Abstract
Background
Immune checkpoint inhibitors including nivolumab, an anti-programmed cell death protein 1 antibody, are recently developed cancer immunotherapy agents. Immune checkpoint inhibitors are known to cause autoimmune-related side effects including endocrine dysfunctions. However, there are few reports on late-onset isolated adrenocorticotropic hormone (ACTH) deficiency caused by nivolumab.
Case presentation
The patient was a 72-year-old female. When she was 64 years old, she was diagnosed with malignant melanoma of the left thigh accompanied by left inguinal lymph node metastases, and she received several courses of chemotherapy for malignant melanoma followed by the resection of these lesions. At 71 years of age, multiple metastases were found and treatment with nivolumab 2 mg/kg every 3 weeks was initiated. Six months later, replacement with levothyroxine was started because of hypothyroidism following mild transient thyrotoxicosis. Eleven months after the beginning of nivolumab, the treatment was discontinued because of tumor expansion. Four months after the discontinuation of nivolumab, general malaise and appetite loss worsened, and 2 months later, hyponatremia (Na; 120–127 mEq/L) and hypoglycemia (fasting plasma glucose; 62 mg/dL) appeared. Her ACTH and cortisol levels were extremely low (ACTH; 9.6 pg/mL, cortisol; undetectable). Challenge tests for anterior pituitary hormones showed that responses of ACTH and cortisol secretion to corticotropin-releasing hormone were disappeared, although responses of other anterior pituitary hormones were preserved. Thus, she was diagnosed with isolated ACTH deficiency. Her symptoms were improved after treatment with hydrocortisone.
Conclusions
The present report showed a case of late-onset isolated ACTH deficiency accompanied by hyponatremia, which was diagnosed 6 months after the discontinuation of nivolumab. The effects of nivolumab last for a long time and the side effects of nivolumab can also appear several months after discontinuation of the drug. Repeated monitoring of serum sodium levels may be a beneficial strategy to find the unexpected development of adrenal insufficiency even after discontinuation of nivolumab.
Keywords: Anti-programmed cell death protein 1 antibody, Anti-cytotoxic T-lymphocyte-associated protein 4 antibody, Nivolumab, Isolated adrenal deficiency, Thyroid dysfunctions
Background
Immune checkpoint inhibitors including ipilimumab and tremelimumab, anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies, and nivolumab and pembrolizumab, anti-programmed cell death protein 1 (PD-1) antibodies, are recently developed cancer immunotherapy agents which activate T lymphocytes and enhance immune responses to cancers [1–4]. In addition, these drugs also cause immune responses to some specific organs and cause immune-related adverse events such as colitis, rash, and hepatitis [5, 6]. Immune checkpoint inhibitors also cause endocrine dysfunctions such as thyroid dysfunctions [2, 7–9], hypophysitis [2, 9, 10], and type 1 diabetes [11–14] during its treatment period.
We report a case of isolated adrenocorticotropic hormone (ACTH) deficiency, which was diagnosed 6 months after the discontinuation of nivolumab, in a patient with malignant melanoma.
Case presentation
The patient was a 72-year-old female. When she was 64 years old, a poorly-marginated black legion was found in her left thigh, which was gradually enlarged. Three years after the appearance of the skin legion, skin biopsy was performed in our hospital and she was diagnosed with malignant melanoma. Positron emission computed tomography showed left inguinal lymph node metastases. She was treated with DAVFeron therapy (dacarbazine; 120 mg/m2/day at day 1–5, nimustine; 60 mg/m2/day at day 1, vincristine; 0.6 mg/m2/day at day 1, and interferon β; 3 million units/day at day 1–5), which was followed by resection of the skin legion and intra-pelvic lymph node dissection. At 71 years of age, liver metastases and intra-pelvic lymph node metastases appeared, thus treatment with nivolumab 2 mg/kg every 3 weeks was initiated (day X).
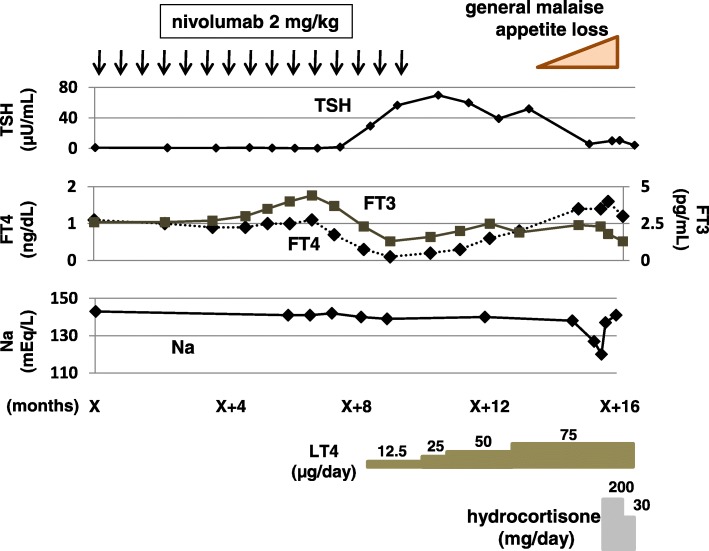
Six months after the day X, biochemical examination of blood revealed mild thyrotoxicosis, which did not need any medical treatment (Fig. 1). After that, hypothyroidism accompanied by general malaise appeared [thyroid-stimulating hormone (TSH); 29.3 μU/mL, free T3 (FT3); 2.3 pg/mL, and free T4 (FT4); 0.3 ng/dL] (Fig. 1). Anti-thyroperoxidase antibody and anti-thyroglobulin antibody were negative. She was diagnosed with primary hypothyroidism associated with nivolumab. Replacement with levothyroxine (LT4) was started, the dose was gradually increased to 75 μg/day, and thereafter her hypothyroidism was well-controlled (Fig. 1).
Fig. 1.
Clinical course and changes in the thyroid hormones, serum sodium levels
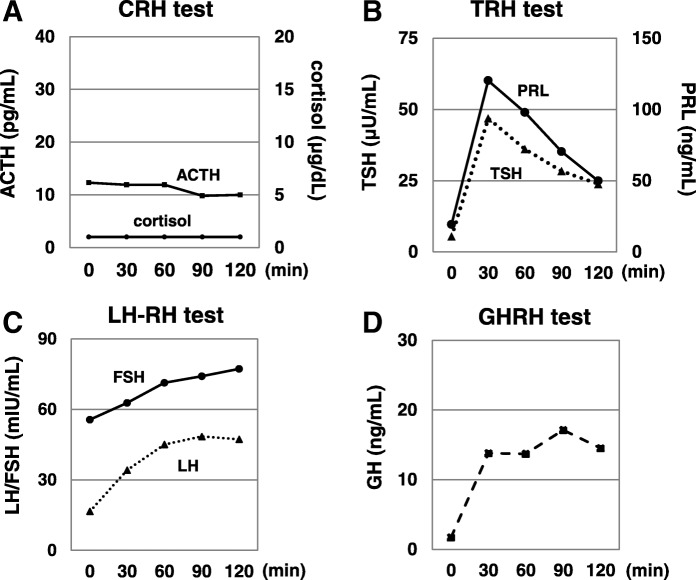
Eleven months after the day X, the treatment was discontinued because of expansion of liver metastases. After that, best supportive care was performed to her disease. Four months after the discontinuation of nivolumab, general malaise and appetite loss appeared. Two months later, she was admitted to our hospital because these symptoms were worsened, which were accompanied by hyponatremia (Na 120–127 mEq/L) and hypoglycemia (fasting plasma glucose 62 mg/dL). Her ACTH and cortisol levels were low (9.6 pg/mL and undetectable, respectively). Challenge tests were performed to examine the secretion of anterior pituitary hormones. The responses of ACTH and cortisol to corticotropin-releasing hormone were disappeared, although the responses of other anterior pituitary hormones were preserved (Fig. 2). Thereby, she was diagnosed with isolated ACTH deficiency. Any lesion to cause hypopituitarism was not observed in the brain including the hypothalamus and the pituitary gland by enhanced computed tomography and magnetic resonance imaging. Her symptoms, hyponatremia, and hypoglycemia were rapidly improved after replacement of hydrocortisone (Fig. 1).
Fig. 2.
Results of challenge tests for anterior pituitary hormones. PRL: prolactin; LH: luteinizing hormone; FSH: follicle-stimulating hormone; GH: growth hormone; CRH: corticotropin-releasing hormone; TRH: thyrotropin-releasing hormone; LH-RH: luteinizing hormone-releasing hormone; GHRH: growth hormone-releasing hormone
Discussion and conclusions
Anti-CTLA-4 antibodies and anti-PD-1 antibodies are immunotherapy agents to interfere with tumor cell growth and survival by activating immune responses of T cells to cancer cells by blockade of signals via CTLA-4 and PD-1, respectively [15]. However, these drugs can also cause autoimmune disorders such as endocrine dysfunctions including hypopituitarism [2, 9, 10] and thyroid dysfunctions [2, 7–9]. The frequencies of hypophysitis were 0.3% and 11–15% in patients treated with nivolumab (anti-PD-1 antibody) [2] and ipilimumab (anti-CTLA-4 antibody) [16–18], respectively. In addition, several case reports reported a relationship between treatment with nivolumab and isolated ACTH deficiency [19–24]. These findings suggests that administration of nivolumab was associated with the thyroid dysfunctions and the isolated ACTH deficiency in our patient.
A notable finding in this case was that isolated ACTH deficiency appeared 6 months after the discontinuation of nivolumab. One clinical study showed that tumor progression of malignant melanoma was prevented for at least 16 weeks after discontinuation of nivolumab in 12 out of 17 patients [25]. Furthermore, Kimura et al. reported that two times administration of nivolumab for non-small cell lung cancer progressively shrank the primary legion of the tumor for 6 months [26]. These reports indicate that the anti-tumor effects of nivolumab last even after the discontinuation of the drug. In addition, Teramoto et al. reported that fulminant type 1 diabetes mellitus occured 6 weeks after the discontinuation of nivolumab [27]. This observation suggests that immune-related side effects can also develop after discontinuation of immune checkpoint inhibitors and that careful observation for endocrine disorders is recommended even after discontinuation of these agents.
Monitoring of serum sodium levels might help to detect unexpected adrenal insufficiency in patients treated with nivolumab. General malaise and appetite loss is considered as signs of adrenal insufficiency. Indeed, these symptoms appeared 2 months before the development of hyponatremia in this case, suggesting that careful observation or medical check for latent adrenal insufficiency need to be started when those signs are observed. Hyponatremia is one of the important laboratory findings of overt adrenal deficiency. Cho et al. reported that serum sodium levels were clearly decreased in three of the four patients with nivolumab-associated isolated ACTH deficiency [23], indicating that measurement of serum sodium levels may be useful to find adrenal insufficiency in patients treated with immune checkpoint inhibitors in clinical practice. In addition, hypopituitarism occurred 12–36 weeks after the initiation of nivolumab [19–24] and endocrine impairment potentially develops after discontinuation of the drug. These findings suggest that repeated monitoring of serum sodium levels, at least in the situation after the appearance of general malaise and appetite loss, may be effective to find the development of adrenal insufficiency in patients treated with nivolumab.
In conclusion, the present report showed a case of nivolumab-induced late-onset isolated ACTH deficiency accompanied by hyponatremia. The effects of immune checkpoint inhibitors last for a long time. This case teaches us that repeated monitoring of serum sodium levels may be a beneficial strategy to find the unexpected development of adrenal insufficiency in patients treated with immune checkpoint inhibitors even after these agents are discontinued.
Acknowledgments
The authors thank the patient and her family for their participation.
Funding
This research did not receive any grants from any funding agencies.
Availability of data and materials
The data that support the findings of this study are stored in Shimane University Hospital (Shimane, Japan), and available from the corresponding author on reasonable request.
Abbreviations
- ACTH
adrenocorticotropic hormone
- CRH
corticotropin-releasing hormone
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- FSH
follicle-stimulating hormone
- FT3
free T3
- FT4
free T4
- GH
growth hormone
- GHRH
growth hormone-releasing hormone
- LH
luteinizing hormone
- LH-RH
luteinizing hormone-releasing hormone
- LT4
levothyroxine
- PD-1
programmed cell death protein 1
- PRL
prolactin
- TRH
thyrotropin-releasing hormone
- TSH
thyroid-stimulating hormone
Authors’ contributions
AT, IK and M Yamamoto interpreted the data and drafted the manuscript. TS revised the manuscript. AT, MM, ST, IK, SK, M Yamauchi, M Yamamoto and TS participated in the endocrinological treatment and collected the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication of this case report was obtained from the family of the patient after her death. A copy of the consent form is available for review by the editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayumu Takeno, Phone: +81-853-20-2183, Email: atakeno@med.shimane-u.ac.jp.
Masahiro Yamamoto, Email: masa-ya@med.shimane-u.ac.jp.
Miwa Morita, Email: miwaota@med.shimane-u.ac.jp.
Sayuri Tanaka, Email: s-tanaka@med.shimane-u.ac.jp.
Ippei Kanazawa, Email: ippei.k@med.shimane-u.ac.jp.
Mika Yamauchi, Email: yamauchi@med.shimane-u.ac.jp.
Sakae Kaneko, Email: kanekos2@med.shimane-u.ac.jp.
Toshitsugu Sugimoto, Email: sugimoto@med.shimane-u.ac.jp.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction Cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 6.Kahler KC, Hassel JC, Heinzerling L, Loquai C, Mossner R, Ugurel S, Zimmer L, Gutzmer R. Cutaneous side effects Committee of the Work Group Dermatological O: management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662–681. doi: 10.1111/ddg.13047. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka R, Fujisawa Y, Maruyama H, Nakamura Y, Yoshino K, Ohtsuka M, Fujimoto M. Nivolumab-induced thyroid dysfunction. Jpn J Clin Oncol. 2016;46(6):575–579. doi: 10.1093/jjco/hyw036. [DOI] [PubMed] [Google Scholar]
- 8.Narita T, Oiso N, Taketomo Y, Okahashi K, Yamauchi K, Sato M, Uchida S, Matsuda H, Kawada A. Serological aggravation of autoimmune thyroid disease in two cases receiving nivolumab. J Dermatol. 2016;43(2):210–214. doi: 10.1111/1346-8138.13028. [DOI] [PubMed] [Google Scholar]
- 9.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361–1375. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 10.Faje A. Hypophysitis: evaluation and management. Clin Diabetes Endocrinol. 2016;2:15. doi: 10.1186/s40842-016-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, Herold KC. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–e57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 13.Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, Hirsch IB. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38(9):e137–e138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7(6):915–918. doi: 10.1111/jdi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, Nachtigall L. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078–4085. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 17.Albarel F, Gaudy C, Castinetti F, Carre T, Morange I, Conte-Devolx B, Grob JJ, Brue T. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172(2):195–204. doi: 10.1530/EJE-14-0845. [DOI] [PubMed] [Google Scholar]
- 18.Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21(4):749–755. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okano Y, Satoh T, Horiguchi K, Toyoda M, Osaki A, Matsumoto S, Tomaru T, Nakajima Y, Ishii S, Ozawa A, et al. Nivolumab-induced hypophysitis in a patient with advanced malignant melanoma. Endocr J. 2016;63(10):905–912. doi: 10.1507/endocrj.EJ16-0161. [DOI] [PubMed] [Google Scholar]
- 20.Fujimura T, Kambayashi Y, Furudate S, Kakizaki A, Hidaka T, Haga T, Hashimoto A, Morimoto R, Aiba S. Isolated adrenocorticotropic hormone deficiency possibly caused by nivolumab in a metastatic melanoma patient. J Dermatol. 2017;44(3):e13–e14. doi: 10.1111/1346-8138.13532. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa M, Oashi K. Case of hypophysitis caused by nivolumab. J Dermatol. 2017;44(1):109–110. doi: 10.1111/1346-8138.13437. [DOI] [PubMed] [Google Scholar]
- 22.Kitajima K, Ashida K, Wada N, Suetsugu R, Takeichi Y, Sakamoto S, Uchi H, Matsushima T, Shiratsuchi M, Ohnaka K, et al. Isolated ACTH deficiency probably induced by autoimmune-related mechanism evoked with nivolumab. Jpn J Clin Oncol. 2017;47(5):463–466. doi: 10.1093/jjco/hyx018. [DOI] [PubMed] [Google Scholar]
- 23.Cho KY, Miyoshi H, Nakamura A, Kurita T, Atsumi T. Hyponatremia can be a powerful predictor of the development of isolated ACTH deficiency associated with nivolumab treatment [letter to the editor] Endocr J. 2017;64(2):235–236. doi: 10.1507/endocrj.EJ16-0596. [DOI] [PubMed] [Google Scholar]
- 24.Narahira A, Yanagi T, Cho KY, Nakamura A, Miyoshi H, Hata H, Imafuku K, Kitamura S, Shimizu H. Isolated adrenocorticotropic hormone deficiency associated with nivolumab therapy. J Dermatol. 2017;44(4):e70. doi: 10.1111/1346-8138.13571. [DOI] [PubMed] [Google Scholar]
- 25.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura H, Sone T, Murata A, Koba H, Tambo Y, Hara J, Abo M, Kasahara K. Long-lasting shrinkage in tumor mass after discontinuation of nivolumab treatment. Lung Cancer. 2017;108:7–8. doi: 10.1016/j.lungcan.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Teramoto Y, Nakamura Y, Asami Y, Imamura T, Takahira S, Nemoto M, Sakai G, Shimada A, Noda M, Yamamoto A. Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient. J Dermatol. 2017;44(5):605–606. doi: 10.1111/1346-8138.13486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are stored in Shimane University Hospital (Shimane, Japan), and available from the corresponding author on reasonable request.