Abstract
Objective
To describe the outcomes of MTX and biologic DMARD (bDMARD) treatment in patients with RA and assess unmet needs in patients who fail treatment, using real-world data from the Norwegian DMARD (NOR-DMARD) registry.
Methods
Data included RA treatment courses from January 2007 until July 2016. Patients received MTX monotherapy (in MTX-naïve patients), bDMARD monotherapy, bDMARDs + MTX, or bDMARDs + other conventional synthetic DMARDs (csDMARDs). DAS28-4(ESR) was used to measure remission (<2.6) and inadequate response (>3.2) across all groups at Months 6 and 12. Estimated ACR20/50/70 and EULAR good and good/moderate response rates (based on DAS28-4[ESR] score) for bDMARDs were modelled at Months 6 and 12 using logistic mixed regression. DAS28-4(ESR) scores and changes from baseline, and rates and reasons for discontinuation, were evaluated for all groups over 24 months.
Results
The 2778 treatment courses in this analysis included 714 MTX monotherapy, 396 bDMARD monotherapy, 1460 bDMARDs + MTX and 208 bDMARDs + other csDMARDs. Of patients with DAS28-4(ESR) data at Months 6 and 12 (25.0–34.1%), 33.9–47.2% did not switch treatment and were inadequate-responders at Month 12. There were no significant differences in efficacy between bDMARD groups (bDMARD monotherapy, or bDMARDs + MTX or other csDMARDs). Lack of efficacy was the most common reason for stopping treatment across all groups (13.7–22.1% over 24 months).
Conclusion
An unmet treatment need exists for patients still experiencing inadequate response to MTX monotherapy and bDMARDs as monotherapy or in combination with MTX/other csDMARDs after 12 months.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01581294.
Keywords: rheumatoid arthritis, real-world data, registry, NOR-DMARD, biologic disease-modifying antirheumatic drugs, MTX
Rheumatology key messages
Many RA patients registered with NOR-DMARD have inadequate responses to MTX and bDMARDs +/- MTX/csDMARDs.
Despite inadequate response to current treatment, many RA patients do not switch to alternative treatments.
An unmet need exists for RA patients with inadequate response to current treatment.
Introduction
RA is a chronic autoimmune disease characterized by systemic inflammation, persistent synovitis and joint destruction. The goal of treatment in patients with RA is persistent clinical remission or, if remission cannot be achieved, low disease activity, particularly in patients with established disease [1, 2].
According to current recommendations, treatment should be initiated with conventional synthetic DMARDs (csDMARDs) [2] as early as possible after diagnosis [1]. MTX is the most commonly prescribed csDMARD, yet it has limited efficacy in some patients [3, 4]. In patients with an inadequate response (IR) to MTX or other csDMARDs, the addition of a second csDMARD or a biologic DMARD (bDMARD), including TNF inhibitors (TNFi), is recommended [2] within 3–6 months [1].
The administration of bDMARDs is recommended with csDMARDs or as monotherapy when csDMARDs are not tolerated [1]. Although the efficacy of bDMARDs with MTX is well-established, 19–37% of patients in European registries receive bDMARDs as monotherapy [5–9], indicating a discrepancy between practice recommendations and real-world clinical use. Furthermore, many patients who receive bDMARDs do not achieve remission or experience a loss of response over time [10, 11]. TNFi-IR patients may switch to a second bDMARD; however, debate exists on whether patients should use an alternative TNFi or a bDMARD with an alternative mechanism of action [11–14].
In this study, we analysed real-world data from the Norwegian DMARD (NOR-DMARD) registry to describe the outcomes of MTX and bDMARD therapy and assess the need for further treatment options for patients with RA.
Methods
Study design and data collection
Data were obtained from the NOR-DMARD registry (NCT01581294), which includes longitudinal data related to patients with inflammatory arthropathies from five clinical centres in Norway [15]. The registry was established in December 2000 and until 2012 included patients initiating treatment with both csDMARDs and bDMARDs. The revised NOR-DMARD protocol, implemented from 2012 onwards, includes bDMARDs only. The data have since been merged into a single database. The main objectives of the NOR-DMARD registry are to study the effectiveness of bDMARD treatments for inflammatory joint diseases in clinical practice by measuring disease activity and health-related quality of life, including physical function and, secondly, to study the long-term safety of such treatments.
This analysis included patients with RA of any severity in the NOR-DMARD registry from 1 January 2007 until 1 July 2016, with follow-up until 31 December 2016. RA was diagnosed using the 1987 ACR revised criteria [16] under the first NOR-DMARD protocol and both the 1987 ACR revised criteria and 2010 ACR/EULAR classification criteria [17] under the revised protocol. Information on patients starting csDMARDs (including MTX, leflunomide, sulfasalazine, hydroxychloroquine, ciclosporin, azathioprine and gold) was captured from 2007 to 2011, after which the protocol changed to include only patients starting bDMARDs. csDMARD treatment regimens included MTX monotherapy in MTX-naïve patients as well as other csDMARD therapy. The use of bDMARDs (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, abatacept, anakinra, rituximab, tocilizumab and ustekinumab [approved for psoriatic arthritis patients only]) was recorded from 2007 until December 2016. Each time a patient switched treatment (e.g. from bDMARD monotherapy to bDMARDs in combination with MTX or other csDMARDs), the current treatment course was considered as terminated in the analysis, registration was ended and a new treatment course was started. Patients may have had different treatment courses, and each treatment course had separate follow-up visits. For the purposes of this analysis, ‘patient’ denotes an individual with a unique treatment course. For each treatment course, the previous use of either csDMARDs or bDMARDs was registered.
The NOR-DMARD registry has been approved by the Norwegian Data Inspectorate and Regional Ethics Committee of Eastern Norway. Patients provided written informed consent prior to inclusion. This analysis did not require additional ethical approval.
Assessments
Patients receiving MTX
MTX treatment outcomes were assessed in MTX-naïve patients who initiated MTX monotherapy (MTX monotherapy group). Assessments included the proportion of MTX-IR patients (defined as patients with DAS in 28 joints, ESR [DAS28-4(ESR)]>3.2 despite MTX treatment) at Month 6, the proportions of MTX-IR patients remaining on MTX at Months 6 and 12 despite being inadequate-responders (DAS28-4[ESR]>3.2) and despite having high disease activity (HDA; DAS28-4[ESR]>5.1), estimated ACR20, ACR50 and ACR70 response rates (the proportions of patients achieving an improvement from baseline of ⩾20%, ⩾50% and ⩾70%, respectively, in the number of tender/swollen joints combined with ⩾3 of the five other ACR components) at Months 6 and 12, estimated EULAR-defined good and good/moderate response rates (good response rate: proportion of patients achieving improvements from baseline in DAS28-4[ESR]>1.2 with current DAS28-4[ESR]⩽3.2; moderate response rate: proportion of patients achieving improvements from baseline in DAS28-4[ESR] >0.6 to ⩽1.2 with current DAS28-4[ESR]⩽3.2 or >3.2 to ⩽5.1, or improvements from baseline >1.2 with current DAS28-4[ESR] >3.2 to ⩽5.1 or >5.1) [18] at Months 6 and 12, and DAS28-4(ESR) score and change from baseline in DAS28-4(ESR) score over 24 months. Reasons for stopping MTX monotherapy over 24 and 60 months were also assessed.
Patients receiving bDMARDs
bDMARD treatment outcomes were assessed in patients receiving bDMARDs as monotherapy (bDMARD monotherapy group), bDMARDs in combination with MTX (bDMARDs + MTX group) and bDMARDs in combination with csDMARDs other than MTX (bDMARDs + other csDMARDs group). Assessments included the estimated ACR20, ACR50 and ACR70 response rates and estimated EULAR-defined good and good/moderate response rates at Months 6 and 12. Rates of remission (DAS28-4[ESR]<2.6) were assessed at Months 6 and 12, together with rates of sustained remission (patients in remission at both Months 6 and 12). DAS28-4(ESR) scores, change from baseline in DAS28-4(ESR) score and time to stopping therapy were assessed over 24 months; reasons for stopping therapy were assessed over 24 and 60 months.
Tocilizumab may be more effective as monotherapy than other bDMARDs [19, 20]. A sub-analysis that excluded patients receiving tocilizumab was performed to test for differences in the estimated ACR and EULAR response rates at Months 6 and 12 and DAS28-4(ESR) scores over 24 months.
Statistical analyses
Analyses were based on patients with ⩾1 post-baseline efficacy assessment. Baseline data are presented using descriptive statistics, with mean and standard deviation presented for continuous non-skewed variables, median and inter-quartile (25th and 75th percentiles) ranges for skewed variables and proportions (%) for categorical data. Assessments of response rates were analysed using logistic mixed regression models with patient-specific random intercept, both unadjusted and adjusted for sex, age, baseline DAS28-4(ESR) score, time from diagnosis to baseline and previous use of bDMARDs. There was no imputation for missing data. Assessments of continuous disease activity measures (DAS28-4[ESR]) were analysed using linear mixed models with patient-specific intercept, both unadjusted and adjusted with the same covariates as for the logistic mixed regression models. Estimates from both models are presented using marginal means.
All descriptive data are presented as observed, while the mixed models used for both categorical and continuous variables give unbiased estimates under the assumption of data missing at random.
All analyses were conducted using Stata version 14.1 (StataCorp LLC).
Results
Patients
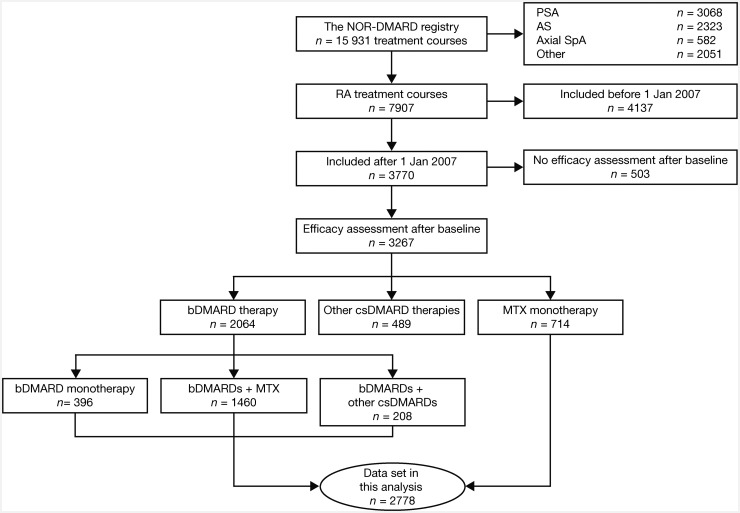
These analyses included 2778 treatment courses for RA, which were recorded after 1 January 2007 and included patients who received MTX monotherapy (n = 714) or bDMARD therapy (n = 2064) (Fig. 1). Patient demographics and baseline disease characteristics are presented in Table 1. The majority of patients across all treatment groups were female. The mean DAS28-4(ESR) score across all treatment groups was ⩾4.5, equating to a moderate level of baseline disease activity. Etanercept was the most common bDMARD in both the monotherapy and combination therapy groups (Supplementary Table S1, available at Rheumatology online).
Fig. 1.
Patient disposition
NOR-DMARD: Norwegian DMARD.
Table 1.
Patient demographics and disease characteristics of patients in the NOR-DMARD registry (2007–2016)
| Treatment coursea | |||||
|---|---|---|---|---|---|
| MTX monotherapy | bDMARD monotherapy | bDMARDs + MTX | bDMARDs + other csDMARDs | P valueb | |
| (n = 714) | (n = 396) | (n = 1460) | (n = 208) | ||
| Age, mean (s.d.), years | 55.9 (13.2) | 53.9 (13.1) | 53.4 (14.0) | 52.2 (13.5) | 0.37 |
| Sex, female, n (%) | 477 (66.8) | 318 (80.3) | 1079 (73.9) | 174 (83.7) | <0.001 |
| RA disease duration, median (IQR), years | 0.0 (0.0, 0.3) | 10.1 (3.5, 16.3) | 7.8 (2.6, 16.2) | 8.4 (4.6, 17.3) | 0.030 |
| RF positive, n (%) | 445 (62.3) | 204 (51.5) | 737 (50.5) | 113 (54.3) | 0.36 |
| Anti-CCP positive, n (%) | 452 (63.3) | 180 (45.5) | 685 (46.9) | 101 (48.6) | 0.37 |
| Erosive disease, n (%) | 225 (31.5) | 171 (43.2) | 638 (43.7) | 98 (47.1) | 0.25 |
| TJC28, median (IQR) | 5.0 (2.0, 10.0) | 6.0 (2.0, 13.0) | 5.0 (2.0, 10.0) | 5.0 (2.0, 9.0) | 0.003 |
| SJC28, median (IQR) | 4.0 (2.0, 8.0) | 4.0 (2.0, 8.0) | 4.0 (1.0, 8.0) | 4.0 (2.0, 8.0) | 0.55 |
| DAS28-4(ESR), mean (s.d.) | 4.6 (1.4) | 4.9 (1.5) | 4.5 (1.5) | 4.6 (1.4) | <0.001 |
| mHAQ, mean (s.d.) | 0.6 (0.5) | 0.8 (0.5) | 0.7 (0.5) | 0.7 (0.5) | <0.001 |
| PtGA, mean (s.d.) | 46.0 (24.5) | 57.2 (25.2) | 50.0 (25.7) | 47.4 (24.5) | <0.001 |
| CRP, mg/l, median (IQR) | 8.0 (3.0, 20.5) | 7.0 (3.0, 23.0) | 6.0 (3.0, 17.0) | 6.0 (3.0, 17.0) | 0.18 |
| ESR, mm/h, median (IQR) | 21.0 (11.0, 35.0) | 25.0 (13.0, 37.0) | 20.0 (10.0, 35.0) | 21.0 (11.0, 32.0) | 0.004 |
Patients who switched treatments are included in ≥1 treatment course.
Overall test of equality among bDMARD groups.
bDMARD: biologic DMARD; csDMARDs: conventional synthetic DMARDs; DAS28-4(ESR): DAS in 28 joints, ESR; IQR: interquartile range; mHAQ: modified Health Assessment Questionnaire; NOR-DMARD: Norwegian DMARD; PtGA: patient global assessment; SJC28: number of joints (out of 28) that are swollen; TJC28: number of joints (out of 28) that are tender.
MTX therapy
Overall, 714 treatment courses included MTX-naïve patients starting MTX monotherapy. At Month 6, improvements in efficacy outcomes including estimated ACR and EULAR response rates and DAS28-4(ESR) scores were seen with MTX monotherapy (Supplementary Fig. S1, available at Rheumatology online); however, 34 patients (4.8%) switched to another treatment and 68 patients (9.5%) dropped out of the registry prior to Month 6. Of the 424 patients with available DAS28-4(ESR) data at Month 6, 178 (42.0%) were MTX-IR (DAS28-4[ESR]>3.2). At Month 12, estimated ACR and EULAR response rates were either similar or slightly increased vs Month 6 (Supplementary Fig. S1A, available at Rheumatology online), and 110/355 (31.0%) patients with available DAS28-4(ESR) data were MTX-IR. Of 239 patients with DAS28-4(ESR) data at Month 6 and Month 12, 86 (36.0%) were MTX-IR at Month 6, and 51 (21.3%) remained MTX-IR at Month 12. Additionally, by Month 12, 30 patients with an earlier response to MTX were MTX-IR at Month 12 (a total of 81/239 [33.9%] inadequate-responders at Month 12). Furthermore, 4/239 (1.7%) had HDA (DAS28-4[ESR]>5.1) at both time points. Mean DAS28-4(ESR) and changes from baseline in DAS28-4(ESR) scores showed no further improvements beyond Month 12 (Supplementary Fig. S1B and S1C, available at Rheumatology online). The most frequent reason for stopping MTX monotherapy over 24 and 60 months was lack of efficacy (n = 98 [13.7%] and 117 [16.4%], respectively) (Table 2 and Supplementary Table S2, available at Rheumatology online).
Table 2.
Proportion of NOR-DMARD registry patients stopping therapy over 24 months
| Reason, n (%) | MTX monotherapy (n = 714) | bDMARD monotherapy (n = 396) | bDMARDs + MTX (n = 1460) | bDMARDs + other csDMARDs (n = 208) | All patients (n=2778) |
|---|---|---|---|---|---|
| Lack of efficacy | 98 (13.7) | 82 (20.7) | 284 (19.5) | 46 (22.1) | 510 (18.4) |
| Adverse events | 59 (8.3) | 63 (15.9) | 133 (9.1) | 10 (4.8) | 265 (9.5) |
| Lack of efficacy and adverse events | 8 (1.1) | 7 (1.8) | 25 (1.7) | 4 (1.9) | 44 (1.6) |
| Patient’s decision | 6 (0.8) | 8 (2.0) | 24 (1.6) | 4 (1.9) | 42 (1.5) |
| Lost to follow-up | 14 (2.0) | 2 (0.5) | 13 (0.9) | 0 (0.0) | 29 (1.0) |
| Remission | 7 (1.0) | 3 (0.8) | 7 (0.5) | 2 (1.0) | 19 (0.7) |
| Other (death, pregnancy, unknown, other reasons) | 79 (11.1) | 44 (11.1) | 122 (8.4) | 32 (15.4) | 277 (10.0) |
| Total rate | 271 (38.0) | 209 (52.8) | 608 (41.6) | 98 (47.1) | 1186 (42.7) |
bDMARD: biologic DMARD; csDMARDs: conventional synthetic DMARDs; NOR-DMARD: Norwegian DMARD.
bDMARD therapy
Of the 2064 treatment courses including bDMARDs, the majority (70.7%) involved MTX as co-medication, while 19.2% involved monotherapy and 10.1% involved other csDMARDs as co-medication (Table 1). Across each treatment group, the most common reasons for stopping bDMARD therapy were lack of efficacy, followed by adverse events (Table 2 and Supplementary Table S2, available at Rheumatology online). Of 183 patients receiving bDMARD monotherapy who had previously received MTX and who had provided a reason for discontinuation, 136 (74.3%), 35 (19.1%) and 12 (6.6%) discontinued MTX due to adverse events, lack of efficacy and unknown/other reasons, respectively.
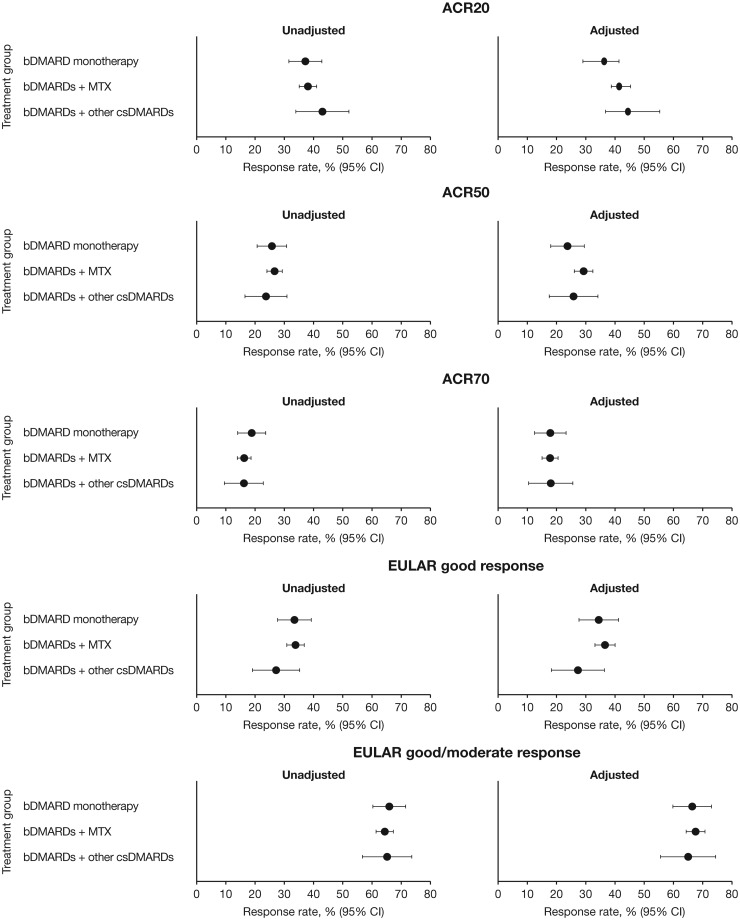
There were no significant differences in estimated ACR20, ACR50, ACR70 and EULAR good and good/moderate responses at Month 6 (P > 0.05; Fig. 2) when bDMARDs were administered as monotherapy or in combination with MTX or csDMARDs, even after adjusting for age, sex, baseline disease activity, disease duration and previous use of bDMARDs. Numerically higher estimated ACR20, ACR50 and EULAR good response rates were observed in the bDMARDs + MTX vs the bDMARD monotherapy group in both the unadjusted and adjusted analyses. Results at Month 12 generally followed the same trend as Month 6 with no significant differences between treatment groups (Supplementary Fig. S2, available at Rheumatology online). In unadjusted and adjusted analyses, all estimated response rates increased from Month 6 to Month 12 in patients receiving bDMARDs + MTX, bDMARD monotherapy (except ACR70 and EULAR good/moderate response [adjusted analysis only]) and bDMARD + other csDMARDs (except ACR20 and EULAR good/moderate response [unadjusted analysis only]) (Fig. 2, Supplementary Fig. S2, available at Rheumatology online).
Fig. 2.
Estimated rates of ACR20/50/70 and EULAR good and good/moderate responses at 6 months
bDMARD monotherapy: n = 396; bDMARDs + MTX: n = 1460; bDMARDs + other csDMARDs: n = 208. Adjusted means have been adjusted for sex, age, baseline DAS28-4(ESR) score, time from diagnosis to baseline and use of previous bDMARD treatment. ACR20/50/70: proportion of patients achieving ≥20%, ≥50% or ≥70% improvement, respectively, in the number of tender and swollen joints and ≥3 of 5 ACR components. EULAR good response: proportion of patients achieving improvements from baseline in DAS28-4(ESR)>1.2 in patients with DAS28-4(ESR)≤3.2 at current visit. EULAR moderate response: proportion of patients achieving improvements from baseline in DAS28-4(ESR) >0.6 to ≤1.2 in patients with DAS28-4[ESR]≤3.2 or >3.2 to ≤5.1 at current visit, or improvements from baseline >1.2 in patients with DAS28-4(ESR) >3.2 to ≤5.1 or DAS28-4(ESR)<5.1 at current visit. bDMARD: biologic DMARD; csDMARDs: conventional synthetic DMARDs.
Sub-analyses that excluded tocilizumab yielded similar results, although differences were observed in the estimated rates for good EULAR responses at Month 6 in the adjusted analysis (P < 0.05; Supplementary Figs. S3 and S4, available at Rheumatology online). The estimated rates were generally slightly lower and similar trends in improvements to Month 12 were observed vs the analyses including tocilizumab.
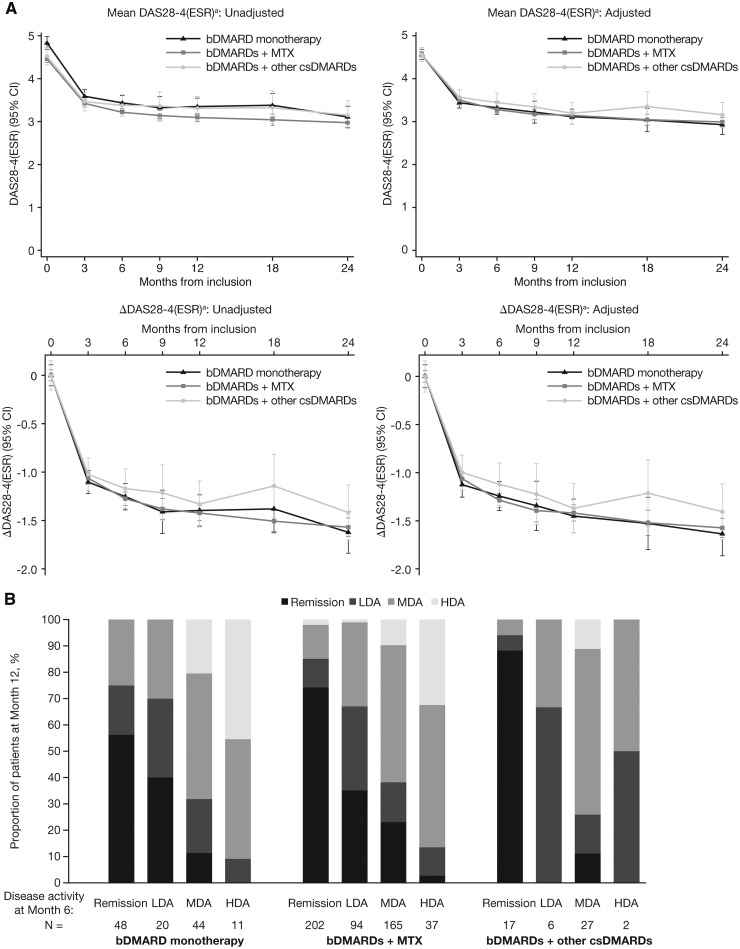
Mean DAS28-4(ESR) scores and changes in DAS28-4(ESR) scores over 24 months were generally similar across bDMARD groups, with no significant differences between groups by Month 6 (P > 0.05) (Fig. 3A). When tocilizumab was excluded, a significant difference (P < 0.05) between groups at Month 6 was observed in the unadjusted analysis; bDMARDs + MTX showed the greatest improvement (Supplementary Fig. S5, available at Rheumatology online).
Fig. 3.
Disease activity as measured by DAS28-4(ESR) in patients with RA receiving bDMARDs
(A) Estimated marginal mean DAS28-4(ESR) score and mean changes from baseline in DAS28-4(ESR) score in patients receiving bDMARDs over 24 months, and (B) proportion of bDMARD-treated patients achieving DAS28-4(ESR)-defined remission, LDA, MDA and HDA at Month 12 according to disease activity at Month 6. abDMARD monotherapy: n = 396; bDMARDs + MTX: n = 1460; bDMARDs + other csDMARDs: n = 208. Δ: change from baseline; bDMARD: biologic DMARD; csDMARDs: conventional synthetic DMARDs; DAS28-4(ESR): DAS in 28 joints, ESR; HDA: high disease activity; LDA: low disease activity; MDA: moderate disease activity.
Remission (DAS28-4[ESR]<2.6) at Month 6 was achieved by 48/123 (39.0%) bDMARD monotherapy patients, 150/498 (30.1%) bDMARDs + MTX patients and 17/52 (32.7%) bDMARDs + other csDMARDs patients. In the same treatment groups (patients with data at both time points), remission was achieved by 40/123 (32.5%), 222/498 (44.6%) and 18/52 (34.6%) patients at Month 12.
Remission was sustained from Month 6 to Month 12 by a greater proportion of patients receiving bDMARDs + other csDMARDs (15/17; 88.2%) than bDMARD monotherapy (27/48; 56.3%) or bDMARDs + MTX (150/202; 74.3%) (Fig. 3B). However, in patients with DAS28-4(ESR) data at Month 6 and Month 12, 58/123 (47.2%) bDMARD monotherapy patients, 24/52 (46.2%) bDMARDs + other csDMARDs patients and 195/498 (39.2%) bDMARDs + MTX patients remained on the same treatment and were still inadequate-responders at Month 12.
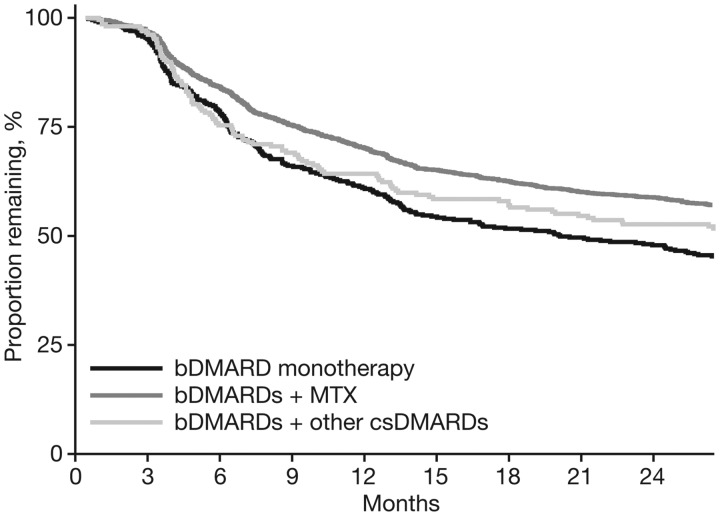
Log-rank tests showed significant differences between the time to stopping therapy among the different bDMARD groups (P < 0.001; Fig. 4 and Supplementary Fig. S6, available at Rheumatology online). Retention was highest over 24 and 60 months with bDMARDs + MTX and lowest with bDMARD monotherapy.
Fig. 4.
Time to stopping therapy of bDMARD-treated patients over 24 months
P < 0.001 (time to stopping therapy is different among treatment groups). bDMARD: biologic DMARD; csDMARDs: conventional synthetic DMARDs.
Discussion
This analysis of real-world data from the NOR-DMARD registry aimed to describe the outcomes associated with MTX and bDMARD therapy and assess the need for further treatment options for patients with RA.
A notable proportion (31.0%) of MTX-naïve patients who received MTX monotherapy had a DAS28-4(ESR)-defined IR at Month 12 and even more patients stopped therapy over 24 and 60 months (38.0% and 62.9%, respectively), primarily from lack of efficacy. Efficacy endpoints including estimated ACR20, ACR50 and ACR70 response rates and EULAR good and good/moderate response rates increased from Month 6 to Month 12. DAS28-4(ESR) scores and changes from baseline also improved to Month 12 but were stable from Month 12 to Month 24.
The bDMARD groups were generally similar in terms of efficacy outcomes, although there appeared to be a trend in the adjusted data toward numerically better scores in the bDMARDs + MTX vs the bDMARD monotherapy group. At Month 12, 32.5–44.6% of patients from the bDMARD groups achieved remission, whereas 39.2–47.2% had remained on their current treatment and were inadequate-responders. Among patients receiving bDMARDs, rates of stopping therapy over 24 and 60 months were greatest with bDMARD monotherapy and lowest with bDMARDs + MTX.
MTX is the most prescribed first-line DMARD for RA and its widespread use has prompted the development of clinical practice recommendations by the 3E Initiative [21]. However, in line with these data from the NOR-DMARD registry, other studies have shown that not all patients respond to MTX; for example, in a study of tofacitinib vs MTX in MTX-naïve patients, <20% of MTX-treated patients achieved low disease activity at Month 24 [3]. Although the reasons for not changing therapy despite IR were not recorded in the NOR-DMARD registry, a previous analysis in Australian RA patients with moderate-to-high disease activity despite active treatment (75% were receiving MTX) revealed that the most common reasons for not switching treatment included irreversible joint damage (19.7%), patient preference (14.7%) and rheumatologist-driven under-treatment (9.9%) [22]. Patients were reluctant to switch if they were comfortable with the level of disease activity or had concerns about potential toxicity of new treatments [22]; patients also tended to prefer oral to injectable medications [23, 24]. Furthermore, patients and rheumatologists may have different perceptions of disease activity, leading to potential disagreements during shared decision-making [25], and thresholds for switching RA treatments may alter over time [26]. Treat-to-target recommendations published in 2010 recommend that the treatment of RA be based on shared decision-making between the patient and physician [27], and a previous study reported that NOR-DMARD-registered RA patients with moderate disease activity perceived their state as acceptable [28]; therefore, patients may have remained on the same treatment by choice despite an IR. The publication of these recommendations may have affected the treatment practices for RA during the period covered by this analysis.
Over 24 months, the rate of stopping MTX monotherapy in the NOR-DMARD registry (38.0%) was generally similar to that previously reported in an Austrian database (44.3%) [29]; in both, this was most often due to lack of efficacy or adverse events. The rate of stopping MTX monotherapy in the NOR-DMARD registry was lower than stopping bDMARD therapies over 24 months (38.0% vs 41.6–52.8%) and similar over 60 months (62.9% vs 53.2–63.1%).
Following an IR to csDMARDs (e.g. MTX), EULAR recommends the addition of a bDMARD to the treatment regimen [1]. Despite this and the demonstrated efficacy of bDMARDs [30], in clinical practice eligible patients are not always advanced to bDMARDs [31]. This may, in part, be due to the subcutaneous or intravenous routes of administration for bDMARDs, which are disliked by some patients [31], and may also be a result of the higher cost of bDMARD treatment with similar perceived efficacy and safety, on a group level, as MTX [1, 32, 33]. Etanercept was the most prescribed bDMARD in the NOR-DMARD registry, as with other European DMARD registries including DANBIO, the Danish biologics registry [8], the British Society for Rheumatology Biologics Register (together with adalimumab) [5], and RABBIT, the German biologics register [7]. Certolizumab pegol use was higher in the NOR-DMARD registry vs other European countries (13.8% vs 1.1% and 3.4% in the Swiss and Danish registries, respectively [8, 9]).
Prior evidence suggests that TNFi may have increased efficacy when administered with MTX [34], and it is recommended that bDMARDs be administered with csDMARDs [1]; however, 19.2% of patients in the NOR-DMARD registry received bDMARD monotherapy. bDMARD monotherapy is also common in other European countries such as Denmark, Switzerland, the UK, Germany and France [5–9]. Patients with RA in Switzerland who were prescribed bDMARD monotherapy tended to be slightly older and have longer disease durations and higher disease activity than patients prescribed combination therapy [9]; the same patterns were observed in the NOR-DMARD registry. Patients may have had intolerances, contraindications or comorbidities that precluded the use of csDMARDs in combination with bDMARDs; additionally, if monotherapy is effective, the prescribing of combination therapy is not necessary [19, 35].
Indeed, the NOR-DMARD registry data did not show statistical differences between bDMARD monotherapy, bDMARDs in combination with MTX, and bDMARDs with other csDMARDs, in terms of estimated ACR20, ACR50 and ACR70 response rates and EULAR good and good/moderate response rates at Months 6 and 12. However, rates of estimated ACR20, ACR50 and EULAR good response did show a numerical trend towards greater improvement in bDMARDs + MTX patients vs bDMARD monotherapy patients, consistent with prior research that demonstrated the superiority of bDMARD combination therapy over monotherapy [9, 36, 37]. DAS28-4(ESR) scores and improvements from baseline were similar across bDMARD treatment groups. In the NOR-DMARD registry, patients who received bDMARD monotherapy had higher disease activity and worse patient-reported outcomes at baseline than those receiving bDMARD combination therapy, which may have enhanced the observed treatment effect in the bDMARD monotherapy group. The results may also have been confounded by pooling of data from different bDMARDs, with different mechanisms of action and that might have differing efficacy when used as monotherapy or with csDMARDs. Further, this analysis included patients from all lines of therapy, which may have affected the results, as it has recently been shown that line of therapy affects the differences in clinical effectiveness between bDMARD monotherapy and bDMARDs + MTX, with differences largest in second-line (bDMARD-naïve) and smallest in fourth-line therapy patients (IR to ⩾2 bDMARDs) [37].
At Month 12, a substantial proportion of bDMARD-treated patients in the NOR-DMARD registry were inadequate-responders. Patients receiving bDMARD monotherapy had the highest remission rate at Month 6 and the lowest remission rate at Month 12 vs those receiving bDMARDs + MTX or other csDMARDs. Furthermore, a higher percentage of patients receiving combination therapy sustained remission vs monotherapy. A greater proportion of patients receiving bDMARD monotherapy stopped treatment vs those receiving combination therapy, primarily due to lack of efficacy.
A sub-analysis was performed to exclude patients receiving tocilizumab, which is known to be more effective as monotherapy than other bDMARDs [19, 20]. Efficacy remained similar among the bDMARD groups, although EULAR good response rates (adjusted) at Month 6 and mean DAS28-4(ESR) scores over 24 months were significantly different. However, the proportion of patients receiving tocilizumab in the NOR-DMARD registry was low (4.8%). Furthermore, the results may be affected by patient compliance, which was not accounted for in this analysis, as some patients may have used bDMARDs as monotherapy despite these being prescribed as combination therapy.
Limitations of this analysis include a lack of a control group and potential bias (due to lack of randomization and recording of information at the discretion of the investigator), which are inherent in observational analyses. The off-label use of several of the drugs (e.g. infliximab and golimumab as monotherapy) may have also affected the results; patients are more likely to achieve better EULAR responses when receiving infliximab + MTX vs monotherapy [38], and the use of MTX with golimumab improves persistence vs monotherapy [39]. Additionally, there was unmeasured confounding in the analysis. The analysis was also limited by a lack of information regarding the switching of patients between treatments, the reasons why patients with insufficient responses did not switch treatment or why patients received bDMARD monotherapy, and ‘other’ reasons for patients stopping therapy. Comparisons between time points must be made with caution as the number of patients with measurements varied at different time points. Furthermore, handling of missing data was limited by the assumption of data missing at random, which cannot be verified.
In conclusion, this analysis of real-world data demonstrates that although there are many differing treatment strategies available to patients with moderate to severe RA, there exists a substantial unmet treatment need for patients who still experience IR to MTX and bDMARDs, without clinical remission, after up to 12 months of treatment.
Funding: This work was supported by Pfizer Inc. Data collection in NOR-DMARD was partly funded through unrestricted grants from AbbVie, BMS, MSD, Pfizer (Wyeth), Roche and UCB. Medical writing support was provided by Christina Viegelmann, PhD, at CMC Connect, a division of Complete Medical Communications Ltd, Glasgow, UK, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461–464).
Disclosure statement: I.C.O. has received fees for speaking from Pfizer Inc. T.K.K. has received fees for speaking and/or consulting from AbbVie, BMS, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Hospira, Merck-Serono, MSD, Novartis, Orion Pharma, Pfizer Inc, Roche, Sandoz and UCB and has received research funding to Diakonhjemmet Hospital from AbbVie, BMS, MSD, Pfizer Inc, Roche and UCB. E.L. has received fees for speaking and/or consulting from AbbVie, BMS, Celgene, Hospira, Pfizer Inc and UCB. R.V., G.W. and S.S. are employees and shareholders of Pfizer Inc.
Supplementary Material
References
- 1. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 3. Fleischmann RM, Huizinga TW, Kavanaugh AF. et al. Efficacy of tofacitinib monotherapy in methotrexate-naive patients with early or established rheumatoid arthritis. RMD Open 2016;2:e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wells AF, Westhovens R, Reed DM. et al. Abatacept plus methotrexate provides incremental clinical benefits versus methotrexate alone in methotrexate-naive patients with early rheumatoid arthritis who achieve radiographic nonprogression. J Rheumatol 2011;38:2362–8. [DOI] [PubMed] [Google Scholar]
- 5. Soliman MM, Ashcroft DM, Watson KD. et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011;70:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mariette X, Gottenberg JE, Ravaud P, Combe B.. Registries in rheumatoid arthritis and autoimmune diseases: data from the French registries. Rheumatology 2011;50:222–9. [DOI] [PubMed] [Google Scholar]
- 7. Listing J, Strangfeld A, Rau R. et al. Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low–results from RABBIT, the German biologics register. Arthritis Res Ther 2006;8:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jørgensen TS, Kristensen LE, Christensen R. et al. Effectiveness and drug adherence of biologic monotherapy in routine care of patients with rheumatoid arthritis: a cohort study of patients registered in the Danish biologics registry. Rheumatology 2015;54:2156–65. [DOI] [PubMed] [Google Scholar]
- 9. Gabay C, Riek M, Scherer A, Finckh A.. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology 2015;54:1664–72. [DOI] [PubMed] [Google Scholar]
- 10. Marchesoni A, Zaccara E, Gorla R. et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci 2009;1173:837–46. [DOI] [PubMed] [Google Scholar]
- 11. Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ.. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56:13–20. [DOI] [PubMed] [Google Scholar]
- 12. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL.. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology 2014;53:1664–8. [DOI] [PubMed] [Google Scholar]
- 13. Kim HL, Lee MY, Park SY. et al. Comparative effectiveness of cycling of tumor necrosis factor-α (TNF-α) inhibitors versus switching to non-TNF biologics in rheumatoid arthritis patients with inadequate response to TNF-α inhibitor using a Bayesian approach. Arch Pharm Res 2014;37:662–70. [DOI] [PubMed] [Google Scholar]
- 14. Chastek B, Becker LK, Chen CI, Mahajan P, Curtis JR.. Outcomes of tumor necrosis factor inhibitor cycling versus switching to a disease-modifying anti-rheumatic drug with a new mechanism of action among patients with rheumatoid arthritis. J Med Econ 2017;20:464–73. [DOI] [PubMed] [Google Scholar]
- 15. Kvien TK, Heiberg LE. et al. A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol 2005;23:S188–94. [PubMed] [Google Scholar]
- 16. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 18. Fransen J, van Riel PL.. Outcome measures in inflammatory rheumatic diseases. Arthritis Res Ther 2009;11:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaufmann J, Feist E, Roske AE, Schmidt WA.. Monotherapy with tocilizumab or TNF-alpha inhibitors in patients with rheumatoid arthritis: efficacy, treatment satisfaction, and persistence in routine clinical practice. Clin Rheumatol 2013;32:1347–55. [DOI] [PubMed] [Google Scholar]
- 20. Jansen JP, Buckley F, Dejonckheere F, Ogale S.. Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs—a systematic review and network meta-analysis. Health Qual Life Outcomes 2014;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Visser K, Katchamart W, Loza E. et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 2009;68:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tymms K, Zochling J, Scott J. et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res (Hoboken) 2014;66:190–6. [DOI] [PubMed] [Google Scholar]
- 23. Alten R, Krüger K, Rellecke J. et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence 2016;10:2217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolfe F, Michaud K.. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients' treatment choices. Arthritis Rheum 2007;56:2135–42. [DOI] [PubMed] [Google Scholar]
- 25. Studenic P, Radner H, Smolen JS, Aletaha D.. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum 2012;64:2814–23. [DOI] [PubMed] [Google Scholar]
- 26. Zhang J, Shan Y, Reed G. et al. Thresholds in disease activity for switching biologics in rheumatoid arthritis patients: experience from a large U.S. cohort. Arthritis Care Res (Hoboken) 2011;63:1672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smolen JS, Aletaha D, Bijlsma JW. et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heiberg T, Kvien TK, Mowinckel P. et al. Identification of disease activity and health status cut-off points for the symptom state acceptable to patients with rheumatoid arthritis. Ann Rheum Dis 2008;67:967–71. [DOI] [PubMed] [Google Scholar]
- 29. Aletaha D, Stamm T, Kapral T. et al. Survival and effectiveness of leflunomide compared with methotrexate and sulfasalazine in rheumatoid arthritis: a matched observational study. Ann Rheum Dis 2003;62:944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rein P, Mueller RB.. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther 2017;4:247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor PC, Alten R, Gomez-Reino JJ. et al. Comparison of biologic disease-modifying anti-rheumatic drug rheumatoid arthritis treatment dynamics across five European Union countries. Rheumatology 2016;55(Suppl 1):i84, abstract 057. [Google Scholar]
- 32. Smolen JS, Burmester GR, Combe B. et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet 2016;388:2763–74. [DOI] [PubMed] [Google Scholar]
- 33. Schiff M, Weinblatt ME, Valente R. et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 2014;73:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manders SH, Kievit W, Jansen TL. et al. Effectiveness of tumor necrosis factor inhibitors in combination with various csDMARD in the treatment of rheumatoid arthritis: data from the DREAM registry. J Rheumatol 2016;43:1787–94. [DOI] [PubMed] [Google Scholar]
- 35. Rintelen B, Zwerina J, Herold M. et al. Validity of data collected in BIOREG, the Austrian register for biological treatment in rheumatology: current practice of bDMARD therapy in rheumatoid arthritis in Austria. BMC Musculoskelet Disord 2016;17:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inui K, Koike T.. Combination therapy with biologic agents in rheumatic diseases: current and future prospects. Ther Adv Musculoskelet Dis 2016;8:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reed GW, Gerber RA, Shan Y. et al. TNFI and tofacitinib monotherapy and comparative effectiveness in clinical practice: results from CORRONA Registry. Ann Rheum Dis 2017;76(Suppl 2):60, abstract THU0132. [Google Scholar]
- 38. Hyrich KL, Symmons DP, Watson KD, Silman AJ, British Society for Rheumatology Biologics Register. Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another disease-modifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:1786–94. [DOI] [PubMed] [Google Scholar]
- 39. Kanbori M, Suzuka H, Yajima T. et al. Postmarketing surveillance evaluating the safety and effectiveness of golimumab in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2018;28:66–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.