Abstract
In ankylosing spondylitis (AS), structural damage that occurs as a result of syndesmophyte formation and ankylosis of the vertebral column is irreversible. Structural damage is currently assessed by conventional radiography and scoring systems that reliably assess radiographic structural damage are needed to capture the differential effects of drugs on structural damage progression. The validity of the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) as a primary outcome measure in evaluating the effect of AS treatments on radiographic progression rates was assessed in this review. The mSASSS has not been used, to date, as a primary outcome measure in a prospective randomized controlled clinical trial of biologic therapy in AS. This review of the medical literature confirmed that the mSASSS is the most validated and widely used method for assessing radiographic progression in AS, correlating with worsening measures of disease signs and symptoms, spinal mobility and physical function, with a 2-year interval being required to ensure sufficient sensitivity to change.
Keywords: ankylosing spondylitis, imaging, mSASSS, radiography, scoring, spondyloarthritis, radiograph
Rheumatology key messages
In AS, structural damage occurring because of syndesmophyte formation and ankylosis is irreversible.
Scoring systems that reliably assess radiographic structural damage in AS are needed to assess drugs’ effects.
The mSASSS is the most validated and widely used measure for assessing AS radiographic progression.
Background
AS is a chronic inflammatory disease that typically affects the axial skeleton and the entheses. The hallmark clinical manifestations of AS include inflammatory back pain and stiffness, with syndesmophytes and spinal ankylosis being the most characteristic features. Spinal ossification may lead to reduced physical function and quality of life. Disability (limitation of physical function and spinal mobility) related to disease activity or inflammation may be reversible, and controlling inflammation or disease activity may not only have direct effects on patient-reported outcomes but also prevent further progression of structural damage. Structural damage can cause permanent limitation in spinal mobility and physical function [1]. Thus, drugs that are effective in both treating spinal inflammation and protecting against radiographic progression may have a beneficial impact on long-term physical function.
The aim of this scoping review is to describe the validity of the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) as a primary outcome measure in evaluating the efficacy of treatments for AS in terms of structural damage progression; a systematic review was not conducted. In brief, a literature search was conducted in PubMed, using the key search terms AS, structural progression and associated terms intended to identify publications relating to radiographic outcome measures, such as the mSASSS, that are used to assesses the impact of treatment on radiographic structural progression in patients with AS. Additionally, authors made suggestions regarding pertinent publications for potential inclusion in the review. The search demonstrated that the mSASSS has been used in a large number of AS studies to assess structural damage progression on spinal radiographs with different therapeutic interventions, including NSAIDs and biologic agents, in either retrospective cohort comparisons, prospective controlled trials of non-biologic therapies or prospective trials of biologic agents with no controlled comparison for long-term radiographic endpoints. However, the mSASSS has never been used as a primary outcome measure in a prospective randomized clinical study of a biologic therapy that includes a comparator.
Natural history and disease progression
Syndesmophyte formation and ankylosis of the vertebral column are pathognomonic structural changes in AS and are currently best visualized by conventional radiography of the axial skeleton, including the SI joints and the whole vertebral spine [2, 3]. Osteodestructive changes occur less frequently and may include erosions [2]. Erosions and sclerosis are early signs of radiographic progression that may precede the development of syndesmophytes, which can eventually grow and bridge adjacent vertebrae, ultimately leading to spinal fusion or the characteristic bamboo spine in some patients [3, 4].
It has been hypothesized that progression of AS, at least in part, involves inflammation that progresses to fatty degeneration, as assessed by MRI, and ultimately to new bone formation in the form of syndesmophytes [5–7]. The link between baseline levels of inflammation and the development of structural damage in the SI joints is supported by a study that explored the risk factors for progression of non-radiographic to radiographic axial SpA [8]. Longitudinal analysis of 12-year data from the Outcome in Ankylosing Spondylitis International Study (OASIS) cohort, which found that disease activity assessed by the Ankylosing Spondylitis Disease Activity Score (ASDAS) was longitudinally associated with radiographic progression, provides the strongest evidence to date that inflammation leads to new bone formation [9].
Radiographic scoring methods in AS
A number of scoring methods are available for assessing radiographic damage in AS. These include the BASRI, a grading system that evaluates radiographs of the anteroposterior view of the pelvis, the anteroposterior and lateral views of the lumbar spine and the lateral view of the cervical spine [10]; the Stoke Ankylosing Spondylitis Spinal Score (SASSS) that evaluates posterior and anterior corners of the lumbar spine for erosions, sclerosis, squaring, syndesmophytes and total bony bridging [11]; the mSASSS, which is a modification of the SASSS and evaluates only the anterior edges of both the lumbar and cervical spine on a lateral view [12]; and the Radiographic Ankylosing Spondylitis Spinal Score (RASSS), a newer scoring method that includes vertebral segments assessed in the mSASSS and additionally the lower vertebrae of the thoracic spine (T10–T12), scoring structural damage entirely based on new bone formation [13].
A limitation of all the available radiographic scoring systems may be the inability to assess involvement of the facet joints [14, 15]. Furthermore, the thoracic spine is not assessed using the BASRI, SASSS or mSASSS scoring methods; this is largely due to technical reasons, such as superimposition of the lungs on the thoracic vertebrae in plain radiographs [10, 11]. As MRI studies have shown the lower half of the thoracic spine to be most frequently affected by active inflammation and structural changes in patients with AS [16], the sensitivity of these scoring systems is limited by the fact that radiographic changes in the thoracic spine are not measured. The RASSS scoring method tried to overcome this limitation by adding the three lower thoracic vertebrae to the score, and the original study describing the RASSS demonstrated that this method had an increased sensitivity to change, compared with mSASSS [13]. However, another study comparing the performance of the RASSS to the mSASSS concluded that the contribution of the thoracic spine was negligible and did not warrant the additional time that is required in scoring, especially given that thoracic spine radiographs are technically challenging to obtain routinely in the clinical setting and may not be of the quality needed for adequate assessment in a clinical trial [17].
A study comparing three of these methods (i.e. BASRI, SASSS and mSASSS) concluded that mSASSS is the most appropriate method by which to score radiographic progression in AS clinical trials. The BASRI and SASSS had lower sensitivity to change relative to mSASSS, and while the BASRI had the advantage of reduced scoring time, it yielded the highest radiation exposure to patients and lowest sensitivity to change [18]. Consequently, the Assessment of SpondyloArthritis international Society and OMERACT group have endorsed the mSASSS as the preferred scoring method for radiographic progression in AS [18, 19].
More recently, a published study of 98 patients with AS assessed a novel scoring method—the Combined Ankylosing Spondylitis Spine Score, which evaluates all vertebral bodies captured in the mSASSS along with the cervical facet joints from C2 to C7. Based on the OMERACT filter, the Combined Ankylosing Spondylitis Spine Score performed similarly to the mSASSS with respect to feasibility and discrimination, while demonstrating greater truth value (i.e. Is the measure truthful, and does it measure what is intended? How valid is the measure?) because of its ability to assess a broader range of structural changes and to capture an increased number of AS patients with progression [20].
Scoring of the mSASSS
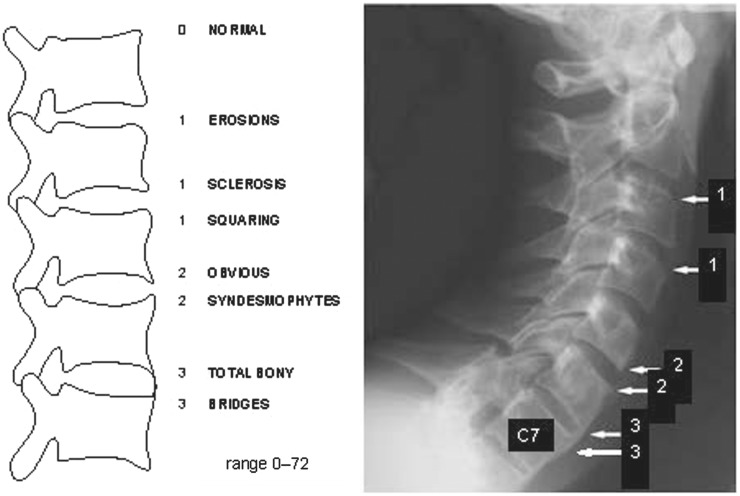
The mSASSS is a well-validated scoring method for quantifying chronic structural changes on conventional radiographs. The methodology originally described by Creemers et al. recommended scoring each upper and lower vertebral edge as follows (see sample diagram in Fig. 1): 0 = no abnormality; 1 = erosion and/or sclerosis and/or squaring; 2 = syndesmophyte (non-bridging); 3 = total bony bridging between upper and lower vertebral edges (ankylosis), with the exception that the third cervical vertebra C3 is not scored for squaring. The total mSASSS (range: 0–72) is the sum of scores calculated for the 24 vertebral edges included in the lumbar and cervical scoring system, based on lateral radiographic views of the vertebrae [12].
Fig. 1.
Sample mSASSS scoring (from 0 to 3) for spinal vertebral edges
Reproduced from imaging of axial spondyloarthritis including ankylosing spondylitis, J Braun & X Baraliakos, Ann Rheum Dis 70(Suppl 1): i97–i103, 2011. With permission from BMJ Publishing Group Ltd.
Progression of structural damage in AS patients may be presented as the absolute change in mSASSS, the proportion of patients with progression exceeding a particular cut-off value [e.g. an increase in mSASSS of >0 or >0.5 U if using the mean scores of two readers, or an increase above the smallest detectable change (SDC)] [21], or the proportion of patients with new syndesmophyte(s). It is important to present the data in cumulative probability plots to show the coherence of the data and to take the negative changes into account [22]. A 2-year interval has been established as the shortest follow-up time to detect progression in an acceptable number of patients based on the reliability and sensitivity to change of the mSASSS [19, 23], although some research has advocated an interval as short as 1 year [12].
Scoring radiographs in AS clinical trials
To avoid reader bias, readers should be blinded as to the patient, time point and treatment assignment. Typically, two or more independent readers evaluate radiographs separately, with an average score used in the analysis. Films may be grouped for each patient but presented with readers blinded to the time point of the radiographs (paired scoring) or in chronological order of the radiographs. While research has suggested that reading films chronologically increases the ability to detect changes in comparison with paired reading, this method has the potential to result in an over-estimation of progression based on the readers’ expectation [24]. It is questionable whether this is a disadvantage, however, provided that readers remain blinded to patient and treatment information. Adjudication of discrepant change scores between two readers by a third reader is a method commonly used to reduce variability of the final overall mSASSS. An even better alternative would be to use three blinded readers, which increases the precision and obviates the need for adjudication.
A percentage of the radiographs may also be scored a second time by the same reader for use in determining intra-rater variability, while inter-reader variability may be determined from the two readers’ scores of all radiographs. The reliability of scoring is assessed by calculating inter- and intra-observer correlations by the intraclass correlation coefficient; additional information may be obtained by calculating the SDC and using Bland and Altman analysis on the progression intervals of mSASSS [25, 26].
The SDC represents the smallest change that can be detected beyond measurement error and is calculated as SDC = (1.96 * s.d.diff)/(√k * √2), where s.d. diff is the standard deviation (s.d.) of the set of differences in change scores of two readers and k is the number of readers whose change scores are used [21]. The SDC can be used to determine whether the progression in an individual patient is larger than measurement error. The SDC values reported from studies that scored radiographs in a blinded manner range from 1.8 to 2.8 mSASSS units [2, 27]. Two studies that scored radiographs in chronological order reported SDC values of 2.3 and 2.9 U [26, 28]. An increase of 2 U may reflect the formation of at least one new syndesmophyte at any vertebral edge or the development of erosions, sclerosis, and/or squaring in at least two vertebral edges [12].
Use of mSASSS as an outcome measure in clinical studies
Radiographic damage in AS generally takes several years to progress [23]. Highly variable rates of radiographic structural progression have been identified in individuals with AS [26, 29]. One longitudinal study of AS patients naïve to TNF inhibitors that scored radiographs in chronological order reported that ∼25% of patients showed no progression, 25% showed a high level of progression (at least one 2-year interval with progression of ⩾5 mSASSS units) and the remaining patients showed progression rates of ∼2 mSASSS units over 2 years [26]. A constant rate of 0.98 mSASSS units/year was reported over the 12-year study period, with 60% of all AS patients developing at least one new syndesmophyte over that timeframe [26]. Another longitudinal study of the Groningen Leeuwarden AS cohort that includes TNF inhibitor-treated patients with severe AS and that also scored radiographs in chronological order estimated a mean progression rate of 1.7, 1.6 and 1.1 mSASSS units during time periods of 0–2, 2–4 and 4–6 years, respectively [30].
At a group level, mean changes in mSASSS from baseline to year 2 that have been reported in studies using a blinded approach to scoring radiographs range from 0.2 to 1.5 U in NSAID-treated AS patients [31–34] and 0.4–1.3 U in TNF inhibitor-treated patients (Table 1) [28, 34–38]. In a separate study that used a blinded approach to scoring radiographs and stratified patients according to whether they had syndesmophytes at baseline, the mSASSS from baseline to year 2 was 2.6 U in patients with syndesmophytes compared with 0.8 in patients without [2]. Using a chronological approach to reading radiographs, a mean change in mSASSS from baseline to year 2 of 1.6 has been reported for TNF inhibitor-treated patients [30].
Table 1.
Summary of mSASSS changes after 2 years of treatment in studies assessing effects on progression of radiographic damage in patients with AS
| Clinical Study | Treatment/cohort | Baseline characteristics | Radiograph scoring approach | SDC, mSASSS units | % patients as reported with progression or non-progression (mSASSS cutoff, units) at year 2 | Mean change in mSASSS from baseline to year 2, mean (s.d.) or 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Number of patientsa | Time since diagnosis, yearsb, mean (s.d.) or median (IQR) | mSASSS value, mean (s.d.) | ||||||
| NSAIDs Randomized trials | ||||||||
|
Diclofenac (on-demand) | 60 | 17.0 (12.6)* | 16.4 (18.2) | Blinded | NR | Non-progression (≤0 U) in ∼55% of both treatment groupsc | 0.8 (0.2, 1.4) |
| Diclofenac (continuous) | 62 | 12.8 (11.3) | 10.9 (15.5) | 1.3 (0.7, 1.9) | ||||
| 2-year RCT (2005) [31] and post hoc analysis (2012) [40] | Celecoxibd (on-demand) | 74 | 10.2 (9.3) | 9.3 (15.2) | Blinded | NR |
|
1.5 (2.5)* |
| Celecoxibd (continuous) | 76 | 13.0 (10.2) | 7.9 (14.7) | 0.4 (1.7) | ||||
| Observational studies | ||||||||
|
Low intake NSAID (NSAID index <50) | 64 | 5.0 (2.9) | 5.7 (11.6) | Blinded | NR | Progression (≥2 U) in 8.3% (high intake NSAID) vs 21.9% (low intake NSAID) of AS patients | 1.0 (2.8)* |
| High intake NSAID (NSAID index ≥50) | 24 | 5.5 (2.7) | 6.7 (7.7) | 0.02 (1.39) | ||||
| Analysis of German cohorts, including GESPIC, naïve/not naïve to TNF inhibitors (GESPIC; 2007) [2] | German cohorts | 116 | 11.0 (8.2) | 9.3 (14.0) | Blinded | 2.0 |
|
2.6 (4.0) in patients with syndesmophyte at BL vs 0.8 (1.4) in patients without* |
| TNF inhibitors Randomized trials (without radiographic control arm) | ||||||||
|
Golimumab 50 mg | 111 | 5.2 (1.6– 11.6)e | 11.7 (16.4) | Blinded | NR | Progression (≥2 U) in 26.1% of golimumab 50 mg group vs 28.7% of golimumab 100 mg group at 4 yearsf | 0.9 (2.7) |
| Golimumab 100 mg | 122 | 5.2 (1.5–13.3)e | 13.5 (18.9) | 0.9 (3.9) | ||||
| Historical control cohort comparison | ||||||||
| Comparison of 2-year pooled data from the ATLAS study (NCT00085644) and a Canadian AS study (M03-606; NCT00195819) vs an historic anti-TNF-naïve cohort (2009) [37] | Adalimumab | 307 | 11.2 (9.3) | 19.8 (19.3)* | Blinded | NR | Non-progression (≤0 U) in ∼55% of adalimumab group vs ∼58% of OASIS cohorte | 0.8 (2.6) |
| OASIS | 169 | 11.3 (8.7) | 15.8 (17.6)* | 0.9 (3.3) | ||||
| Comparison of 2-year data from RCT (NCT00356356) vs an historical cohort naïve to TNF inhibitors (2008) [34] | Etanercept | 257 | 10.0 (8.5) | 16.0 (18.3) | Blinded | NR | Non-progression (≤0 U) in ∼55% of etanercept group vs ∼55% of OASIS cohortc | 0.9 (2.5) |
| OASIS | 175 | 11.0 (8.5) | 14.0 (17.6) | 1.0 (3.2) | ||||
| Comparison of 2-year data from ASSERT, a double-blind RCT, vs an historic cohort naïve to TNF inhibitors (2008) [36] | Infliximab | 156 | 10.2 (8.7) | 17.7 (17.9) | Blinded | NR | Progression in infliximab group vs OASIS cohort:
|
0.9 (2.6) |
| OASIS | 165 | 11.3 (8.6) | 15.8 (18.1) | 1.0 (3.2) | ||||
| Comparison of 2-year data from RCT vs a cohort naïve to TNF inhibitors (GESPIC; 2005) [38] | Infliximab | 41 | 15.5 (3–35)e,* | 12.2* | Blinded | NR | Progression (≥1 U) in 17% of infliximab group vs 12% of GESPIC cohort | 0.4 (2.7) |
| GESPIC | 41 | 5.5 (1–10)e,* | 5.9* | 0.7 (2.8) | ||||
| Observational studies | ||||||||
| Infliximab, etanercept, or adalimumab | 163 | 6 (1–15)g | 10.0 (15.5)h | Chronological order | 2.3 | Progression (>SDC) in 25% | 1.6 (2.8) | |
| Infliximab, etanercept, or adalimumab | 176 | 5 (1–14)e | 11 (5–24)e | Blinded | NR | Progression:
|
1.3 (3.2) | |
| IL-17 inhibitor | ||||||||
| Randomized trials (without radiographic control arm) | ||||||||
|
Secukinumab iv-75 mg | 82 | 7.8 (8.9) | 10.8 (16.7) | Blinded | 2.8 | Non-progression (mSASSS<SDC) in >80% of AS patientsi | 0.3 (3.0) |
| Secukinumab iv-150 mg | 86 | 6.6 (7.0) | 9.6 (16.6) | 0.3 (1.9) | ||||
Significant differences (P < 0.05) between treatment groups (after adjustment for radiographic status at baseline in GESPIC).
Number of patients with baseline and year 2 radiographic images.
Data for TNF inhibitors, with the exception of golimumab, are disease duration in years.
Visual estimation based on cumulative probability plots.
Patients who discontinued celecoxib (due to inefficiency or adverse event) were allowed to continue the study with any other NSAID, but were instructed to maintain the allocated dosing strategy (on-demand or continuous treatment only); ∼73% of patients in both groups used celecoxib during the entire study period. eValues are median (interquartile range).
Data unavailable at 2 years.
A different mSASSS scoring approach was performed in these studies using patients from the GLAS cohort.
Baseline data for all patients in study (n = 210).
As defined by SDC at 80% level of agreement (i.e. 1.838).
ASSERT: Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy; ATLAS: Adalimumab Trial Evaluating Long-Term Efficacy and Safety for Ankylosing Spondylitis; BL: baseline; GESPIC: German AS cohort (GESPIC); GLAS: Groningen Leeuwarden AS; IQR: interquartile range; mSASSS: modified Stoke Ankylosing Spondylitis Spinal Score; NR: not reported; OASIS: Outcome in Ankylosing Spondylitis International Study; OR: odds ratio; RCT: randomized controlled trial; SDC: smallest detectable change.
The mean change in mSASSS may serve as a primary end point measure in randomized and controlled prospective studies comparing different interventions, but to date this has not been performed. There is no universally agreed upon mSASSS cut-off value defining radiographic progression and it is debatable whether choosing a high or low mSASSS threshold is preferable. While a number of studies have defined progression as an increase of ⩾2 mSASSS units over 2 years [33, 35, 39], other studies have defined radiographic progression as any change in mSASSS of >0 U over time [31, 37]. Spinal radiographic progression over 2 years has been further classified, according to Baraliakos et al. [29], as slow (<2 mSASSS units), moderate (2–5 mSASSS units) or fast (>5 mSASSS units). Table 1 provides a summary of mSASSS changes after 2 years of treatment with NSAIDs and biologic therapies in studies assessing effects on progression of radiographic damage in patients with AS.
Nonsteroidal anti-inflammatory drugs and structural progression
The mSASSS has been used as an outcome measure to evaluate the ability of NSAIDs and biologic agents to inhibit structural progression in AS, with varying results (Table 1). There is some evidence from a randomized controlled trial (RCT) to suggest a potential benefit of NSAIDs in preventing structural progression in AS, with continuous use of celecoxib having an inhibitory effect on structural progression over a 2-year time period [31], although data from a more recent RCT with diclofenac failed to corroborate this [32]. Post hoc analysis of the celecoxib trial demonstrated that patients with elevated vs normal levels of acute-phase reactants, including CRP, and ESR saw the most benefit from treatment with continuous celecoxib, in terms of radiographic progression [40]. Analysis of the German Spondyloarthritis Inception Cohort of patients with AS also provides support for NSAIDs retarding radiographic spinal progression, with high NSAID intake being associated with lower mSASSS progression over 2 years, as compared with low NSAID intake, particularly in those with syndesmophytes or abnormal CRP levels at baseline [41].
TNF-inhibitors and structural progression
While TNF-inhibitors have been reported to improve the signs and symptoms of AS [42], there are no data available to date from prospectively controlled and randomized studies to demonstrate a reduction in spinal radiographic progression in AS with anti-TNF treatment (Table 1). It is not considered ethical to expose patients with AS to ineffective treatment in a 2-year placebo-controlled trial. Thus, data on radiographic progression with different anti-TNF inhibitors have been compared with data from historical control cohorts, such as OASIS, whose participants are naïve to treatment with biologic agents. These historically controlled cohort comparisons have shown no significant benefit of anti-TNF therapies on structural progression after 2 years (Table 1) [34, 36, 37].
Multiple observational studies have suggested that TNF inhibitors have either no effect or a possible effect on structural progression; however, it is difficult to draw firm conclusions from these studies, since a comparison with similarly active but untreated patients was lacking [30, 35, 41]. Data from prospective longitudinal studies suggest that TNF inhibitors may have a delayed beneficial effect on, or deceleration of, radiographic progression, but it is unknown if this effect is attributable to confounding factors that may influence radiographic progression. These studies were observational and include follow-up data from the Groningen Leeuwarden AS cohort out to 8 years [30], a North American cohort out to 9 years [range1.5–9 years; mean 2.87 (1.17)] [43], and from extension studies out to 4 years (GO-RAISE, RAPID-axSpA) [35, 44]. The findings of one such study [43] have been criticized over methodological concerns [45]. Two recent studies, one of which has been published as an abstract, adjusted for confounding by indication and provide circumstantial evidence that TNF inhibitors may have some inhibitory effect on radiographic progression; this effect appears to be mediated by reducing disease activity [46, 47].
IL-17A inhibitors
Recent radiographic results of biologic treatment that blocked a different cytokine pathway in patients with AS have been reported from the MEASURE 1 study, a randomized trial of secukinumab (an IL-17A inhibitor). Two-year data from the trial demonstrated a mean change in mSASSS of 0.3 U [27]. However, it is difficult to interpret the significance of the finding and the findings from other similarly designed studies of TNF inhibitors, due to the uncontrolled nature of the studies.
Risk factors for radiographic progression in AS
The most widely studied factor in relation to radiographic progression is the level of inflammation. An association between radiographic progression and higher baseline CRP levels has been demonstrated in several shorter-term studies [9, 33, 40]. It is only recently that a significant longitudinal association could be demonstrated between radiographic progression and disease activity in AS, with ASDAS being the most significant measure, in a 12-year follow-up of the OASIS cohort [9]. This was confirmed in an analysis of the German Spondyloarthritis Inception Cohort cohort [48]. However, the evidence for BASDAI alone as a risk factor for structural damage progression has been less consistent, with one study demonstrating an association [10], while others have not [33, 49].
Several baseline risk factors have been associated with the formation of new bone in the spine of patients with AS, the most significant of which appears to be the presence of radiographic damage at baseline, as assessed by mSASSS or by the number of syndesmophytes [9, 28, 29]. Results of a retrospective cohort study showed syndesmophyte formation in 44% of patients with radiographically detectable damage (syndesmophytes or ankylosis) at baseline, compared with only 19% in patients without syndesmophytes at baseline [2]. A more recent prospective longitudinal cohort study indicated the risk of radiographic progression was 4-fold higher in patients with pre-existing syndesmophytes [28].
The presence of fatty lesions on MRI is also reported to be an important risk factor in the development of structural damage [5–7].
Younger age and shorter symptom duration have also been established as risk factors for radiographic progression [9]. Other risk factors for structural damage progression include male gender, smoking and having a physically demanding job [9, 26, 28, 43, 50].
When evaluating spinal radiographic progression, the risk factors above need to be borne in mind, as analyses should be adjusted for patient characteristics that have the potential to influence radiographic progression [30].
Relationship between mSASSS and spinal mobility/physical function in AS
According to the stratified model of health outcomes published by Machado et al. [51], spinal mobility is determined by structural damage and inflammation of the spine, while physical function is determined by spinal mobility and disease activity.
An association between structural damage, as measured using mSASSS, and impaired spinal mobility, as assessed by multiple disease outcomes, has been established at a group level in several studies (supplementary Table S1, available at Rheumatology online); however, at an individual patient level, the relationship between spinal radiographic findings and spinal mobility is variable [52–55]. An analysis of the Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy cohort demonstrated that spinal mobility impairment in AS is independently determined both by reversible spinal inflammation, as measured by MRI, and irreversible spinal damage, as measured by mSASSS [56].
Analysis of the OASIS cohort has demonstrated that functional impairment in AS is independently associated with both patient-reported signs and symptoms of disease activity and the degree of structural damage in both the cervical and lumbar spine, as assessed by the mSASSS [9]. Radiographic abnormalities including syndesmophytes, in addition to erosions, sclerosis and squaring of vertebral edges, were found to contribute to impaired physical function [1].
Other imaging techniques to monitor structural disease progression in AS
Although less validated, other imaging techniques can also prove useful in monitoring changes in AS patients (disease activity and/or structural damage), including MRI and CT; the strengths and weaknesses of these techniques are summarized in Table 2. MRI can be used to detect inflammatory changes in the spine [57, 58] and has been compared with radiography in the assessment of chronic structural changes in the spine [59] and SI joints [60]. There are several emerging MRI techniques that may prove useful in assessing mineralized bone and subsequent new bone formation; for example, using chemical shift-encoded MRI to map transverse relaxation rate (R2*) as a potential marker of bone marrow composition and structure [61].
Table 2.
Strength and weaknesses of spinal imaging techniques in patients with AS
| Imaging technique | Strengths | Weaknesses |
|---|---|---|
| Conventional radiography [3, 57] |
|
|
| MRI [3, 57] |
|
|
| CT [3, 57] |
|
|
CT provides a sensitive method for assessing structural changes in the spine and has the advantage over conventional radiography of being able to detect structural changes in the thoracic spine [62]. At present, conventional radiography is considered the preferred method for detecting structural progression in AS [57], but this may change in the near future with the use of low-dose CT scanning, which may offer a more sensitive means than conventional radiography of assessing structural damage. This modality has the increased sensitivity of CT for structural changes in the spine, with a 10-fold lower radiation dose than conventional CT and, thus, has the potential for being more sensitive than conventional radiography in detecting structural changes in AS. However, the radiation dose is still 10-fold higher than with conventional radiographs, but with new imaging software this may be reduced. Future studies comparing assessments with low-dose CT vs mSASSS would be of interest.
A number of spine scoring systems have been developed for use with MRI in AS, such as the Ankylosing Spondylitis spine Magnetic Resonance Imaging-activity score, the Berlin modification of the Ankylosing Spondylitis spine Magnetic Resonance Imaging-activity and the Spondyloarthritis Research Consortium of Canada score, all of which assess spinal inflammation [63–65], as well as the Canada-Denmark scoring system, which can also be used to assess structural changes on MRI [66]. The CT syndesmophyte scoring system is a validated method to assess bone formation on spinal CT scans of the spine [67]. Use of the currently available imaging measures to monitor structural damage progression may be challenging in clinical practice due to the paucity of experienced readers; thus, development of an automated means of assessing spinal structural damage may be of value.
Conclusions
Interventions that slow or halt the progression of irreversible structural damage in AS are expected to confer clinical benefits in terms of delaying loss of function and improving quality of life. Preventing the progression of structural damage in the spine is, therefore, an important goal in the treatment of AS [45]. This review explores the validity of the mSASSS as a primary outcome measure to evaluate the efficacy of treatments for AS in terms of structural damage progression.
A scoping review of the literature showed that there are a number of scoring methods available for assessing radiographic damage in AS, although the advantages of the mSASSS over other scoring methods such as the BASRI and SASSS to measure radiographic progression in AS clinical trials have been noted [18]. Moreover, multiple studies have demonstrated the association between the mSASSS and worsening measures of AS signs and symptoms, spinal mobility and physical function [9]. The inability of the mSASSS (and, similarly, the BASRI and SASSS) to monitor radiographic changes in the thoracic and other parts of the spine is a limitation that restricts sensitivity [38]. Nonetheless, the mSASSS is deemed the preferred scoring system of spinal radiographs in comparison to the newer RASSS method, which includes the thoracic spine [17], and the mSASSS has been endorsed by OMERACT and Assessment of SpondyloArthritis international Society experts as an appropriate clinical research outcome measure, given its reliability, sensitivity to change and proven feasibility for use [19]. Although other imaging techniques such as MRI and CT can prove useful in detecting structural damage in patients with AS, and while several years are required to detect structural changes by conventional radiography, X-ray assessments are the most widely validated modality for monitoring structural progression in AS. Comparisons of structural assessments using X-rays to other imaging modalities, such as low-dose CT, would be of interest.
The mSASSS has never been used as a primary outcome measure in prospective, controlled and randomized clinical trials of biologics with a comparator arm out to 2 years. Despite the large number of completed and ongoing studies in AS that have used the mSASSS, there are no data from prospective studies that provide a long-term controlled comparison to confirm that treatment with any biologic is associated with a reduction in spinal structural progression in patients with AS [34, 36–38]. Therefore, well-designed head-to-head studies are needed to establish the role of biologic therapies in slowing the progression of structural damage in AS, for which the mSASSS can be used as a primary outcome measure.
Supplementary Material
Acknowledgements
The authors thank Aisling O’Keeffe, PhD of Novartis Ireland Ltd, Dublin, Ireland for providing medical writing support, which was funded by Novartis, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). D.vd.H., J.B., A.D., X.B., R.L., H.B.R., B.P. and A.R. participated in the development of the manuscript, including advising on which papers should be reviewed and cited. All authors agreed on the content, reviewed drafts and approved the final version.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: D.vd.H. has been a consultant for AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb (BMS), Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB and is an employee of Imaging Rheumatology BV. J.B. has received grant/research support from Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB, has been a consultant for Abbvie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB and received speakers fees from AbbVie (Abbott), Amgen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB. A.D. has received grant/research support from Eli Lilly, GSK, Janssen, Novartis, Pfizer, Sun Pharma, UCB and has been a consultant for AbbVie, Eli Lilly, GlaxoSmithKline (GSK), Janssen, Novartis, Pfizer, UCB. X.B. has received grant/research support from AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, Werfen, been a consultant for AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, Werfen, received speakers fees from AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, UCB, Werfen. R.L. has received grant/research support from Abbvie (Abbott), Amgen, Centocor, Novartis, Pfizer, Roche, Schering, UCB, been a consultant for Abbvie (Abbott), Ablynx, Amgen, Astra-Zeneca, Bristol Myers Squibb, Celgene, Eli-Lilly, Janssen, Gilead, Galapagos, Glaxo-Smith-Kline, Novartis, Novo Nordisk, Merck, Pfizer, Roche, Schering, TiGenix, UCB, is an employee of Rheumatology Consultancy BV and received speakers fees from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Merck, Pfizer, Roche, Schering, UCB. H.B.R. is an employee of Novartis Pharma AG and a shareholder of Novartis. B.P. and A.R. are employees of Novartis Pharmaceuticals Corporation and shareholders of Novartis.
References
- 1. Landewé R, Dougados M, Mielants H, van der Tempel H, van der Heijde D.. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863–7. [DOI] [PubMed] [Google Scholar]
- 2. Baraliakos X, Listing J, Rudwaleit M. et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007;66:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostergaard M, Lambert RG.. Imaging in ankylosing spondylitis. Ther Adv Musculoskelet Dis 2012;4:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramiro S, van Tubergen A, van der Heijde D. et al. Brief report: erosions and sclerosis on radiographs precede the subsequent development of syndesmophytes at the same site: a twelve-year prospective followup of patients with ankylosing spondylitis. Arthritis Rheumatol 2014;66:2773–9. [DOI] [PubMed] [Google Scholar]
- 5. Baraliakos X, Heldmann F, Callhoff J. et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis 2014;73:1819–25. [DOI] [PubMed] [Google Scholar]
- 6. Maksymowych WP, Morency N, Conner-Spady B, Lambert RG.. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 2013;72:23–8. [DOI] [PubMed] [Google Scholar]
- 7. Machado PM, Baraliakos X, van der Heijde D, Braun J, Landewé R.. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016;75:1486–93. [DOI] [PubMed] [Google Scholar]
- 8. Dougados M, Sepriano A, Molto A. et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramiro S, van der Heijde D, van Tubergen A. et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 10. MacKay K, Mack C, Brophy S, Calin A.. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum 1998;41:2263–70. [DOI] [PubMed] [Google Scholar]
- 11. Averns HL, Oxtoby J, Taylor HG. et al. Radiological outcome in ankylosing spondylitis: use of the Stoke Ankylosing Spondylitis Spine Score (SASSS). Br J Rheumatol 1996;35:373–6. [DOI] [PubMed] [Google Scholar]
- 12. Creemers MC, Franssen MJ, van't Hof MA. et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun J.. Development of a radiographic scoring tool for ankylosing spondylitis only based on bone formation: addition of the thoracic spine improves sensitivity to change. Arthritis Rheum 2009;61:764–71. [DOI] [PubMed] [Google Scholar]
- 14. Mahmoud I, Gafsi L, Saidane O. et al. Limit of the available spine radiologic scoring methods in ankylosing spondylitis when the facet joint is the only structure involved. Egyptian Rheumatol 2016;38:203–7. [Google Scholar]
- 15. de Vlam K, Mielants H, Veys EM.. Involvement of the zygapophyseal joint in ankylosing spondylitis: relation to the bridging syndesmophyte. J Rheumatol 1999;26:1738–45. [PubMed] [Google Scholar]
- 16. Baraliakos X, Landewé R, Hermann KG. et al. Inflammation in ankylosing spondylitis: a systematic description of the extent and frequency of acute spinal changes using magnetic resonance imaging. Ann Rheum Dis 2005;64:730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramiro S, van Tubergen A, Stolwijk C. et al. Scoring radiographic progression in ankylosing spondylitis: should we use the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) or the Radiographic Ankylosing Spondylitis Spinal Score (RASSS)? Arthritis Res Ther 2013;15:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wanders AJ, Landewé RB, Spoorenberg A. et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 2004;50:2622–32. [DOI] [PubMed] [Google Scholar]
- 19. van der Heijde D, Landewe R.. Selection of a method for scoring radiographs for ankylosing spondylitis clinical trials, by the Assessment in Ankylosing Spondylitis Working Group and OMERACT. J Rheumatol 2005;32:2048–9. [PubMed] [Google Scholar]
- 20. Maas F, Arends S, Brouwer E. et al. Incorporating assessment of the cervical facet joints in the modified Stoke ankylosing spondylitis spine score is of additional value in the evaluation of spinal radiographic outcome in ankylosing spondylitis. Arthritis Res Ther 2017;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruynesteyn K, Boers M, Kostense P, van der Linden S, van der Heijde D.. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis 2005;64:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landewé R, van der Heijde D.. Radiographic progression depicted by probability plots: presenting data with optimal use of individual values. Arthritis Rheum 2004;50:699–706. [DOI] [PubMed] [Google Scholar]
- 23. Spoorenberg A, de Vlam K, van der Linden S. et al. Radiological scoring methods in ankylosing spondylitis. Reliability and change over 1 and 2 years. J Rheumatol 2004;31:125–32. [PubMed] [Google Scholar]
- 24. Wanders A, Landewé R, Spoorenberg A. et al. Scoring of radiographic progression in randomised clinical trials in ankylosing spondylitis: a preference for paired reading order. Ann Rheum Dis 2004;63:1601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 26. Ramiro S, Stolwijk C, van Tubergen A. et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015;74:52–9. [DOI] [PubMed] [Google Scholar]
- 27. Braun J, Baraliakos X, Deodhar A. et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2017;76:1070–7. [DOI] [PubMed] [Google Scholar]
- 28. Maas F, Spoorenberg A, Brouwer E. et al. Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-α blocking therapy: a prospective longitudinal observational cohort study. PLoS One 2015;10:e0122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baraliakos X, Listing J, von der Recke A, Braun J.. The natural course of radiographic progression in ankylosing spondylitis–evidence for major individual variations in a large proportion of patients. J Rheumatol 2009;36:997–1002. [DOI] [PubMed] [Google Scholar]
- 30. Maas F, Arends S, Brouwer E. et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res 2017;69:1011–9. [DOI] [PubMed] [Google Scholar]
- 31. Wanders A, Heijde D, Landewé R. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005;52:1756–65. [DOI] [PubMed] [Google Scholar]
- 32. Sieper J, Listing J, Poddubnyy D. et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43. [DOI] [PubMed] [Google Scholar]
- 33. Poddubnyy D, Haibel H, Listing J. et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012;64:1388–98. [DOI] [PubMed] [Google Scholar]
- 34. van der Heijde D, Landewé R, Einstein S. et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31. [DOI] [PubMed] [Google Scholar]
- 35. Braun J, Baraliakos X, Hermann KG. et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis 2014;73:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Heijde D, Landewé R, Baraliakos X. et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. [DOI] [PubMed] [Google Scholar]
- 37. van der Heijde D, Salonen D, Weissman BN. et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baraliakos X, Listing J, Rudwaleit M. et al. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor alpha antibody infliximab. Ann Rheum Dis 2005;64:1462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baraliakos X, Listing J, Brandt J. et al. Radiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-alpha antibody infliximab. Rheumatology 2007;46:1450–3. [DOI] [PubMed] [Google Scholar]
- 40. Kroon F, Landewé R, Dougados M, van der Heijde D.. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:1623–9. [DOI] [PubMed] [Google Scholar]
- 41. Poddubnyy D, Rudwaleit M, Haibel H. et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 2012;71:1616–22. [DOI] [PubMed] [Google Scholar]
- 42. Sepriano A, Regel A, van der Heijde D. et al. Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 2017;3:e000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haroon N, Inman RD, Learch TJ. et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Heijde D, Baraliakos X, Hermann KA. et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Machado P. Anti-tumor necrosis factor and new bone formation in ankylosing spondylitis: the controversy continues. Arthritis Rheum 2013;65:2537–40. [DOI] [PubMed] [Google Scholar]
- 46. Gensler LS, Reveille JD, Lee M. et al. High dose nonsteroidal anti-inflammatory drugs (NSAIDs) and tumor necrosis factor inhibitor use results in less radiographic progression in ankylosing spondylitis – a longitudinal analysis. In: ACR/AHRP Annual Meeting, Washington DC, 2016. Abstract 1956.
- 47. Molnar C, Scherer A, Baraliakos X. et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018;77:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poddubnyy D, Protopopov M, Haibel H. et al. High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis 2016;75:2114–8. [DOI] [PubMed] [Google Scholar]
- 49. van Tubergen A, Ramiro S, van der Heijde D. et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 2012;71:518–23. [DOI] [PubMed] [Google Scholar]
- 50. Ramiro S, Landewé R, van Tubergen A. et al. Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with ankylosing spondylitis: a longitudinal analysis. RMD Open 2015;1:e000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Machado P, Landewé R, Braun J. et al. A stratified model for health outcomes in ankylosing spondylitis. Ann Rheum Dis 2011;70:1758–64. [DOI] [PubMed] [Google Scholar]
- 52. Viitanen JV, Kokko ML, Heikkilä S, Kautiainen H.. Neck mobility assessment in ankylosing spondylitis: a clinical study of nine measurements including new tape methods for cervical rotation and lateral flexion. Br J Rheumatol 1998;37:377–81. [DOI] [PubMed] [Google Scholar]
- 53. Viitanen JV, Kokko ML, Lehtinen K, Suni J, Kautiainen H.. Correlation between mobility restrictions and radiologic changes in ankylosing spondylitis. Spine (Phila Pa 1976) 1995;20:492–6. [DOI] [PubMed] [Google Scholar]
- 54. Kennedy LG, Jenkinson TR, Mallorie PA. et al. Ankylosing spondylitis: the correlation between a new metrology score and radiology. Br J Rheumatol 1995;34:767–70. [DOI] [PubMed] [Google Scholar]
- 55. Wanders A, Landewé R, Dougados M. et al. Association between radiographic damage of the spine and spinal mobility for individual patients with ankylosing spondylitis: can assessment of spinal mobility be a proxy for radiographic evaluation? Ann Rheum Dis 2005;64:988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Machado P, Landewé R, Braun J. et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465–70. [DOI] [PubMed] [Google Scholar]
- 57. Braun J, Golder W, Bollow M, Sieper J, van der Heijde D.. Imaging and scoring in ankylosing spondylitis. Clin Exp Rheumatol 2002;20 (6 Suppl 28):S178–84. [PubMed] [Google Scholar]
- 58. Bochkova AG, Levshakova AV, Bunchuk NV, Braun J.. Spinal inflammation lesions as detected by magnetic resonance imaging in patients with early ankylosing spondylitis are more often observed in posterior structures of the spine. Rheumatology 2010;49:749–55. [DOI] [PubMed] [Google Scholar]
- 59. Braun J, Baraliakos X, Golder W. et al. Analysing chronic spinal changes in ankylosing spondylitis: a systematic comparison of conventional X rays with magnetic resonance imaging using established and new scoring systems. Ann Rheum Dis 2004;63:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Diekhoff T, Hermann KA, Greese J. et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis 2017;76:1502–8. [DOI] [PubMed] [Google Scholar]
- 61. Bray TJP, Bainbridge A, Punwani S, Ioannou Y, Hall-Craggs MA.. Simultaneous quantification of bone edema/adiposity and structure in inflamed bone using chemical shift-encoded MRI in spondyloarthritis. Magn Reson Med 2018;79:1031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tan S, Yao J, Flynn JA, Yao L, Ward MM.. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis 2015;74:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Braun J, Baraliakos X, Golder W. et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003;48:1126–36. [DOI] [PubMed] [Google Scholar]
- 64. Haibel H, Rudwaleit M, Brandt HC. et al. Adalimumab reduces spinal symptoms in active ankylosing spondylitis: clinical and magnetic resonance imaging results of a fifty-two-week open-label trial. Arthritis Rheum 2006;54:678–81. [DOI] [PubMed] [Google Scholar]
- 65. Maksymowych WP, Inman RD, Salonen D. et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. [DOI] [PubMed] [Google Scholar]
- 66. Lambert RGW, Pedersen SJ, Maksymowych W, Chiowchanwisawakit P, Ostergaard M.. Active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis – definitions, assessment system, and reference image set. J Rheumatol 2009;36 (Suppl 84):3–17. [Google Scholar]
- 67. de Bruin F, de Koning A, van den Berg R. et al. Development of the CT Syndesmophyte Score (CTSS) in patients with ankylosing spondylitis: data from the SIAS cohort. Ann Rheum Dis 2018;77:371–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.