Abstract
Besides their innate ability to rapidly produce effector cytokines and kill virus-infected or transformed cells, natural killer (NK) cells display a strong capability to adapt to environmental modifications and to differentiate into long-lived, hyperfunctional populations, dubbed memory or memory-like NK cells. Despite significant progress in the field of NK cell-based immunotherapies, some factors including their short life span and the occurrence of a tumor-dependent functional exhaustion have limited their clinical efficacy so that strategies aimed at overcoming these limitations represent one of the main current challenges in the field. In this scenario, the exploitation of NK cell memory may have a considerable potential. This article summarizes recent evidence in the literature on the peculiar features that render memory NK cells an attractive tool for antitumor immunotherapy, including their long-term survival and in vivo persistence, the resistance to tumor-dependent immunosuppressive microenvironment, the amplified functional responses to IgG-opsonized tumor cells, and in vitro expansion capability. Along with highlighting these issues, we speculate that memory NK cell-based adoptive immunotherapy settings would greatly take advantage from the combination with tumor-targeting therapeutic antibodies (mAbs), as a strategy to fully unleash their clinical efficacy.
1. Introduction
NK cells represent a pivotal player of innate antitumor immune responses. They can eradicate neoplastic cells by a targeted release of cytotoxic granules containing perforin and granzymes and/or death receptor-mediated killing [1]. Moreover, NK cells can signal to other immune cells by producing cytokines and chemokines, such as IFN-γ, TNFα, IL-6, GM-CSF, and CCL5 in response to target cells or cytokine stimulation [1, 2]. In particular, NK-derived IFN-γ stands as a well-recognized key immunoregulatory factor in the shaping of antitumor adaptive immune responses, by modulating dendritic cell (DC) and T cell responses [3–5]. Further, NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) is a main immune-dependent mechanism by which tumor-targeting therapeutic mAbs mediate tumor cell killing [6–8].
NK cell functional response to tumor cells encounter is triggered by a variety of activating receptors, some of which (e.g., NKG2D and DNAM-1) recognize stress-induced ligands expressed on malignantly transformed cells; additionally, NK cells are potently activated by CD16 or FcγRIIIa (low-affinity Fc receptor for IgG)-dependent recognition of IgG-opsonized targets. In contrast, individual NK cells express, in different combinations, several inhibitory receptors (e.g., CD94/NKG2A and killer immunoglobulin-like receptors (KIR)) that recognize MHC class I molecules. In addition to modulate functional responsiveness, NK cell inhibitory receptors are critical for promoting their education [9].
The perspective of NK cells as exquisitely innate effectors is challenged by the recent appreciation that NK cells can adapt their functional program in response to environmental factors, through the generation of long-lasting specialized NK cell populations with enhanced effector functions, named adaptive or memory NK cells [10–12].
The first demonstration of antigen-specific recall responses by NK cells was in the setting of hapten-induced contact hypersensitivity, where CXCR6+ liver-derived murine NK cells could mediate antigen-specific contact hypersensitivity independently from B and T cells [13, 14].
Doubtless, memory NK cell populations have been mostly extensively characterized in the setting of cytomegalovirus (CMV) infection. Murine CMV (MCMV) infection induces immunological memory independent of T and B cells [15, 16]. Protection in these models is mediated by Ly49H+ NK cells, which upon recognition of m157 viral antigen (Ly49H ligand) proliferate and persist in lymphoid and nonlymphoid organs. Upon reinfection, these memory NK cells undergo secondary expansion, rapidly degranulate, and release cytokines, leading to a protective immune response, and also provide protection to newborn mice challenged with MCMV, upon adoptive transfer [16].
Accordingly, human CMV (HCMV) deeply impacts on NK cell compartment; in such context, memory NK cells have been initially identified in healthy HCMV-seropositive individuals, mainly on the basis of high expression levels of CD94/NKG2C activating receptor and CD57 terminal differentiation marker [17–21]. Such NKG2C+ memory NK cells can constitute up to 70% of the total NK cell population and can persist at high frequency for years [22–25].
Similar to Ly49H in mice, NKG2C is a member of the C-type lectin superfamily and associates to the adaptor protein DAP12 [26]. NKG2C forms heterodimers with CD94 and binds to the nonclassical MHC class I molecule HLA-E bound to HLA-E-stabilizing peptides [27].
HCMV-associated NK cells exhibit a distinct surface receptor expression pattern, consisting of a reduced expression of NKG2A (the inhibitory receptor counterpart of NKG2C), NKp30, NKp46, and CD16, as well as an increased expression of ILT2 (LIR-1a) [11, 19]. Further, it has been reported that ex vivo memory NK cells display an oligoclonal KIR pattern, with a bias for self-specific members both in healthy donors and chronic hepatitis patients [18, 24].
These features, along with additional phenotypic hallmarks, including the preferential expression of the activating receptor CD2, together with the reduced expression of the inhibitory receptor Siglec-7 [28], collectively aid in the identification of this unique and discrete NK cell population.
A link between HCMV and memory NK cell expansion is supported by the finding of an increase in CD94/NKG2C+ NK cells following the HCMV reactivation or infection in patients receiving hematopoietic stem cell transplant [22, 23, 29–31] and strengthened by the recent identification of HCMV-encoded antigen UL40, as the HLA-E ligand that drives the in vitro expansion and differentiation of memory NKG2C+ NK cells [32]; however, a potential role of other receptors besides NKG2C in the recognition and response to HCMV infection and in the skewing of an identical cellular program has been proposed [33].
Seminal independent studies have identified an immune-receptor tyrosine-based activation motif (ITAM)-bearing FcεRIγ adaptor protein-deficient NK cell subset in HCMV-seropositive individuals, endowed with a specific epigenetic signature, mostly overlapping with the CD94/NKG2C+ population [19–21, 34, 35]. FcεRIγ chain deficiency became an important feature of memory NK cell population, together with the specific downregulation of PLZF and IKZF2 transcription factors, as well as the variable loss of the intracellular signaling molecules DAB2, SYK, and EAT-2.
Memory NK cells also display a distinctive genome-wide methylation profile that confers an overall epigenetic profile very similar to that of memory CD8+ T cells, thus providing a molecular basis for the adaptive features of these cells. In particular, the promoter regions of FcεRIγ, EAT-2, SYK, and PLZF genes are highly methylated in memory NK cells, compared to conventional NK cells (cNK). Likewise, the promoter regions of IL-12 and IL-18 receptor subunit genes, which are regulated by PLZF, are also highly methylated, accounting for a reduced ability to respond to bystander activation by these cytokines [12, 21].
Another major epigenetic hallmark of memory NKG2C+ NK cells is represented by a significant demethylation of the conserved noncoding sequence (CNS) 1 in the IFNG locus, which remains stable in progeny, similar to what occurs in memory Th1 cells [25]. This molecular signature provides a mechanism to explain the potent IFN-γ production in response to the stimulation through a selective recognition repertoire. Indeed, the engagement of NKG2C by HLA-E-expressing target cells potently activates memory NK cells and leads to polyfunctional responses characterized by degranulation as well as TNFα and IFN-γ production [18]. Further, memory NK cells can be efficiently stimulated by the cross-linking of CD16 through the recognition of Ab-coated virus-infected cells [19, 21, 33, 34].
Long-lived memory-like NK cells can also be generated in noninfectious or antigen-independent settings. Specifically, in vitro stimulation of mouse splenic NK cells with IL-12 and IL-18, prior to transfer into a naive host, generated a pool of cells with enhanced IFN-γ production in response to cytokines, activating receptor ligands or tumor targets [36, 37], without any enhanced cytotoxicity. Similar to murine memory-like NK cells, when human NK cells are preactivated with IL-12, IL-15, and IL-18 and subsequently rested for several days, they display an increased IFN-γ production upon restimulation with cytokines or target cells compared with control population and such enhanced activity is maintained following an extensive cell division [38, 39].
2. In Vivo Evidence of Memory NK Cell Antitumor Activity
Preclinical and clinical observations suggest that memory NK cell activities could be advantageous in tumor settings and may contribute to relapse protection, in the context of hematopoietic malignancies.
Several studies reported a longer relapse-free survival after allogeneic stem cell transplantation in acute myeloid leukemia (AML) or chronic myeloid leukemia (CML) patients experiencing HCMV reactivation [40–43]. Moreover, the expansion of NKG2C+CD57+ memory NK cells in leukemic patients that reactivated CMV following allo-hematopoietic stem cell transplant (HSCT) is associated with a significantly reduced rate of relapse [44], suggesting that the recognition of HLA-E+ leukemic blasts by memory NKG2C+ NK cells expanded in response to HCMV infection may have beneficial effect through the eradication of minimal residual disease.
Furthermore, consistent with the finding that murine cytokine-preactivated memory-like NK cells maintain enhanced antitumor activity after adoptive transfer [38], a single injection of human memory-like NK cells significantly reduced the leukemia burden and improved the overall survival compared with that of control NK cells, in a xenograft model of leukemia [45]. Similarly, an independent study also found effective control of melanoma growth by cytokine-preactivated human NK cells in a melanoma xenograft model in NOD scid gamma (NGS) mice. The enhanced antitumor effects mediated by memory-like NK cells might result from their augmented cytotoxicity, high IFN-γ production capacity, and persistence in large numbers in the host [46].
More importantly, a phase I clinical trial harnessing cytokine-induced memory-like NK cells was recently performed in patients with relapsed or refractory AML [45], which consisted in the adoptive transfer of donor-derived NK cells preactivated with IL-12, IL-15, and IL-18, following fludarabine/cyclophosphamide-mediated lymphodepletion. Tracking donor memory-like NK cells in recipients revealed that they underwent in vivo expansion. As expected, donor memory-like NK cells displayed higher frequencies of IFN-γ+ cells with respect to recipient NK cells when challenged ex vivo with K562 leukemia cells. Notably, five out of nine evaluable patients showed a clinical response, including four complete remissions, which compares favorably with previous studies utilizing purified NK cells without cytokine preactivation [47].
3. Unique Features of Memory NK Cells Exploitable in Cancer Immunotherapy
3.1. Long-Term Survival and In Vivo Persistence
A crucial aspect of memory or memory-like NK cells is a longer life span, with respect to conventional populations, along with the capability to mediate persistent responses.
While NK cells are considered short-lived effectors of innate immunity, with an estimated half-life of 14 days [48, 49], HCMV-induced CD94/NKG2C+ NK cells exhibit a persistence of several months in the absence of detectable viremia and were stably maintained at elevated frequency for years, in some healthy individuals [22, 23, 30]. Moreover, after the umbilical cord blood transplantation in patients with hematopoietic malignancies, CMV reactivation leads to a long-lasting increase of NKG2C+ NK cells [29, 31].
Recent studies, involving patients with either paroxysmal nocturnal hemoglobinuria or GATA2 deficiency, demonstrate that memory NK cells selectively persist in these patients in spite of a reduction of conventional NK cell populations, supporting an independent survival and self-renewal pathway for the homeostatic maintenance of CD56+ NK cells with an adaptive phenotype [50, 51].
Mechanistic studies demonstrated that human memory NK cells express higher levels of antiapoptotic Bcl-2 that marked an epigenetically unique population persisting for at least 35 months [21, 34]. The authors speculate that, analogously to long-lived and self-renewing memory T cells, the reduced expression of PLZF could support memory NK cell longevity.
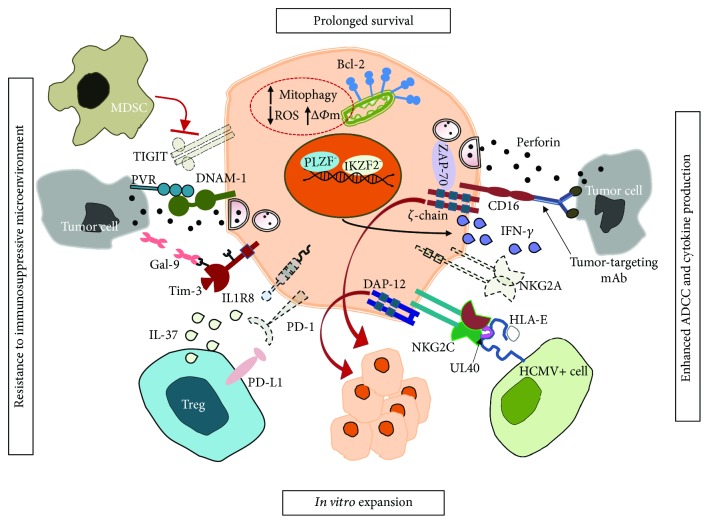
More recently, an isoform of AT-rich interaction domain 5 (ARID5B) transcription factor has been found selectively upregulated in memory NK cells and involved in promoting mitochondrial membrane potential, oxidative metabolism, survival, and IFN-γ production [52]. Collectively, such evidence provides molecular basis for memory NK cell longevity (Figure 1).
Figure 1.
NKG2C+ memory NK cell features exploitable in cancer immunotherapy. The dotted lines indicate reduced receptor expression.
The MCMV infection model gave important insights on memory NK cell longevity. The analysis of the proliferation kinetics and persistence of MCMV-driven Ly49H+ NK cells showed that following a contraction phase, a long-lived and self-renewing memory cell pool persists for several months after infection in a variety of peripheral tissues, where it displays an enhanced response to secondary challenge [16]. The downregulation of the prosurvival molecule Bcl-2 and Bim-mediated proapoptotic signaling during the contraction phase regulate the size of the memory cell pool [53, 54]. Further, the survival of memory NK cells during the contraction phase after MCMV infection requires mitophagy of dysfunctional mitochondria, through an Atg3-dependent mechanism [55].
3.2. Resistance to Tumor-Dependent Immunosuppressive Microenvironment
Although NK cells are expected to target malignant cells and to play an important role in the immune surveillance against tumors, it is now appreciated that the suppressive components in the tumor microenvironment dampen the NK cell efficacy [56, 57]. Several studies have revealed a central role for Treg in suppressing tumor-infiltrating NK cells [58, 59]; in this context, Treg-mediated suppression of ADCC has been shown to correlate with a lower clinical efficacy of therapeutic tumor-targeting mAbs [60]. Treg can act both by secreting immunosuppressive cytokines (TGFβ, IL-10, and IL-35) and by expressing inhibitory receptors (e.g., CTLA4 and PD-1) on their surface. Recent data uncover a new mechanism for Treg-mediated suppression of NK cells, based on the production of IL-37 which promotes the downregulation of the T cell immunoglobulin and mucin-domain containing-3 (Tim-3), that may behave as a stimulatory receptor in NK cells [61], and the upregulation of PD-1. Compared with cNK cells, whose proliferation, IFN-γ production, and cytotoxicity were efficiently inhibited by Treg, memory NK cells were found to be inherently resistant to Treg-mediated suppression, as they expressed low levels of IL-37 receptor, IL1R8, and PD-1, along with high levels of Tim-3 [62].
Further, NK cells express an inhibitory receptor called T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT), which also marks exhausted CD8+ tumor-infiltrating lymphocytes (TIL) [63]. TIGIT along with CD96 (also known as TACTILE) are coinhibitory receptors which by recognizing the same ligands of DNAM-1, namely, PVR (CD155) and nectin-2 [64, 65], counterbalance DNAM-1 activation at the NK-target synapse. Similar to T cells, in vitro blockade of TIGIT enhances cytokine secretion and cytotoxicity in NK cells [66–68] (Figure 1).
Recently, NK cell inhibition by myeloid-derived suppressor cells (MDSC) was shown to rely on TIGIT-PVR axis and was consequently abrogated upon TIGIT blockade. Also in this case, memory NK cells were found to be resistant to MDSC-mediated suppression in patients with cancer [68].
mAb-mediated interference with MHC class I-specific inhibitory receptors of NK cells can represent a strategy to potentiate their antitumor functions. In this regard, NKG2A blockade by means of a specific mAb (IPH2201, monalizumab) is currently being evaluated for a variety of tumor types in combination, for instance, with tumor-targeting mAbs [69]. The lack of NKG2A inhibitory receptor on memory NK cells makes such cells inherently resistant to HLA-E-expressing tumor-mediated inhibition and represents another advantage for the possible exploitation of these cells.
3.3. Amplified Functional Responses to IgG-Opsonized Targets
CD16 represents a prototype NK activating receptor; its engagement by IgG-opsonized targets is sufficient to trigger ADCC, as well as the production of proinflammatory cytokines and chemokines, such as IFN-γ, TNFα, IL-6, GM-CSF, and CCL5 [1, 2, 70, 71]. Human CD16 exhibits two extracellular Ig domains, a short cytoplasmic tail and a transmembrane domain that enables its association with ITAM-containing CD3ζ and FcεRIγ chains [72], which guarantee Syk- and ZAP-70-dependent signal transduction [2].
Notably, CD16-triggered ADCC and phagocytosis, performed by NK cells and macrophages, respectively, are among the main immune-dependent mechanisms by which tumor-targeting therapeutic mAbs mediate tumor cell killing [6–8].
A key feature of memory NK cells is their capability to mediate amplified Ab-dependent functional responses in terms of degranulation and cytokine production [19–21, 33, 34]. In particular, memory NK cells exhibit a greatly enhanced ability to produce IFN-γ, as a consequence of hypomethylated IFNG regulatory region [25], in response to activation via CD16, thus providing a prompt and powerful response against Ab-opsonized target cells. Indeed, despite the lower CD16 expression, they have been shown to more efficiently mediate polyfunctional responses, e.g., degranulation and IFN-γ production, upon stimulation via Ab-opsonized targets. The apparent conflict between higher CD16-triggered functional responses and lower surface receptor levels may be explained by the exclusive coupling of CD16 to CD3ζ chain in memory NK cells that, thanks to ITAM motif quantitative differences (3 ITAM in CD3ζ vs. 1 ITAM in FcεRIγ), may lead to more robust and efficient biochemical signals [70] (Figure 1). Moreover, the residual levels of CD3ζ chain may preserve the CD2/CD58 costimulatory interaction [73].
The enhanced response to CD16 stimulation has been well documented in response to antiviral IgG-opsonized infected cells and, of relevance here, to tumor-targeting therapeutic mAb-opsonized tumor cells [19, 21, 33, 34, 74]. Moreover, hyperresponsiveness to anti-CD20 mAb-opsonized tumor cells was also observed in in vitro expanded memory NK cells [74].
The capability of memory NK cells to activate in response to tumor cells has not been satisfactorily demonstrated yet. The reduction of NKp46 levels may explain the reduced ability of fresh and in vitro cultured memory NK cells to mediate effector functions in response to stimulation with K562 target cells, being its recognition largely dependent on this receptor [75]. However, NKG2C+ memory NK cells from HCMV-reactivating patients efficiently produced IFN-γ upon K562 stimulation [23, 44], indicating that the upregulation of other activating receptors may compensate for NKp46 defect. For example, CD2 ligand CD58, widely expressed by tumor B cells, has been shown to costimulate memory NK cell responses [33].
3.4. In Vitro Expansion Capability
In vitro expansion of NKG2C+ memory NK cells can be achieved by coculturing NK cells with CMV-infected fibroblasts or HLA-E-transfected cell lines [24, 76]. In these conditions, the interaction between CD94/NKG2C and its cellular ligand HLA-E, in combination with inflammatory cytokines, such as monocyte-derived IL-12, was critical for their expansion [77]. More recently, an HLA-E+ feeder cell-based protocol was shown to induce the selective in vitro expansion of memory NK cells that exhibited a profound skewing toward the expression of a single self-KIR, depending on the donor HLA-C genotype. These cells showed a high NKG2C-dependent cytotoxic potential against allogeneic pediatric acute lymphoblastic leukemia primary blasts [78], previously shown to be refractory to killing by allogeneic NK cells or NK92 NK cell line [79]. These data envisage a potential exploitation of memory NK cell alloreactivity in the context of novel adoptive cell therapy strategies.
Different lines of evidence highlight that primary HCMV infection drives the priming and proliferation of memory NK cells in a NKG2C-dependent manner [10–12, 76, 77]. HCMV-driven memory NK cell pool can be maintained by a variety of different viral super infections. In particular, an expanded population of memory NK cells was detected in EBV-, HBV-, HCV-, and HIV-seropositive individuals, only when patients were also seropositive for HCMV [18, 80]. It is therefore conceivable that Ab-mediated immune responses may drive the proliferation and maintenance of an already existing pool of memory NK cells, in some viral disease settings. Indeed, the capability of CD16-initiated signals to regulate NK cell proliferation and death, under selected conditions, has been shown [81, 82].
Seminal in vitro studies offered a mechanistic explanation for the role of virus-specific Abs in sustaining memory NK cell expansion and established a pivotal role for CD16 binding to antiviral IgG-opsonized cells to induce the proliferation of this specific subset [20, 21].
In this context, our recent data [74] demonstrate the unique capability of anti-CD20 therapeutic mAb-opsonized targets to drive the selective in vitro expansion of memory NK cells from HCMV-seropositive healthy donors. Indeed, we developed an effective in vitro culture system, consisting of a 9-day coculture of PBMC with irradiated lymphoblastoid Raji cells opsonized with anti-CD20 therapeutic mAbs, in IL-2-containing medium (Figure 1). Importantly, in vitro expanded memory NK cells, as their freshly isolated counterpart, displayed amplified CD16-polyfunctional responses upon stimulation with anti-CD20-opsonized tumor cells. It is conceivable that CD16-dependent memory NK cell proliferation also relies on multiple accessory signals, conveyed by cell-cell contacts and soluble mediators. In our system, ligands expressed by Raji lymphoblastoid B cells may provide accessory proliferative signals to memory NK cells; among them, CD2 ligand CD58, has been shown to costimulate memory NK cell responses [33]. Moreover, monocyte-derived IL-12, probably stimulated through FcγR engagement by anti-CD20-opsonized targets, likely mediates a critical contribution through the upregulation of CD25, as demonstrated by a recent report [77].
An extensive cell division, along with an induced expression of a functional high-affinity IL-2 receptor (IL-2R) αβγ, is also observed in cytokine-induced memory-like NK cells [39].
4. Perspectives
Based on their peculiarities, memory NK cell exploitment in adoptive therapy strategies is considered a particularly attractive tool in anticancer therapeutic perspective and is already a reality. Indeed, phase I clinical trials based on adoptive transfer of cytokine-induced memory-like NK cells for patients with relapsed or refractory AML [45], or in vitro expanded NK cells with an inducible adaptive phenotype in advanced cancer [83], are ongoing. The possibility to enhance memory NK cell antitumor functions through genetic manipulation has been suggested by a recent work showing that CAR-transduced terminally differentiated/adaptive NK cells exhibit superior effector functions when compared to other NK subsets [84].
Future studies are needed to uncover relevant aspects of memory NK cell biology in order to optimize their clinical application. A better definition of the phenotypic and functional heterogeneity in terms of tumor recognition capability, the possibility to in vitro manipulate or selectively expand memory NK cells endowed with selected receptor repertoire, the GMP-compliant adaptation of the procedure for their in vitro expansion will be instrumental for the better exploitment of NK cell memory for the ultimate benefit of treating cancer patients.
Importantly, the enhanced responsiveness and expansion capability in response to mAb-coated tumors may guide future attempts to combine strategies based on the adoptive transfer of in vitro expanded memory NK cells and tumor-targeting therapeutic mAbs, whose clinical responses are burdened by a significant proportion of relapses. Indeed, the promotion of an endogenous long-lasting adaptive antitumor immune response that may be highly relevant in maintaining long-term protection is becoming a major goal for improving the efficacy of mAb-based therapies.
It is worth investigating the possible contribution of memory NK cells to the development of the so-called “vaccinal effect” of therapeutic mAbs. Indeed, thanks to their amplified capability to produce cytokines upon mAb-opsonized tumor recognition, memory NK cells could participate to the development of adaptive antitumor immune responses, required for the long-term protection of mAb-treated patients [8, 85, 86].
Acknowledgments
CC received a grant from the Italian Ministry of University and Research (MIUR) SIR 2014 (RBSI14022M). SB is a recipient of an Italian Association for Cancer Research (AIRC) fellowship.
Conflicts of Interest
The authors declare no conflict of interest for this article.
References
- 1.Lanier L. L. Up on the tightrope: natural killer cell activation and inhibition. Nature Immunology. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long E. O., Sik Kim H., Liu D., Peterson M. E., Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual Review of Immunology. 2013;31(1):227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín-Fontecha A., Thomsen L. L., Brett S., et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nature Immunology. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 4.Walzer T., Dalod M., Robbins S. H., Zitvogel L., Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 5.Schuster I. S., Coudert J. D., Andoniou C. E., Degli-Esposti M. A. “Natural regulators”: NK cells as modulators of T cell immunity. Frontiers in Immunology. 2016;7:p. 235. doi: 10.3389/fimmu.2016.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor R. P., Lindorfer M. A. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Current Opinion in Immunology. 2008;20(4):444–449. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner G. J. Building better monoclonal antibody-based therapeutics. Nature Reviews Cancer. 2015;15(6):361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battella S., Cox M. C., Santoni A., Palmieri G. Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. Journal of Leukocyte Biology. 2016;99(1):87–96. doi: 10.1189/jlb.5VMR0415-141R. [DOI] [PubMed] [Google Scholar]
- 9.Anfossi N., André P., Guia S., et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Cerwenka A., Lanier L. L. Natural killer cell memory in infection, inflammation and cancer. Nature Reviews Immunology. 2016;16(2):112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 11.Rölle A., Brodin P. Immune adaptation to environmental influence: the case of NK cells and HCMV. Trends in Immunology. 2016;37(3):233–243. doi: 10.1016/j.it.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Tesi B., Schlums H., Cichocki F., Bryceson Y. T. Epigenetic regulation of adaptive NK cell diversification. Trends in Immunology. 2016;37(7):451–461. doi: 10.1016/j.it.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary J. G., Goodarzi M., Drayton D. L., von Andrian U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nature Immunology. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 14.Paust S., Gill H. S., Wang B. Z., et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature Immunology. 2010;11(12):1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr M. T., Sun J. C., Hesslein D. G. T., et al. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. The Journal of Experimental Medicine. 2009;206(4):807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J. C., Beilke J. N., Lanier L. L. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumá M., Angulo A., Vilches C., Gómez-Lozano N., Malats N., López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 18.Béziat V., Dalgard O., Asselah T., et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. European Journal of Immunology. 2012;42(2):447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 19.Hwang I., Zhang T., Scott J. M., et al. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. International Immunology. 2012;24(12):793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J., Zhang T., Hwang I., et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlums H., Cichocki F., Tesi B., et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Verges S., Milush J. M., Schwartz B. S., et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proceedings of the National Academy of Sciences. 2011;108(36):14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley B., Cooley S., Verneris M. R., et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beziat V., Liu L. L., Malmberg J. A., et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luetke-Eversloh M., Hammer Q., Durek P., et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathogens. 2014;10(10, article e1004441) doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanier L. L., Corliss B., Wu J., Phillips J. H. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8(6):693–701. doi: 10.1016/S1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 27.Braud V. M., Allan D. S. J., O'Callaghan C. A., et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 28.Hammer Q., Romagnani C. About training and memory: NK-cell adaptation to viral infections. Advances in Immunology. 2017;133:171–207. doi: 10.1016/bs.ai.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Della Chiesa M., Falco M., Podesta M., et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- 30.Foley B., Cooley S., Verneris M. R., et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C+ NK cells are transplantable and expand in vivo in response to recipient CMV antigen. The Journal of Immunology. 2012;189(10):5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muccio L., Bertaina A., Falco M., et al. Analysis of memory-like natural killer cells in human cytomegalovirus-infected children undergoing αβ+T and B cell-depleted hematopoietic stem cell transplantation for hematological malignancies. Haematologica. 2016;101(3):371–381. doi: 10.3324/haematol.2015.134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammer Q., Rückert T., Borst E. M., et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nature Immunology. 2018;19(5):453–463. doi: 10.1038/s41590-018-0082-6. [DOI] [PubMed] [Google Scholar]
- 33.Liu L. L., Landskron J., Ask E. H., et al. Critical role of CD2 co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Reports. 2016;15(5):1088–1099. doi: 10.1016/j.celrep.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., Scott J. M., Hwang I., Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. The Journal of Immunology. 2013;190(4):1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muntasell A., Pupuleku A., Cisneros E., et al. Relationship of NKG2C copy number with the distribution of distinct cytomegalovirus-induced adaptive NK cell subsets. The Journal of Immunology. 2016;196(9):3818–3827. doi: 10.4049/jimmunol.1502438. [DOI] [PubMed] [Google Scholar]
- 36.Cooper M. A., Elliott J. M., Keyel P. A., Yang L., Carrero J. A., Yokoyama W. M. Cytokine-induced memory-like natural killer cells. Proceedings of the National Academy of Sciences. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keppel M. P., Yang L., Cooper M. A. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. The Journal of Immunology. 2013;190(9):4754–4762. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni J., Miller M., Stojanovic A., Garbi N., Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. The Journal of Experimental Medicine. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romee R., Schneider S. E., Leong J. W., et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behrendt C. E., Rosenthal J., Bolotin E., Nakamura R., Zaia J., Forman S. J. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biology of Blood and Marrow Transplantation. 2009;15(1):54–60. doi: 10.1016/j.bbmt.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elmaagacli A. H., Steckel N. K., Koldehoff M., et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 42.Green M. L., Leisenring W. M., Xie H., et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito S., Pophali P., CO W., et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplantation. 2013;48(10):1313–1316. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cichocki F., Cooley S., Davis Z., et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30(2):456–463. doi: 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romee R., Rosario M., Berrien-Elliott M. M., et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Science Translational Medicine. 2016;8(357, article 357ra123) doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni J., Hölsken O., Miller M., et al. Adoptively transferred natural killer cells maintain long-term antitumor activity by epigenetic imprinting and CD4+ T cell help. OncoImmunology. 2016;5(9, article e1219009) doi: 10.1080/2162402X.2016.1219009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachanova V., Cooley S., Defor T. E., et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Wallace D. L., de Lara C. M., et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutz C. T., Karapetyan A., al-Attar A., et al. Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. The Journal of Immunology. 2011;186(8):4590–4598. doi: 10.4049/jimmunol.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corat M. A. F., Schlums H., Wu C., et al. Acquired somatic mutations in PNH reveal long-term maintenance of adaptive NK cells independent of HSPCs. Blood. 2017;129(14):1940–1946. doi: 10.1182/blood-2016-08-734285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlums H., Jung M., Han H., et al. Adaptive NK cells can persist in patients with GATA2 mutation depleted of stem and progenitor cells. Blood. 2017;129(14):1927–1939. doi: 10.1182/blood-2016-08-734236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cichocki F., Wu C. Y., Zhang B., et al. ARID5B regulates metabolic programming in human adaptive NK cells. The Journal of Experimental Medicine. 2018;215(9):2379–2395. doi: 10.1084/jem.20172168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaulieu A. M., Zawislak C. L., Nakayama T., Sun J. C. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nature Immunology. 2014;15(6):546–553. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min-Oo G., Bezman N. A., Madera S., Sun J. C., Lanier L. L. Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. The Journal of Experimental Medicine. 2014;211(7):1289–1296. doi: 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Sullivan T. E., Johnson L. R., Kang H. H., Sun J. C. BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory. Immunity. 2015;43(2):331–342. doi: 10.1016/j.immuni.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capuano C., Romanelli M., Pighi C., et al. Anti-CD20 therapy acts via FcγRIIIA to diminish responsiveness of human natural killer cells. Cancer Research. 2015;75(19):4097–4108. doi: 10.1158/0008-5472.CAN-15-0781. [DOI] [PubMed] [Google Scholar]
- 57.Hasmim M., Messai Y., Ziani L., et al. Critical role of tumor microenvironment in shaping NK cell functions: implication of hypoxic stress. Frontiers in Immunology. 2015;6:p. 482. doi: 10.3389/fimmu.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smyth M. J., Teng M. W. L., Swann J., Kyparissoudis K., Godfrey D. I., Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. The Journal of Immunology. 2006;176(3):1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Research. 2017;27(1):109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jie H. B., Schuler P. J., Lee S. C., et al. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Research. 2015;75(11):2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gleason M. K., Lenvik T. R., McCullar V., et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarhan D., Hippen K. L., Lemire A., et al. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunology Research. 2018;6(7):766–775. doi: 10.1158/2326-6066.CIR-17-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinet L., Smyth M. J. Balancing natural killer cell activation through paired receptors. Nature Reviews Immunology. 2015;15(4):243–254. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 64.Bottino C., Castriconi R., Pende D., et al. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. The Journal of Experimental Medicine. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dougall W. C., Kurtulus S., Smyth M. J., Anderson A. C. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunological Reviews. 2017;276(1):112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 66.Stanietsky N., Simic H., Arapovic J., et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proceedings of the National Academy of Sciences. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanietsky N., Rovis T. L., Glasner A., et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. European Journal of Immunology. 2013;43(8):2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarhan D., Cichocki F., Zhang B., et al. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Research. 2016;76(19):5696–5706. doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muntasell A., Ochoa M. C., Cordeiro L., et al. Targeting NK-cell checkpoints for cancer immunotherapy. Current Opinion in Immunology. 2017;45:73–81. doi: 10.1016/j.coi.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Trinchieri G., Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nature Immunology. 1993;12(4-5):218–234. [PubMed] [Google Scholar]
- 71.Capuano C., Pighi C., Molfetta R., et al. Obinutuzumab-mediated high-affinity ligation of FcγRIIIA/CD16 primes NK cells for IFNγ production. OncoImmunology. 2017;6(3, article e1290037) doi: 10.1080/2162402X.2017.1290037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Letourneur O., Kennedy I. C., Brini A. T., Ortaldo J. R., O'Shea J. J., Kinet J. P. Characterization of the family of dimers associated with Fc receptors (Fc epsilon RI and Fc gamma RIII) The Journal of Immunology. 1991;147(8):2652–2656. [PubMed] [Google Scholar]
- 73.Grier J. T., Forbes L. R., Monaco-Shawver L., et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. Journal of Clinical Investigation. 2012;122(10):3769–3780. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capuano C., Battella S., Pighi C., et al. Tumor-targeting anti-CD20 antibodies mediate in vitro expansion of memory natural killer cells: impact of CD16 affinity ligation conditions and in vivo priming. Frontiers in Immunology. 2018;9:p. 1031. doi: 10.3389/fimmu.2018.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sivori S., Pende D., Bottino C., et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. European Journal of Immunology. 1999;29(5):1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Gumá M., Budt M., Sáez A., et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 77.Rölle A., Pollmann J., Ewen E. M., et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. Journal of Clinical Investigation. 2014;124(12):5305–5316. doi: 10.1172/JCI77440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L. L., Béziat V., Oei V. Y. S., et al. Ex vivo expanded adaptive NK cells effectively kill primary acute lymphoblastic leukemia cells. Cancer Immunology Research. 2017;5(8):654–665. doi: 10.1158/2326-6066.CIR-16-0296. [DOI] [PubMed] [Google Scholar]
- 79.Romanski A., Bug G., Becker S., et al. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Experimental Hematology. 2005;33(3):344–352. doi: 10.1016/j.exphem.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Zhou J., Amran F. S., Kramski M., et al. An NK cell population lacking FcRγ is expanded in chronically infected HIV patients. The Journal of Immunology. 2015;194(10):4688–4697. doi: 10.4049/jimmunol.1402448. [DOI] [PubMed] [Google Scholar]
- 81.Warren H. S., Kinnear B. F. Quantitative analysis of the effect of CD16 ligation on human NK cell proliferation. The Journal of Immunology. 1999;162(2):735–742. [PubMed] [Google Scholar]
- 82.Lee H. R., Son C. H., Koh E. K., et al. Expansion of cytotoxic natural killer cells using irradiated autologous peripheral blood mononuclear cells and anti-CD16 antibody. Scientific Reports. 2017;7(1):p. 11075. doi: 10.1038/s41598-017-09259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cichocki F., Valamehr B., Bjordahl R., et al. GSK3 inhibition drives maturation of NK cells and enhances their antitumor activity. Cancer Research. 2017;77(20):5664–5675. doi: 10.1158/0008-5472.CAN-17-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oei V. Y. S., Siernicka M., Graczyk-Jarzynka A., et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunology Research. 2018;6(4):467–480. doi: 10.1158/2326-6066.CIR-17-0207. [DOI] [PubMed] [Google Scholar]
- 85.Abès R., Gélizé E., Fridman W. H., Teillaud J. L. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116(6):926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- 86.Pampena M. B., Levy E. M. Natural killer cells as helper cells in dendritic cell cancer vaccines. Frontiers in Immunology. 2015;6:p. 13. doi: 10.3389/fimmu.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]