʹNTR of their genomic RNA. mutant DENV-4 virus ∆30 was used as a backbone to generate a chimeric DENV-2 virus ∆30 by substitution of the prM and E genes.
Dengue vaccine involving DNA technology
Another variety of current dengue vaccine candidates involves recombinant DNA technology. There are recombinant subunit vaccines requiring expression systems such as plasmid DNA, heterologous vectors, virus-like particles based on the co-expression of the prM and E proteins or only displaying EDIII of the four serotypes, and purified recombinant viral antigens [34,43,47–50,75–77]. Most dengue vaccine candidates are in preclinical studies or still in Phase I clinical trials.
Discussion
Vaccination on the human population is one of the key strategies to prevent the risk of dengue virus transmission from a human host to a mosquito vector [52,53]. The increasing incidence of co-infection with all four serotypes underlines the urgent need for a tetravalent dengue vaccine. This vaccine must be capable of inducing long-term protective immunity against all four dengue serotypes, regardless of the age of the vaccine recipient or their dengue serostatus at the time of vaccination. Dengvaxia® is the first tetravalent vaccine licensed against dengue that has been approved in about 20 countries in Asia and Latin America. WHO recently imposed severe restrictions on the use of Dengvaxia®, seriously calling into question the continuation of mass dengue vaccination programs in dengue-endemic countries around the world. At the end of 2017, vaccination with Dengvaxia® was suspended in the Philippines due to adverse events in the vaccinated population. Validation of other dengue vaccination strategies is therefore a high priority. The new vaccines must ensure indisputable efficacy in protecting against all dengue serotypes and circulating viral strains regardless of the age and immunological status of those at risk of infection. Currently, TDV and LAV Delta 30 are the two most advanced alternatives to CYD-TDV. Nevertheless, the final verdict remains to be pronounced as the evaluation of the protective efficacy linked to each strategy is still in progress. As an alternative to the LAV strategy, the Walter Reed Army Research Institute (WRAIR), FIOCRUZ and GlaxoSmithKine (GSK) recently joined forces in the development of a dengue purified tetravalent inactivated vaccine (DPIV) that has been administered in two separate doses to a group of adults [78]. Adjuvant formulations were supplied by the manufacturer, GSK. Results from the Phase 1 clinical study showed that adjuvanted DPIV is well tolerated and immunogenic, justifying the initiation of a Phase 2 trial in the near future.
Intriguing changes in human susceptibility to dengue disease have been recently documented in different endemic regions. So far, the classic pattern of dengue fever endemicity is that the pediatric population was most likely to experience dengue infection due to the absence of protective immunity. However, recent epidemiological data would tend to clearly nuance this assertion [79]. A set of systematic reviews based on outbreaks from 2000 pointed out a gradual shift in the burden of dengue from the children to the adult population among five of the seven hyperendemic regions [58,80–85]. A large systematic review and meta-analysis including 96 studies reporting outbreaks from 1999 to 2015 in all seven WHO regions was published [2]. In this work, Guo et al. confirmed this trend and reported that it became more pronounced in the last 5 years. Indeed, they described that the mean age of infected people for this period was around 30 years, and increased to 34 years in outbreaks occurring later than 2010. Recently, cases of serious dengue fever have been reported in Puerto Rico, Singapore and Bangladesh highlighting that the adage of the adult population being at low risk of severe dengue outcomes may no longer be true [86–88]. In the current epidemic of DENV-2 in La Reunion island (Indian ocean) that started in early 2018 [89], most hospitalized cases of dengue disease are people aged 15 to 65, while only a small number of pediatric hospitalizations have been reported to date. Furthermore, studies of dengue disease in the elderly have already warned about the increasing burden of disease in this population. Indeed, it has been shown that the elderly present atypical clinical features with fewer symptoms that could lead to misdiagnosed dengue cases [90,91]. Combined with a higher risk of co-morbidities and an increased case-fatality rate, there is a need to refine diagnostic criteria for reliable classification and assessment of the risk of progression of a severe disease [92]. Such changes in epidemiological trends challenge the current management strategies and raise the need to adjust and/or develop new vaccination strategies more appropriate to such a target population.
Funding Statement
This work was supported by the Conseil Régional de La Réunion [SYNERGY: RE0001902];MEESR [école doctorale STS, Université de La Réunion].
Acknowledgments
We thank the members of PIMIT for helpful discussions. We want to thank all of those who have contributed our understanding of dengue disease and we also want to apologize in advance if we have missed any important references that we should have included in this review. This work was supported by the ZIKAlert project (European Union-Region Reunion programme under grant agreement n° SYNERGY: RE0001902). SB has PhD degree scholarship from Reunion Island University (Ecole Doctorale STS), funded by the French ministry MEESR.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Higa Y.Dengue vectors and their spatial distribution. Trop Med Health. 2011;39:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guo C, Kim TK, Pinto AFM, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2017;7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vong S, Khieu V, Glass O, et al. Dengue incidence in urban and rural cambodia: results from population-based active fever surveillance, 2006–2008. PLoS Negl Trop Dis. 2010;4:e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Screaton G, Mongkolsapaya J, Yacoub S, et al. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol. 2015;15:745–759. [DOI] [PubMed] [Google Scholar]
- [7].Chapman EG, Costantino DA, Rabe JL, et al. The structural basis of pathogenic subgenomic flavivirus RNA (sfRNA) production. Science. 2014;344:307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clarke BD, Roby JA, Slonchak A, et al. Functional non-coding RNAs derived from the flavivirus 3ʹ untranslated region. Virus Res. 2015;206:53–61. [DOI] [PubMed] [Google Scholar]
- [9].Simmons CP, Farrar JJ, Nguyen VVC, et al. Dengue. N Engl J Med. 2012;366:1423–1432. [DOI] [PubMed] [Google Scholar]
- [10].Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vannice KS, Durbin A, Hombach J, et al. Status of vaccine research and development of vaccines for dengue. Vaccine. 2016;34:2934–2938. [DOI] [PubMed] [Google Scholar]
- [12].Salje H, Cummings DA, Rodriguez-Barraquer I, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018;557:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Duong V, Lambrechts L, Paul RE, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A. 2015;112:14688–14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ten Bosch QA, Fernández-Borges N, Younas N, et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14:e1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cahill ME, Conley S, DeWan AT, et al. Identification of genetic variants associated with dengue or west nile virus disease: a systematic review and meta-analysis. BMC Infect Dis. 2018;18:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gupta S, Agarwal A, Kumar A, et al. Genome-wide analysis to identify HLA factors potentially associated with severe dengue. Front Immunol. 2018;9:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jaenisch T, Cornick JE, Harris SR, et al. Clinical evaluation of dengue and identification of risk factors for severe disease: protocol for a multicentre study in 8 countries. BMC Infect Dis. 2016;16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Whitehorn J, Smouse SL, Tau NP, et al. Genetic variants of MICB and PLCE1 and associations with the laboratory features of dengue. BMC Infect Dis. 2017;17:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Flores-Mendoza LK, Estrada-Jiménez T, Sedeño-Monge V, et al. IL-10 and socs3 are predictive biomarkers of dengue hemorrhagic fever. Mediators Inflamm. 2017;2017:5197592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nhi DM, Huy NT, Ohyama K, et al. A proteomic approach identifies candidate early biomarkers to predict severe dengue in children. PLoS Negl Trop Dis. 2016;10:e0004435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nikolayeva I, Bost P, Casademont I, et al. A blood RNA signature detecting severe disease in young dengue patients at hospital arrival. J Infect Dis. 2018;217:1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vicente CR, Cornick JE, Harris SR, et al. Serotype influences on dengue severity: a cross-sectional study on 485 confirmed dengue cases in Vitória, Brazil. BMC Infect Dis. 2016;16:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. [DOI] [PubMed] [Google Scholar]
- [24].Wahala WMPB, Kraus AA, Haymore LB, et al. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wahala WMPB, De Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3:2374–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. [DOI] [PubMed] [Google Scholar]
- [27].Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85:410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vatti A, Monsalve DM, Pacheco Y, et al. Original antigenic sin: a comprehensive review. J Autoimmun. 2017;83:12–21. [DOI] [PubMed] [Google Scholar]
- [29].Whitehead SS, Blaney JE, Durbin AP, et al. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. [DOI] [PubMed] [Google Scholar]
- [30].Elong Ngono A, Chen H-W, Tang WW, et al. Protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine. 2016;13:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weiskopf D, Angelo MA, Bangs DJ, et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J Virol. 2015;89:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chu H, George SL, Stinchcomb DT, et al. CD8+ T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis. 2015;212:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lam JH, Reynard S, Fizet A, et al. Dengue vaccine-induced CD8+ T cell immunity confers protection in the context of enhancing, interfering maternal antibodies. JCI Insight. 2017;2(24):94500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vannice KS, Roehrig JT, Hombach J. Next generation dengue vaccines: a review of the preclinical development pipeline. Vaccine. 2015;33:7091–7099. [DOI] [PubMed] [Google Scholar]
- [35].Thomas SJ, Rothman AL. Trials and tribulations on the path to developing a dengue vaccine. Vaccine. 2015;33 Suppl 4(Suppl 4):D24–D31. [DOI] [PubMed] [Google Scholar]
- [36].Barban V, Mateo M, Page A, et al. Improvement of the dengue virus (DENV) nonhuman primate model via a reverse translational approach based on dengue vaccine clinical efficacy data against DENV-2 and −4. J Virol. 2018;92(12):e00440-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Milligan GN, Sarathy VV, Infante E, et al. A dengue virus type 4 model of disseminated lethal infection in AG129 mice. PLoS One. 2015;10:e0125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Milligan GN, Sarathy VV, White MM, et al. A lethal model of disseminated dengue virus type 1 infection in AG129 mice. J Gen Virol. 2017;98:2507–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sarathy VV, White M, Li L, et al. Characterization of a murine model of non-lethal, symptomatic dengue virus infection. Sci Rep. 2018;8:4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sarathy VV, Oksanen HM, Bamford JKH. Characterization of lethal dengue virus type 4 (DENV-4) TVP-376 infection in mice lacking both IFN-α/β and IFN-γ receptors (AG129) and comparison with the DENV-2 AG129 mouse model. J Gen Virol. 2015;96:3035–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Flipse J, Smit JM, Simmons CP. The complexity of a dengue vaccine: a review of the human antibody response. PLoS Negl Trop Dis. 2015;9:e0003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Screaton G, Mongkolsapaya J. Which dengue vaccine approach is the most promising, and should we be concerned about enhanced disease after vaccination? the challenges of a dengue vaccine. Cold Spring Harb Perspect Biol. 2017. DOI: 10.1101/cshperspect.a029520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Torresi J, Ebert G, Pellegrini M. Vaccines licensed and in clinical trials for the prevention of dengue. Hum Vaccines Immunother. 2017;13:1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Guy B, Lang J, Saville M, et al. Vaccination against dengue: challenges and current developments. Annu Rev Med. 2016;67:387–404. [DOI] [PubMed] [Google Scholar]
- [45].Pantoja P, Pérez-Guzmán EX, Rodríguez IV, et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun. 2017;8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sariol CA, Nogueira ML, Vasilakis N. A tale of two viruses: does heterologous flavivirus immunity enhance zika disease? Trends Microbiol. 2017. DOI: 10.1016/j.tim.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Slon Campos JL, Poggianella M, Marchese S, et al. DNA-immunisation with dengue virus E protein domains I/II, but not domain III, enhances Zika, West Nile and yellow fever virus infection. PLoS One. 2017;12:e0181734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rather IA, Kim TK, Pinto AFM, et al. Prevention and control strategies to counter dengue virus infection. Front Cell Infect Microbiol. 2017;7:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Martin J, Hermida L, Xia W-Y, et al. Dengue vaccine: an update on recombinant subunit strategies. Acta Virol. 2016;60:3–14. [DOI] [PubMed] [Google Scholar]
- [50].Liu Y, Liu J, Cheng G, et al. Vaccines and immunization strategies for dengue prevention. Emerg Microbes Infect. 2016;5:emi201674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Halstead SB, Russell PK, Sun G, et al. Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine. 2016;34:1643–1647. [DOI] [PubMed] [Google Scholar]
- [52].Pang T, Mak TK, Gubler DJ, et al. Prevention and control of dengue-the light at the end of the tunnel. Lancet Infect Dis. 2017;17:e79–e87. [DOI] [PubMed] [Google Scholar]
- [53].Schwartz LM, Halloran ME, Durbin AP, et al. The dengue vaccine pipeline: implications for the future of dengue control. Vaccine. 2015;33:3293–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol. 2015;14(nrmicro.2015.2):45. [DOI] [PubMed] [Google Scholar]
- [55].Vannice KS, Edge C, Dunleavy U, et al. Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines. Vaccine. 2018;36:3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Arredondo-García J. L., Hadinegoro SR, Reynales H, et al. Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2018;24:755–763. [DOI] [PubMed] [Google Scholar]
- [57].Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018. DOI: 10.1056/NEJMoa1800820 [DOI] [PubMed] [Google Scholar]
- [58].Villar LA, Rojas DP, Besada-Lombana S, et al. Epidemiological trends of dengue disease in colombia (2000-2011): a systematic review. PLoS Negl Trop Dis. 2015;9:e0003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guy B, Noriega F, Ochiai RL, et al. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev Vaccines. 2017;16:1–13. [DOI] [PubMed] [Google Scholar]
- [60].Capeding MR, Tran NH, Hadinegoro SRS, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet Lond Engl. 2014;384:1358–1365. [DOI] [PubMed] [Google Scholar]
- [61].Butrapet S, Huang CY-H, Pierro DJ, et al. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. J Virol. 2000;74:3011–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Huang CY-H, Butrapet S, Pierro DJ, et al. Chimeric dengue type 2 (vaccine strain pdk-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000;74:3020–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kinney RM, Linder M, Wengler G, et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230:300–308. [DOI] [PubMed] [Google Scholar]
- [64].Huang CY-H, Butrapet S, Tsuchiya KR, et al. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77:11436–11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Osorio JE, Huang CY-H, Kinney RM, et al. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine. 2011;29:7251–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rupp R, Luckasen GJ, Kristein JL, et al Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a phase 1b randomized study. Vaccine. 2015;33:6351–6359. [DOI] [PubMed] [Google Scholar]
- [67].Osorio JE, Page N, Cortese MM, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis. 2014;14:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis. 2016;213:1562–1572. [DOI] [PubMed] [Google Scholar]
- [69].Jackson LA, Edge C, Dunleavy U, et al. A phase 1 study of safety and immunogenicity following intradermal administration of a tetravalent dengue vaccine candidate. Vaccine. 2018;36:3976–3983. [DOI] [PubMed] [Google Scholar]
- [70].Swanstrom JA, Henein S, Plante JA, et al. Analyzing the human serum antibody responses to a live attenuated tetravalent dengue vaccine candidate. J Infect Dis. 2018;217:1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Whitehead SS, Falgout B, Hanley KA, et al. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3ʹ untranslated region is highly attenuated and immunogenic in monkeys. J Virol. 2003;77:1653–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bustos-Arriaga J, Edge C, Dunleavy U, et al. Decreased accumulation of subgenomic RNA in human cells infected with vaccine candidate DEN4Δ30 increases viral susceptibility to type I interferon. Vaccine. 2018;36:3460–3467. [DOI] [PubMed] [Google Scholar]
- [73].Durbin AP, McArthur J, Marron JA, et al. The live attenuated dengue serotype 1 vaccine rDEN1Δ30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006;2:167–173. [DOI] [PubMed] [Google Scholar]
- [74].Kirkpatrick BD, Breen M, Choyke P, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med. 2016;8:330ra36. [DOI] [PubMed] [Google Scholar]
- [75].Danko JR, Kochel T, Simmons M, et al. Safety and immunogenicity of a tetravalent dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am J Trop Med Hyg. 2018;98:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gottschamel J, Lössl A, Ruf S, et al. Production of dengue virus envelope protein domain III-based antigens in tobacco chloroplasts using inducible and constitutive expression systems. Plant Mol Biol. 2016;91:497–512. [DOI] [PubMed] [Google Scholar]
- [77].Coller B-AG, Clements DE, Bett AJ, et al. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine. 2011;29:7267–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Diaz C, Jarman RG, Febo I, et al. Phase I randomized study of a tetravalent dengue purified inactivated vaccine in healthy adults from puerto rico. Am J Trop Med Hyg. 2018;98:1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lin RJ, Lee TH, Leo YS. Dengue in the elderly: a review. Expert Rev Anti Infect Ther. 2017;15:729–735. [DOI] [PubMed] [Google Scholar]
- [80].Bravo L, Roque VG, Brett J, et al. Epidemiology of dengue disease in the philippines (2000–2011): a systematic literature review. PLoS Negl Trop Dis. 2014;8:e3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dantés HG, Farfán-Ale JA, Sarti E, et al. Epidemiological trends of dengue disease in mexico (2000–2011): a systematic literature search and analysis. PLoS Negl Trop Dis. 2014;8:e3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].L’Azou M, Taurel A-F, Flamand C, et al. Recent epidemiological trends of dengue in the french territories of the Americas (2000–2012): a systematic literature review. PLoS Negl Trop Dis. 2014;8:e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Teixeira MG, Siqueira JB Jr, Ferreira GLC, et al. Epidemiological trends of dengue disease in brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013;7:e2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Limkittikul K, Brett J, L’Azou M, et al. Epidemiological trends of dengue disease in thailand (2000–2011): a systematic literature review. PLoS Negl Trop Dis. 2014;8:e3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mohd-Zaki AH, Brett J, Ismail E, et al. Epidemiology of dengue disease in malaysia (2000–2012): a systematic literature review. PLoS Negl Trop Dis. 2014;8:e3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rigau-Pérez JG, Vorndam AV, Clark GG, et al. The dengue and dengue hemorrhagic fever epidemic in Puerto Rico, 1994-1995. Am J Trop Med Hyg. 2001;64:67–74. [DOI] [PubMed] [Google Scholar]
- [87].Rahman M, Rahman K, Siddque AK, et al. First outbreak of dengue hemorrhagic fever, Bangladesh. Emerg Infect Dis. 2002;8:738–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lee VJ, Lye DCB, Sun Y, et al. Predictive value of simple clinical and laboratory variables for dengue hemorrhagic fever in adults. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2008;42:34–39. [DOI] [PubMed] [Google Scholar]
- [89].Surveillance de la dengue à la Réunion Point épidémiologique au 23 avril 2018./2018/Océan Indien/Tous les numéros/Points épidémiologiques/Publications et outils/Accueil. [cited2018July26]. Available from: http://invs.santepubliquefrance.fr/Publications-et-outils/Points-epidemiologiques/Tous-les-numeros/Ocean-Indien/2018/Surveillance-de-la-dengue-a-la-Reunion.-Point-epidemiologique-au-23-avril-2018
- [90].Lee -C-C, Hsu H-C, Chang C-M, et al. Atypical presentations of dengue disease in the elderly visiting the ED. Am J Emerg Med. 2013;31:783–787. [DOI] [PubMed] [Google Scholar]
- [91].Rowe EK, Leo Y-S, Wong JGX, et al. Challenges in dengue fever in the elderly: atypical presentation and risk of severe dengue and hospita-acquired infection. PLoS Negl Trop Dis. 2014;8:e2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].García-Rivera EJ, Rigau-Pérez JG. Dengue severity in the elderly in Puerto Rico. Rev Panam Salud Publica Pan Am J Public Health. 2003;13:362–368. [DOI] [PubMed] [Google Scholar]


